Abstract
Skeletal muscle regeneration is a physiological process that occurs in adult skeletal muscles in response to injury or disease. Acute injury-induced skeletal muscle regeneration is a widely used, powerful model system to study the events involved in muscle regeneration as well as the mechanisms and different players. Indeed, a detailed knowledge of this process is essential for a better understanding of the pathological conditions that lead to skeletal muscle degeneration, and it aids in identifying new targeted therapeutic strategies. The present work describes a detailed and reproducible protocol to induce acute skeletal muscle regeneration in mice through a single intramuscular injection of cardiotoxin (CTX). CTX belongs to the family of snake venom toxins and causes myolysis of myofibers, which eventually triggers the regeneration events. The dynamics of skeletal muscle regeneration is evaluated by histological analysis of muscle sections. The protocol also illustrates the experimental procedures for dissecting, freezing, and cutting the Tibialis Anterior muscle, as well as the routine Hematoxylin & Eosin staining that is widely used for subsequent morphological and morphometric analysis.
Keywords: Developmental Biology, Issue 119, Cardiotoxin, Acute Skeletal Muscle Regeneration, Tibialis Anterior Muscle Isolation, Fresh Frozen Muscle Technique, Hematoxylin & Eosin staining, Myofibers Cross Sectional Area.
Introduction
Mammalian adult skeletal muscles are formed by groups of fascicules of multinucleated muscle cells (myofibers) that are specialized for contraction. Each myofiber is an elongated syncytium, surrounded by the sarcolemma (plasmatic membrane) and containing myofibrils, which are made up of regularly and repeatedly organized contractile proteins (actin and myosin filaments). In adult life and in resting conditions, skeletal muscles have a very low turnover of their myonuclei1; indeed, the myonuclei, which are located at the periphery of the myofiber, under the sarcolemma, are arrested in the G0 phase of the cell cycle and are unable to proliferate1,2.
Skeletal muscles have the peculiar ability to regenerate following damage, reaching homeostasis after several events of tissue remodeling that are tightly related to each other. After an acute injury or trauma, degeneration is induced, followed by regeneration processes that involve different cell populations, including a resident population of muscle cells, the satellite cells (SCs). Indeed, in the absence of any environmental stimuli, the satellite cells are in a quiescent state and are located in a specialized niche between the sarcolemma and the basal lamina3,4. Following an injury or disease, SCs become activated, proliferate, migrate to the damaged areas, and eventually differentiate, giving rise to newly forming myofibers5. Activated SCs establish cross-talk with different cell populations, mainly inflammatory cells, which are recruited in the site of trauma6-8. This cross-talk allows the cells to follow a regulated paradigm by which molecular signals drive structural modifications, eventually leading to homeostasis9. Besides SCs, inflammatory and interstitial cells, angiogenic processes, and re-innervation events are also involved, acting in a coordinated manner to repair this highly organized and specialized structure.
There is great interest in studying different aspects of skeletal muscle regeneration, not only to understand the physiology of the muscle, but also to improve therapeutic strategies that require deeper knowledge of the whole process. Several experimental approaches are currently available to study the identity and function of the different cell populations, the signaling pathways, and the molecular mechanisms involved. Mouse models of acute injury represent a powerful tool to investigate many aspects of this process. Different commonly used techniques to induce acute muscle damage allow researchers to follow the regeneration process in vivo, from the very early stages to the end of the process. This protocol describes the steps from the intramuscular injection of snake venom-derived cardiotoxin (CTX), which induces myolysis and triggers the regeneration process, up to the analysis of tissue samples. Following CTX injection, mice can be sacrificed at different time points depending on experimental requirements, and the skeletal muscles can be dissected and processed for further analysis. Finally, we describe the staining protocol of tissue sections to perform morphological observations and basic quantitative analyses. This protocol allows for the study of acute skeletal muscle regeneration in vivo in a highly reproducible manner10.
Protocol
All experiments were conducted in strict accordance with the institutional guidelines for animal research and approved by the Department of Public Health, Animal Health, Nutrition and Food Safety of the Italian Ministry of Health in accordance with the law on animal experimentation. Cervical dislocation procedures may vary from institution to institution based on IACUC or its equivalent requirements.
