Abstract
Patients with CKD on hemodialysis exhibit increased cardiovascular risk. Fibrin clot structure and clot lysis are crucially involved in development of cardiovascular events, but little is known about the influence of clot density on outcome in patients on hemodialysis. We determined fibrin clot structure parameters and effect on mortality in a prospective cohort of 171 patients on chronic hemodialysis (mean±SD age =59±11 years old; 54% men) using a validated turbidimetric assay. Kaplan–Meier analysis revealed that patients on hemodialysis with a denser clot structure had increased all–cause and cardiovascular mortality risks (log rank P=0.004 and P=0.003, respectively). Multivariate Cox regression models (adjusted for age, diabetes, sex, and duration of dialysis or fibrinogen, C-reactive protein, and complement C3) confirmed that denser clots are independently related to mortality risk. We also purified fibrinogen from healthy controls and patients on hemodialysis using the calcium–dependent IF-1 mAb against fibrinogen for additional investigation using mass spectrometric analysis and electron microscopy. Whereas purified fibrinogen from healthy controls displayed no post-translational modifications, fibrinogen from patients on hemodialysis was glycosylated and guanidinylated. Clots made of purified fibrinogen from patients on hemodialysis exhibited significantly thinner fibers compared with clots from fibrinogen of control individuals (mean±SD =63±2 and 77±2 nm, respectively; P<0.001). In vitro guanidinylation of fibrinogen from healthy subjects increased the formation of thinner fibers, suggesting that difference in fiber thickness might be at least partially due to post-translational modifications. Thus, in patients on hemodialysis, a denser clot structure may be a potent independent risk factor for mortality.
Keywords: dialysis patients, uremia, chronic kidney disease, clot, mortality, coagulopathy
Patients with CKD on hemodialysis (HD) exhibit a high risk for cardiovascular events as well as an altered coagulation with both increased thrombotic and bleeding risks.1 The development of an occlusive vascular thrombus represents the final step in the atherothrombotic process and may lead to deleterious events, such as myocardial infarction and stroke.2 Fibrin clot structure as well as thrombolysis play crucial roles in determining atherothrombotic risk, and in patients with normal renal function, clots with a compact structure and impaired fibrinolysis are associated with premature and more severe cardiovascular disease.3,4 In addition, there is increasing evidence from experimental as well as epidemiologic data that inflammation is closely linked to coagulation and clot structure in various populations.5
A substantial decrease in GFR has been reported to negatively affect hemostasis (uremic coagulopathy) in different ways, including increased production and/or decreased clearance of procoagulatory proteins, inflammatory cytokines, and augmented oxidative stress.1,6,7 So far, only a limited number of small studies investigated clot properties in patients with CKD, suggesting an association between ESRD and unfavorable alterations of fibrin clot structure and function.8,9 Fibrin clots from plasma of patients on HD display a significantly reduced permeability, faster protofibril formation, and a denser clot structure compared with healthy control individuals.8 In addition, data on subjects with acute coronary syndrome and mild CKD indicated an association between impaired GFR and fibrin clot alterations.10 However, to date, the number of patients analyzed is limited, and there is a lack of data on whether clot structure is an independent risk factor for mortality in patients with CKD. Therefore, this study investigated whether clot structure is associated with outcome in patients on HD.
Results
Baseline Characteristics
We prospectively analyzed 171 patients on prevalent HD. In the overall population, clot density assessed by clot final turbidity was 0.32±0.11 arbitrary units, and mean clot lysis time was 7285±6067 seconds. Clinical baseline characteristics according to clot density below and above the median are shown in Table 1. Patients with a higher clot density had a shorter dialysis vintage compared with those with a lower clot density (5.7±3.9 versus 7.0±4.9 years; P=0.05); otherwise, groups were not significantly different with respect to age, sex, diabetes, smoking status, BP, and body mass index. In addition, there was no difference in clot lysis time between these groups.
Table 1.
