Abstract
Objectives: The aim of the study was to examine the trend in incidence and prevalence of dementia, use and spending of antidementia and antipsychotic drugs among dementia patients. Methods: Using 2006-2012 Medicare claims data, we identified individuals with diagnosis of dementia and collected their pharmacy claims in 2006-2012. We built regression models to test the trend in number of prescriptions and spending on antidementia, antipsychotic, and other drugs. Results: The prevalence of dementia did not change during our study period. Spending on antidementia and antipsychotic drugs creased to increase in 2011, following the patent expiration of Aricept, Zyprexa, and Seroquel; and total pharmaceutical spending did not change in 2006-2012. Use of antidementia drugs increased during our study period; however, the off-label use of antipsychotic drugs did not decrease. Discussion: Pharmaceutical spending associated with dementia may not be as concerning for Medicare as previously thought; nevertheless, policies that discourage the nonevidence-based off-label use of drugs are warranted.
Keywords: dementia, health services, medications, policy
Introduction
Dementia is a progressive cognitive impairment that affects primarily individuals 65 years of age and older. As the population ages, the prevalence of dementia will increase. Researchers have estimated that between 8 and 13 million will be affected by dementia in the United States by 2050 (Brookmeyer, Gray, & Kawas, 1998; Brookmeyer, Johnson, Ziegler-Graham, & Arrighi, 2007; Hebert, Scherr, Bienias, Bennett, & Evans, 2003; Sloane et al., 2002). The most common form of dementia, Alzheimer’s disease (AD), accounts for almost half of the dementia cases (Centers for Medicare & Medicaid Services [CMS] Chronic Conditions Data Warehouse, 2013). Although there is no cure for AD, four antidementia drugs are available to delay the cognitive impairment progression. However, these drugs have not been approved by the Food and Drug Administration (FDA) to treat other forms of dementia. In addition, many patients with dementia are commonly treated with antipsychotic drugs which may alleviate noncognitive symptoms, such as agitation, aggression, and psychosis. However, antipsychotic medications have not been approved by the FDA to treat dementia, and thus, their use among dementia patients is referred as off-label use. Off-label use of a prescription drug is defined as the use of a prescription drug in a manner or for an indication other than the regulatory agencies, in this case the FDA, has approved (Stafford, 2008). Because the use of antipsychotic medications has been shown to increase the risk of death among patients with dementia, the off-label use of antipsychotic drugs among this population is a major policy concern (Gill et al., 2007; Langballe et al., 2014).
Evaluating the time trend in pharmaceutical spending associated with dementia in Medicare is important for four main reasons: (a) annual costs of medical care per patient attributable to dementia can be as high as US$28,000, the majority of which are paid by Medicare and Medicaid (Hurd, Martorell, Delavande, Mullen, & Langa, 2013); (b) after nursing home expenditures, pharmaceutical spending is the second largest component of the costs of care for patients with dementia (Bharmal et al., 2012); (c) as described above, the use of antipsychotics and antidementia drugs in patients with dementia is not backed by evidence; and (d) previous studies that studied pharmaceutical expenditures for AD used data before 2007, and we need some updates using most recent data. For instance, using 2003-2004 Medicare data, Zhao, Kuo, Weir, Kramer, and Ash (2008) found that the annual pharmaceutical spending of patients with AD was US$4,056, over US$1,700 more than the pharmaceutical spending of similar Medicare beneficiaries without AD. Using 2002 commercial insurance data, Frytak and colleagues (2008) estimated the annual pharmaceutical spending per patient among a sample of managed health plan enrollees at US$1,879, around US$750 more than that the spending of a comparable control cohort without AD. Pharmaceutical spending attributable to AD may have decreased after the patent expiration of Aricept (brand name for donepezil) in 2010, Risperdal (brand name for risperidone) in 2007, Zyprexa (brand name for olanzapine) in 2011, and Seroquel (brand name for quetiapine) in 2012 (Frytak et al., 2008; Zhao et al., 2008). Furthermore, the use of pharmaceuticals may have changed as new evidence appears on the effectiveness and safety profile of antidementia and antipsychotic drugs (Lopez et al., 2009; Tariot et al., 2004). For example, the use of antipsychotic medications may have decreased after the introduction of a boxed warning in the packages of conventional antipsychotics in 2008 to alert on the increased mortality associated with their use (FDA, 2008).