1. Cardiotoxin Injection in the Tibialis Anterior Muscle
Prepare a 10 μM working solution of cardiotoxin (CTX) by diluting the cardiotoxin stock solution in sterile Phosphate-Buffered Saline (PBS) or in water before starting the procedure. Note: CTX is soluble in water at 1 mg/mL. Prepare a 70 μM stock solution. Aliquot and store at -20 °C. CTX used for this protocol is a mixture of cardiotoxins having a molecular weight of approximately 7,000 Da. CAUTION: It is suggested to wear double gloves, a hygiene mask, and safety glasses (alternatively to safety glasses, it is recommended to work under a hood) and to be very careful to avoid any contact with the solution, even if diluted.
Anesthetize the mice by intraperitoneal injection of ketamine and xylazine (80 mg/kg and 10 mg/kg of body mass) or other approved available anesthetic.
With the mouse face up, spray the hind limbs with 70% ethanol. To visualize the exact location of the Tibialis Anterior (TA) muscle, with the help of a scalpel, cut the hair away at the anterior part of the lower leg, under the knee. Note: The TA muscle arises from the proximal body of the tibia, going down lateral to the bone and ending at the ankle. Its long tendon extends into the foot across the ankle (see Figure 1A).
Draw ~ 100 μL of the solution into the syringe by pulling the plunger back slowly.
Remove air bubbles, holding the syringe upright (with the needle upward). Gently pull the plunger down and tap the side of the syringe to help any air bubbles attached to the inner part of the tube come upwards. Slowly press the plunger to release large air bubbles. A few drops of liquid will come out the needle. At this point, make sure to pick the liquid up again into the tube and not disperse it, given the toxicity of cardiotoxin.
Withdraw the solution into the syringe until the desired dose is reached. Inject 20 μL of CTX solution for each TA muscle.
Insert the needle in the center of the TA by following the position of the Tibia bone as guidance, from the tendon towards the knee. Since the TA is a thin tissue, perform the injection without going too deep (insert the needle 2/3 mm deep, at approximately a 10° to 20° angle ), and avoid going beyond the muscle itself (Figure 1A).
Do not pull out the needle immediately after the injection, but wait 2 - 3 s to prevent leakage of CTX drops from the muscle.
After the procedure, place the cage on a heated plate (at 37 °C) until the mice are awake, since the anesthetic and the ethanol used reduce body temperature.
2. Tibialis Anterior Isolation
Note: Muscles can be isolated at different time points after cardiotoxin injection according to experimental requirements.
Sacrifice the mouse by cervical dislocation.
Spray the hindlimbs with 70% ethanol. During the procedure, the animal can be fixed to a support to hold it down.
Cut the skin at the level of the proximal tendon (above the foot), insert one of the blades of the scissors under the skin, and cut the skin upwards, in the direction of the knee. With the help of forceps, pull up the skin and expose the muscle entirely. Carefully remove all of the skin and the hair from the area of interest.
Eliminate the fascia by slowly pinching or cutting (with thin-tip tweezers or with fine micro dissecting scissors) the extreme periphery of the muscle on the opposite side of the tibia (Figure 1B). Once broken, gently remove the fascia with the help of thin-tip tweezers; avoid damaging the underlying muscle. Note: As with other muscles and organs, the TA is covered by fascia (deep fascia of the leg), a thin sheet of connective tissue that lies deep under the skin and that needs to be removed to easily isolate the underlying muscle.
Visualize the distal TA tendon and the Extensor Digitorum tendon above the foot, which are juxtaposed. The tendon of the Extensor Digitorum Longus (EDL) muscle is located slightly below the TA tendon. Tendons of adult mice are visible to the naked eye.
- Remove the TA muscle following one of the two following techniques:
- Cut both tendons with fine micro dissecting scissors and pull them towards the proximal side, holding the tendons with forceps. Separate the two tendons, pulling them gently in opposite directions to separate the muscles.
- With the help of thin-tip tweezers, separate the distal TA tendon from the Extensor Digitorum tendon by passing the tip below the tendon itself (Figure 1C, upper picture). Gently slide the tweezers below the TA to separate it from the underlying muscles (Figure 1C, bottom picture).