Clinical characteristics of the study population with low and high levels of final turbidity
| Variable | All Patients, n=171 | FT<0.32, AU, n=85 | FT≥0.32, AU, n=86 | P Value |
|---|---|---|---|---|
| Sex, n (men/women) | 92/79 | 47/38 | 45/41 | 0.76 |
| Age, yr | 59±11 | 58±12 | 61±11 | 0.09 |
| Dialysis vintage, yr | 6.3±4.4 | 7.0±4.9 | 5.7±3.9 | 0.05 |
| Diabetes, n (%) | 21 (12) | 9 (11) | 12 (14) | 0.64 |
| Type 1 | 3 | 2 | 1 | 0.62 |
| Type 2 | 18 | 7 | 11 | 0.46 |
| Smokers, n (%) | 56 (33) | 30 (35) | 26 (30) | 0.52 |
| Ischemic heart disease, n (%) | 36 (21) | 15 (18) | 21 (24) | 0.35 |
| Peripheral artery disease, n (%) | 10 (6) | 3 (4) | 7 (8) | 0.33 |
| Congestive heart failure, n (%) | 21 (12) | 11 (13) | 10 (12) | >0.99 |
| Access thrombosis, n (%) | 61 (36) | 34 (40) | 27 (31) | 0.27 |
| Access, n (fistula/graft) | 164/7 | 80/5 | 84/2 | 0.28 |
| BP | ||||
| Systolic, mmHg | 139±22 | 140±23 | 139±21 | 0.79 |
| Diastolic, mmHg | 83±13 | 84±14 | 82±11 | 0.26 |
| Anthropometrics | ||||
| BMI, kg/m2 | 23.4±3.8 | 23.8±3.7 | 23.1±3.7 | 0.27 |
| Dialysis parameter Kt/V | 1.28±0.20 | 1.29±0.21 | 1.27±0.20 | 0.41 |
| Laboratory variables | ||||
| Protein, g/L | 66.8±4.6 | 66.8±4.5 | 66.9±4.6 | 0.94 |
| Hemoglobin, g/dl | 9.4±1.6 | 9.3±1.6 | 9.4±1.6 | 0.76 |
| CRP, nmol/L | 751±1127 | 578±1161 | 919±1074 | 0.05 |
| C3, g/L | 0.69±0.37 | 0.59±0.32 | 0.79±0.38 | <0.001 |
| Fibrinogen, μmol/L | 26.5±16.5 | 18.8±10.3 | 33.5±18.2 | <0.001 |
| Medication | ||||
| Platelet inhibition | 48 (28) | 25 (29) | 23 (27) | 0.74 |
| ESA | 110 (64) | 55 (65) | 55 (64) | >0.99 |
| ESA dose per week | 5512±1699 | 5672±1741 | 5365±1659 | 0.41 |
| Vitamin D | 124 (73) | 62 (73) | 62 (72) | >0.99 |
| ACE inhibitor | 59 (35) | 25 (29) | 34 (40) | 0.20 |
| Clot measures | ||||
| Clot lysis time, s | 7285±6067 | 7071±6340 | 7507±5802 | 0.77 |
| Clot FT (AU) | 0.32±0.107 | 0.237±0.052 | 0.403±0.079 | <0.001 |
Data are presented as mean±SD or a percentage for categorical data. FT, final turbidity; AU, arbitrary unit; BMI, body mass index; ESA, erythropoietin-stimulating agent; ACE, angiotensin-converting enzyme inhibitor.
Clot Density and Inflammatory Markers
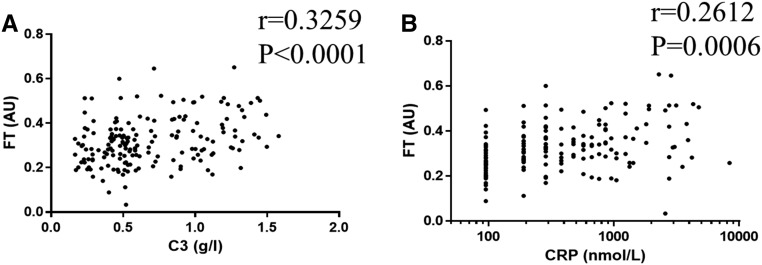
Because experimental as well as epidemiologic data suggest that alterations of clot structure are linked to inflammation in various patient populations,5,11 we analyzed the association of clot density with biochemical markers of inflammation. Patients on HD with a denser clot structure exhibited higher levels of both C-reactive protein (CRP) and complement C3 (919±1074 versus 578±1161 nmol/L; P=0.05 and 0.79±0.38 versus 0.59±0.32 g/L; P<0.001, respectively) (Table 1). Moreover, C3 as well as CRP levels significantly correlated with clot density (r=0.33; P<0.001 and r=0.26; P<0.001, respectively) (Figure 1).
Figure 1.
Clot density correlates with complement C3 and CRP plasma levels. Correlation of (A) complement C3 and (B) CRP plasma levels with clot density as assessed by clot final turbidity (FT; n=171). AU, arbitrary unit.
As expected, patients with a denser clot structure showed higher fibrinogen levels (Table1), and fibrinogen levels were significantly associated with clot density (r=0.53; P<0.001) as well as a prolongation of clot lysis (r=0.21; P<0.01).
