Abstract
Lenalidomide, with or without prednisone, is an active therapy for patients with myelofibrosis (MF). We provide an update of a phase II study of lenalidomide plus prednisone in patients with MF, after median follow up of 9 years. Forty patients were enrolled in the study and all patients were evaluable for response. Response to the treatment was reevaluated using IWG response criteria published in 2013: quality of response improved over time and overall response rate was 35%. Response in splenomegaly was seen in 39% of patients and anemia response in 32%. The median time to treatment failure (TTF) in all patients was 8.2 months and the median duration of response was 34.6 months. Response was highly durable in some patients: six patients (15%) had TTF for more than 60 months (5 years) and three patients are still on the treatment beyond 109 months (9 years). Complete and partial responses were seen in one and five patients, respectively, but achieving deeper response was not necessary for the response to be durable. New clinical studies are needed to explore safe and well tolerated lenalidomide-based combination strategies for patients with MF.
Keywords: Myelofibrosis, lenalidomide, prednisone, complete response, anemia, splenomegaly
Introduction
Myelofibrosis (MF) is a clonal stem-cell disorder characterized by bone marrow fibrosis, osteosclerosis, and pathological angiogenesis that are thought to be largely mediated by proinflammatory, fibrogenic, and angiogenic cytokines [1]. The discovery of the JAK2V617F mutation promoting constitutive activation in JAK-STAT pathway as a key pathogenesis of this disease, led to development of JAK inhibitors, including ruxolitinib [2]. Two large randomized trials comparing ruxolitinib to placebo or best available therapy resulted in its approval by the Food and Drug Administration (FDA) in 2011[3, 4]. Ruxolitinib is particularly effective in reducing spleen size and improving MF-related systemic symptoms. Also, pooled analysis of these two studies showed that patients who were randomized to ruxolitinib had significantly longer overall survival (OS) compared to those who received placebo or best available therapy [5]. However, there is no curable treatment for MF at the present time, except for potential cure following allogeneic stem cell transplant, but this treatment modality is not generally considered for older patients. The median age at diagnosis of MF is 66 years [6]; therefore other effective treatments, with different mechanisms to JAK inhibition, are needed.
Lenalidomide is one of the immunomodulatory agents (IMiDs), which have anti-inflammatory and anti-angiogenic activity, with potential to modify the bone marrow environment and possibly reduce fibrosis; clinically it may improve anemia and thrombocytopenia in patients with MF. It may also reduce the spleen size in some patients. In our previous report of this phase II study of lenalidomide plus prednisone, after median follow up of 22 months (range: 6–27), 30% of the patients responded to the treatment with improved blood cell counts and reduced splenomegaly [7]. Selected patients also achieved histopathologic and molecular responses. Here, we report an update of this phase II trial, which also addresses the patients who benefited from therapy for over 5 years.
Patients and Methods
This study was a prospective single arm phase II study with primary endpoint being objective clinical response rate [7]. Patients were eligible if they had untreated or previously treated MF with intermediate or high-risk disease requiring therapy according to the Lille scoring system [8]. The details of eligibility criteria were previously described [7]. The diagnosis of MF was established according to the WHO classification [9, 10]. A total of 40 consecutive patients with untreated (N=10) or previously treated (N=30) MF were enrolled in this trial between July 2006 and March 2007. The details of the treatment regimen, patient evaluation, and assessment of JAK2V617F mutation status were previously described [7]. Briefly, lenalidomide 10 mg/day (5mg/day for patients whose platelet count were lower than 100 × 109/L) was given orally in 28 days cycles on a 21-day on/7-day off schedule for at least 6 months unless significant toxicities were observed. Thereafter, lenalidomide was continued until progression and/or toxicity warranted treatment discontinuation. Dose modification of lenalidomide based on toxicities and response were previously described [7]. Prednisone was given orally at 30mg/day during cycle 1, 15mg/day during cycle 2, and 15mg every other day during cycle 3 and then it was discontinued. For this report, all responses were re-assessed according to revised International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria published in 2013 [11]. The study, however, did not include an assessment of baseline symptoms and symptomatic improvement over time during therapy based on a validated tool (e.g. MPN - symptom associated form - total symptom score (MPN-SAF-TSS)) [12]; therefore, we did not include symptomatic improvement in the overall response analysis. The only major differences between IWG-MRT response criteria published in 2013 to those published in 2006 (that were used in our previous report on this study [7]) are the lowering of blood counts required for PR and CR and the requirement for bone marrow histologic remission without complete normalization of blood counts. For example, in the 2006 criteria, PR required Hg ≥ 110 g/L, platelets ≥ 100 × 109/L, absolute neutrophil count ≥ 1.0 × 109/L and no higher than the upper normal limit (UNL), and a normal leukocyte differential in the absence of bone marrow histologic remission. In the 2013 criteria, a PR requires bone marrow histologic remission (age adjusted normocellularity; <5% blasts; ≤ grade 1 MF) and Hg ≥ 85 g/L but < 100 g/L and < UNL; neutrophil count ≥ 1 × 109/L and < UNL; platelets ≥ 50, but < 100 × 109/L and < UNL; <2% immature myeloid cells. We did not evaluate cytogenetic/molecular responses in current paper. The study was performed in accordance with the declaration of Helsinki and approved by the institutional review board of MD Anderson Cancer Center.
