Abstract
Norbornene can be polymerized by a variety of mechanisms, including insertion polymerization whereby the double bond is polymerized and the bicyclic nature of the monomer is conserved. The resulting polymer, polynorbornene, has a very high glass transition temperature, Tg, and interesting optical and electrical properties. However, the polymerization of functional norbornenes by this mechanism is complicated by the fact that the endo substituted norbornene monomer has, in general, a very low reactivity. Furthermore, the separation of the endo substituted monomer from the exo monomer is a tedious task. Here, we present a simple protocol for the polymerization of substituted norbornenes (endo:exo ca. 80:20) bearing either a carboxylic acid or a pendant double bond. The process does not require that both isomers be separated, and proceeds with low catalyst loadings (0.01 to 0.02 mol%). The polymer bearing pendant double bonds can be further transformed in high yield, to afford a polymer bearing pendant epoxy groups. These simple procedures can be applied to prepare polynorbornenes with a variety of functional groups, such as esters, alcohols, imides, double bonds, carboxylic acids, bromo-alkyls, aldehydes and anhydrides.
Keywords: Chemistry, Issue 120, Polymer, Catalysis, Polynorbornene, Diels-Alder reaction, water-soluble polymer, thermoset, glass transition temperature
Introduction
Norbornene, NBE, the Diels-Alder adduct of ethylene and cyclopentadiene (obtained by "cracking" of dicyclopentadiene (DCPD)), is readily polymerized using either free-radical polymerization,1 cationic polymerization,2 ring-opening metathesis polymerization3 and catalytic insertion polymerization.4,5,6,7 Unlike the other mechanisms, the catalytic insertion polymerization leads to the formation of a very high glass-transition temperature (Tg) polymer whereby the bicyclic backbone of NBE is conserved. A variety of catalysts such as metallocene catalysts and late transition metal catalysts can be used to promote the polymerization of NBE.4,5,6,7 However, due to its low solubility and due to difficulties associated with the processing of a very high Tg polymer, the PNBE homopolymer has, to our knowledge, never found any use.
Functional polynorbornenes (PNBEs) have been the object of considerable scrutiny for the last 20 years, because they combine the high Tg imparted by the bicyclic rigid repeat unit as well as desirable properties endowed by their functionalities.8,9,10 NBE monomers are obtained from rather simple and inexpensive feedstocks, using a one-step Diels-Alder reaction between cyclopentadiene and a functionalized dienophile. However, the Diels-Alder reaction leads to two stereoisomers, endo and exo, which have very different reactivities.11,12 In fact, the endo stereoisomer is less reactive than exo form and deactivates the catalyst.11,12 Thus, in the past, the preparation of functional polynorbornenes usually required the separation of the endo and exo stereoisomers, and only the exo stereoisomer was used. Such a separation procedure was time-consuming, and led to the accumulation of unreacted endo stereoisomers as undesirable waste.
Recently we have shown that the polymerization of functionalized NBEs containing both stereoisomers is in fact feasible.13 We have thus been able to prepare a variety of substituted PNBEs, containing functional groups such as esters, anhydrides, aldehydes, imides, alcohols and double bonds. Due to their high Tg and functionality, these polymers show desirable properties. We describe here two methods to prepare functional polymers. The first one leads to the synthesis of the water soluble polymer poly(5-norbornene-2-carboxylic acid), PNBE(CO2H), using a cationic Pd catalyst (Figure 1).13,14 The same polymerization method can be used to prepare functional PNBEs with various pendant functionalities, such as esters, alcohols, imides, bromo-alkyls, aldehydes and anhydrides. In our hands, this cationic Pd catalyst cannot be used for NBEs containing pendant double bonds such as 5-vinyl-2-norbornene. In this case, a partial insertion of the pendant double bond during the polymerization leads to the formation of a cross-linked material. Therefore, we present here a second method dedicated to the formation of poly(5-vinyl-2-norbornene), PNBE(vinyl), using Pd2(dba)3:AgSbF6:PPh3 as an in situ catalyst.14 The pendant vinyl groups of the polymer are then further epoxidized, to lead to the formation of PNBE(epoxy) (Figure 1). Both PNBE(CO2H) and PNBE(epoxy) have been found to lead to the formation of thermoset resins with a Tg as high as 350 °C.14 Thus, the simple method described here allows one to efficiently prepare polymers with a very high Tg and having a variety of functional groups, which can be used for numerous applications.