Clot Density and Mortality
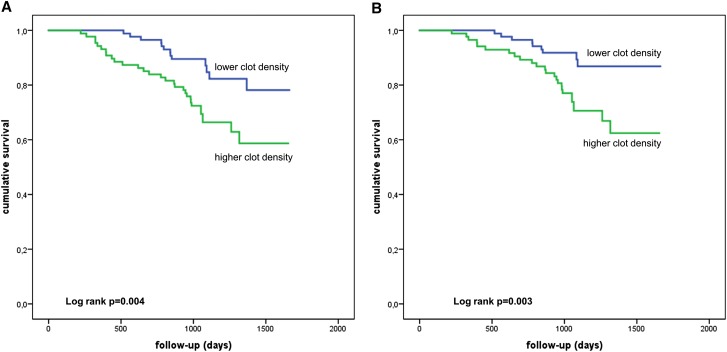
Over a mean follow-up of 3.0±0.85 years, 41 deaths occurred in the 171 patients on HD (cardiovascular, 32; malignancy, seven; and other, two). Death occurred more often in patients with denser clot structure above the median compared with patients with a less dense clot structure below the median (all-cause mortality: 28 versus 13 events and cardiovascular mortality: 23 versus nine events). Kaplan–Meier curves followed by a log rank test showed that all-cause and cardiovascular mortality (Figure 2) were significantly increased in patients on HD with a denser clot structure compared with those with a clot density below the median (log rank P=0.004 and P=0.003, respectively). In addition, we performed analyses for tertiles and quartiles of clot density and found again that higher clot density was associated with both all-cause and cardiovascular mortality (Supplemental Figures 1 and 2).
Figure 2.
A denser clot structure is related to mortality in patients on dialysis. Kaplan–Meier analysis of (A) all-cause and (B) cardiovascular mortality in patients with denser or less dense clot structure (grouped according to median; log rank test: P=0.004 and P=0.003, respectively).
Among the parameters analyzed, a denser clot structure (final turbidity ≥0.32) was associated with the highest mortality risk in this population (Table 2). A higher clot density was associated with increased risks for all-cause and cardiovascular death, with hazard ratios (HRs) of 2.54 and 3.02, respectively (95% confidence interval [95% CI], 1.31 to 4.91; P<0.01 and 95% CI, 1.39 to 6.53; P<0.01, respectively) (Table 3, model 1). Adjustment for age, sex, and duration of dialysis as well as presence of diabetes did not influence the significance of the prognostic value of a higher clot density, resulting in HRs of 2.36 and 2.80 for all-cause and cardiovascular mortality, respectively (95% CI, 1.21 to 4.61; P=0.01 and 95% CI, 1.28 to 6.14; P=0.01) (Table 3, model 2). In addition, adjustment for the biochemical markers CRP, C3, and fibrinogen still revealed significantly elevated all–cause and cardiovascular mortality risks of a higher clot density, with HRs of 2.55 and 3.20, respectively (95% CI, 1.23 to 5.33; P=0.01 and 95% CI, 1.35 to 7.60; P<0.01, respectively) (Table 3, model 3). When assessing clot structure as a continuous variable, a denser clot structure was associated with increased all–cause and cardiovascular mortality risks (Table 4). All covariates in Table 1 were included into adjusted models, and higher clot density remained associated with increased all–cause and cardiovascular mortality (Supplemental Table 1).
Table 2.
Univariate analysis on mortality risk (all cause and cardiovascular)
| Variable | All-Cause Mortality | Cardiovascular Mortality | ||||
|---|---|---|---|---|---|---|
| Risk Coefficient | 95% CI | P Value | Risk Coefficient | 95% CI | P Value | |
| Denser clot structure (FT≥0.32) | 2.54 | 1.31 to 4.91 | <0.01 | 3.02 | 1.39 to 6.53 | <0.01 |
| Denser clot structure (continuous variable) | 73.7 | 4.7 to 1154 | <0.01 | 67.9 | 3.0 to 1545 | <0.01 |
| Sex | 1.07 | 0.66 to 1.75 | 0.78 | 1.07 | 0.62 to 1.85 | 0.82 |
| Age | 1.05 | 1.02 to 1.07 | <0.001 | 1.06 | 1.03 to 1.09 | <0.001 |
| Diabetes | 1.17 | 0.56 to 2.46 | 0.68 | 1.33 | 0.60 to 2.96 | 0.49 |
| Dialysis vintage | 0.99 | 0.94 to 1.04 | 0.75 | 0.99 | 0.93 to 1.05 | 0.67 |
| CRP | 1.03 | 1.02 to 1.04 | <0.001 | 1.04 | 1.03 to 1.05 | <0.001 |
| Fibrinogen | 1.03 | 0.98 to 1.09 | 0.27 | 1.04 | 0.99 to 1.10 | 0.14 |
| C3 | 1.66 | 0.71 to 3.91 | 0.24 | 1.38 | 0.52 to 3.69 | 0.52 |
FT, final turbidity.
Table 3.
