Abstract
Background
Although we have previously reported that intravenous resveratrol administration inhibits the nociceptive neuronal activity of spinal trigeminal nucleus caudalis neurons, the site of the central effect remains unclear. The aim of the present study was to examine whether acute intravenous resveratrol administration in the rat attenuates central glutamatergic transmission of spinal trigeminal nucleus caudalis neurons responding to nociceptive mechanical stimulation in vivo, using extracellular single-unit recordings and microiontophoretic techniques.
Results
Extracellular single-unit recordings using multibarrel electrodes were made from the spinal trigeminal nucleus caudalis wide dynamic range neurons responding to orofacial mechanical stimulation in pentobarbital anesthetized rats. These neurons also responded to iontophoretic application of glutamate, and the evoked neuronal discharge frequency was significantly increased in a current-dependent and reversible manner. The mean firing frequency evoked by the iontophoretic application of glutamate (30, 50, and 70 nA) was mimicked by the application of 10 g, 60 g, and noxious pinch mechanical stimulation, respectively. The mean firing frequency of spinal trigeminal nucleus caudalis wide dynamic range neurons responding to iontophoretic application of glutamate and N-methyl-D-aspartate were also significantly inhibited by intravenous administration of resveratrol (2 mg/kg) and the maximal inhibition of discharge frequency was observed within 10 min. These inhibitory effects lasted approximately 20 min. The relative magnitude of inhibition by resveratrol of the glutamate-evoked spinal trigeminal nucleus caudalis wide dynamic range neuronal discharge frequency was similar to that for N-methyl-D-aspartate iontophoretic application.
Conclusion
These results suggest that resveratrol suppresses glutamatergic neurotransmission of the spinal trigeminal nucleus caudalis neurons responding to nociceptive mechanical stimulation via the N-methyl-D-aspartate receptor in vivo, and resveratrol may be useful as a complementary or alternative therapeutic agent for the treatment of trigeminal nociceptive pain.
Keywords: Iontophoretic application, resveratrol, trigeminal spinal nucleus caudalis, single-unit recording, alternative medicine
Background
Recent reports have described the use of complementary and alternative medicine (CAM), such as herbal medicines and acupuncture, for the treatment of persistent clinical chronic pain.1–3 Additionally, the potential effects of diet and dietary supplementation on conditions associated with pain have been the focus of considerable research.4–6 Resveratrol (trans-3,4′,5-trihydroxystilbene) is a plant polyphenol7,8 and has several beneficial biological actions, including anti-oxidative, anti-inflammatory, neuroprotective, anticancer, and cardioprotective effects.8–11 Because resveratrol has no known toxic side effects,12 it could be a candidate CAM for the therapeutic treatment of nociceptive and inflammatory pain.13 For orofacial pain, it is well known that, in addition to the upper cervical (C1–C2) dorsal horn, the spinal trigeminal nucleus caudalis (SpVc) is an important relay station for trigeminal nociceptive inputs from inflammation and tissue injury.14–16 Previous studies have demonstrated that wide dynamic range (WDR) neurons in the SpVc region have an important role in the mechanism underlying hyperalgesia/allodynia and/or referred pain associated with orofacial pain.17–19 Recently, we have reported that intravenous resveratrol administration suppresses SpVc WDR neuronal excitability via both peripheral and central mechanisms. Therefore, resveratrol could be a CAM used for the treatment of trigeminal nociceptive pain;20 however, the precise site of the central effects of resveratrol still needs to be determined.
Previous reports suggest that resveratrol modulates the neuronal excitability of the central nervous system. For example, in a hippocampal slice preparation, resveratrol significantly suppressed the glutamate-induced currents in post-synaptic CA1 pyramidal neurons21 that contribute to excitatory synaptic transmission. There are reports that resveratrol decreases action potential duration and L-type Ca2+ currents in excitable tissues,22,23 suggesting that presynaptic Ca2+ channels may contribute to the suppression of nociceptive transmission. In the trigeminal system, intravenous injection of both N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the excitatory responses of C1-C2 spinal neuronal activity to electrical stimulation of the sagittal sinus.24 This also supports the report that iontophoretic application of NMDA and non-NMDA glutamate receptor antagonists attenuates tooth-pulp-evoked C1 neuronal excitation.25 We previously demonstrated, using extracellular single-unit recordings and microiontophoretic techniques, that activation of postsynaptic glutamate receptor-evoked C1 neuronal excitation was modulated by α2-adrenergic receptor agonists.26 Since the intrathecal administration of NMDA receptor antagonists inhibits nociceptive behavior,27,28 it can be assumed that the NMDA receptor signaling system contributes to spinal nociceptive transmission. These observations led us to hypothesize that intravenous resveratrol administration would attenuate the noxious mechanical stimulation-induced glutamatergic neurotransmission in SpVc neurons through a central mechanism, possibly a postsynaptic NMDA receptor mechanism. However, the local effects of resveratrol on trigeminal neuronal activity in vivo in response to nociceptive mechanical stimulation remain to be determined. Therefore, the aim of the present study was to investigate whether acute intravenous resveratrol administration attenuates central postsynaptic glutamatergic neuronal transmission in the SpVc neurons responding to nociceptive mechanical stimulation in vivo, by using extracellular single-unit recording and microiontophoretic application combined with multibarrel electrodes.