- Hold the tweezers under the muscle to keep it slightly raised, cut the TA tendon, and gently pull it upwards until only the area below the knee is attached (Figure 1D, left picture). Cut the TA below the knee, following the edge of the muscle with the scissors (Figure 1D, right picture). Note: If the muscle remains slightly attached to the sides, use fine micro dissecting scissors to help detach it. Note: If the forceps do not easily fit below the TA, it is possible that the fascia has not been completely removed, and it could still be visible on top of the muscle. In this case, completely remove the fascia before proceeding.
3. Fresh Frozen Muscle Technique
Place a small amount of tragacanth gum or a similar adhesive freezing compound (e.g., Optimal Cutting Temperature (OCT) compound) on a slice of cork. Label the cork's slice on the reverse side before freezing, if necessary (Figure 2A).
In order to obtain transverse sections of the muscle, sink the TA into the tragacanth gum by inserting the distal tendon and leaving about 3/4 of the muscle outside, making sure to have the muscle in a perpendicular position with respect to the cork (Figure 2A).
Fill an aluminum can (alternatively, a metal cup or a glass beaker), with isopentane. Suspend the can of isopentane in a Dewar containing liquid nitrogen, not allowing the liquid nitrogen to enter into the can. Note: Isopentane needs to reach the proper temperature (from -150 to -160 °C) for optimal freezing of the tissue. The correct temperature is reached when some white solid particles form at the bottom of the can and the isopentane becomes slightly viscous. Note: Do not freeze the sample before the formation of the solid particles, as it can lead to freezing artifacts. On the other hand, if the isopentane becomes completely solid, leave it at room temperature until it is thawed, making sure that some white particles are still present.
Using Mixter forceps, rapidly dip the cork with the muscle into the isopentane, keeping the muscle positioned downwards, and make sure to release the cork when it is completely immersed in the liquid. In this way, while the specimen floats, only the reverse side of the cork should be visible in the liquid.
Leave the sample in the isopentane for 1 min.
Using Mixter forceps (or similar forceps), take the muscle and immerse it in liquid nitrogen for at least 2 min.
Wrap the specimens individually in labeled aluminum foil and immediately place them in dry ice (-70 °C), or directly store them in a -80 °C freezer. Note: Throughout the procedure, from steps 3.4 to 3.7, it is extremely important to maintain the correct temperature and to prevent the specimens from thawing (e.g., during the transfer to the -80 °C freezer). The rising temperature results in uncontrolled thawing and refreezing of the water microcrystals that are present within the tissue and eventually causes the formation of artifacts or the breakdown of muscle fibers (visualized on the tissue sections as large white holes in the center of the fiber).
4. Cryostat Sectioning of Frozen Muscles
Set the cryostat chamber temperature to -20 to -22 °C.
Put the specimen stub, the blade, and the specimen in the cryostat chamber, allowing them to equilibrate for at least 30 min.
Place a small amount of freezing compound (e.g., OCT compound) on the specimen stub and immerse the cork with the specimen before the freezing compound solidifies. In the meantime, place the blade in the blade holder.
Align the specimen and the blade using the provided screws to orient the specimen holder with the blade holder (Figure 2B).
Set the section thickness to 10 μm. Proceed to cut the tissue. For each turn of the drive wheel, the specimen holder advances a controlled distance towards the blade.
Place the sections on a polarized slide (8 to 10 section per slide) (Figure 2C).
After sectioning, store the slides at -80 °C. Note: The specimen can be stored in a -80 °C freezer and reused for further sectioning, if properly handled and stored.
5. Routine Histological Staining (Hematoxylin & Eosin Stains)
Note: Several histological stains can be performed on muscle sections according to the analysis. A routine histological stain for morphological and morphometric analysis is the Hematoxylin & Eosin (H&E) bichromic stain. The hematoxylin stains the nuclei a deep purple. Nuclear staining is counterstained with eosin (pink/red), which stains eosinophilic structures, such as the myofibers in the cytoplasm.
Completely air-dry frozen slides with sections for 10 - 15 min at room temperature, lodge the slides in a staining tray, and put the tray in a staining trough.