Statistical analysis
The Fisher exact tests were used for the descriptive statistical analyses on categorical variables and Mann-Whitney U-test for continuous variables. Time to treatment failure (TTF) was defined as the time from commencement of treatment to discontinuation of treatment from any cause. The associations between baseline characteristics to response were calculated using odds ratio (OR) and 95% confidence intervals (95%CI) by logistic regression. OS was defined as the time from commencement of treatment to death from any cause. TTF, response duration, and OS were constructed using the Kaplan-Meier method [13]. All analyses were performed using STATA version 13.1 (StataCorp LP, College Station, TX), with the significance level set at the 5%.
Results
Patient characteristics
The median age at the study enrollment was 62 (range: 41–86) years (Table 1). Median time from diagnosis to the treatment was 10 months (range: 0–269 months) and 30 patients (75%) were previously treated. Twenty-three patients had an enlarged spleen ≥ 5 cm (measured by physical examination), and 22 had anemia with hemoglobin less than 10 g/dl (seven were transfusion dependent). Prior treatment included hydroxyurea in 14 (35%), azacitidine in 6 (15%), steroids in 5 (13%), thalidomide in 4 (10%) and others (27%). Of the 40 patients, the JAK2V617F mutation was detected in 20 patients; two had a MPL515 mutation, three had a CALR mutation, and the others (n=15) were JAK2V617F mutation negative but not tested for either MPL515 or CALR mutations. The median duration of follow up for all surviving patients at the time of this analysis was 109 months (range: 23–116+ months); median OS for all patients was 46.9 months (95%CI: 38–74.9 months).
Table 1.
Patients characteristics
| Characteristics | All | Responder | Non-responder | p-value | |
|---|---|---|---|---|---|
| N | 40 | 14 | 26 | ||
| Median age | (range) | 62 (41–86) | 69 (47–83) | 63 (41–86) | 0.387 |
| WBC count (x109/l) | Median (range) | 8.7 (1.1–89) | 10.5 (2.3–35.3) | 8.7 (1.1–89) | 0.379 |
| Hemoglobin (g/l) | Median (range) | 9.8 (7.8–17.3) | 9.6 (7.8–17.3) | 10.2 (8.4–15.4) | 0.327 |
| Platelet count (x109/l) | Median (range) | 237 (8–1183) | 217 (68–1183) | 242 (8–1005) | 0.590 |
| Peripheral blood blasts (%) | Median (range) | 0 (0–17) | 0 (0–2) | 1 (0–17) | 0.003 |
| Bone marrow blasts (%) | Median (range) | 2 (0–14) | 1 (0–4) | 2 (0–14) | 0.715 |
| Spleen size BCM (cm) | Median (range) | 10 (0–25) | 11 (0–22) | 5 (0–25) | 0.836 |
| Previously treated | 30 (75%) | 10 (71%) | 20 (77%) | 0.178 | |
| No. of prior therapies | Median(range) | 1 (1–4) | 1 (1–3) | 1 (1–4) | 0.324 |
| JAK2V671F mutation | 20 (50%) | 9 (64%) | 11 (42%) | 0.320 | |
| Karyotype | Diploid | 24 (60%) | 7 (50%) | 17 (65%) | 0.198 |
| Abnormal | 16 (40%) | 7 (50%) | 9 (35%) |
Abbreviations: WBC, white blood cell; BCM, below costal margin
Overall response, response duration and toxicity