Methods
The experiments described herein were approved by the Animal Use and Care Committee of Azabu University and were performed in accordance with the guidelines of the International Association for the Study of Pain.29 Every effort was made to minimize the number of animals used and their suffering.
Extracellular single-unit recording of WDR neuronal activity in the SpVc with multibarrel electrodes
Electrophysiological recordings were made in 15 adult male Wistar rats weighing 250–290 g. Rats were anesthetized with pentobarbital sodium (45 mg/kg, i.p.) and anesthesia was maintained with additional doses of pentobarbital sodium (2–3 mg/kg/h) through a jugular vein cannula, as required. The level of anesthesia was confirmed by the absence of the corneal reflex and a lack of response to paw pinching. Rectal temperature was maintained at 37.0 ± 0.5℃ with a homeothermic blanket during recording. Rats were placed in a stereotaxic apparatus, and the activity of a single neuron from the SpVc region, according to the stereotaxic coordinates of Paxinos and Watson,30 was recorded extracellularly.
Single neuronal activity was recorded by means of a three-barreled glass micropipette filled with 2% pontamine sky blue with 0.5 M sodium acetate as described in our previous studies.17,18 Neuronal activity was amplified (DAM 80; World Precision Instruments, Sarasota, FL), filtered (0.3–10 kHz), monitored with an oscilloscope (SS-7672; Iwatsu, Tokyo, Japan), and recorded on a polygraph (8M14; NEC-Sanei, Tachikawa, Japan) for subsequent off-line analysis using Power Lab and Chart 5 software (AD Instruments, Oxford, UK). The technique of iontophoretic application was the same as described in our previous reports.17,18,25,31 One of the two lateral barrels of the micropipette contained 160 mmol/L NaCl which was used for balancing currents to prevent the occurrence of tip polarization artifacts. The remaining lateral barrel contained NMDA (200 mmol/L in 160 mmol/L NaCl, pH 8.5) or L-glutamate (100 mmol/L in 160 mmol/L NaCl, pH 8.5; Nacalai Tesque, Kyoto, Japan), as described previously.18,26 The currents for ejecting, retaining, and balancing were provided by a constant current unit (Dia Medical, DPI-25, Japan). The drugs were ejected with 30–70 nA cationic currents, and 10–25 nA retaining currents were used.
Experimental protocols
Extracellular recordings of SpVc WDR unit activity were made as follows. Mechanical stimulation was used as a stimulus to identify the repetitive field quickly and to avoid sensitization of peripheral receptors. Single units that responded to stimulation of the left side of the orofacial skin (whisker pad) with a brush and a set of von Frey hairs (Semmes-Weinstein Monofilaments; North Coast Medical, Gilroy, CA) were identified. Noxious pinch stimulation, which evoked a pain sensation when applied to a human subject, was applied to the orofacial area using forceps. After identification of WDR SpVc neurons responding to stimulation of the whisker pad, we determined whether there was spontaneous discharge. The threshold for mechanical stimulation was determined using non-noxious and noxious mechanical stimulation (5 s) with von Frey hairs (1, 4, 6, 10, 15, 26, and 60 g). The mechanical receptive field of neurons was mapped by probing the facial skin with von Frey hairs, and then outlined on a life-sized drawing of a rat on tracing paper. The WDR neuronal discharges induced by mechanical stimulation were quantified by subtracting background activity from evoked activity. Spontaneous discharge frequencies were determined over a period of 2–5 min. If no discharge was recorded, the cell was deemed a silent neuron. The mean firing rate of SpVc WDR neurons evoked by mechanical stimulation was compared before and after resveratrol administration. The focus of the present study was on the effects of resveratrol on SpVc WDR neuronal activity, but we did not examine nociceptive-specific neurons.32 For this reason, we must stress that WDR neurons in the SpVc region contribute to the mechanism of hyperalgesia and/or referred pain associated with orofacial pain.16,19 Post-stimulus histograms (bin = 100 ms) were generated in response to each stimulus.