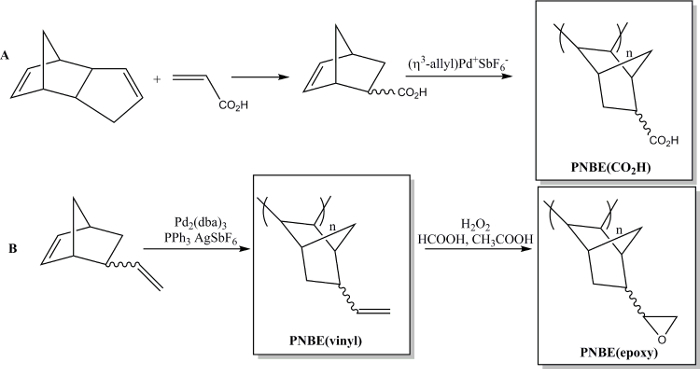 Figure 1: Functional PNBEs prepared by Pd catalyzed polymerization. (A) preparation of PNBE(CO2H), (B) preparation of PNBE(vinyl) and PNBE(epoxy). The dashed bond indicates a mixture of endo and exo isomers. Please click here to view a larger version of this figure.
Figure 1: Functional PNBEs prepared by Pd catalyzed polymerization. (A) preparation of PNBE(CO2H), (B) preparation of PNBE(vinyl) and PNBE(epoxy). The dashed bond indicates a mixture of endo and exo isomers. Please click here to view a larger version of this figure.
Protocol
1. Preparation of Poly(5-norbornene-2-carboxylic acid), PNBE(CO2H)
- Preparation of the monomer NBE(CO2H)
- Weigh out acrylic acid (AA) (327 g, 4.5 mol, 2 eq.) and hydroquinone (4.9 g, 4.5 x 10-2 mol, 0.02 eq.) and add them to a 2 L round-bottom flask equipped with a condenser and a magnetic stir bar. Heat the flask at 150 °C using a silicone oil bath.
- Once reflux is settled, add DCPD (300 g, 2.3 mol, 1 eq.) in a single portion, and then increase the temperature to 170 °C.
- Leave the reaction at this temperature for 16 h. Observe the color change from clear to yellow-brown.
- Purification of NBE(CO2H)
- Replace the condenser with a simple distillation setup (one plateau) connected to a condenser in which cold water is circulated.
- Put the reaction setup under a vacuum set to approx. 1 mmHg. Heat the mixture at 100 °C, and collect a clear liquid (ca. 40 mL) that can be discarded.
- Replace the collection flask with a 500 mL round bottom flask. Heat the oil bath to 155 °C, and observe the dropwise distillation of NBE(CO2H) (317 g, 2.3 mol. Yield = 98%). The distillation takes over 7 h.
- Analyze the colorless liquid by 1H NMR to assess purity as well as endo:exo proportions (Figure 2, bottom).15 The endo:exo ratio changes with the time used for distillation as well as with the heating time used for the preparation of the crude NBE(CO2H). Typically, endo:exo ratios between 50:50 and 80:20 are obtained (60:40 in this case).
- Polymerization of NBE(CO2H)
- Place 300 g (2.3 mol, 5,000 eq.) of NBE(CO2H) in a 500 mL round bottom flask equipped with a magnetic stir bar. Degas the liquid by bubbling nitrogen for 30 min.
- Weigh allylpalladium(II) chloride dimer, [PdCl(C3H5)]2 (76 mg, 4.2 x 10-1 mmol, 1 eq. of Pd) and add it to the solution. Add silver antimonate AgSbF6 (180 mg, 5.2 x 10-1 mmol, 1.2 eq.).