Mortality (all cause and cardiovascular) according to clot density (higher versus lower clot density)
| Clot Density | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All cause | ||||||
| FT<0.32 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| FT≥0.32 | 2.54 (1.31 to 4.91) | <0.01 | 2.36 (1.21 to 4.61) | 0.01 | 2.55 (1.23 to 5.33) | 0.01 |
| Cardiovascular | ||||||
| FT<0.32 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
| FT≥0.32 | 3.02 (1.39 to 6.53) | <0.01 | 2.80 (1.28 to 6.14) | 0.01 | 3.20 (1.35 to 7.60) | <0.01 |
HR was calculated with Cox proportional hazard models. FT, final turbidity.
Model 1: crude.
Model 2: adjustment for age, diabetes, sex, and duration of dialysis.
Model 3: adjustment for CRP, C3, and fibrinogen.
Table 4.
Mortality (all cause and cardiovascular) according to clot density (clot density as continuous variable)
| Clot Density | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All cause | 73.7 (4.7 to 1154) | <0.01 | 24.3 (1.5 to 399) | 0.03 | 48.1 (1.6 to 1459) | 0.03 |
| Cardiovascular | 67.9 (3.0 to 1545) | <0.01 | 16.7 (0.7 to 376) | 0.08 | 29.7 (0.6 to 1382) | 0.08 |
HR was calculated with Cox proportional hazard models.
Crude.
Adjusted for age, diabetes, sex, and duration of dialysis.
Model 2 with adjustment for CRP, C3, and fibrinogen.
Finally, we matched patients with higher versus lower clot structure (20 patients in each group) for CRP, sex, age, dialysis vintage, diabetes, and smoking (Supplemental Table 2) and still found in subjects with a denser clot structure a tendency for increased all–cause and cardiovascular mortality risks (log rank P=0.10 and P=0.09, respectively) (Supplemental Figure 3).
Clot lysis time, dichotomized as being above and below the median, was not associated with an increased mortality risk (log rank P=0.53). Clot density was not associated with vascular access thrombosis (log rank P=0.41) (Supplemental Figure 4).
Post-Translational Modifications of Purified Fibrinogen
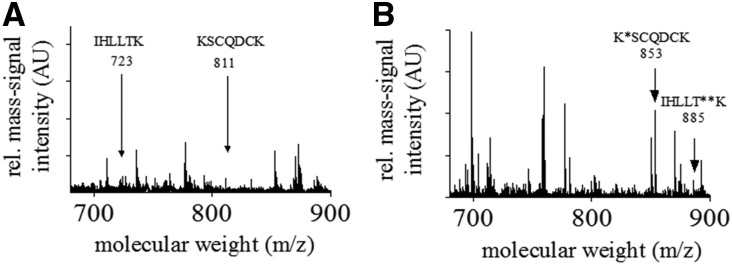
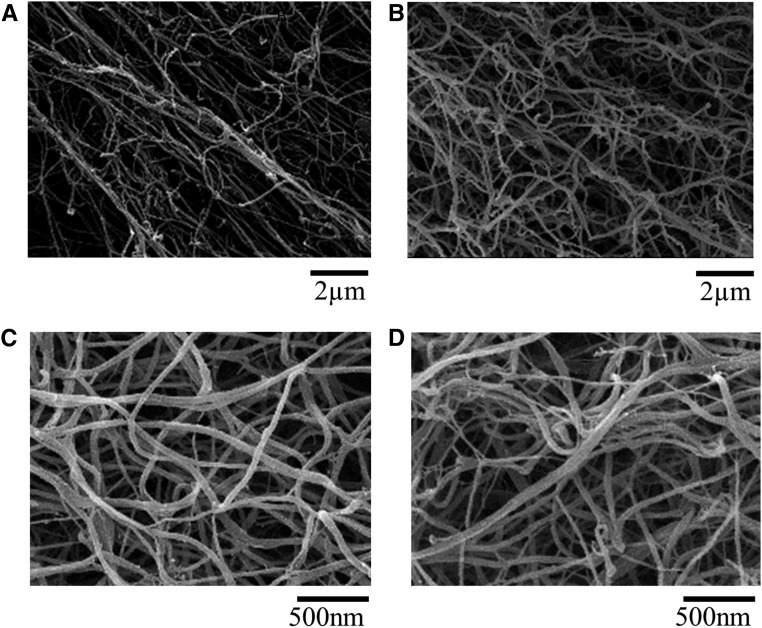
Given that post-translational modifications of fibrinogen may account for changes in clot structure and clot density, we performed matrix–assisted laser desorption/ionization (MALDI) time of flight (TOF)-TOF mass spectrometry (MS) on fibrinogen isolated from ten healthy individuals and ten patients on HD. Although we were not able to detect any post-translational modifications in fibrinogen isolated from healthy controls (Figure 3A), fibrinogen isolated from patients on HD displayed glycosylation and guanidinylation (Figure 3B). To further analyze a possible effect of the post-translational modification on clot structure, we performed scanning electron microscopy of fibrin clots in the presence of FXIII, thrombin, and CaCl2. Fibrin fibers in clots made of purified fibrinogen from patients on HD were significantly thinner compared with those from control individuals (63±1.9 and 77±2.2 nm, respectively; P<0.001) (Figure 4) and less porous (0.11±0.01 and 0.53±0.05 μm2, respectively; P<0.001), which is known to associate with an increased cardiovascular risk. Although the effect of nonenzymatic glycation of fibrinogen on clot properties has already shown by others,12–14 the effect of guanidinylation on clot structure remains unexplored. Therefore, we tested whether in vitro guanidinylation alters fiber thickness in clots of healthy controls. As shown in Figure 4, in vitro guanidinylation of fibrinogen from healthy subjects resulted in an increased formation of thinner fibers, leading to an overall 30%±5% reduction of fiber thickness compared with unguanidinylated fibrinogen.