The effects of resveratrol (2 mg/kg, i.v., equivalent to 10 mmol/L), injected through a cannula into the jugular vein, were evaluated 5, 10, 20, and 30 min after administration because peak effect and recovery were thought to occur during this period. Resveratrol was dissolved in dimethyl sulfoxide. The stock solution was stored at −20℃ in small aliquots until use and diluted in saline. After the identification of SpVc WDR neurons responding to mechanical stimulation, we determined the optimum currents of iontophoretically applied glutamate and NMDA. In brief, to assess the effectiveness of iontophoretically applied glutamate and NMDA on the firing frequency of SpVc WDR neurons, we varied the current intensity (30, 50, and 70 nA; 5 s) to enable us to determine the iontophoretic conditions. Iontophoretic application of glutamate (>60 nA) showed a maximal increase in spinal neuronal activity at a minimal current, as described in our previous study.31 The responses of SpVc WDR neuron activity to the iontophoretic application of glutamate or NMDA (50 nA; 5 s) were compared before and after intravenous resveratrol. SpVc WDR neuronal activity after the iontophoretic application of glutamate or NMDA was recorded three times at intervals of 5 s.
Identification of recording sites
Recording sites of SpVc WDR neuronal activity were identified as described previously.16,17 Briefly, at the end of the recording sessions, rats were deeply anesthetized, and anodal DC currents (30 μA, 5 min) were passed through a recording micropipette. The rats were then perfused transcardially with saline and 10% formalin. Frozen coronal sections (30 µm) were cut and stained with hematoxylin–eosin. Recording sites were identified from the blue spots, and the path of the electrode track was constructed in combination with micromanipulator readings.
Data analysis
Values are expressed as the mean ± SEM. Statistical analyses were performed using two-way repeated-measures analysis of variance followed by Tukey–Kramer or Dunnett’s post hoc tests for electrophysiological data. P < 0.05 was considered significant.
Results
Effect of iontophoretic application of glutamate on the excitability of SpVc WDR neurons responding to mechanical stimulation of the orofacial area
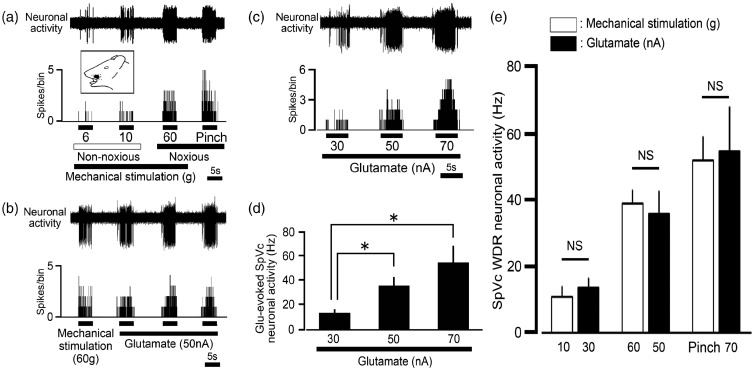
Figure 1(a) shows a typical example of SpVc WDR neurons responding to non-noxious and noxious mechanical stimulation of the whisker pad. Since graded mechanical stimulation applied to the most sensitive area of the receptive field showed increased firing frequency of SpVc neurons as proportional to stimulus intensity, these neurons were recorded as belonging to the category of WDR neurons, as described previously.20,33,34 Their recording sites were found in layers I–III (n = 14, 63%) and IV–V (n = 5, 27%) in the dorsolateral region of SpVc (obex −1.0 ∼ −2.0 mm). In this study, we found that 19 SpVc WDR neurons responding to non-noxious and noxious mechanical stimulation of the whisker pad were also activated by iontophoretic application of glutamate (Figure 1(b)). Glutamate-evoked discharge was reproducible at intervals of 5 s as illustrated in Figure 1(b). The firing frequency evoked by the iontophoretic application of glutamate was current-dependently increased (30, 50, and 70 nA; Figure 1(c)). The mean discharge frequencies of SpVc WDR neurons evoked by iontophoretic glutamate application significantly increased in a current-dependent manner (Figure 1(d); P < 0.05, n = 4). There were no significant differences between the discharge frequencies evoked by iontophoretic glutamate (30, 50, and 70 nA) and mechanical stimulation (10 g, 60 g, and noxious pinch), respectively (Figure 1(e)). We also observed that iontophoretic application of vehicle (160 mmol/l, NaCl, pH 4.5) had no significant effect on SpVc WDR neuronal activity, as previously described (n = 4, data not shown).16
Figure 1.