- Under stirring, dissolve the Pd salt by heating at 70 °C, and maintain the temperature at 70 °C under a slight nitrogen flux. After 7 to 8 h, the stirring stops due to a viscosity increase.
- Stop the reaction after 36 h.
- Cool the round bottom flask with liquid nitrogen. With a spatula, break the polymer into small pieces.
- In a 2 L beaker equipped with a magnetic stir bar, add 750 mL of ethyl acetate. Add the polymer chunks to the ethyl acetate under vigorous stirring. Continue stirring for 2 h.
- Filter the solution over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter).
- Wash the polymer with ethyl acetate three times (500 mL each washing). Dry the polymer (123 g, 9.4 x 10-1 mol, yield = 41%) in a vacuum oven set at 50 °C for 12 h.
2. Preparation of PNBE(vinyl)
- Polymerization of NBE(vinyl)
- Degas toluene (ca. 200 mL) and NBE(vinyl) (ca. 200 mL) by bubbling with N2 for 30 min and place them in a glove box.
- Within the glove box, load toluene (100 g) in a 250 mL round bottom flask.
- Add Pd2(dba)3 (76 mg, 1.6 x 10-1 mmol, 1 eq. of Pd), AgSbF6 (68 mg, 2.0 x 10-1 mmol, 1.2 eq.) and triphenylphosphine, PPh3 (43 mg, 1.6 x 10-1 mmol, 1 eq.) successively to the toluene solution.
- Heat the mixture to 70 °C until complete dissolution occurs. It occurs within 10 min.
- Add 100 g (8.0 x 10-1 mol, 5,000 eq.) of NBE(vinyl) to this purple solution.
- Stir at 70 °C for 72 h.
- Remove the solution from the glovebox, and transfer the viscous black solution to a 1 L glass bottle containing a magnetic stir bar.
- Add toluene (200 mL) and stir.
- Add silica powder (silica gel 40-63 µm, 10 g). Stir at room temperature for 16 h.
- Stop stirring and let the powder settle for at least 2 h in order for the silica particles to sedimentate.
- Filter the solution over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter). Avoid pouring sedimented silica particles in the Buchner funnel.
- Rinse the silica particles with toluene (50 mL) and filter it through the Buchner funnel.
- Add methanol (1.2 L) to a 4 L beaker equipped with a magnetic stir bar.
- Add all of the toluene solution containing the polymer to the methanol gradually under vigorous stirring, and continue stirring for 30 min.
- Filter the polymer over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter). Wash the polymer with three aliquots of methanol (400 mL each). Change the filter paper between each washing.
- Dry the polymer (75 g, 6.3 x 10-1 mol, yield = 78%) under vacuum at room temperature overnight.
3. Preparation of PNBE(epoxy)
- Epoxidation of PNBE(vinyl)
- Add 150 g of dichloromethane to a 500 mL round bottom flask equipped with a magnetic stirrer and a condenser.
- Add PNBE(vinyl) (15 g, 1.3 x 10-1 mol, 1 eq.) with stirring until complete dissolution.
- Place the flask in an ice-bath and let it cool for 15 min.
- In a separate container, mix together formic acid (30 g, 6.5 x 10-1 mol, 5 eq.) and acetic acid (5 g, 8.3 x 10-2 mol, 0.6 eq.). Add the combined acids to the polymer solution.
- Let it cool for 15 min.
- Add hydrogen peroxide aqueous solution (30%) (75 g, 6.5 x 10-1 mol, 5 eq.) to the polymer solution.
- Stir for 18 h. The ice bath does not need to be removed, as the temperature will gradually increase to ambient temperature.
- Take a small sample, precipitate the polymer with acetone, and analyze it by 1H NMR in CDCl3.15 If the signal for the double bond (δ = 4.5-6.0 ppm) is sufficiently decreased (Figure 3), pass to the next step. Typically, the ratio of the integral of the double bonds to the other protons is less than 1:20 (1:83 in Figure 3). Otherwise, continue the reaction.
- Add acetone (750 mL) to a 4 L beaker equipped with a magnetic stir bar.
- Add the polymer solution to the acetone gradually under vigorous stirring for 15 min.