Stain for 1 min with hematoxylin.
Lay the staining trough under a fairly weak tap water jet for 10 min to wash out the hematoxylin.
Immerse the slides for 5 min in 95% ethanol, if an alcoholic solution of eosin will be used (skip this step in case of an aqueous eosin solution).
Counterstain for 1 min with eosin solution.
Rinse with water to eliminate excessive eosin solution. Note: Optimal timing of staining with either hematoxylin or eosin should be defined for each new batch.
Dehydrate the samples in a graded ethanol series: 50% and 70% (a few seconds each). Rapidly immerse in 95% ethanol, followed by 2 changes in 100% ethanol, 5 min each.
Clear in 2 changes of xylene, each 5 min or longer. This step must be carried out under a chemical hood.
- Mount the slides with a xylene-based mounting medium, applying a few drops of mounting medium on the slide and covering with a coverslip. The mounting medium can also be applied to the coverslip and the coverslip put on the slide.
- Avoid forming air bubbles between the slide and the coverslip. To eliminate air bubbles, keep the slide vertical (after mounting) and squeeze out the excess mounting medium and air bubbles by gently pressing on the coverslip with blunt forceps (or a similar blunt tool).
Keep the slides under the hood for a few hours or overnight to let the xylene evaporate completely before proceeding to the analysis.
Perform the morphological and morphometric analysis of H&E-stained regenerating muscle sections by using non-overlapping serial images of at least one whole muscle section per mouse. Capture images with a bright-field microscope, with 20X magnification. Note: A minimum number of 5 mice is recommended to obtain statistical significance of the measurements. Note: Image analysis software is available for this purpose, such as the free download software ImageJ (http://imagej.nih.gov/ij/).
Representative Results
H&E staining allows for the evaluation of the morphology of the regeneration process at specific time points during skeletal muscle regeneration. Figure 3 shows the time course analysis performed on injured TA muscles of wild type mice. Muscles have been isolated at 3, 7, 15, and 30 days after CTX injection, as schematized in Figure 3A. Representative pictures of H&E-stained transverse sections show the dynamics of skeletal muscle repair over time (Figure 3B-E). At day 3 after injury, the structural architecture of the muscle is completely destroyed, and both degenerated myofibers (necrotic area) and mononucleated cells are clearly visible (Figure 3B). This heterogeneous group of cells mainly consists of transient amplifying satellite cells (myoblasts), inflammatory cells recruited from blood11, and interstitial and endothelial cells that participate in the regeneration process, which is triggered at the site of injury. In this context, new regenerating myofibers are generated by fusion and differentiation of myoblasts. The main feature of the regenerating area is the presence of small basophilic myofibers with centrally located nuclei and a dark-stained cytoplasm (Figure 3C). At this stage, inflammation is still visible, although it progressively decreases. The size of regenerating myofibers increases over time, giving rise to eosinophilic regenerated myofibers characterized by centrally located nuclei (Figure 3D, E). The regenerated myofibers are visible at later stages of regeneration and until the end of the process. The healthy myofibers appear highly organized and close to each other, with a pink cytoplasm and with the nuclei placed at the periphery of the fibers, under the sarcolemma. These myofibers are usually present far from the site of CTX injection and injury, and they more rarely represent areas where the regeneration is definitively complete. Indeed, regenerated fibers are characterized by the presence of centrally located nuclei that move to the periphery only several months after injury.
Digital images of the stained muscle sections are captured by adding the scale bars for spatial calibration, which is necessary to accurately measure the areas of interest by outlining the contours of the interested areas. The measurement of the myofiber cross-sectional area (CSA) is a reliable morphometric analysis, which is widely used to quantify the differences in the regeneration trend between different groups of mice. This analysis requires the measurement of areas of centrally nucleated myofibers, including at least 500 and up to 1,000 fibers per section. Both the average of the regenerating and regenerated myofiber areas and the Gaussian distribution of these areas are indicators of the regeneration process. Increased myofiber CSA is generally associated with an improved and/or accelerated regenerative response. On the contrary, failure of proper regeneration is associated with a decrease in CSA. Here, we reported the myofiber CSA analysis, indicated as both the average (Figure 3F) and distribution (Figure 3G) of myofiber CSA at different time points. The analysis highlights how the distribution of myofiber areas changes over time and how the CSA shifts towards larger-sized fibers as the process of regeneration proceeds.