Glutamate iontophoretic application-evoked neuronal discharges of spinal trigeminal nucleus caudalis (SpVc) wide dynamic range (WDR) neurons responding to mechanical stimulation of the orofacial area. (a) Typical example of SpVc WDR neuronal activity evoked by non-noxious (6, 10 g) and noxious mechanical stimulation (60 g, noxious pinch) of the orofacial skin. Upper trace: SpVc WDR neuronal activity. Lower trace: poststimulus histogram. Inset: Receptive field of the whisker pad in the orofacial area. Blackened area indicates the location and size of the receptive field. (b) Iontophoretic application of glutamate (50 nA, 5 s) evoked reproducible neuronal discharges from SpVc WDR neurons (interval of 10 s) responding to noxious mechanical stimulation of the orofacial area (60 g, von Frey hair, 5 s). (c) Typical example of glutamate iontophoretic application-induced SpVc WDR neuronal activity (30, 50 and 70 nA; 5 s). (d) Summary of the effect of iontophoretic application of glutamate (30, 50, 70 nA; 5 s) on evoked neuronal discharges of SpVc WDR neurons. P < 0.05, 30 nA vs. 50 nA and 70 nA. (e) Comparison of neuronal discharges of SpVc WDR neurons evoked by iontophoretic application of glutamate (30, 50, 70 nA; 5 s) and mechanical stimulation. NS, not significant.
Effect of intravenous administration of resveratrol on the iontophoretic application of glutamate-evoked neuronal discharge
Figure 2(a) shows a typical example of the effect of intravenous administration of resveratrol on SpVc WDR neuronal excitability in response to iontophoretic application of glutamate. Glutamate-evoked discharges of SpVc WDR neurons were inhibited by resveratrol administration (2 mg/kg, i.v.) in a reversible manner. As shown in Figure 2(b), the mean firing frequency of SpVc WDR neurons in response to iontophoretic application of glutamate was significantly inhibited by intravenous administration of resveratrol (2 mg/kg) and maximal inhibition of discharge frequency was seen within 10 min (P < 0.05, n = 4). These inhibitory effects were reversed after approximately 20 min (Figure 2(a) and (b)). No significant change was observed in the mean receptive field size after resveratrol administration, as described previously.20 The relative magnitude of inhibition by resveratrol of SpVc WDR neuronal discharge frequency was significantly greater for 50 nA than 30 nA glutamate iontophoretic application (67% ± 5% vs. 40% ± 5%, respectively; P < 0.05). Also, the relative magnitude of inhibition by resveratrol of SpVc WDR neuronal discharge frequency was significantly greater for 70 nA than 30 nA glutamate iontophoretic application (71% ± 7% vs. 40% ± 5%, respectively; P < 0.05).
Figure 2.
Effect of intravenous administration of resveratrol on glutamate iontophoretic application-evoked spinal trigeminal nucleus caudalis (SpVc) wide dynamic range (WDR) neuronal discharge frequency. (a) Typical examples of glutamate iontophoretic application-evoked (30, 50, 70 nA; 5 s) neuronal discharges of SpVc WDR neurons responding to mechanical stimulation of the orofacial area before, and 5 and 20 min after, intravenous administration of 2 mg/kg resveratrol. (b) Summary of glutamate iontophoretic application-evoked neuronal discharges of SpVc WDR neurons responding to mechanical stimulation of the orofacial area before, and 5 and 20 min after, intravenous administration of resveratrol. *P < 0.05.
Effect of intravenous administration of resveratrol on the iontophoretic application of NMDA-evoked neuronal discharge frequency
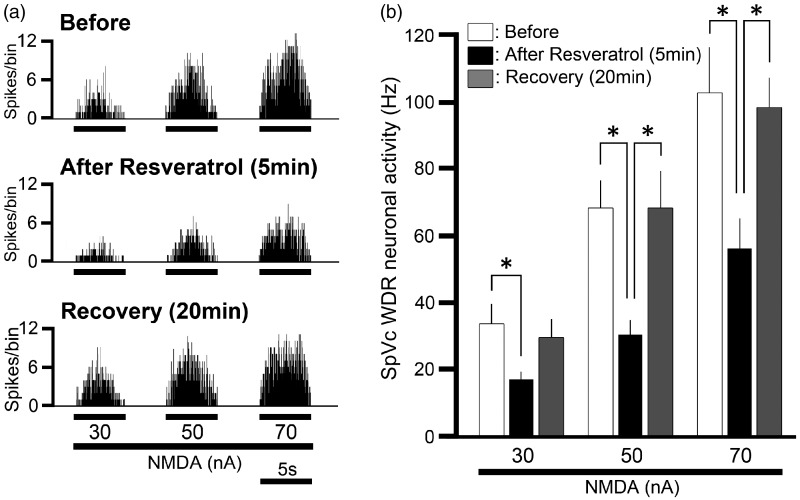
Next, we tested whether intravenous administration of resveratrol affected the iontophoretic application of NMDA-evoked neuronal discharge frequencies. Figure 3(a) shows the effect of intravenous administration of resveratrol on SpVc WDR neuronal excitability in response to iontophoretic application of NMDA. The NMDA-evoked discharges of SpVc WDR neurons were inhibited by resveratrol administration (2 mg/kg, i.v.) in a reversible manner. As shown in Figure 3(b), the mean firing frequency of SpVc WDR neurons responding to iontophoretic application of NMDA were significantly inhibited by intravenous administration of resveratrol (2 mg/kg, P < 0.05), and maximal inhibition of discharge frequency was seen within 10 min. These inhibitory effects lasted approximately 20 min.