- Filter the polymer over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter).
- Wash the polymer four times with acetone (200 mL each time).
- Change the filter paper between each washing.
- Dry the polymer (7.5 g) under vacuum at room temperature overnight.
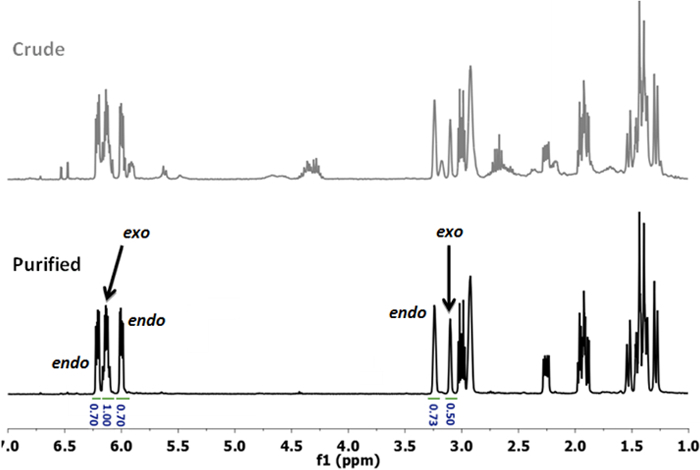 Figure 2: 1H NMR spectra of crude (top) and purified (bottom) NBE(CO2H). The purified product is obtained by simple distillation. Note the peaks which are used to assess the endo:exo ratio. Please click here to view a larger version of this figure.
Figure 2: 1H NMR spectra of crude (top) and purified (bottom) NBE(CO2H). The purified product is obtained by simple distillation. Note the peaks which are used to assess the endo:exo ratio. Please click here to view a larger version of this figure.
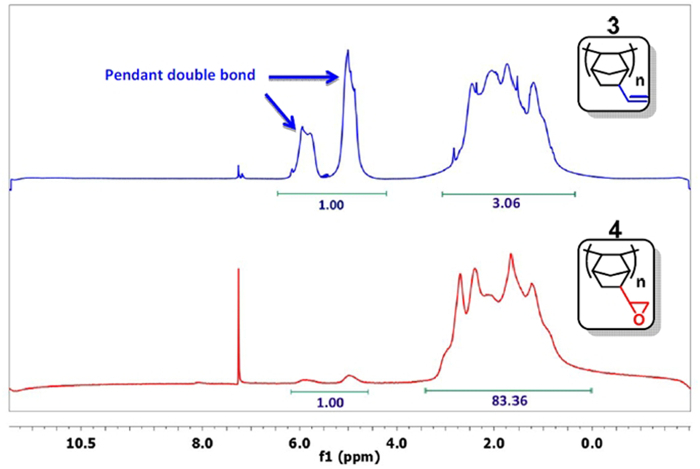 Figure 3: 1H NMR spectra of PNBE(vinyl) (blue) and PNBE(epoxy) (red). Note the ratio 1:3 between the integrals of the vinyl group (δ = 4.5-6.0 ppm) and the other protons in the PNBE(vinyl) spectrum. After reaction with H2O2, the ratio decreases to 1:83, thus confirming that epoxidation of vinyl group has occurred. Please click here to view a larger version of this figure.
Figure 3: 1H NMR spectra of PNBE(vinyl) (blue) and PNBE(epoxy) (red). Note the ratio 1:3 between the integrals of the vinyl group (δ = 4.5-6.0 ppm) and the other protons in the PNBE(vinyl) spectrum. After reaction with H2O2, the ratio decreases to 1:83, thus confirming that epoxidation of vinyl group has occurred. Please click here to view a larger version of this figure.
