Abstract
Combined clinical pretest probability (PTP) and D-dimer testing have great diagnostic value for pulmonary embolism exclusion. To harmonize performance levels of D-dimer assays available on the market, the Clinical and Laboratory Standard Institute (CLSI) has published a guideline, endorsed by the US Food and Drug Administration (FDA). Such guideline specifies the ideal D-dimer assay characteristic and target population. This study was conducted following the CLSI guideline to upgrade the assay-intended use and obtain FDA clearance of STA-Liatest D-Di assay for pulmonary embolism exclusion in patient with low/moderate PTP. This was an international, multicenter, prospective nonrandomized, noninterventional clinical outcome management study conducted in a standard of care setting. D-dimer assay was performed in consecutive, ambulatory outpatients suspected of pulmonary embolism, with low/moderate PTP, and without medical conditions or in clinical settings known to alter default D-dimer values regardless of the presence of thrombosis using a threshold of 0.5 μg/ml (fibrinogen equivalent units) for venous thromboembolism exclusion. Results were used to determine test performance. Of 1141 patients who underwent D-dimer testing, 1060 had valid results and completed study as planned. STA-Liatest D-Di assay performance has exceeded the CLSI/FDA guidance requirements, with a sensitivity of 97.6% (95% confidence interval: 91.7–99.7%) and a negative predictive value of 99.7% (95% confidence interval: 99.0–100%). STA-Liatest D-Di assay has an excellent performance when used in combination with a PTP score in relevant patients and has the potential to minimize the economic healthcare burden avoiding unnecessary and expensive imaging tests.
Keywords: Clinical and Laboratory Standard Institute, D-dimer, Food and Drug Administration, pulmonary embolism, STA-Liatest D-Di
Introduction
Pulmonary embolism is a life-threatening condition with an estimated annual incidence of 0.5–1.2 cases per 1000 individuals [1]. The symptoms and signs of pulmonary embolism are nonspecific, making the diagnosis highly challenging [2]. Importantly, the consequences of a misdiagnosis can be serious. Indeed, a false-negative diagnosis is associated with an increased risk of fatal outcome, whereas a false-positive diagnosis might lead to unnecessary exposure to anticoagulant therapy. Therefore, reliable diagnostic strategies for pulmonary embolism are of high importance.
A well accepted diagnostic strategy for patients with suspected pulmonary embolism consists of combining assessment of clinical pretest probability (PTP) with imaging or D-dimer tests [3]. Ideally, patients are first stratified by pulmonary embolism risk probability, and then those with high PTP scores proceed to imaging testing to confirm the presence of pulmonary embolism, whereas those with low/moderate PTP undergo D-dimer testing to exclude it.
Currently, there are a variety of D-dimer tests available on the market, differing in terms of detection methods, units of measure, and reference threshold values [4,5]. These methodological differences can create confusion, contribute to interassay inconsistency and ultimately cause difficulties in pulmonary embolism diagnosis [6]. The Clinical and Laboratory Standard Institute (CLSI) recognized the need for harmonization of the various D-dimer tests and compiled a guideline to describe the optimal use of D-dimer assays for the exclusion of venous thromboembolism (VTE) in nonhospitalized, ambulatory patients [7]. Importantly, this guideline specifies the recommended assay characteristics, in terms of negative predictive value (NPV) and specificity, according to the CLSI and US Food and Drug Administration (FDA), and the ideal target patient population [7].
The STA-Liatest D-Di (Stago, Asnières sur Seine, France) is a well established, rapid, automated, quantitative immune-turbidimetric assay that has been used for the quantitative determination of D-dimer levels. This assay has been FDA approved and validated for the use as aid in the diagnosis of deep vein thrombosis and pulmonary embolism since 2005. The management study reported in this article was conducted to upgrade the assay-intended use and obtain FDA clearance of the STA-Liatest D-Di assay for the exclusion of pulmonary embolism in patients with low/moderate PTP. Specificity, sensitivity, NPV, positive predictive value (PPV), positive and negative likelihood ratios (PLR and NLR, respectively) of the assay were defined using plasma samples obtained from the ideal target population as defined in the CLSI guideline.