For these reasons, we used 2006-2012 pharmacy claims from a 5% random sample of Medicare Part D beneficiaries to analyze how the use and spending on antidementia and antipsychotic drugs changed between 2006 and 2012, and to quantify the off-label use of antidementia and antipsychotic drugs among Medicare Part D beneficiaries with AD and other forms of dementia. To fully understand the potential impact of our findings, we also examined the time trend in the incidence and prevalence of AD and other forms of dementia among Medicare Part D beneficiaries, and how AD patients and those with other forms of dementia compare.
Method
Data and Sample Selection
We obtained 2006-2012 pharmacy and medical claims for a 5% random sample of Medicare beneficiaries from the CMS. First, we identified individuals who had a diagnosis of AD, related disorders, or senile dementia any time before the end of that year. We used CMS Chronic Conditions Data Warehouse (2014) indicators that trace back the first diagnosis of these conditions to January 1999. The diagnosis of AD is defined as having one claim with primary or secondary International Classification of Diseases, Ninth Revision (ICD-9) code 331.0 (CMS Chronic Conditions Data Warehouse, 2014). The diagnosis of AD related disorders or dementia (other forms of dementia) was defined as having one claim with primary or secondary ICD-9 codes 331.1x, 331.2, 331.7, 290.xx, 294.xx or 797(CMS Chronic Conditions Data Warehouse, 2014). Second, we required beneficiaries to be enrolled in a Part D plan (either stand-alone Part D or Medicare-Advantage Part D) in at least 1 month of the year. The study was declared by the institutional review board at the University of Pittsburgh as exempt because it used existing de-identified data.
Trend in Incidence and Prevalence of Dementia
Dependent variables
Incidence was calculated as the ratio between the number of new patients diagnosed with the condition during the year of analysis, and the number of Medicare Part D beneficiaries in the 5% random sample. Prevalence was calculated as the ratio between the number of total patients, which include new patients and existing patients—who were diagnosed with the condition before January 1 of the year of study—and the number of Medicare Part D beneficiaries in the 5% random sample.
Statistical analysis
We constructed ordinary least squares regression models that included year as a continuous time variable to test the change in incidence and prevalence of AD and other forms of dementia over time.
Patient Characteristics and Spending in 2012 by Type of Dementia
Dependent variables
We compared demographic, clinical characteristics and spending between patients with AD and patients with other forms of dementia. Demographics included age, gender, race, and eligibility for Medicaid coverage. Clinical characteristics included a variable indicating whether the patient died within the year, and indicators for patients with a history of selected CMS priority chronic conditions, including depression, diabetes mellitus, acute myocardial infarction, congestive heart failure, stroke or transient ischemic attack, chronic kidney disease, asthma, chronic obstructive pulmonary disease, and any cancer in the CMS priority list (definitions in Supplemental Table 1). We also compared the spending for antidementia drugs, antipsychotic agents, and other therapeutic classes of mental health drugs such as antidepressants, sedatives, and anticonvulsants between patients with AD and patients with other forms of dementia (the lists of drugs included in each category are shown in Supplemental Table 1).
Statistical analysis
We performed chi-square tests to compare the above-mentioned outcomes between patients with AD and patients with other forms of dementia.
Trend in Pharmaceutical Spending and Use
Dependent variables
Outcomes included total annualized pharmaceutical spending for all drugs of any therapeutic class, annualized spending on antidementia drugs, annualized spending on antipsychotics, annualized off-label spending on antipsychotics, annualized number of monthly prescriptions filled for antidementia drugs, annualized number of monthly prescriptions for antipsychotic medications, and annualized number of monthly off-label prescriptions for antipsychotic medications, all of them measured per patient.
To define annualized pharmaceutical spending for all drugs, we extracted all pharmacy claims for our study sample from January 1, 2006, through December 31, 2012, in the months they were enrolled in a Part D plan, and after their first diagnosis of AD or other forms of dementia. To calculate the mean total pharmaceutical spending per beneficiary per year, we summed the gross cost of all prescriptions for each beneficiary and divided them by the number of months that each beneficiary was enrolled in a Medicare Part D plan, and then multiplied by 12. We adjusted for changes in the price of pharmaceuticals over time, using the producer price pharmaceutical index with 2012 as the reference year (U.S. Department of Labor, 2015). In addition, we calculated the mean annualized number of monthly prescriptions filled for antidementia and antipsychotic drugs. To standardize monthly prescriptions, we divided the days of supply by 30; and prescriptions with less than 30 days of supply were considered as one.