Figure 3.
Post-translational modifications of fibrinogen in uremia. (A) Characteristic mass fingerprint spectrum of tryptic-digested fibrinogen isolated from healthy controls. The arrows indicate characteristic mass signals of peptides of the tryptic-digested fibrinogen. The amino acid sequences of the peptides of interest are given. (B) Characteristic mass fingerprint spectrum of tryptic-digested fibrinogen isolated from a patient with CKD (stage 5). The arrow at 853 m/z indicates the guanidinylated peptide. The arrow at 885 m/z indicates the glycosylated peptide. AU, arbitrary unit. *Guanidinylated lysine of the corresponding amino acid sequence; **glycosylated threonine of the corresponding amino acid sequence.
Figure 4.
Thinner fibrin fibres in uremia and after in vitro guanidinylation. Scanning electron micrographs of clots prepared from pooled fibrinogen of (A) healthy control individuals and (B) patients on HD, (C) purified fibrinogen, which was not modified, and (D) in vitro guanidinylated fibrinogen. P<0.001.
Discussion
This study shows that an increased clot density was independently and strongly associated with overall as well as cardiovascular mortality in patients on HD. In addition, fibrinogen purified from patients on HD showed post-translational modifications and an altered clot structure.
Altered clot properties in terms of compact structure and impaired fibrinolysis are known to associate with an elevated cardiovascular risk.3,4 Alterations of fibrin clot structure have previously been described in plasma samples of patients with CKD and patients with acute coronary syndrome. Patients with CKD (defined as GFR<60 ml/min) displayed less porous fibrin clots and a prolonged clot lysis compared with individuals with a normal GFR.10 Clot permeability, another measure of clot density, was independently associated with eGFR and fibrinogen levels.10 Several studies point toward a prothrombotic profile in CKD: patients with CKD exhibit elevated plasma levels of tissue factor,15–18 the key initiator of the coagulation cascade, as well as increased plasma levels of vWF, which promotes platelet adhesion and serves as a carrier for factor 8.19 In subjects with diabetes, data from our own group suggest an increased incorporation of complement C3 in fibrin clots as well as an increased complement C3 binding to fibrinogen from subjects with diabetes compared with controls, with fibrinogen leading to alterations of clot structure.20 In addition, in a large cohort of patients with type 2 diabetes, complement C3 was the strongest predictor of clot lysis time compared with other factors, such as PAI-1, CRP, or fibrinogen.11 Our study extends the knowledge on altered coagulation in CKD by showing a highly significant association of complement C3 with clot density in patients on HD.
To the best of our knowledge, this is the first study to show that a more compact clot structure in patients on HD is independently associated with a higher all–cause and cardiovascular mortality. This finding was independent of age, diabetes, sex, or duration of dialysis, and the association remained significant after adjustment for CRP, fibrinogen, and complement C3. Recently, Sharma et al.21 investigated the role of the global thrombotic status in patients with ESRD measured by occlusion and lysis time of an occlusive platelet thrombus on mortality. In this study, 216 patients were followed up for nearly a year for major adverse cardiovascular events, showing that impaired thrombolysis but not occlusion time was strongly associated with major adverse cardiovascular events.21 In contrast to this study, we measured clot structure of plasma without platelets, thus excluding a direct effect of CKD-mediated alterations of platelet function. Therefore, our data suggest that not only uremic thrombopathy but also, plasmatic coagulopathy may contribute to the increased mortality risk in patients with CKD. In another study including 33 patients on long-term HD, 30% of patients died within a follow-up of 36 months due to cardiovascular causes. Those patients who died exhibited elevated plasma levels of fibrinogen, Lp(a), and F2-isoprotanes, and plasma clots were less permeable with impaired fibrinolysis; however, a survival analysis using Cox regression analysis was not performed.8
In our cohort, fibrinogen plasma levels were associated with both a denser clot structure and prolongation of clot lysis, but interestingly, fibrinogen levels were not independently associated with mortality risk. Data from the Cardiovascular Health Study showed significantly higher levels of inflammatory proteins and coagulation factors, including fibrinogen, in patients with renal insufficiency.7 Fibrinogen plasma levels have been shown to be elevated in all stages of CKD independent of treatment regime22 and strongly correlate with eGFR,23 and they are associated with a rapid loss of kidney function in patients with CKD.24 Previous data suggest that the increase of fibrinogen plasma levels is at least in part due to reduced elimination of fibrinogen25 and that fibrinogen plasma levels independently predict all-cause mortality and cardiac events in stages 3 and 4 CKD.26–30 However, in our study, fibrinogen was not an associated with mortality, and adding fibrinogen to the multivariate model did not alter our major finding of clot structure as a risk factor. These discrepant observations could be due to differences in the individuals investigated, because we exclusively focused on patients on HD, whereas earlier studies investigated patients with a broader spectrum of CKD stages.