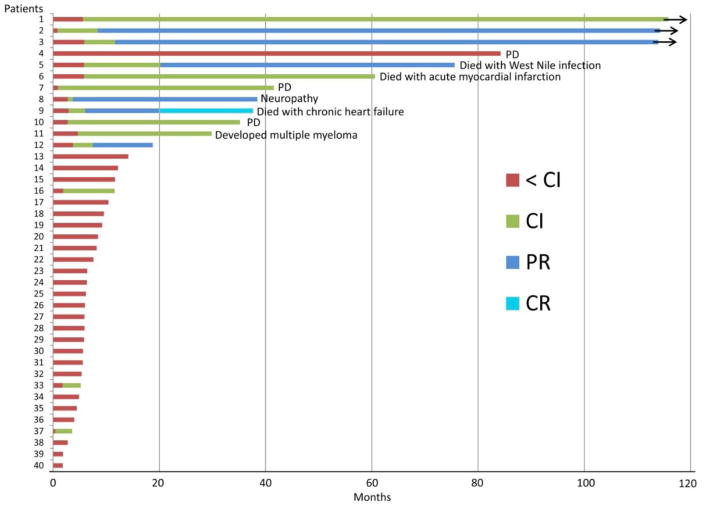
Response to the treatment improved over time (Figure 1), and 14 patients (35%, 95%CI: 21–52%) responded overall. Although not formally assessed using the MPN-SAF-TSS, all patients who achieved a response described improvements in symptoms in a relatively short time, most frequently within 1–2 cycles. There were no significant differences in the baseline characteristics between the patients who responded and those who did not (Table 1). One patient achieved complete response (CR), five patients achieved partial response (PR) and eight patients achieved clinical improvement (CI: three for spleen size and hemoglobin, three for spleen size and two for hemoglobin). In total, 39% (95%CI: 20–61%) of patients showed a response in terms of spleen size reduction, and the overall anemia response was 32% (95%CI: 14–55%). Three of seven patients that were transfusion dependent became transfusion independent. The median time to first response was 2.9 months (range: 0.4–5.9 months). The median TTF was 8.2 months (95%CI: 5.9–11.7 months). The median TTF for responders and non-responders were 37.6 months and 5.9 months, respectively. The median duration of response was 34.6 months (95%CI: 9.7–69.8+ months). At the time of data analysis, 29 patients died, eight patients were alive but off treatment and three patients remain on treatment. Six patients were on therapy for more than 60 months (5 years), and 3 patients are still on the treatment beyond 109 months (Figure 1). The median OS for responders and non-responders were 52.8 months and 45.7 months, respectively (P=0.57). The median OS after treatment failure was 30.2 months.
Figure 1.
Overall duration of therapy and best response for each patient enrolled in the study.
As previously reported [7], the treatment was generally well tolerated with no severe or unexpected toxicity. Among responders, four of 14 patients started lenalidomide at 5 mg daily due to thrombocytopenia (platelets < 100 × 109/L); of the other 10 responding patients who started lenalidomide at 10 mg daily, five patients required dose reductions due to anemia (n=2), thrombocytopenia (n=1), neutropenia (n=1), and elevated liver function test (n=1). Most commonly seen grade 3–4 non-hematological toxicities were fatigue in 27% of patients, followed by diarrhea (15%) and infection (15%). The reason for discontinuation of the treatment included lack of response/progression of disease (n=21), grade 3–4 cytopenia (n=5, 3 anemia, 1 neutropenia, 1 thrombocytopenia), transfer to bone marrow transplant (n=2), thrombotic event (n=1), grade 3 neuropathy (n=1), newly diagnosed atrial fibrillation with dyspnea (n=1), newly diagnosed multiple myeloma (n=1), unable to come to our center for follow up (n=1), and death due to unrelated medical conditions (n=3: West Nile infection, acute myocardial infarction, and chronic heart failure).