We further examined two examples of off-label use of prescription drugs: first, the use of antidementia drugs among patients with forms of dementia other than AD, and second, the use of antipsychotic drugs among dementia patients who did not have a condition for which antipsychotic drugs have been approved by the FDA. To identify this second type of off-label use, we identified beneficiaries who had at least one medical claim with diagnosis codes for those conditions for which antipsychotic drugs have been approved, including schizophrenia, bipolar disorder, and several forms of depressive disorders (ICD-9 codes 295.XX or 296.XX) (FDA, 2009). After excluding these beneficiaries from the sample, we calculated the annualized off-label spending on antipsychotics and the number of monthly off-label prescriptions filled for antipsychotic drugs for each year, as explained above.
Statistical analysis
To test the change over time in the outcomes mentioned above, we constructed ordinary least squares regression models that included quarter as a continuous variable. The models constructed to evaluate the change in spending on antidementia drugs over time also included an indicator variable for the period after January 1, 2010, and an interaction term between the period after January 1, 2010, and quarter. The regression models constructed to test the change in spending on antipsychotic drugs included an indicator variable for the period after January 1, 2012, as well as an interaction term between post-January 1, 2012, and quarter. All analyses were separately performed for beneficiaries with AD and with other forms of dementia, and were conducted with statistical software SAS 9.4 (Cary, North Carolina) and Stata 14.1 (College Station, Texas).
Results
Trend in Incidence and Prevalence of Dementia
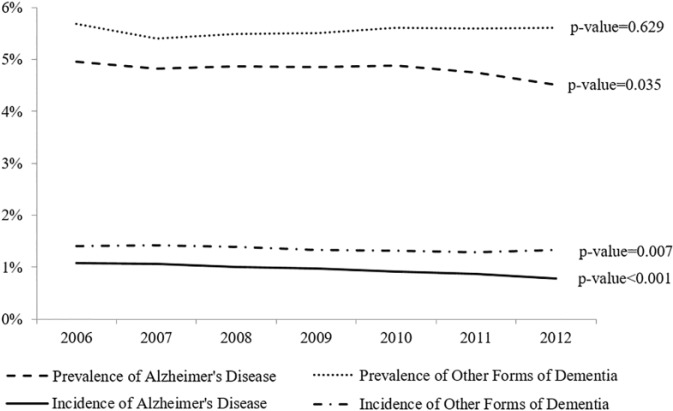
The incidence of AD in Medicare Part D decreased from 1.07% in 2006 to 0.78% in 2012 (Figure 1), with an annual decrease of 0.05% (p value < .001). The incidence of forms of dementia other than AD among Medicare Part D beneficiaries decreased from 1.41% in 2006 to 1.32% in 2012, with an annual decrease of 0.02% (p value = .007). Combined, the incidence of dementia decreased by 0.07% annually (p value < .001). The prevalence of AD among Medicare Part D beneficiaries decreased from 4.96% in 2006 to 4.51% in 2012 (annual decrease of 0.05%, p value = .035); however, the prevalence of other forms of dementia did not change during our study period (annual increase of 0.01%, p value = .629).
Figure 1.
Trend in the prevalence and incidence of Alzheimer’s disease and other forms of dementia.
Note. Incidence was calculated as the ratio between the number of new patients diagnosed with the condition during the year of analysis, and the number of Medicare Part D beneficiaries in the 5% random sample. Prevalence was calculated as the ratio between the number of total patients, which include new patients and existing patients—who were diagnosed with the condition before January 1 of the year of study—and the number of Medicare Part D beneficiaries in the 5% random sample. Patients with other dementia includes patients with Alzheimer’s disease related disorders and senile dementia. The p values indicate the p value for the annual slope change and were obtained from ordinary least squares regression models.
Patient Characteristics and Spending in 2012 by Type of Dementia
Supplemental Table 1 compares patient characteristics and annual pharmaceutical spending between beneficiaries with AD and with other types of dementia. We observed that patients with other forms of dementia were on average older than those with AD, more likely to be male and be eligible for Medicaid coverage. Patients with AD spent slightly more on antipsychotic and antidepressant medications than patients with other forms of dementia. The annual spending on psychiatric drugs per patient was US$1,276 for AD, compared with US$831 for other forms of dementia (p value < .001). In 2012, the total annual pharmaceutical spending per patient was US$3,080 for AD and US$2,952 for other forms of dementia (p value < .001).