Changes in clot structure due to elevated fibrinogen concentrations, interactions with other proteins, or post-translational modifications have been shown to alter fibrin clot properties.31 An increased fiber density or decreased porosity coupled with a decreased individual fiber diameter leads to the formation of a stiffer clot, which is more difficult to lyse and associated with an increased cardiovascular risk.4,32,33 In different groups of patients, including smokers and individuals with Alzheimer disease, studies showed fibrinogen to be oxidated with altered clot properties. Similarly, in diabetes, fibrinogen has been shown to be glycated, with improved glycemic control decreasing glycation and improving clot structure properties.13 In our study, we were able to show fibrinogen glycosylation and guanidinylation in patients on HD. Using scanning electron microscopy, we showed a decreased individual fiber diameter and clot porosity in these samples. Several studies investigated the effect of nonenzymatic glycation of fibrinogen on clot properties. Nonenzymatic glycosylation reduced the susceptibility of fibrin to plasmin degradation.12 Furthermore, in an interventional study, there was an association between reduced fibrinogen glycation with favorable alterations of clot structure, including a decrease in lateral aggregation, increased permeability, and lysis rate. In subjects with diabetes, the proportion of thin fibers in the overall clot is increased compared with in nondiabetic subjects. In addition, improved glycemic control lowers the proportion of thin fibers in these subjects.13 This is of interest, because a very recent study using a combined atomic force microscopy/fluorescence microscopy technique found that glycation had no significant systematic effect on single-fiber modules. However, they showed that thin fibers can be 100 times stiffer than thicker fibers.14 To our knowledge, no data are available on the effect of guanidinylation of fibrinogen. Using electron microscopy, we were able to show an increased formation of thinner fibrin fibers with an overall reduction of fiber diameter, suggesting a causal role of guanidinylation for altered clot structure in HD, which may be of importance in the context of fiber stiffness. Furthermore, in vivo thrombin generation is a dynamic process, in which the thrombin concentration actively changes. In vivo, this may have an effect on clot structure, because lower thrombin concentrations result in thick, loosely woven fibrin strands, whereas high thrombin concentrations produce clots with thin, tightly packed fibrin fibers.34 For all of our experiments, including the plasma samples, a fixed dose of thrombin was used. Therefore, in our study, the alterations in clot structure are not due to variations in thrombin but are due to fibrinogen or theoretically, other plasma factors.
Our study has limitations: it is a monocentric study with only whites at relatively young age and different etiologies of ESRD but with a low frequency of diabetes. In addition, intradialytic heparin dosages are lacking. Our results obtained in patients with a long dialysis vintage cannot be generalized to other cohorts and need validation in cohorts with a shorter dialysis vintage. In addition, we adjusted for several potential confounders but cannot rule out additional factors that might influence the effect of clot density on outcome in patients on HD.
In summary, we show that a denser clot structure is a potent independent risk factor for all-cause and cardiovascular mortality in patients on HD. In addition, fibrinogen of patients on HD is post-translationally modified, resulting in a prothrombotic clot structure with thinner fibrin fibers, which may contribute to the elevated mortality in CKD.
Concise Methods
Study Population and Examination
We prospectively analyzed 171 patients on prevalent HD from the Center for Renal Diseases of the Zvezdara University Medical Center. All patients on chronic HD were eligible to enter the study if they agreed to participate. Patients on warfarin treatment were excluded. The study protocol was approved by the local ethics committees of the Zvezdara University Medical Center and the RWTH Aachen University Hospital, and each patient gave informed consent. Blood samples were taken before dialysis into citrated tubes. Samples were spun down within 2 hours of collection, with plasma stored at −80°C until analysis. A total of 171 plasma samples were available for measurement of plasma protein levels and clot structure analysis. All experiments adhered to the Declaration of Helsinki.