Characteristics of long-term responders treated with lenalidomide >60 months
Six patients continued on the treatment for more than 60 months (patients 1–6; Table 2). Three patients achieved PR, two patients achieved CI (spleen size and anemia), and one patient was in stable disease but had excellent symptomatic improvement. There are no significant differences in the baseline characteristics between these six patients and those who did not respond to the treatment. All but one patient had an enlarged spleen, and four patients were JAK2V617F mutation positive; the other two were either CALR or MPL515 mutation positive. Changes in JAK2V617F allele burden during therapy did not correlate with the response type or durability (data not shown). Two of six patients were taken off the study due to death from other causes (West Nile infection and acute myocardial infarction). One (patient 4) experienced progression of the disease with clonal evolution, and underwent allogeneic stem cell transplant following treatment with ruxolitinib and azacitidine. She is currently alive and in complete molecular response (MPL515 mutation negative) 18 months post-transplant.
Table 2.
Baseline clinical characteristics and outcomes of patients treated with lenalidomide for >60 months
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age at presentation | 55 | 46 | 52 | 59 | 73 | 59 |
| Gender | F | M | M | F | M | M |
| Prior MPN diagnosis | MPN, unclassified (4 yrs) | ET (12 yrs) | PV (7 yrs) | ET (1 yr) | -- | -- |
| Prior treatment | PEG-IFNa-2b, VEGF inhibitor | HU, anagrelide, EPO, IFN | None | HU, anagrelide, EPO | EPO | None |
| Symptoms at treatment initiation | Fatigue | Fatigue | Fatigue, dyspnea, bone pain | Fatigue | Fatigue | Fatigue |
| Baseline Characteristics | ||||||
| IPSS/DIPSS-Plus | Int-2/Int-2 | Int-1/Int-1 | Int-1/Int-1 | Int-1/Int-1 | Int-2/Int-2 | Int-1/Int-2 |
| Circulating blasts (%) | 0 | 0 | 0 | 0 | 0 | 2 |
| Performance status | 1 | 1 | 1 | 1 | 0 | 2 |
| Hemoglobin (g/dL) | 9.5 | 9.7 | 11.6 | 12.4 | 7.8 | 10.3 |
| Platelets (109/L) | 232 | 95 | 156 | 1005 | 202 | 704 |
| LDH (IU/L) | 2461 | 1089 | 968 | 1277 | 1606 | 3055 |
| Mutation status | JAK2V617F | CALR type 1 | JAK2V617F | MPL515 | JAK2V617F | JAK2V617F |
| Spleen size by palpation (cm) | 9 | 11 | 15 | 0 | 10 | 22 |
| Cytogenetics | 47 XX+8, 47 XX+9 | 46 XY | 46 XY | 46 XX | 45X,-Y | 46,XY,t(8;12)(q22;q13) |
| Bone marrow fibrosis grade | MF-2 | MF-2 | MF-2 | MF-2 | MF-2 | MF-2 |
| Clinical Response and Outcomes | ||||||
| Change in bone marrow fibrosis grade | MF-1 | MF-1 | MF-1 | MF-1 | No change | No change |
| Time to achieve response (mo) | ||||||
| Symptom improvement | 1 | 1 | 1 | 2 | 1 | 1 |
| CI (anemia) | 5 | 2 | 1 | -- | 6 | 11 |
| CI (spleen) | 5 | 3 | 6 | -- | 11 | 6 |
| PR | 9 | -- | 20 | |||
| CR | ||||||
| Maximal reduction in splenomegaly, cm (time point on therapy) | 0 (54 mo) | 0 (9 mo) | 0 (12 mo) | -- | 0 (20 mo) | 6 (30 mo) |
| Total treatment duration (mo) | 116+ | 114+ | 114+ | 84 | 76 | 61 |
Abbreviations: MF, myelofibrosis; ET, essential thrombocythemia; PV, polycythemia vera; EPO, erythropoietin; PEG-IFN, pegylated interferon; VEGF, vascular endothelial growth factor; HU, hydroxyurea; IPSS, International Prognostic Scoring System; D-IPSS, Dynamic–International Prognostic Scoring System; LDH, lactate dehydrogenase; CI, clinical improvement; PR, partial remission; PMF, partial molecular remission.