Figure 3.
Effect of intravenous administration of resveratrol on N-methyl-D-aspartate (NMDA) iontophoretic application-evoked spinal trigeminal nucleus caudalis (SpVc) wide dynamic range (WDR) neuronal discharge frequency. (a) Typical examples of NMDA iontophoretic application-evoked (30, 50, 70 nA; 5 s) neuronal discharges of SpVc WDR neurons responding to mechanical stimulation of the orofacial area before, and 5 and 20 min after, intravenous administration of 2 mg/kg resveratrol. (b) Summary of NMDA iontophoretic application-evoked neuronal discharges of SpVc WDR neurons responding to mechanical stimulation of the orofacial area before, and 5 and 20 min after, intravenous administration of resveratrol. *P < 0.05.
Comparison of the inhibition of discharge frequency of SpVc WDR neurons between noxious mechanical stimuli, iontophoretic glutamate, and NMDA
Finally, we compared the magnitude of the inhibitory effect of resveratrol on SpVc WDR neuronal activity evoked by noxious mechanical stimulation (60 g), glutamate (50 nA), and NMDA (50 nA), because the mean firing frequency evoked by 30, 50, and 70 nA iontophoretic glutamate application was mimicked by the application of 10 g, 60 g, and pinch mechanical stimulation, respectively (Figure 1(e)). In this study, the mean magnitude of inhibition by resveratrol of 60 g noxious mechanical stimulation evoked SpVc neuronal discharge frequency was 62.1 ± 2.8% (n = 4). Figure 4 shows the mean magnitude of inhibition of noxious stimulation (60 g)-, glutamate (50 nA)-, and NMDA (50 nA)-induced SpVc WDR neuronal firing by resveratrol. The mean magnitude of inhibition by resveratrol of glutamate-evoked SpVc neuronal discharge frequency was almost equal to that evoked by NMDA iontophoretic application.
Figure 4.
Comparison of the inhibition of discharge frequencies by resveratrol of spinal trigeminal nucleus caudalis (SpVc) wide dynamic range (WDR) neurons evoked by noxious mechanical stimuli, glutamate and N-methyl-D-aspartate (NMDA) iontophoretic application currents. Noxious mechanical stimulation (60 g), glutamate and NMDA iontophoretic application (50 nA each). NS, not significant.
Discussion
Methodological consideration
We have recently reported that acute intravenous resveratrol administration suppresses SpVc WDR neuronal excitability via both peripheral and central mechanisms.20 More recently, we also demonstrated subcutaneous local injection of resveratrol into the peripheral receptive field suppresses the excitability of SpVc neurons to respond to non-noxious and noxious stimulation, possibly by inhibiting sodium channels in the nociceptive nerve terminals of primary sensory neurons (trigeminal ganglion neurons).34 These findings suggest that acute intravenous resveratrol administration in the rat attenuates the excitability of neuronal transmission of SpVc neurons responding to nociceptive mechanical stimulation. However, the precise site of the central effects of resveratrol remains to be determined. In the present study, we aimed to test whether intravenous administration of resveratrol attenuates central postsynaptic glutamatergic transmission of SpVc neurons responding to noxious mechanical stimulation of the receptive field. In this study, we found the following findings: (i) SpVc WDR neurons responding to non-noxious and noxious mechanical stimulation of the whisker pad were also activated by iontophoretic application of glutamate; (ii) the firing frequency of iontophoretic glutamate-evoked spikes was current-dependently increased (30, 50, and 70 nA) and reversible; and (iii) the mean firing frequency evoked by 30, 50, and 70 nA iontophoretic glutamate application was mimicked by the application of 10 g, 60 g, and pinch mechanical stimulation, respectively. When taking these observations together, the iontophoretic application of glutamate is a valid method for testing the central effects of resveratrol and SpVc neuronal excitability.
Resveratrol suppresses the glutaminergic excitatory transmission of SpVc neurons via the NMDA receptor
It is known that resveratrol significantly suppresses glutamate-induced currents in post-synaptic CA1 pyramidal neurons in hippocampal slices, without having any presynaptic effects.21 In the present study, we found that the mean firing frequency of SpVc WDR neurons responding to the iontophoretic application of glutamate was significantly inhibited by the administration of resveratrol (2 mg/kg, i.v.). Maximal inhibition of discharge frequency of SpVc WDR neurons was observed within 10 min, and these inhibitory effects were reversed in approximately 20 min. The time course of the peak effect and recovery following systemic administration of resveratrol was in agreement with our previous data.20 Therefore, it is possible to speculate that resveratrol suppresses the glutamatergic excitatory synaptic transmission of SpVc neurons by inhibiting post-synaptic glutamate receptors.