Follow-up for outcomes was performed by review of the patients’ hospital charts or telephone interviews with the patients’ primary health care providers. Cardiovascular death was diagnosed when death was attributed to myocardial infarction, cardiogenic shock, or stroke. Death occurring outside the hospital for which no other cause was specified was regarded as sudden cardiac death and included in the definition of cardiovascular death.
Laboratory Analyses of Plasma
Plasma fibrinogen and complement C3 levels were determined using commercial ELISA kits (both GenWay Biotech, Inc., San Diego, CA). Serum analysis for high-sensitivity CRP was performed by particle-enhanced immunonephelometry using a standard CardioPhase hsCRP for BNII (Dade Behring Holding GmbH, Liederbach, Germany). Clot turbidity and lysis measurements were conducted as described previously.35 Briefly, for clotting, 25 μl citrated plasma (in duplicates) was added to 75 μl assay buffer (0.05 mol/L Tris-HCl and 0.1 mol/L NaCl, pH 7.4), and 50 μl activation mix (final concentration: 0.03 U/ml thrombin [Chalbiochem] and 7.5 mmol/L calcium in assay buffer) was added to a 96-well plate. Plates were read at 340 nm every 12 seconds for 1 hour in a BIO-TEK 808 Microplate Reader. The turbidimetric lysis assay was carried out as above in the presence of 83 ng/ml tPA (Technoclone) in 75 μl assay buffer. Plates were read every 108 seconds for 9 hours. Variables were recorded, which showed associations with CV risk,4,35 including clot maximum absorbance and lysis time that was measured from full clot formation to 50% lysis.
Fibrinogen Purification
Fibrinogen was precipitated from heparin plasma by affinity chromatography using a calcium–dependent IF-1 mAb as previously described.20,36 Integrity of the samples was analyzed by SDS-PAGE. Fibrinogen concentration was determined at 280 nm using the extinction coefficient ε280=1.506.
Tryptic Digestion of Fibrinogen and MS Analyses
The protein fibrinogen was digested by incubation with 0.2 μg trypsin at 37°C for 24 hours. The resulting tryptic peptides were desalted and concentrated using the ZipTipC18 technology (EMD Millipore) and water with 0.1% trifluoroacetic acid and 80% acetonitrile. The eluate was spread onto the MALDI target plate (MTP-AnchorChip 400/384; Bruker-Daltonic). The subsequent MS analyses were carried out using an MALDI TOF/TOF Mass Spectrometer (Ultraflex III; Bruker-Daltonic). Calibrated and annotated spectra were subjected to the database search Swiss-Prot (http://www.expasy.org/) using the software tool Bruker Bio-Tool 3.2 and the Mascot 2.2 search engine (Matrix Science Ltd., London, United Kingdom), comparing the experimental MS as well as MS/MS datasets and the calculated peptide mass signals for each entry into the sequence database and empirically determined factors to assign a statistical weight to each individual peptide match.
In Vitro Guanidinylation of Fibrinogen
In vitro guanidinylation of pooled fibrinogen from healthy controls (three samples per group) was performed by adapting the method recently described by Rueth et al.37 Briefly, 2 mg/ml aqueous fibrinogen solution was incubated with 1 mol/L o-methylisourea-bisulfate solution (pH 11.0; Sigma-Aldrich) for 3 hours at room temperature. After incubation, the reaction mixture was diluted and dialyzed against PBS.
Scanning Electron Microscopy of Fibrin Clots
Pooled fibrinogen at 0.5 mg/ml from healthy controls and patients on HD (three to five samples per group) was clotted on addition of 0.5 U/ml thrombin and 2.5 mmol/L CaCl2 in the presence of 10 μg/ml FXIII in specially devised small perforated plastic vessels. Samples were incubated at room temperature in a moist chamber for 2 hours, then washed with 0.1 M phosphate buffer (0.026 M NaH2PO4×H2O and 0.174 M Na2HPO4×2H2O, pH 7.4), and subsequently, fixed with 3% glutardialdehyde. Clots were recovered and stepwise dehydrated with an acetone gradient. Air-dried clots were sputter coated with a 12.5-nm gold palladium layer. Samples were analyzed using an environmental scanning electron microscope (ESEM XL 30 FEG; FEI, Eindhoven, The Netherlands) in a high-vacuum environment using acceleration voltage of 10 kV. Fiber diameters and area of the holes of all clots were measured with the image analysis software package ImageJ 1,23y (National Institutes of Health). In all, 100 fibrin fibers per sample and 60 holes were measured.