Methods
Study design and participants
The study was an international, multicenter, prospective nonrandomized, noninterventional clinical outcome management study (DiET study, NCT01221805). The study was conducted in a standard of care setting in consecutive, ambulatory outpatients suspected of having pulmonary embolism. Patients had to be less than 80 years old and willing to participate in the 3-month follow-up evaluation. Strict exclusion criteria were set, following the CLSI guideline [7], to avoid the inclusion of patients with medical conditions or undertaking therapies that are known to influence D-dimer concentrations independently of the occurrence of a thrombotic event. Especially, patients with bone fracture or surgery with general anesthesia longer than 30 min within the previous month, disseminated malignancy or active cancer, sepsis, severe infection, pneumonia within the previous month, pregnancy or postpartum within the previous month, and ongoing anticoagulant drug treatment (at curative or prophylactic dose) started 24 h or more before the D-dimer level is measured were excluded.
Ethical conduct of the study
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with the US Code of Federal Regulation Title 21, Parts 50, 54, and 56. In addition, the study complied with elements relevant to the use of in-vitro diagnostic products within the International Organization for Standardization 14155 ‘Clinical investigation of medical devices for human study participants – Good clinical practice’ and International Conference on Harmonisation E6 ‘Good clinical practice: consolidated guideline’. All patients had to sign an informed consent form, according to local regulations.
Study assessments
Patient management and follow-up
Eligible patients were assessed for pulmonary embolism risk using the Wells’ PTP score [8,9].
Patients with low/moderate PTP were referred to real-time D-dimer testing using the STA-Liatest D-Di assay and managed differently depending on the test's results (Fig. 1): patients with positive D-dimer results were assigned to imaging procedures; patients with negative D-dimer results were considered as not having pulmonary embolism and were followed up after 3 months. The follow-up consisted of a phone interview using a standard questionnaire; if in the course of the 3 months following the initial visit patients experienced a VTE, their medical record was checked. Follow-up was performed by the local principal investigator and reviewed by the study coordinating investigator. No independent adjudication panel was involved in the study, and assay performance was determined on a worst case basis (i.e. false-negative events were always included in the analysis).
Fig. 1.
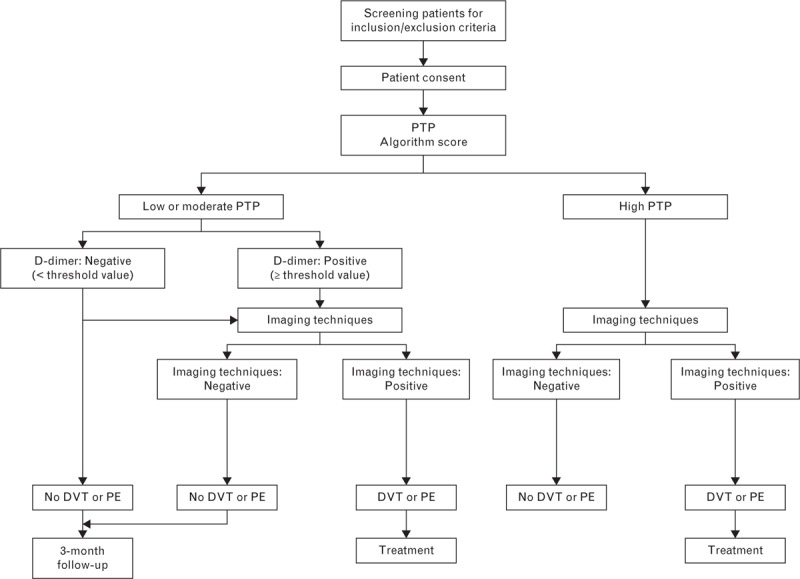
Deep vein thrombosis clinical algorithm. DVT, deep vein thrombosis; PE, pulmonary embolism; PTP, pretest probability.