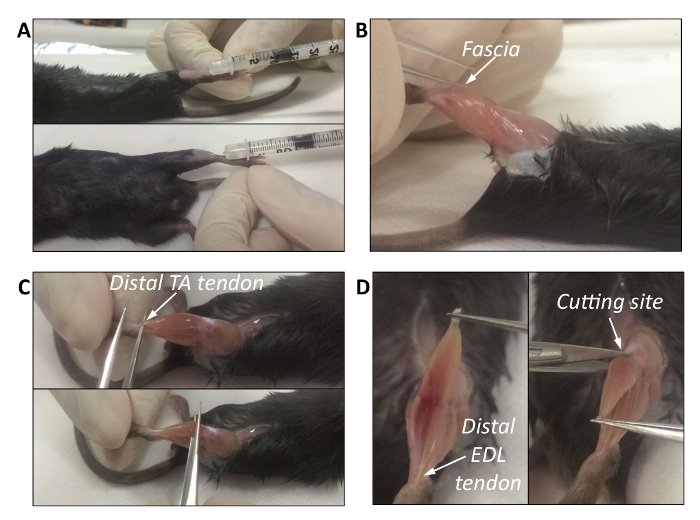 Figure 1. Intramuscular Injection and Isolation of the Tibialis Muscle. A. Intramuscular injection of cardiotoxin in the Tibialis Anterior muscle. B. Removal of muscle fascia. The white arrow indicates the thin-tipped tweezers that pinch the muscle to remove the fascia. C. The top picture shows the anatomical position of the distal TA tendon. The bottom picture shows how to lift the TA and remove it while avoiding muscle damage. D. Left picture: the TA is lifted by pulling the distal tendon upward. The white arrow indicates the distal EDL tendon. Right picture: by holding the tendon with forceps, the top edge of the TA is cut off below the knee. The white arrow indicates the cutting site. Please click here to view a larger version of this figure.
Figure 1. Intramuscular Injection and Isolation of the Tibialis Muscle. A. Intramuscular injection of cardiotoxin in the Tibialis Anterior muscle. B. Removal of muscle fascia. The white arrow indicates the thin-tipped tweezers that pinch the muscle to remove the fascia. C. The top picture shows the anatomical position of the distal TA tendon. The bottom picture shows how to lift the TA and remove it while avoiding muscle damage. D. Left picture: the TA is lifted by pulling the distal tendon upward. The white arrow indicates the distal EDL tendon. Right picture: by holding the tendon with forceps, the top edge of the TA is cut off below the knee. The white arrow indicates the cutting site. Please click here to view a larger version of this figure.
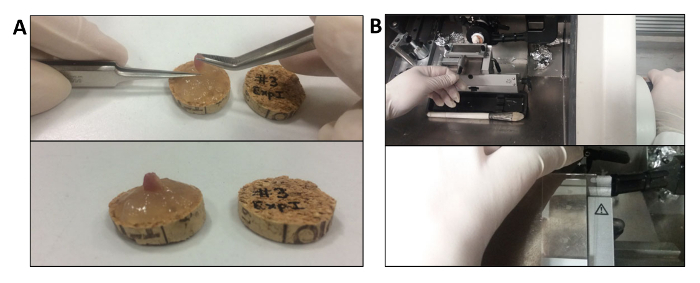 Figure 2. Fresh Freezing Muscle and Cryostat Sectioning of Frozen Muscles. A. Technique of fresh muscle inclusion in tragacanth gum. The TA muscle is immersed in the gum from the tendon, with about 3/4 outside and maintained in a perpendicular position with respect to the cork. B. Representative images of the procedure for cryostat sectioning. The cork is placed in a small amount of freezing compound on the specimen stub. Please click here to view a larger version of this figure.
Figure 2. Fresh Freezing Muscle and Cryostat Sectioning of Frozen Muscles. A. Technique of fresh muscle inclusion in tragacanth gum. The TA muscle is immersed in the gum from the tendon, with about 3/4 outside and maintained in a perpendicular position with respect to the cork. B. Representative images of the procedure for cryostat sectioning. The cork is placed in a small amount of freezing compound on the specimen stub. Please click here to view a larger version of this figure.