Additionally, Gao et al.21 have reported that NMDA receptors are more sensitive to resveratrol than α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid (AMPA) receptors. Since it is known that intrathecal administration of selective NMDA receptor antagonists depresses nociceptive behavior (tail-flick and hot plate test),27,28 it is more likely that NMDA receptors are involved in nociceptive spinal transmission.35 In the present study, we found that the relative magnitude of inhibition by resveratrol of glutamate-evoked SpVc WDR neuronal discharge frequency was similar to that of iontophoretic application of NMDA. Taken together, these findings suggest that it is likely that noxious stimulation-induced NMDA receptor-activated SpVc neuronal excitability was suppressed by the intravenous administration of resveratrol. This notion is also supported by the observation that NMDA receptors are involved in mediating the enhanced responses to noxious peripheral inputs, whereas AMPA receptors are concerned with responses to non-noxious inputs in the spinal dorsal horn of rats with spinal nerve ligation neuropathy.36
Alternatively, it is also reported that resveratrol decreases action potential duration and L-type Ca2+ currents in ventricular myocytes,22,23 suggesting the possibility that resveratrol suppresses the glutamatergic excitatory synaptic transmission of SpVc neurons by inhibiting pre-synaptic Ca2+ channels. In addition, using the hot plate test, Gupta et al.37 have reported that intraperitoneal administration of resveratrol exhibits a dose-dependent antinociceptive effect via an opioidergic mechanism. It is also known that enkephalin inhibits nociceptive transmission through the inhibition of glutamate release in the superficial layer of spinal dorsal horn neurons in a slice preparation.38 We previously reported that activation of μ-opioid receptors inhibits the excitability of rat nociceptive small-diameter trigeminal ganglion neurons projecting to the superficial layer of the upper cervical dorsal horn and this inhibition is mediated by potentiation of voltage-gated K+ currents.39 These results suggest that resveratrol may activate μ-opioid receptors in the presynaptic terminal causing potentiation of voltage-gated K+ currents. These effects inhibit the excitability of neuronal transmission in the SpVc via depression of glutamate release. However, further study is needed to elucidate this possibility.
Functional significance of suppression of nociceptive transmission by the dietary constituent, resveratrol
The majority of spinal dorsal horn neurons are responsive to both NMDA and non-NMDA receptor antagonists in naïve rats, suggesting the coexistence of both types of excitatory amino acid receptors on the spinal neurons of naïve rats.35 In the trigeminal system, intravenous injection of both NMDA and non-NMDA receptor antagonists block the excitatory responses of C1-C2 spinal neurons to electrical stimulation of the sagittal sinus.24 In addition, local iontophoretic application of NMDA and non-NMDA glutamate receptor antagonists attenuates tooth-pulp-evoked C1 neuronal excitation.25 Bereiter and Bereiter40 have also reported that both NMDA and non-NMDA antagonists significantly reduce the number of neurons at the trigeminal spinal nucleus interpolaris/caudalis (SpVi/Vc) and SpVc/C1 transition regions that produce the neuronal excitation marker, c-fos protein, following mustard oil stimulation of the cornea. We previously provided evidence that chronic administration of resveratrol attenuates inflammation-induced mechanical hyperalgesia and that this effect is due primarily to the suppression of SpVc WDR neuron hyperexcitability via inhibition of both peripheral and central cyclooxygenase cascade signaling pathways. Up-regulation of NMDA receptors in the trigeminal spinal nociceptive neurons have been reported under inflammatory and neuropathic conditions,41,42 suggesting that up-regulation of the NMDA receptor signaling pathway plays an important role in the hyperalgesia and allodynia of central sensitization.39 These findings suggest that in inflammatory/neuropathic conditions, systemic resveratrol attenuates the excitability of SpVc neurons via the mechanism of NMDA receptor antagonism.
Recently, an increasing number of CAMs have been used for the treatment of chronic pain.3,43 It is known that patients frequently turn to CAM therapies, such as herbal medicines and acupuncture, for pain control when other medical treatments are ineffective.2,3 There has also been much research concerning the potential influence of diet and dietary supplementation on conditions associated with pain,4,6,44 and a recent paper has reviewed the modulatory mechanism of the dietary constituent, resveratrol, on nociceptive neuronal activity.13 Therefore, our results contribute to the development of analgesic drugs for the treatment of orofacial pathological pain. The findings from our present in vivo study support the idea that resveratrol, as well as being a candidate molecular target for NMDA receptor signaling studies, is a potential CAM for the prevention of nociceptive and inflammatory hyperalgesia.
Conclusions
The present study provides evidence that resveratrol suppresses glutamatergic neurotransmission of the SpVc neurons that respond to nociceptive mechanical stimulation in vivo via an NMDA receptor. These findings suggest that resveratrol may be used as a CAM for the treatment of trigeminal nociceptive pain.