D-dimer testing is not recommended as first-line assay in patients suspected of pulmonary embolism with high PTP [3]. Therefore, patients at high risk were assigned to imaging procedures to confirm pulmonary embolism (Fig. 1), and were neither included in analysis population nor followed up.
D-dimer assay
Plasma samples were collected at the initial visit from patients with low/moderate PTP. Specimen collection, processing, transport and storage were performed according to the CLSI guidelines (H21–A5–2008) [10]. The STA-Liatest D–Di assay was carried out in each local laboratory on fresh plasma samples using STA-R Evolution or STA Compact (Diagnostica Stago, Asnières sur Seine, France) according to the manufacturer's instructions. STA-Liatest D–Di is a rapid, automated, quantitative immuno-turbidimetric assay. D-dimer levels were expressed in fibrinogen equivalent units (FEUs). The STA-Liatest D-Di established exclusion threshold was 0.50 μg/ml FEUs, as demonstrated in previous studies [11–13].
Imaging techniques
Each investigational site used imaging procedures according to standard of care; diagnosis was formulated according to local practice. The procedures used included spiral computed tomography scan, perfusion/ventilation lung scan, and pulmonary angiogram. Imaging procedures were generally performed in patients with high PTP and in patients with low/moderate PTP and positive D-dimer results. Occasionally, depending on local practice, imaging procedures were performed in patients regardless of their D-dimer results.
Statistical methods
All statistical analyses were carried out using SAS Version 9.2 (SAS Institute Inc., Cary, North Carolina, USA). Demographic characteristics and Wells’ PTP scores were analyzed by means of descriptive statistics. Sensitivity, NPV, specificity, PPV, PLR, and NLR 95% confidence intervals (CIs) were computed using the exact binomial method with two-sided α of 0.05.
Impact of missing data was investigated using a combined multiple imputation procedure with bootstrap method.
Results
Participants
Nine centers in Europe and North America participated in the study: one in Canada, two in France, two in Italy, two in Spain, and two in the United States. Patient disposition and management are represented in Fig. 2. Of the initial 1281 patients who signed the informed consent form between November 2011 and August 2013, 1177 patients exhibited low/moderate PTP, 19 had high PTP, and 85 were excluded from the study. Most patients with low/moderate PTP (n = 1141) had valid D-dimer results and were further evaluated for pulmonary embolism, whereas 36 patients were excluded. Of the 361 patients who had positive D-dimer results (≥0.50 μg/ml FEUs), 305 underwent imaging procedure: pulmonary embolism was excluded in 223 patients and confirmed in 82 patients. Of the 780 patients who had negative D-dimer results, 755 were assessed at the 3-month follow-up and 25 were declared lost to follow-up. At the 3-month follow-up, pulmonary embolism was excluded in 753 patients, confirming the D-dimer results. At that stage, only two of 755 (0.26%) patients had experienced pulmonary embolism. Specifically, one of the two patients experienced pulmonary embolism 2 months following the initial visit, during a hospitalization for respiratory and heart decompensation with acute pulmonary edema. The patient had atrial fibrillation, hence was treated with anticoagulant therapy to prevent stroke; the anticoagulant therapy was then interrupted because of a hematoma while the patient was still bedridden. Bedridden patients are known to be at high risk for VTE when they do not receive VTE prophylaxis. Therefore, the pulmonary embolism observed in this patient was attributed to the condition and treatment he underwent during the hospitalization. For the second patient, the false-negative result was most likely because of the use of a hemolyzed plasma sample for the STA-Liatest D-Di assay. Indeed, hemolyzation is a specific condition for sample rejection according to the STA-Liatest D-Di package insert.
Fig. 2.