Discussion
The current study updates outcomes of patients with MF treated with lenalidomide plus prednisone, and confirms that responses are highly durable in some patients: 15% of patients had a TTF of more than 60 months. Interestingly, achieving PR/CR was not necessary for the response to be durable. The median time to first response was three months and all clinically measurable responses occurred within six months. Our results therefore suggest that six months duration of therapy would be sufficient and appropriate to give to see whether the patient can benefit from lenalidomide and prednisone or not.
Mesa and colleagues reported results of phase II study of lenalidomide plus prednisone in 42 patients with MF [14]. In that study, lenalidomide was given at 10 mg/day continuously (without off days) in 28 day cycles. Overall response by IWG-MRT 2006 criteria was seen in 10 patients (24%). This somewhat lower response rate compared to our study could be mostly associated with the difference in the trial designs. The study by Mesa and colleagues aimed to give treatment for three months at first and then treatment was given up to six months in those who were not showing progressive disease or unacceptable toxicities. In contrast, our study planned to give at least six months of therapy and continued as long as possible. Nevertheless, median event free survival among responders in Mesa’s study was 36.6 months, confirming a durable response of lenalidomide in selected patients. We previously evaluated thalidomide and lenalidomide as a single agents in patients with MF, and conducted a pooled analysis of the three trials [15–17], including the current study. Interestingly, there was no significant difference in response between lenalidomide single agent and lenalidomide plus prednisone; however, response duration was significantly longer in patients who received lenalidomide plus prednisone [15]. Of note, lenalidomide single agent was given for a shorter duration than in the current study: three months for all patients and up to 6–24 months for responders. As we showed in the current study, continuation of lenalidomide is important as long as there is a benefit.
A newer IMiD, pomalidomide, has shown activity (in initial studies) in patients with MF [18, 19]. In the first randomized phase II study evaluating different doses of pomalidomide, either alone or in combination with prednisone, 25% of patients with anemia responded [19] by IWG-MRT 2006 criteria. Since responses were seen both with 0.5 mg or 2 mg/day doses regardless of prednisone, a subsequent phase II trial evaluated single agent pomalidomide at 0.5 mg/day dose. Interestingly, response to anemia was only seen in patients who were JAK2V617F positive (24% vs 0%) [18]. Recently, however, a phase III double-blind placebo-controlled randomized trial was conducted [20] and no difference in anemia response between pomalidomide and placebo (both 16%) were recorded using IWG-MRT 2013 criteria, whereas response in platelets was significantly better in patients who received pomalidomide (22% vs 0%). We recently conducted a phase II trial of ruxolitinib in combination with lenalidomide [21]. Ruxolitinib was given as 15 mg twice daily, and lenalidomide was given at 5 mg daily for 21 days on 28 days cycle. Unfortunately, however, this treatment resulted in a high rate of treatment discontinuation, mainly due to hematologic toxicities, most frequently thrombocytopenia. Overall, 31 patients were enrolled in the trial: all required interruption of lenalidomide and 45% of the patients were completely off lenalidomide within three months from start of the treatment. Responses by IWG-MRT 2013 criteria were seen in 55% of the patients; however, the trial was terminated early because of the toxicities. Of note, improvement in anemia was only seen in one patient. This study tells us that even though new drugs are effective as a single agents, and may have different mechanism of action, careful considerations of toxicity profiles of each drug are needed in order to provide superior result without an increase in toxicity.
In conclusion, about a third of patients with MF may respond to lenalidomide plus prednisone, and in selected patients the response is highly durable. However, no differences in the baseline characteristics among patients could be found to explain differences in clinical outcome. Minimal exposure to therapy of 6 months is suggested, with a continuation of therapy for as long as patients tolerate and have clinical benefit. New lenalidomide-based combination therapies should be developed and evaluated within clinical trial settings in order to improve outcome for patients with MF.