Trend in Pharmaceutical Spending and Use
On-label spending and use of antidementia drugs by patients with Alzheimer’s disease
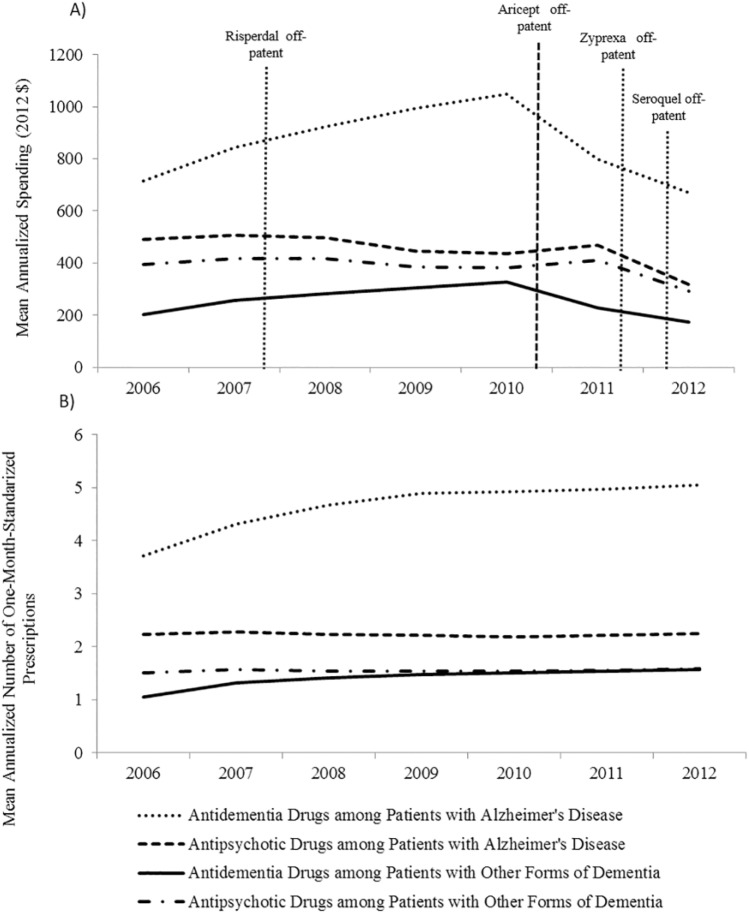
Among patients with AD, mean annual spending on antidementia drugs increased from US$714 in 2006 to US$1,049 in 2010 (Figure 2a), with a quarterly increase of US$6.09 (p value = .002) (Table 1). It peaked in 2010 and decreased to US$669 in 2012. The mean number of monthly prescriptions filled per year by patients with AD showed a steady increase trend, from 3.7 in 2006 to 5.5 in 2012 (Figure 2b), with an annual increase of 0.01, p value = .045 (Table 1).
Figure 2.
Mean annualized pharmaceutical spending and counts of monthly prescriptions filled for antidementia and antipsychotic drugs per patient diagnosed with Alzheimer’s disease or other forms of dementia.
Note. (a) Mean annualized spending for antidementia drugs and antipsychotic agents per patient, by type of dementia and year. (b) Mean annualized number of monthly prescriptions filled for antidementia and antipsychotic drugs per patient, by type of dementia and year. Patients with other dementia include patients with Alzheimer’s disease related disorders and senile dementia.
Table 1.
Results for 2006-2012 Trend in Pharmaceutical Spending and Use of Antidementia and Antipsychotic Drugs.
| Coefficient | p value | |
|---|---|---|
| Alzheimer’s disease patients | ||
| Spending | ||
| Trend in total pharmaceutical spending | −6.41 | 0.134 |
| Spending on antidementia drugs | ||
| Trend | 6.09 | 0.002 |
| Trend change after January 2011 | −15.77 | 0.012 |
| Level change after January 2011 | 252.00 | 0.074 |
| Spending on antipsychotic drugs | ||
| All AD patients | ||
| Trend | −0.84 | 0.248 |
| Trend change after January 2012 | −25.52 | 0.027 |
| Level change after January 2012 | 648.67 | 0.033 |
| AD patients with no diagnosis of FDA-approved indications for antipsychotic drugs | ||
| Trend | −1.22 | 0.047 |
| Trend change after January 2012 | −22.13 | 0.020 |
| Level change after January 2012 | 567.57 | 0.024 |
| Utilization | ||
| Trend number of prescriptions for antidementia drugs | 0.01 | 0.045 |
| Trend number of prescriptions for antipsychotic drugs | ||
| All AD patients | 0.00 | 0.698 |
| AD patients with no diagnosis of FDA-approved indications for antipsychotic drugs | 0.00 | 0.337 |
| Patients with other forms of dementia | ||
| Spending | ||
| Trend in total pharmaceutical spending | −4.10 | 0.276 |
| Spending on antidementia drugs | ||
| Trend | 2.18 | 0.001 |
| Trend change after January 2011 | −6.44 | 0.002 |
| Level change after January 2011 | 109.44 | 0.016 |
| Spending on antipsychotic drugs | ||
| All patients with other forms of dementia | ||
| Trend | −0.11 | 0.861 |
| Trend change after January 2012 | −13.45 | 0.152 |
| Level change after January 2012 | 331.16 | 0.182 |
| Patients with other forms of dementia and no diagnosis of FDA-approved indications for antipsychotic drugs | ||
| Trend | −0.53 | 0.114 |
| Trend change after January 2012 | −8.63 | 0.090 |
| Level change after January 2012 | 217.25 | 0.106 |