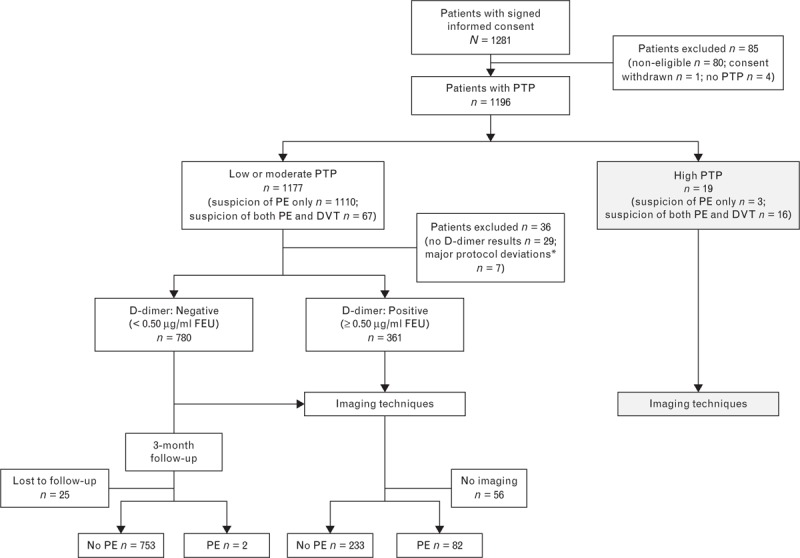
Study flow chart. DVT, deep vein thrombosis; PE, pulmonary embolism; PTP, pretest probability. ∗Major protocol deviations: six procedure-related deviations, one noncompliance with inclusion/exclusion criteria.
STA-Liatest D-Di assay performance
Assay performance was evaluated using samples from patients for whom all assessments planned in the study were conducted and gave valid results (i.e. patients with low/moderate PTP, with valid D-dimer results, reference imaging or 3-month follow-up and without major protocol deviation; n = 1060). In the study population, 44.6% were men, the majority of patients were Caucasian (82.3%), and mean age was 46.7 years (SD 15.2). The large majority of patients did not have pulmonary embolism [976 (92.1%) patients], whereas 84 (7.9%) patients experienced pulmonary embolism.
Assay performance for pulmonary embolism exclusion claim was evaluated combining results obtained in patients with low and moderate PTP, as specified in the CLSI guideline [7] (Table 1). Results were compared with the CLSI recommendations (sensitivity: ≥97% with 95% lower limit CI: ≥90%; NPV: ≥98% with 95% lower limit CI: ≥95%) and FDA clearance requirements (sensitivity: ≥95% with 95% lower limit CI: ≥90%; NPV: ≥97% with 95% lower limit CI: ≥95%). The STA-Liatest D-Di assay performance was in line with both the CLSI and FDA guidance, with a sensitivity of 97.6% (95% lower limit CI: 91.7%) and an NPV of 99.7% (95% lower limit CI: 99.0%).
Table 1.
STA-Liatest D-Di assay performance
| Requirements | |||
| STA-Liatest D-Di (%) | CLSI (%) | FDA (%) | |
| Sensitivity | |||
| Point estimate | 97.6 | ≥97 | ≥95 |
| 95% lower limit of CI | 91.7 | ≥90 | ≥90 |
| 95% upper limit of CI | 99.7 | NA | NA |
| NPV | |||
| Point estimate | 99.7 | ≥98 | ≥97 |
| 95% lower limit of CI | 99.0 | ≥95 | ≥95 |
| 95% upper limit of CI | 100.0 | NA | NA |
CI, confidence interval; CLSI, Clinical and Laboratory Standard Institute; FDA, Food and Drug Administration; NA, not applicable; NPV, negative predictive value.