Highlights.
An update of phase II trial of lenalidomide plus prednisone in patients with myelofibrosis after a median follow-up of nine years is presented.
A third of patients with myelofibrosis responded to lenalidomide plus prednisone.
Response was highly durable in 6 patients who received therapy for more than 5 years.
A minimum of 6 months therapy is suggested to best evaluate clinical benefit.
Acknowledgments
This research is supported in part by the MD Anderson Cancer Center Support Grant CA016672
Footnotes
Conflict of interest
Srdan Verstovsek received research funding from Celgene Corporation for the clinical trial
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23:8520–30. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]
- 2.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 5.Vannucchi AM, Kantarjian HM, Kiladjian JJ, Gotlib J, Cervantes F, Mesa RA, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100:1139–45. doi: 10.3324/haematol.2014.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tefferi A, Lasho TL, Jimma T, Finke CM, Gangat N, Vaidya R, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc. 2012;87:25–33. doi: 10.1016/j.mayocp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quintas-Cardama A, Kantarjian HM, Manshouri T, Thomas D, Cortes J, Ravandi F, et al. Lenalidomide plus prednisone results in durable clinical, histopathologic, and molecular responses in patients with myelofibrosis. J Clin ncol. 2009;27:4760–6. doi: 10.1200/JCO.2009.22.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupriez B, Morel P, Demory JL, Lai JL, Simon M, Plantier I, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood. 1996;88:1013–8. [PubMed] [Google Scholar]
- 9.Swerdlow S, Campo E, Harris N, Swerdlow S, Campo E, Harris N. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. International Agency for Research on Cancer; 2008. [Google Scholar]
- 10.Jaffe E, Harris N, Stein H, Vardiman J. WHO Classification Tumors of Hematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer (IARC); 2001. [Google Scholar]
- 11.Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–8. doi: 10.1182/blood-2013-03-488098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 14.Mesa RA, Yao X, Cripe LD, Li CY, Litzow M, Paietta E, et al. Lenalidomide and prednisone for myelofibrosis: Eastern Cooperative Oncology Group (ECOG) phase 2 trial E4903. Blood. 2010;116:4436–8. doi: 10.1182/blood-2010-05-287417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabbour E, Thomas D, Kantarjian H, Zhou L, Pierce S, Cortes J, et al. Comparison of thalidomide and lenalidomide as therapy for myelofibrosis. Blood. 2011;118:899–902. doi: 10.1182/blood-2010-12-325589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas DA, Giles FJ, Albitar M, Cortes JE, Verstovsek S, Faderl S, et al. Thalidomide therapy for myelofibrosis with myeloid metaplasia. Cancer. 2006;106:1974–84. doi: 10.1002/cncr.21827. [DOI] [PubMed] [Google Scholar]
- 17.Tefferi A, Cortes J, Verstovsek S, Mesa RA, Thomas D, Lasho TL, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006;108:1158–64. doi: 10.1182/blood-2006-02-004572. [DOI] [PubMed] [Google Scholar]
- 18.Begna KH, Mesa RA, Pardanani A, Hogan WJ, Litzow MR, McClure RF, et al. A phase-2 trial of low-dose pomalidomide in myelofibrosis. Leukemia. 2011;25:301–4. doi: 10.1038/leu.2010.254. [DOI] [PubMed] [Google Scholar]
- 19.Tefferi A, Verstovsek S, Barosi G, Passamonti F, Roboz GJ, Gisslinger H, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27:4563–9. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tefferi A, Passamonti F, Barbui T, Barosi G, Begna KH, Cazzola M, et al. Phase 3 Study Of Pomalidomide In Myeloproliferative Neoplasm (MPN)-Associated Myelofibrosis With RBC-Transfusion-Dependence. Blood. 2013;122:394. [Google Scholar]
- 21.Daver N, Cortes J, Newberry K, Jabbour E, Zhou L, Wang X, et al. Ruxolitinib in combination with lenalidomide as therapy for patients with myelofibrosis. Haematologica. 2015;100:1058–63. doi: 10.3324/haematol.2015.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]