| Utilization | ||
| Trend number of prescriptions for antidementia drugs | 0.01 | 0.005 |
| Trend number of prescriptions for antipsychotic drugs | ||
| All patients with other forms of dementia | 0.00 | 0.789 |
| Patients with other forms of dementia and no diagnosis of FDA-approved indications for antipsychotic drugs | 0.00 | 0.330 |
Note. Bold indicates significant results. Results obtained from ordinary least squares regression models. All models included quarter as a continuous time variable. The models constructed to evaluate the change in spending on antidementia drugs over time also included an indicator variable for the period after January 1, 2010, and an interaction term between the period after January 1, 2010, and quarter. The regression models constructed to test the change in spending on antipsychotic drugs included an indicator variable for the period after January 1, 2012, as well as an interaction term between post-January 1, 2012, and quarter. AD = Alzheimer’s disease; FDA = Food and Drug Administration.
The drop in antidementia spending in 2011 was explained by Aricept (brand name for donepezil, the most widely used antidementia drug) going off patent in November 2010: In 2010, brand name donepezil (Aricept) accounted for 45% of the antidementia prescriptions filled by our study sample. In 2011, after its patent expiration, Aricept only represented 3% of the prescriptions filled for antidementia drugs by our study sample. In this year, generic donepezil represented 44% of the antidementia prescriptions filled by our study sample, compared with 0.3% in 2010.
Off-label spending and use of antidementia drugs by patients with other forms of dementia
The mean annualized off-label spending on antidementia drugs among patients with forms of dementia other than AD was US$203 in 2006, and US$327 in 2010, when it peaked; and it decreased to US$174 in 2012 (Figure 2a and Table 1). The mean number of monthly prescriptions filled off-labelly for antidementia drugs per year among patients with other forms of dementia increased from 1.05 in 2006 to 1.57 in 2007 (Figure 2b), with a quarterly increase of 0.01, p value = .005 (Table 1).
Off-label spending and use of antipsychotic drugs by patients with dementia
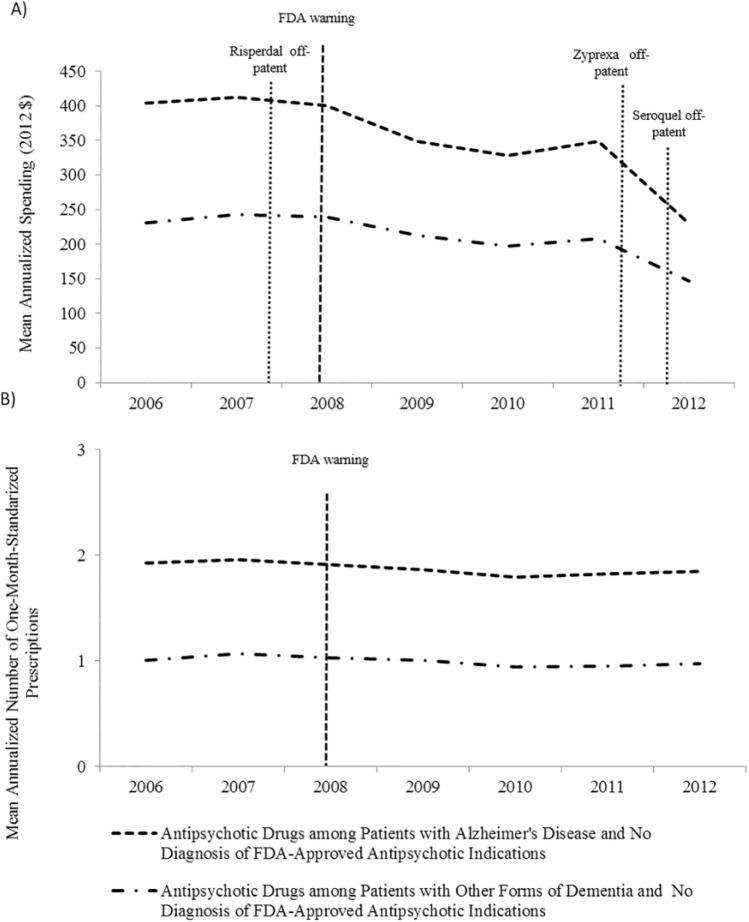
Figure 3 shows the change in off-label spending and number of prescriptions filled for antipsychotic drugs after excluding beneficiaries with conditions for which antipsychotic drugs have been approved. We found that the mean annual off-label spending on antipsychotic drugs by AD patients decreased US$1.22 per quarter between 2006 and 2011, and US$23.35 per quarter in 2012, after the patent expiration date of Zyprexa and Seroquel (Figure 3a and Table 1). However, off-label spending on antipsychotic drugs by patients with other forms of dementia did not significantly decrease during our study period (quarterly decrease of 0.53, p value = .114) (Figure 3a and Table 1). The mean number of prescriptions filled off-labelly for antipsychotic drugs remained relatively steady for two groups (Figure 3b and Table 1).