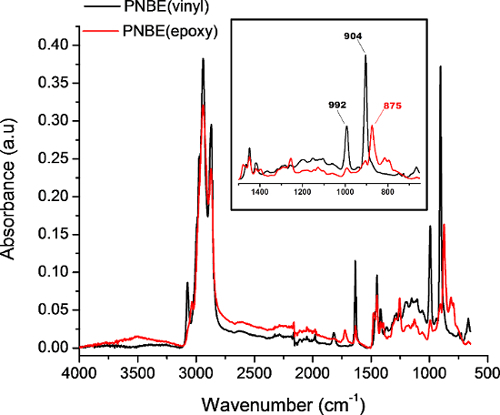 Figure 4: FTIR spectra of PNBE(vinyl) (black) and PNBE(epoxy) acquired in attenuated total reflectance mode. Insert shows a zoom of the characteristic bands of PNBE(vinyl) and PNBE(epoxy). The 902 cm-1 and 992 cm-1 bands correspond to the C=C-H out-of-plane bending, whereas the 875 cm-1 band corresponds to the epoxide ring deformation. Please click here to view a larger version of this figure.
Figure 4: FTIR spectra of PNBE(vinyl) (black) and PNBE(epoxy) acquired in attenuated total reflectance mode. Insert shows a zoom of the characteristic bands of PNBE(vinyl) and PNBE(epoxy). The 902 cm-1 and 992 cm-1 bands correspond to the C=C-H out-of-plane bending, whereas the 875 cm-1 band corresponds to the epoxide ring deformation. Please click here to view a larger version of this figure.
Representative Results
The NBE monomers are prepared by simple Diels-Alder reaction of DCPD and a suitable dienophile, for example acrylic acid (AA). Normally, DCPD is cracked to yield cyclopentadiene (CPD) before reaction.17 Freshly cracked CPD is then engaged in the Diels-Alder reaction. However, in this protocol, both cracking and Diels-Alder steps are performed concomitantly, in a one-pot reaction. Thus, as soon as CPD is formed, it reacts with AA to yield 5-norbornene-2-carboxylic acid, NBE(CO2H) (Figure 1A). After reaction, the crude NBE(CO2H) is purified by simple distillation (Figure 2). This procedure has been repeated with a variety of dienophiles (maleic anhydride13, allyl bromide18, allyl alcohol18, 1-octene18) in a consistent manner (high yields, purification by distillation (Figure 2). The reaction with butadiene as dienophile was not attempted, due to the inherent difficulties associated with the manipulation of butadiene. Interestingly, this reaction is solvent-less.
The polymers are obtained by catalyzed polymerization (Figure 1A and 1B) and collected under the form of dry powders. They are analyzed by 1H NMR, FTIR spectroscopy, and gel permeation chromatography (GPC). The 1H NMR analysis of the polymer (Figure 3) is used to diagnose the presence of leftover solvent and monomer (which appear as sharp peaks, in contrast to the broad resonance of the polymer). The 1H NMR analysis of PNBE(vinyl) (Figure 3 blue) and PNBE(epoxy) (Figure 3 red) permit the confirmation of the epoxidation of PNBE(vinyl). Indeed, the decreasing ratio between the integrals of the vinyl group and the other protons in the PNBE(epoxy) spectrum (1:3 to 1:83) proves the excellent conversion of the reaction (97% in this case). The FTIR spectroscopy can also be used to confirm the outcome of the epoxidation reaction, as shown by the apparition of a characteristic peak at 875 cm-1 (Figure 4) and the disappearance of the 904 and 992 cm-1 which are observed in the PNBE(vinyl) polymer. The GPC is used to confirm the molecular weight distribution of the polymers. Typical Mn for PNBE(CO2H) and PNBE(epoxy) are 100,000 g/mol and 20,000 g/mol, with a PDI comprised between 1.5 and 2.0.
Discussion
The method proposed here is simple, and readily amenable to scale-up. All chemicals could be used as received without any purification. Note that performing the reaction at a lower scale (e.g. scales ≤1 g) usually yields lower yields due to an unavoidable loss of material during the handling and the collection.