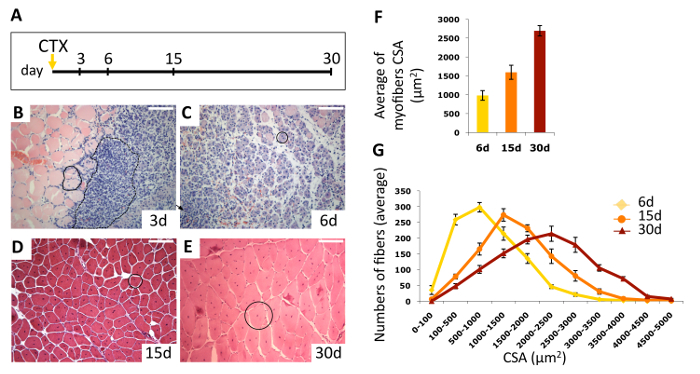 Figure 3. Time Course Analysis of Skeletal Muscle Regeneration. A. Experimental scheme of acute skeletal muscle injury. B-E. Representative pictures of Hematoxylin & Eosin stained sections of cardiotoxin (CTX)-treated muscles at indicated days after injury. B. The black continuous line (on the left side of the picture), encloses a couple of necrotic fibers, likely invaded by inflammatory cells. The dashed line (right side of the picture) marks an area of infiltrating mononucleated cells. C. A single immature myofiber with centrally located nucleus is indicated by the black circle. D-E. Large regenerated eosinophilic myofibers, with centrally located nuclei are marked by black circles. Scale bars represent 100 mm. F. Average of centrally nucleated myofiber size values in TA muscle sections. Values are mean ± SEM, 5 mice/group. G. Myofiber cross-sectional area (CSA) distribution at 6, 15, and 30 days after CTX injection. Values are mean ± SEM, 5 mice/group. Please click here to view a larger version of this figure.
Figure 3. Time Course Analysis of Skeletal Muscle Regeneration. A. Experimental scheme of acute skeletal muscle injury. B-E. Representative pictures of Hematoxylin & Eosin stained sections of cardiotoxin (CTX)-treated muscles at indicated days after injury. B. The black continuous line (on the left side of the picture), encloses a couple of necrotic fibers, likely invaded by inflammatory cells. The dashed line (right side of the picture) marks an area of infiltrating mononucleated cells. C. A single immature myofiber with centrally located nucleus is indicated by the black circle. D-E. Large regenerated eosinophilic myofibers, with centrally located nuclei are marked by black circles. Scale bars represent 100 mm. F. Average of centrally nucleated myofiber size values in TA muscle sections. Values are mean ± SEM, 5 mice/group. G. Myofiber cross-sectional area (CSA) distribution at 6, 15, and 30 days after CTX injection. Values are mean ± SEM, 5 mice/group. Please click here to view a larger version of this figure.
Discussion
Here, we describe a protocol to induce acute injury in skeletal muscle (i.e., the intramuscular injection of CTX). It is widely used as a powerful tool to study the dynamics of skeletal muscle regeneration in vivo. CTX injection induces the degeneration of muscle fibers, which is caused by the depolarization of the sarcolemma and the contraction of the fibers12, and triggers the cascade of events that leads to muscle regeneration. Skeletal muscles are dissected at desired time points after the injection and injury, according to experimental requirements, and used for subsequent histological analyses. The dissected muscles can either be frozen, after inclusion in cryo-protectant media, or included in paraffin. However, these procedures require a step of pre-fixation with paraformaldehyde, which can generate artifacts and can eventually affect the subsequent analyses. Freezing the tissue directly can prevent this problem. Of note, many primary antibodies work exclusively or more effectively on fresh frozen muscle sections13, which makes this procedure more reliable. The morphometric analysis is usually performed on time course experiments, which allow for the evaluation of differences in the regeneration processes between groups of mice with the same age and with specific genetic mutations and/or pharmacological treatments. Although the protocol of CTX-induced damage is highly reproducible per se, a possible limitation is due to the operator-dependent variability of CTX injection. To overcome this limitation, it is preferable that the entire time course experiment is performed by the same operator.