Figure 3.
Mean annualized pharmaceutical spending and counts of monthly prescriptions for antidementia and antipsychotic drugs per patient diagnosed with Alzheimer’s disease or other forms of dementia and no diagnosis of FDA-approved indications of antipsychotic drugs.
Note. (a) Mean annualized off-label spending for antipsychotic agents per patient, by type of dementia and year. (b) Mean annualized number of monthly prescriptions filled off-labelly for antipsychotic drugs per patient, by type of dementia and year. FDA = Food and Drug Administration.
Analysis performed after excluding from the sample patients with at least one claim with diagnosis codes ICD-9 295.XX or 296.XX in the year of analysis. In the 5% random sample, the proportion of patients with AD (and other forms of dementia) who had a diagnosis of any FDA-approved indication for antipsychotic use was 4.6% (6.2%) in 2006, 4.9% (6.3%) in 2007, 4.9% (6.3%) in 2008, 5.1% (6.5%) in 2009, 5.9% (7.3%) in 2010, 6.0% (7.5%) in 2011, and 6.2% (7.5%) in 2012.
The drop in antipsychotic spending in 2012 followed the patent expiration date of two commonly prescribed brand name antipsychotics—Zyprexa (brand name for olanzapine) in October 2011 and Seroquel (brand name for quetiapine) in March 2012. In 2011, prescriptions for Zyprexa and Seroquel accounted for 9% and 35% of the total prescriptions for antipsychotic agents filled by our study sample, compared with 1% and 4% in 2012. In 2012, prescriptions for generic olanzapine and quetiapine represented 11% and 36% of the total number of antipsychotic prescriptions filled by our study sample.
Total pharmaceutical spending for all drugs used by patients with dementia
Supplemental Figure 1 shows the trend in total pharmaceutical spending between 2006 and 2012 by type of dementia. Total pharmaceutical spending per year did not significantly change during our study period among patients with AD (quarterly decrease of US$6.41, p value = .134) or among those with other forms of dementia (quarterly decrease of US$4.10, p value = .276) (Table 1).
Discussion
In this article, we analyzed how the use and spending on antidementia and antipsychotic drugs changed between 2006 and 2012. There are four main findings. First, the incidence of dementia decreased between 2006 and 2012; however, the prevalence did not change during our study period. Second, the number of monthly prescriptions filled for antidementia drugs increased not only among AD patients but also among those with other forms of dementia, for whom these drugs have not been approved. However, spending on antidementia drugs creased to increase in 2010, after the patent expiration of date of Aricept. Third, the off-label use of antipsychotic agents did not decrease during our study period, but the spending decreased in 2011, following the patent expiration date of Zyprexa and Seroquel. Fourth, in 2012, the pharmaceutical spending of patients with AD and other forms of dementia was US$3,080 and US$2,952; which is 30% and 25% greater than in the general Medicare population, respectively (Trish, Joyce, & Goldman, 2014).
Our study contributes to the current literature in the following three areas. First, we separately analyzed pharmaceutical spending for AD and other forms of dementia. This is important because antidementia drugs are only approved to treat AD but no other forms of dementia. Second, we are the first to study the time trend in spending on antipsychotics after the FDA included boxed warnings in the package of these medications to alert on the increased mortality associated with their use. Third, previous studies only presented pharmaceutical spending associated with dementia using data before 2007, but we updated results through 2012. This is important because, as explained in the “Introduction” section, the patents of some of the most commonly used antidementia and antipsychotic drugs have expired in the last decade.