The catalysts are formed in situ upon the reaction of commercial Pd compounds with cationizing agents. In our hands, the yield of the reaction as well as the characteristics of the polymer (e.g. molecular weight distribution) are not changed whether one starts with a well-defined catalyst such as (ɳ3-allyl)PdS2+ SbF6- (S = nitromethane) or with the in situ catalytic system. For the polymerization of NBE(vinyl), only an in situ form of the catalyst is currently known. We did not observe any reactivity difference between Pd2(dba)3 and Pd(dba)2, but the use of the chloroform adduct, Pd2(dba)3. CHCl3 is not recommended (lower yields are obtained). The polymerization reaction is also slightly air sensitive. Compared to a reaction entirely performed in the glove-box (O2 <5 ppm), we obtained similar results in terms of yield and molecular weights when the reaction is performed with roughly degassed reagents (degassed by bubbling with N2, the catalyst still being weighed in the glove-box). The monomers do not need to be dried before use, but the presence of intentionally added water is not recommended, as water coordinates the catalyst and poisons the reaction. In fact, the catalyst (ɳ3-allyl)Pd(OH2)2+ SbF6- was found to be inactive.
The polymerization of NBE(CO2H) stops at 45% yield due to the formation of a solid (vitrification). The residual monomer is extracted with ethyl acetate. Ethyl acetate can be separated from monomer by rotary evaporation and the collected monomer can be engaged in a subsequent polymerization. The endo:exo ratio in the collected monomer is slightly different from the endo:exo ratio of the initial monomer. As we demonstrated in a previous work,13 the catalyst isomerizes the endo isomer to the exo isomer during polymerization but polymerization of the collected monomer proceeds in a similar fashion as the initial monomer.
With the exception of ethyl acetate, no other organic solvent is used for the preparation of PNBE(CO2H), starting from DCPD and acrylic acid. It is possible to replace the ethyl acetate extraction step by a Soxhlet extraction with water. This procedure also allows the separation of the unreacted monomer, leading to a reaction totally free of solvent, but it is tedious (the Soxhlet extraction lasted for two days in our hands).
The polymerization of NBE(vinyl) proceeds with 80% yield with a catalyst loading of 0.02 mol%. With a catalyst loading of 0.01 mol%, the yield decreases to 45%. The reaction stops due to the decomposition of the catalyst, and therefore, the yield is not improved by using longer reaction times. During the purification of the polymer, silica is added in order to remove residual metals. With this procedure, the polymer is virtually free of Ag, Sb and contains less than 15 ppm Pd, as measured by ICP.
The epoxidation of the polymer is performed by peracetic and performic acids formed in situ with H2O2 and the corresponding carboxylic acids. The filtration steps (3.1.11 and 3.1.12) should be performed without any interruption, and as rapidly as possible. Indeed, at this stage, the solid PNE(epoxy) is in contact with a large amount of acid and water, which leads to the ring opening of the epoxy functionality. To assess for the presence of ring-opened functionalities in the polymer, one can simply assess the solubility of the polymer in dimethylformamide (DMF). The ring-opened polymer is not soluble in DMF whereas PNBE(epoxy) is soluble in DMF.
We believe this method is significant because of the important role played by functional PNBEs in the fabrication of photoresists which are, for example, suitable for use in 157 nm photolithography.19 Functional PNBEs can also be employed for the construction of diblock copolymers.20 Due to their saturated hydrocarbon backbone, such polymers are interesting as low dielectric materials for microelectronics.21Recently, we also demonstrated that these materials lead to very high Tg thermosets14 as well as ionomers with possible applications for fuel-cell membranes.18 In view of the wealth of possible applications, the method presented here is of interest for a broad community.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge funding from Fonds de Recherche du Québec - Nature et Technologies, from Conseil Recherches en Sciences Naturelles et Génie (program INNOV) and PrimaQuébec.