As 25 patients were lost of follow-up, we conducted a multiple imputation with the following parameters: Wells’ PTP Score, age, race, and sex for all study participants lost to follow-up for whom reference was missing. Total 10 imputations were done. In all 10 models there arose no false negatives. We can thus conclude that under a realistic imputation scenario sensitivity remains the same, whereas NPV increases slightly (because of an additional 18 true-negative study participants). Therefore, the 25 patients lost to follow-up did not impact the results.
Additional performance parameters comprised specificity (77.2%; 95% CI: 74.4–79.8%), PPV (26.9%; 95% CI: 22.0–32.2%), PLR (4.27; 95% CI: 3.79–4.82%), and NLR (0.03; 95% CI: 0.01–0.12%).
Discussion
The use of D-dimer assay in conjunction with a validated PTP score [8,12,14–17] is currently the diagnostic strategy for excluding pulmonary embolism recommended by renowned organizations such as the American College of Chest Physicians and the European Society of Cardiology [3,18,19].
The CLSI pointed out the limitations of D-dimer assays, including the lack of standardization across the various assays. The CLSI published a guideline to define the required D-dimer assay characteristics and target population [7]. This guideline attempts to overcome the aforementioned limitations by implementing high-performance criteria that are required for an assay used in combination with a validated PTP score to be cleared for well tolerated and efficient VTE diagnosis exclusion [20]. To our knowledge, this is the first international management study conducted after the publication of the CLSI guideline and in compliance with CLSI and FDA recommendations with the purpose of evaluating the pulmonary embolism exclusion diagnosis performances of a D-dimer assay used in combination with a validated PTP. This study involved nine sites between Europe and North America and enrolled the largest study population to date (>1100 patients) [17,21,22].
The use of D-dimer testing is not recommended for patients with high PTP scores, who should rather undergo imaging testing. In addition, it is well known that the usefulness of D-dimer assay in clinical practice is reduced in patients with medical conditions or in clinical settings that result in D-dimer values below or above established cutoffs regardless of the presence of thrombosis (e.g. cancer, infections, pregnancy, advanced age, and anticoagulant treatment). For these patients, results of D-dimer assay could be either false negative or false positive, leading to inaccurate diagnosis or need of further testing. In light of these considerations, in this study we adopted very strict eligibility criteria, as defined in the CLSI guideline, to select the ideal population that would benefit the most of the D-dimer testing [7]. Therefore, although all patients with suspected pulmonary embolism entered the study, only those with low/moderate PTP and not exhibiting conditions known to alter the default levels of D-dimers were included in the analysis. The results of our study clearly showed the excellent performance of the STA-Liatest D-Di assay in the study population showing very high sensitivity and NPV. The assay's sensitivity and NPV (97.6%, 95% CI lower limit 91.7% and 99.7%, 95% CI lower limit 99.0%) exceeded both the CLSI and FDA requirements [7]. Importantly, these results led clearance by FDA for the use of the STA-Liatest D-Di assay in conjunction with a validated clinical score for the exclusion of pulmonary embolism on 03 September 2014.
It is noteworthy that in the group of patients with low/moderate PTP and negative D-dimer results only two patients had experienced a pulmonary embolism at the 3-month follow-up. Interestingly, neither case was a real-negative result nor was because of factors intrinsic to the test. As mentioned, for one patient the pulmonary embolism event was likely independent from the chest pain reported at the initial visit, and for the other patient the false-negative result was because of an improper use of the STA-Liatest D-Di assay. Therefore, pulmonary embolism was successfully and safely excluded for virtually all patients with low/moderate PTP who exhibited negative D-dimer results.
The D-dimer assay specificity observed in this study (i.e. 77.2%; 95% CI: 74.4–79.8%) was higher compared with other studies [23], affirming the relevance of this assay in the selected study population. Indeed, specificity of the test is known to be lower in elderly patients, cancer patient, patients with previous VTE, and in pregnant and postpartum women indicating once more the limited use of D-dimer assays in these patients [24].