The extent of muscle regeneration can be quantified as percentage of healthy fibers, necrotic areas and inflammation, and regenerating and regenerated areas over the total area. However, this approach is mostly used when studying events of chronic damage, such as in dystrophic mdx mice, rather than in models of acute injury. Indeed, while dystrophic muscles are characterized by asynchronous events of degeneration and regeneration, acute injury is followed by well-defined and consequential events. One limitation of these analyses, which require an empirical identification of the morphological features described above, is that they are not completely unbiased. To support experimental conclusions, the morphological analysis should always be complemented with further analysis, such as immunofluorescence analysis of specific molecular markers, to support experimental conclusions. For this reason, it is preferred to use parallel analysis to unequivocally interpret the results. For instance, regenerating myofibers are positively stained for embryonic Myosin Heavy Chain (eMyHC) that is expressed specifically in newly formed myofibers. Thus, quantification of eMyHC-stained myofibers can be performed side-by-side with the morphological analysis.
The CSA analysis is a more reliable quantification and can be performed either on H&E-stained sections or on muscle sections stained with laminin, which marks the edge of the muscle fibers; quantification is performed using appropriate macros of ImageJ, as described above. In both cases, it is always necessary to first assess the quality of the sections and to exclude areas of tissue that appear deformed or curled.
Beside the morphological analyses of the tissues, H&E-stained sections allow for the identification of other specific features, such as the presence of fibrosis and/or fatty tissue. Indeed, fibrosis derives from the excessive accumulation of extracellular matrix that occurs either if the regeneration is impaired or in chronic diseases14. Indeed, extracellular matrix accumulation is visible as pale material deposited between the regenerated myofibers in H&E-histological staining. Multiple rounds of CTX injection can be used to mimic chronic disease in which the myofibers undergo waves of degeneration and regeneration and scar tissue accumulates aberrantly. However, more reliable protocols are available to induce muscle fibrosis15, and specific histological staining can be performed to identify and quantify these structures, such as Masson's Trichrome or Sirius Red staining. Formation of fatty tissue is clearly visible in H&E-stained sections as rounded white structures between the myofibers, giving an important indication of the presence of fatty tissue, which is quantified by Oil Red staining. Finally, skeletal muscle sections obtained following the protocol described can be used to perform immunofluorescence analysis using specific antibodies and protocols.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank the Animal House and the Integrated Microscopy Facilities of IGB-CNR. This work has benefited from research funding from the European Community's Seventh Framework Programme in the project ENDOSTEM (Activation of vasculature associated stem cells and muscle stem cells for the repair and maintenance of muscle tissue, grant agreement number 241440), the Italian Ministry of Education-University-Research (MIUR-PRIN2 010-2011) to G.M. and S.B. and PON Cluster IRMI to G.M., and the CARIPLO foundation to G.M. and S.B.
References
- Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35(8):1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Roca I, Requena J, Edel MJ, Alvarez-Palomo AB. Myogenic Precursors from iPS Cells for Skeletal Muscle Cell Replacement Therapy. J Clin Med. 2015;4(2):243–259. doi: 10.3390/jcm4020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142(9):1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 1985;91(1985):534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Saclier M, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31(2):384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab. 2013;304(5):E453–E465. doi: 10.1152/ajpendo.00553.2012. [DOI] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013;14(12):1062–1072. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costamagna D, Costelli P, Sampaolesi M, Penna F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/805172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chuang ST, Lee CY, Wei JW. Role of cardiotoxin and phospholipase A in the blockade of nerve conduction and depolarization of skeletal muscle induced by cobra venom. Br J Pharmacol. 1972;44(4):752–764. doi: 10.1111/j.1476-5381.1972.tb07313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, et al. Tissue triage and freezing for models of skeletal muscle disease. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]
- Mann CJ, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1(1) doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessina P, et al. Novel and optimized strategies for inducing fibrosis in vivo: focus on Duchenne Muscular Dystrophy. Skelet Muscle. 2014;4(1):7. doi: 10.1186/2044-5040-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