References
- Gaylord NG, Mandal BM, Martan M. Peroxide-induced polymerization of norbornene. J. Polym. Science, Polym. Lett. Ed. 1976;14(9):555–559. [Google Scholar]
- Janiak C, Lassahn PG. The vinyl homopolymerization of norbornene. Macromol. Rapid Comm. 2001;22(7):479–493. [Google Scholar]
- Bielawski CW, Grubbs RH. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007;32(1):1–29. [Google Scholar]
- Blank F, Janiak C. Metal catalysts for the vinyl/addition polymerization of norbornene. Coord. Chem. Rev. 2009;253(7-8):827–861. [Google Scholar]
- Kaminsky W, Boggioni L, Tritto I. Cycloolefin polymerization. Polymer Science: A Comprehensive Reference, 10 Volume Set. 2012;3:843–873. [Google Scholar]
- Boggioni L, Tritto I. State of the art of cyclic olefin polymers. MRS Bull. 2013;38(3):245–251. [Google Scholar]
- Goodall B. Cycloaliphatic polymers via late transition metal catalysis. In: Rieger B, Baugh L, Kacker S, Striegler S, editors. Late Transition Metal Polymerization Catalysis. Wiley-VCH Verlag GmbH; 2003. pp. 101–154. [Google Scholar]
- Zhou W, He X, Chen Y, Chen M, Shi L, Wu Q. Vinyl-addition copolymerization of norbornene and polar norbornene derivatives using novel bis(β-ketoamino)Ni(II)/B(C6F5)3/AlEt3 catalytic systems. J. Appl. Polym. Sci. 2011;120(4):2008–2016. [Google Scholar]
- Müller K, Jung Y, Yoon DY, Agarwal S, Greiner A. Vinyl-type polymerization of alkylester-substituted norbornenes without endo/exo separation. Macromol. Chem. Phys. 2010;211(14):1595–1601. [Google Scholar]
- Boffa LS, Novak BM. Copolymerization of polar monomers with olefins using transition-metal complexes. Chem. Rev. 2000;100(4):1479–1494. doi: 10.1021/cr990251u. [DOI] [PubMed] [Google Scholar]
- Funk JK, Andes CE, Sen A. Addition Polymerization of Functionalized Norbornenes: The Effect of Size Stereochemistry, and Coordinating Ability of the Substituent. Organometallics. 2004;23(8):1680–1683. [Google Scholar]
- Hennis AD, Polley JD, et al. Novel, efficient, palladium-based system for the polymerization of norbornene derivatives: Scope and mechanism. Organometallics. 2001;20(13):2802–2812. [Google Scholar]
- Commarieu B, Claverie JP. Bypassing the lack of reactivity of endo-substituted norbornenes with the catalytic rectification-insertion mechanism. Chem. Sci. 2015;6(4):2172–2182. doi: 10.1039/c4sc03575e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commarieu B, Potier J, et al. Ultrahigh Tg epoxy thermosets based on insertion polynorbornenes. Macromoecules. 2016;49(3):920–925. [Google Scholar]
- Pirrung MC. The Synthetic Organic Chemist's Companion. John Wiley & Sons, Inc; 2007. [Google Scholar]
- Kanao M, Otake A, Tsuchiya K, Ogino K. Stereo-selective synthesis of 5-norbornene-2-exo-carboxylic acid-Rapid isomerization and kinetically selective hydrolysis. Int. J. Org. Chem. 2012;2(1):26–30. [Google Scholar]
- Huertas D, Florscher M, Dragojlovic V. Solvent-free Diels-Alder reactions of in situ generated cyclopentadiene. Green Chem. 2009;11(1):91–95. [Google Scholar]
- Pierre F, Commarieu B, Tavares AC, Claverie J. High Tg sulfonated insertion polynorbornene ionomers prepared by catalytic insertion polymerization. Polymer. 2016;86:91–97. [Google Scholar]
- Woo HG, Li H. Advanced functional materials, Chapter 1.6.8,30. Vol. 1. Hangzhou: Zheijiang University Press; 2011. [Google Scholar]
- Kim D-G, Bell A, Register Ra. Living vinyl addition polymerization of substituted norbornenes by a t-Bu3P-Ligated Methylpalladium Complex. ACS Macro Letters. 2015;4(3):327–330. doi: 10.1021/acsmacrolett.5b00079. [DOI] [PubMed] [Google Scholar]
- Seung H, S A, Baek K, Sang S. Intech Silagui,M.A.,ed.,, editor. Low Dielectric Materials for Microelectronics. Dielectric Material. 2012. pp. 59–76.