It is important to point out that the absence of an independent adjudication panel for the events occurring during the 3-month follow-up period did not bias the study results, as the assay performance was determined on the basis of a worst case. With this approach, false-negative events were always included in the analyses, though they might have been excluded by an independent adjudication panel. Similarly, as described in the results, the 25 patients lost to follow-up did not impact the results.
Our study suffered from several limitations. First, three imaging techniques, having different sensitivities and specificities were used. Indeed, the different centers participating in the study used their routine imaging techniques. However, even though performance differences can be observed across the different imaging techniques, the CLSI guideline does not recommend the use of one particular method over the others. As a consequence, the different imaging techniques used in the study were considered equivalent for pulmonary embolism diagnosis. Second, of 361 patients with positive D-dimer only 305 underwent imaging. The explanation was that patients were screened for possible pulmonary embolism diagnosis upon admission in the emergency room. As soon as pulmonary embolism was considered as a possible diagnosis, and Wells’ score resulted in a low or moderate probability of pulmonary embolism diagnosis, D-Dimer testing was ordered. One of the possible situations associated with a low or moderate probability of pulmonary embolism is the existence of a possible alternative diagnosis. Indeed, in 56 patients with positive D-dimer, the pulmonary embolism diagnosis was excluded because of an alternative diagnosis before imaging techniques can be carried out. There was no rationale for investigating furthermore those patients with specific pulmonary embolism imaging as elevated D-dimer is not specific of pulmonary embolism (as confirmed by the low PPV observed in this study). Third, because of local practices, patients with low or moderate PTP may, in a few cases, have undergone imaging techniques. In those patients, result of negative D-dimer was confirmed by negative imaging techniques. This did not therefore influence the results of the study.
To conclude, the STA-Liatest D-Di assay, when used in combination with the Wells’ score, has an NPV and sensitivity that exceed the CLSI and FDA requirements for pulmonary embolism exclusion. Importantly, our results suggest that in clinical practice D-dimer testing should be used only in relevant patients. This would not only maximize the assay performance but also minimize the economic healthcare burden avoiding unnecessary and expensive imaging tests.
Acknowledgements
The authors would like to thank Scinopsis for the support received in writing this article. Requests for reprints should be made to G.P.
The study was funded by Diagnostica Stago.
Conflicts of interest
C.A. has received speaker honoraria from Diagnostica Stago. G.P. has received lecture fees from Diagnostica Stago. The remaining authors have no conflicts of interest.
Footnotes
Haifeng Wu deceased.
References
- 1.Mos IC, Douma RA, Erkens PM, Kruip MJ, Hovens MM, van Houten AA, et al. Diagnostic outcome management study in patients with clinically suspected recurrent acute pulmonary embolism with a structured algorithm. Thromb Res 2014; 133:1039–1044. [DOI] [PubMed] [Google Scholar]
- 2.Hwang HG, Schulman S. Respiratory review of 2013: pulmonary thromboembolism. Tuberc Respir Dis (Seoul) 2013; 75:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141:e351S–e418S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Nisio M, Squizzato A, Rutjes AW, Buller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost 2007; 5:296–304. [DOI] [PubMed] [Google Scholar]
- 5.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004; 140:589–602. [DOI] [PubMed] [Google Scholar]