Our article is subject to two main limitations. First, we did not study separately beneficiaries enrolled in Medicare-Advantage Part D plants and Stand-Alone Plans. Medicare-Advantage Part D plans are incentivized to maximize the value of the pharmaceuticals prescribed because these plans benefit from medical expenses offsets. As a result, off-label use of antidementia and antipsychotic agents may be less common about beneficiaries enrolled in Medicare-Advantage Part D plans than those in Stand-Alone Plans. In further research, this analysis could be stratified by type of Part D plan, and could be extended to other health care settings Second, using Medicare claims to identify dementia may not be entirely accurate (Taylor, Ostbye, Langa, Weir, & Plassman, 2009). In our study, we used the CMS Chronic Conditions Data Warehouse definition of AD and of other forms of dementia, which identify correctly around 75% and 80% of the patients, respectively (Taylor, Fillenbaum, & Ezell, 2002). These definitions are less likely to identify patients with less severe dementia, who incur smaller medical expenses. For this reason, the use of Medicare claims data results in an overestimation of the costs of care per person attributed to dementia (Taylor et al., 2009). Nevertheless, the impact of the use of Medicare claims data in the estimation of the costs of care associated with dementia should not change over time, and therefore, it should not affect our main results for the change in use and spending on antidementia and antipsychotic drugs over time.
Our results have three main policy implications: First, with pharmaceutical spending being the second largest component of the costs of care for patients with dementia (Bharmal et al., 2012), there were concerns about increasing pressure for Medicare Part D spending for patients with dementia (Fowler et al., 2013). We found that the total pharmaceutical spending of patients with AD was US$3,080, US$725 more than the general Medicare population. This is consistent with the incremental pharmaceutical spending associated with AD reported by Frytak and colleagues (2008) using data from a population of managed health plan enrollees in 2002 (US$751). However, we found that spending on antidementia drugs ceased to increase after Aricept went off patent and that total pharmaceutical spending did not change during our study period. As a result, the total pharmaceutical spending of patients with dementia may not be as concerning for Medicare Part D as previously thought. Second, even though the total pharmaceutical spending in dementia may not be especially concerning for Medicare, the fact that a considerable proportion of this spending (7.5% for Alzheimer patients and 11% for other forms of dementia) was spent on the off-label use of antidementia and antipsychotic drugs is highly concerning. This is because these forms of off-label use are not supported by evidence, and, in some cases, may be harmful. For example, the off-label use of antipsychotic drugs among patients with dementia has been shown to increase the risk of death (Gill et al., 2007; Langballe et al., 2014). To prevent this potentially harmful off-label use, FDA introduced a black box warning in atypical antipsychotics in 2005 and conventional antipsychotics in 2008 (FDA, 2008). However, we found that the number of prescriptions filled for antipsychotics among patients with dementia did not decrease between 2006 and 2012. This suggests that black-boxed warnings are not an effective strategy to reduce the potentially harmful off-label use, and therefore, more innovative policy interventions are needed to mitigate the use of drugs that are not supported by clinical evidence. For example, one possible policy intervention is to align pharmaceutical reimbursement schemes with the value of off-label use for each specific treatment in a subgroup of population. Third, the combination therapy of antidementia drugs has been shown to delay nursing home admission among patients with AD (Lopez et al., 2009), and lead to downstream cost savings in nursing home expenditures (Touchon et al., 2014). However, in our data, we found that the use of antidementia drugs among AD patients only experienced a slight increase. This again suggests that the new evidence on drug effectiveness does not affect the use of drugs quickly and some value-based policy interventions may be helpful.
Conclusion
The use of antidementia drugs increased among patients with AD; but also among those with other forms of dementia, for whom these drugs have not been approved. The off-label use of antipsychotic agents did not decrease during our study period. However, spending on antidementia and antipsychotic drugs creased to increase in 2010 and 2011, respectively, after the patent expiration of date of Aricept, Zyprexa, and Seroquel. Total pharmaceutical spending among patients with dementia did not change during our study period, and in 2012, it was approximately 30% higher than pharmaceutical spending among the general Medicare population. Around 9% of the annual pharmaceutical costs incurred by patients with dementia were spent on the off-label use of drugs, which is not supported by evidence and could even be harmful.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge funding from the Institute of Medicine (HHSP22320042509XI), Commonwealth Foundation, the National Institute of Mental Health (No. R21 MH100721), and “La Caixa” Foundation, Spain.