Authors’ Contributions
ST, YK, and NU performed the electrophysiological and histological experiments. KU, YS, and KI interpreted the data and helped finalize the manuscript. MT participated in the design of the present study and wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest, with respect to the research, authorship or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, or publication of this article.
References
- 1.Rao JK, Mihaliak K, Kroenke K, Bradley J, Tierney WM, Weinberger M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med 1999; 131: 409–16. [DOI] [PubMed] [Google Scholar]
- 2.Konvicka JJ, Meyer TA, McDavid AJ, Roberson CR. Complementary/alternative medicine use among chronic pain clinic patients. J Periaesth Nurs 2008; 23: 17–23. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg EI, Genao I, Chen I, Mechaber AJ, Wood JA, Faselius CJ. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med 2008; 9: 1065–72. [DOI] [PubMed] [Google Scholar]
- 4.Shir Y, Raja SN, Weissman CS, Campbell JN, Seltzer Z. Consumption of soy diet before nerve injury preempts the development of neuropathic pain in rats. Anesthesiology 2001; 95: 1238–44. [DOI] [PubMed] [Google Scholar]
- 5.Ernest E. Complementary medicine. Curr Opin Rheumatol 2003; 15: 151–5. [DOI] [PubMed] [Google Scholar]
- 6.Tall JM, Raja SN. Dietary constituents as novel therapeutics for pain. Clin J Pain 2004; 20: 19–26. [DOI] [PubMed] [Google Scholar]
- 7.Fremont L. Biological effects of resveratrol. Life Sci 2000; 66: 663–7. [DOI] [PubMed] [Google Scholar]
- 8.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J 2003; 17: 1975–85. [DOI] [PubMed] [Google Scholar]
- 9.Leiro J, Arranz JA, Sanmmartin ML, Quezada E, Orallo F. Effect of cis-resveratrol on genes involved in nuclear factor Kappa B signaling. Int Immunopharmacol 2005; 5: 393–406. [DOI] [PubMed] [Google Scholar]
- 10.Bermúdez-Ocaña DY, Ambriz-Tututi M, Pérez-Severiano F, Granados-Soto V. Pharmacological evidence for participation of NO-cyclic GMP-PKG-K+ channel pathway in antiallodynic action of resveratrol. Pharmacol Biochem Behav 2006; 84: 535–42. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Severiano F, Bermúdez-Ocaña DY, López-Sánchez P, Ríos C, Granados-Soto V. Spinal nerve ligation reduces nitric oxide synthase activity and expression: effect of resveratrol. Pharmacol Biochem Behav 2008; 90: 742–7. [DOI] [PubMed] [Google Scholar]
- 12.Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol 2007; 74: 533–44. [DOI] [PubMed] [Google Scholar]
- 13.Takeda M, Takehana S, Sekiguchi K, Kubota Y, Shimazu Y. Modulatory mechanisms of nociceptive neuronal activity by dietary constituent resveratrol. Int J Mol Sci 2016; 17: 1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata K, Tashiro A, Tsuboi Y, et al. Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J Neurophysiol 1999; 82: 1244–53. [DOI] [PubMed] [Google Scholar]
- 15.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 2000; 11: 57–91. [DOI] [PubMed] [Google Scholar]
- 16.Takeda M, Takahashi M, Mastumoto S. Suppression of neurokinin-1 receptor in trigeminal ganglia attenuates central sensitization following inflammation. J Peri Nerv Syst 2012; 17: 169–81. [DOI] [PubMed] [Google Scholar]
- 17.Takeda M, Tanimoto T, Matsumoto S. Change in mechanical receptive field properties induced by GABAA receptor activation in the trigeminal spinal nucleus caudalis neurons in rats. Exp Brain Res 2000; 134: 409–16. [DOI] [PubMed] [Google Scholar]
- 18.Takeda M, Tanimoto T, Ito M, Nasu M, Matsumoto S. Role of capsaicin-sensitive afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Exp Brain Res 2005; 16: 107–17. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa T, Takeda M, Tanimoto T, Matsumoto S. Convergence of nociceptive information from temporomandibular joint and tooth-pulp afferents on C1 neurons in the rat. Life Sci 2004; 75: 1465–78. [DOI] [PubMed] [Google Scholar]
- 20.Takehana S, Sekiguchi K, Inoue M, Ito Y, Kubota Y, Yui K, et al. Systemic administration of resveratrol suppress the nociceptive neuronal activity of trigeminal spinal nucleus caudalis in rats. Brain Res Bull 2016; 120: 117–22. [DOI] [PubMed] [Google Scholar]
- 21.Gao Z-B, Chen X-Q, Hu G-Y. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res 2006; 1111: 41–7. [DOI] [PubMed] [Google Scholar]