- 6.Freyburger G, Labrouche S. Comparability of D-dimer assays in clinical samples. Semin Vasc Med 2005; 5:328–339. [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute (CLSI). Quantitative D-dimer for exclusion of venous thromboembolic disease; approved guideline. CLSI document H59-A (ISBN 1-56238-747-2). Clinical and Laboratory Standard Institute, 940 West Valley Road, Suite 1400, Wayne, PA 19087 USA, 2011. [Google Scholar]
- 8.Wells PS, Anderson DR, Ginsberg J. Assessment of deep vein thrombosis or pulmonary embolism by the combined use of clinical model and noninvasive diagnostic tests. Semin Thromb Hemost 2000; 26:643–656. [DOI] [PubMed] [Google Scholar]
- 9.Wells PS. Integrated strategies for the diagnosis of venous thromboembolism. J Thromb Haemost 2007; 5 Suppl 1:41–50. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (CLSI). Collection, transport, and processing of blood specimens for testing plasma-based coagulation assay and molecular hemostasis assays; approved guideline - fifth edition. CLSI document H21-A5 (ISBN 1-56238-657-3). Clinical and Laboratory Standard Institute, 940 West Valley Road, Suite 1400, Wayne, PA 19087 USA, 2008. [Google Scholar]
- 11.van der Graaf F, van den Borne H, van der Kolk M, de Wild PJ, Janssen GW, van Uum SH. Exclusion of deep venous thrombosis with D-dimer testing: comparison of 13 D-dimer methods in 99 outpatients suspected of deep venous thrombosis using venography as reference standard. Thromb Haemost 2000; 83:191–198. [PubMed] [Google Scholar]
- 12.Schrecengost JE, LeGallo RD, Boyd JC, Moons KG, Gonias SL, Rose CE, Jr, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490. [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt W, Palareti G, Legnani C, Gringel E. Comparative evaluation of D-dimer assays for exclusion of deep venous thrombosis in symptomatic outpatients. Thromb Res 2003; 112:25–32. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DR, Wells PS, Stiell I, MacLeod B, Simms M, Gray L, et al. Management of patients with suspected deep vein thrombosis in the emergency department: combining use of a clinical diagnosis model with D-dimer testing. J Emerg Med 2000; 19:225–230. [DOI] [PubMed] [Google Scholar]
- 15.Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–171. [DOI] [PubMed] [Google Scholar]
- 16.Kline JA, Courtney DM, Kabrhel C, Moore CL, Smithline HA, Plewa MC, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost 2008; 6:772–780. [DOI] [PubMed] [Google Scholar]
- 17.Scarvelis D, Palareti G, Toulon P, Wells PS, Wu JR. HemosIL D-dimer HS assay in the diagnosis of deep vein thrombosis and pulmonary embolism. Results of a multicenter management study. J Thromb Haemost 2008; 6:1973–1975. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069. [DOI] [PubMed] [Google Scholar]
- 19.Konstantinides S, Torbicki A. Management of venous thrombo-embolism: an update. Eur Heart J 2014; 35:2855–2863. [DOI] [PubMed] [Google Scholar]
- 20.Reber G, de Moerloose P. Kitchen S, Olson JD, Preston FE. Standardization of D-dimer testing. Quality in laboratory hemostasis and thrombosis. Hoboken, NJ: Wiley-Blackwell Ltd; 2009. 99–109. [Google Scholar]
- 21.Legnani C, Cini M, Scarvelis D, Toulon P, Wu JR, Palareti G. Multicenter evaluation of a new quantitative highly sensitive D-dimer assay, the Hemosil D-dimer HS 500, in patients with clinically suspected venous thromboembolism. Thromb Res 2010; 125:398–401. [DOI] [PubMed] [Google Scholar]
- 22.de Moerloose P, Palareti G, Aguilar C, Legnani C, Reber G, Peetz D. A multicenter evaluation of a new quantitative highly sensitive D-dimer assay for exclusion of venous thromboembolism. Thromb Haemost 2008; 100:505–512. [DOI] [PubMed] [Google Scholar]
- 23.Boeer K, Siegmund R, Schmidt D, Deufel T, Kiehntopf M. Comparison of six D-dimer assays for the detection of clinically suspected deep venous thrombosis of the lower extremities. Blood Coagul Fibrinolysis 2009; 20:141–145. [DOI] [PubMed] [Google Scholar]
- 24.Righini M, Perrier A, De Moerloose P, Bounameaux H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost 2008; 6:1059–1071. [DOI] [PubMed] [Google Scholar]