References
- Bharmal M. F., Dedhiya S., Craig B. A., Weiner M., Rosenman M., Sands L. P., . . .Thomas J. (2012). Incremental dementia-related expenditures in a Medicaid population. American Journal of Geriatric Psychiatry, 20, 73-83. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R., Gray S., Kawas C. (1998). Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health, 88, 1337-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H. M. (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & Dementia, 3, 186-191. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse. (2013). Medicare beneficiary prevalence for chronic conditions for 2002 through 2011. Retrieved from https://www.ccwdata.org/web/guest/medicare-charts/medicare-chronic-condition-charts
- Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse. (2014). 27 chronic condition algorithm. Retrieved from https://www.ccwdata.org/web/guest/condition-categories
- Food and Drug Administration. (2008). Postmarket drug safety information for patients and providers: Conventional antipsychotics. Retrieved from http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm
- Food and Drug Administration. (2009). Zyprexa prescribing information. Retrieved from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020592
- Fowler N., Chen Y.-F., Thurton C., Men A., Rodriguez E., Donohue J. (2013). The impact of Medicare prescription drug coverage on the use of antidementia drugs. BMC Geriatrics, 13(1), Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frytak J. R., Henk H. J., Zhao Y., Bowman L., Flynn J. A., Nelson M. (2008). Health service utilization among Alzheimer’s disease patients: Evidence from managed care. Alzheimer’s & Dementia, 4, 361-367. [DOI] [PubMed] [Google Scholar]
- Gill S. S., Bronskill S. E., Normand S. L., Anderson G. M., Sykora K., Lam K., . . .Rochon P. A. (2007). Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine, 146, 775-786. [DOI] [PubMed] [Google Scholar]
- Hebert L. E., Scherr P. A., Bienias J. L., Bennett D. A., Evans D. A. (2003). Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Archives of Neurology, 60, 1119-1122. [DOI] [PubMed] [Google Scholar]
- Hurd M. D., Martorell P., Delavande A., Mullen K. J., Langa K. M. (2013). Monetary costs of dementia in the United States. New England Journal of Medicine, 368, 1326-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langballe E. M., Engdahl B., Nordeng H., Ballard C., Aarsland D., Selbaek G. (2014). Short- and long-term mortality risk associated with the use of antipsychotics among 26,940 dementia outpatients: A population-based study. American Journal of Geriatric Psychiatry, 22, 321-331. [DOI] [PubMed] [Google Scholar]
- Lopez O. L., Becker J. T., Wahed A. S., Saxton J., Sweet R. A., Wolk D. A., . . .Dekosky S. T. (2009). Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. Journal of Neurology, Neurosurgery, & Psychiatry, 80, 600-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane P. D., Zimmerman S., Suchindran C., Reed P., Wang L., Boustani M., Sudha S. (2002). The public health impact of Alzheimer’s disease, 2000-2050: Potential implication of treatment advances. Annual Review of Public Health, 23, 213-231. [DOI] [PubMed] [Google Scholar]
- Stafford R. S. (2008). Regulating off-label drug use—Rethinking the role of the FDA. New England Journal of Medicine, 358, 1427-1429. [DOI] [PubMed] [Google Scholar]
- Tariot P. N., Farlow M. R., Grossberg G. T., Graham S. M., McDonald S., Gergel I. (2004). Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: A randomized controlled trial. Journal of the American Medical Association, 291, 317-324. [DOI] [PubMed] [Google Scholar]
- Taylor D. H., Jr., Fillenbaum G. G., Ezell M. E. (2002). The accuracy of Medicare claims data in identifying Alzheimer’s disease. Journal of Clinical Epidemiology, 55, 929-937. [DOI] [PubMed] [Google Scholar]
- Taylor D. H., Jr., Ostbye T., Langa K. M., Weir D., Plassman B. L. (2009). The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. Journal of Alzheimer’s Disease, 17, 807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchon J., Lachaine J., Beauchemin C., Granghaud A., Rive B., Bineau S. (2014). The impact of memantine in combination with acetylcholinesterase inhibitors on admission of patients with Alzheimer’s disease to nursing homes: Cost-effectiveness analysis in France. European Journal of Health Economics, 15, 791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trish E., Joyce G., Goldman D. P. (2014). Specialty drug spending trends among Medicare and Medicare Advantage enrollees, 2007-11. Health Affairs, 33, 2018-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Labor. (2015). Bureau of Labor Statistics Producer Price Index Industry Data. Retrieved from http://data.bls.gov/timeseries/PCU325412325412
- Zhao Y., Kuo T.-C., Weir S., Kramer M., Ash A. (2008). Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Services Research, 8(1), Article 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.