- 22.Liew R, Stagg MA, MacLeod KT, Collins P. The red wine polyphenol, resveratrol, exerts acute direct actions on guinea-pig ventricular myocytes. Eur J Pharmacol 2005; 519: 1–8. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LP, Yin JX, Liu Z, Zhang Y, Wang QS, Zhao J. Effect of resveratrol on L-type calcium current in rat ventricular myocytes. Acta Pharmacol Sin 2006; 27: 179–83. [DOI] [PubMed] [Google Scholar]
- 24.Storer RJ, Goadzby PJ. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience 1999; 90: 1371–6. [DOI] [PubMed] [Google Scholar]
- 25.Takeda M, Tanimoto T, Matsumoto S. Effects of N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists on excitation of the tooth-pulp-evoked C1 spinal neurons in the rat. Exp Brain Res 1999; 128: 303–8. [DOI] [PubMed] [Google Scholar]
- 26.Takeda M, Tanimoto T, Takahashi M, Kadoi J, Nasu M, Matsumoto S. Activation of α-adrenoreceptors suppress the excitability of C1 spinal neurons having convergent inputs from tooth pulp and superior sagittal sinus in rats. Exp Brain Res 2006; 174: 210–20. [DOI] [PubMed] [Google Scholar]
- 27.Cahusac PMB, Evans RH, Hill RG, Rodriquez RE, Smith DAS. The behavioural effects of an N-methylaspartate receptor antagonist following application to the lumbar spinal cord of conscious rats. Neuropharmacology 1984; 23: 719–24. [DOI] [PubMed] [Google Scholar]
- 28.Aanosen LH, Lei S, Wilcox GL. Phencyclidine selectively blocks a spinal action of N-methyl-D- aspartate in mice. Neurosci Lett 1987; 67: 191–7. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–10. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd ed New York, NY: Academic Press, 1986. [Google Scholar]
- 31.Tanimoto T, Takeda M, Nishikawa T, Matsumoto S. The role of 5-hydroxytryptamine3 receptors in the vagal afferent activation-induced inhibition of the first cervical dorsal horn spinal neurons projected from tooth pulp in the rat. J Pharmacol Exp Ther 2004; 311: 803–10. [DOI] [PubMed] [Google Scholar]
- 32.Ness TJ, Randich A. Intravenous lidocaine inhibits nociceptive reflex and spinal neurons in the rat. Anesthesiology 2000; 92: 1685–91. [DOI] [PubMed] [Google Scholar]
- 33.Sekiguchi K, Takehana S, Shibuya E, Matsuzawa N, Hidaka S, Kanai Y, et al. Resveratrol attenuates inflammation-induced hyperexcitability of trigeminal spinal nucleus caudalis neurons associated with hyperalgesia in rats. Mol Pain 2016; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimazu Y, Shibuya E, Takehana S, Sekiguchi K, Oshima K, Kamata H, et al. Local administration of resveratrol inhibits excitability of nociceptive wide-dynamic range neurons in rat trigeminal spinal nucleus caudalis. Brain Res Bull 2016; 124: 262–8. [DOI] [PubMed] [Google Scholar]
- 35.Aanosen LH, Lei S, Wilcox GL. Excitatory amino acid receptors and nociceptive neurotransmission in rat spinal cord. Pain 1990; 41: 309–21. [DOI] [PubMed] [Google Scholar]
- 36.Leem JW, Choi EJ, Park ES, Paik KS. N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists differentially suppress dorsal horn neuron responses to mechanical stimuli in rats with peripheral nerve injury. Neurosci Lett 1996; 211: 37–40. [DOI] [PubMed] [Google Scholar]
- 37.Gupta YK, Sharma M, Briyal S. Antinociceptive effect of trans-resveratrol in rats: involvement of an opioidergic mechanism. Method Find Exp Clin Pharmacol 2004; 26: 667–2. [DOI] [PubMed] [Google Scholar]
- 38.Hori Y, Endo K, Takahashi T. Presynaptic inhibitory action of encephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J Physiol 1992; 450: 673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda M, Tanimoto T, Kdoi J, Nasu M, Matsumoto S. Opioidergic modulation of excitability of rat trigeminal root ganglion neuron projections to the superficial layer of cervical dorsal horn. Neuroscience 2004; 125: 995–1008. [DOI] [PubMed] [Google Scholar]
- 40.Bereiter DA, Bereiter DF. N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists reduced fos-like immunoreactivity in central trigeminal nucleus after corneal stimulation in the rat. Neuroscience 1996; 73: 249–58. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, et al. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain 2009; 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Lim G, Mao J, Sung B, Mao J. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain 2009; 141: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, Wilkey SA, et al. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med 2001; 135: 262–8. [DOI] [PubMed] [Google Scholar]
- 44.Rivat C, Richebé P, Laboureyras E, Laulin JP, Havouis R, Noble F, et al. Polyamine deficient diet to relieve pain hypersensitivity. Pain 2008; 137: 125–37. [DOI] [PubMed] [Google Scholar]