Statistical Analyses
Continuous variables were summarized by means and corresponding SDs. Comparisons of the values of continuous variables between two groups were made using an unpaired t test; for more than two groups, one-way ANOVA was used. Categorical variables were summarized by relative frequencies. The chi-squared test was used for studying associations between various categorical variables. Relative risk (odds ratio) and 95% CIs for numerous variables were calculated by univariate logistic regression. The Kaplan–Meier method was used to estimate the cumulative survival. To analyze the crude effect of clot density on all-cause and cardiovascular mortality, comparisons between survival curves were made by log rank test. Cox regression was used to determine the effect on all-cause and cardiovascular mortality. The multivariable–adjusted Cox regression analysis accounted for age, sex, diabetes, dialysis vintage, high-sensitivity CRP, fibrinogen, and C3 as possible confounders. Confounders were selected on the basis that they were known to influence mortality per se or inflammatory markers (CRP, fibrinogen, or C3). Statistical analysis was performed with SPSS, version 21.0.0.0. P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG) grant HE 5666/1-2 (to K.S.) and a grant from the Interdisciplinary Centre for Clinical Research within the Faculty of Medicine at the Rheinisch-Westfälische Technische Hochschule Aachen University (K7-2, K7-3, and K7-7 to K.S., J.F., N.M., and G.S.). In addition, this work was supported DFG grant OPBF074, the Hans Lamers-Stiftung, and the European Foundation for the Study of Diabetes (to N.M.). The work leading to this paper received funding from the European Community's Seventh Framework Program under grant agreement no. PEOPLE-ITN-GA-2013-608332 to Clinical and system –omics for the identification of the MOlecular DEterminants of established Chronic Kidney Disease (iMODE-CKD) from the European Union.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016030336/-/DCSupplemental.
References
- 1.Schlieper G, Hess K, Floege J, Marx N: The vulnerable patient with chronic kidney disease. Nephrol Dial Transplant 31: 382–390, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Hess K, Grant PJ: Inflammation and thrombosis in diabetes. Thromb Haemost 105[Suppl 1]: S43–S54, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Fatah K, Silveira A, Tornvall P, Karpe F, Blombäck M, Hamsten A: Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb Haemost 76: 535–540, 1996 [PubMed] [Google Scholar]
- 4.Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, Blanchet B, Dumaine R, Gianetti J, Payot L, Weisel JW, Montalescot G: Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol 26: 2567–2573, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Levi M, van der Poll T: Inflammation and coagulation. Crit Care Med 38[Suppl 2]: S26–S34, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Roberts MA, Hare DL, Ratnaike S, Ierino FL: Cardiovascular biomarkers in CKD: Pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis 48: 341–360, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Undas A, Kolarz M, Kopeć G, Tracz W: Altered fibrin clot properties in patients on long-term haemodialysis: Relation to cardiovascular mortality. Nephrol Dial Transplant 23: 2010–2015, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Sjøland JA, Sidelmann JJ, Brabrand M, Pedersen RS, Pedersen JH, Esbensen K, Standeven KF, Ariëns RA, Gram J: Fibrin clot structure in patients with end-stage renal disease. Thromb Haemost 98: 339–345, 2007 [PubMed] [Google Scholar]
- 10.Undas A, Nycz K, Pastuszczak M, Stompor T, Zmudka K: The effect of chronic kidney disease on fibrin clot properties in patients with acute coronary syndrome. Blood Coagul Fibrinolysis 21: 522–527, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Hess K, Alzahrani SH, Price JF, Strachan MW, Oxley N, King R, Gamlen T, Schroeder V, Baxter PD, Ajjan RA: Hypofibrinolysis in type 2 diabetes: The role of the inflammatory pathway and complement C3. Diabetologia 57: 1737–1741, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Brownlee M, Vlassara H, Cerami A: Nonenzymatic glycosylation reduces the susceptibility of fibrin to degradation by plasmin. Diabetes 32: 680–684, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Pieters M, Covic N, van der Westhuizen FH, Nagaswami C, Baras Y, Toit Loots D, Jerling JC, Elgar D, Edmondson KS, van Zyl DG, Rheeder P, Weisel JW: Glycaemic control improves fibrin network characteristics in type 2 diabetes - a purified fibrinogen model. Thromb Haemost 99: 691–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Sigley J, Pieters M, Helms CC, Nagaswami C, Weisel JW, Guthold M: Fibrin fiber stiffness is strongly affected by fiber diameter, but not by fibrinogen glycation. Biophys J 110: 1400–1410, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlak K, Tankiewicz J, Mysliwiec M, Pawlak D: Tissue factor/its pathway inhibitor system and kynurenines in chronic kidney disease patients on conservative treatment. Blood Coagul Fibrinolysis 20: 590–594, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Mezzano D, Tagle R, Pais E, Panes O, Pérez M, Downey P, Muñoz B, Aranda E, Barja P, Thambo S, González F, Mezzano S, Pereira J: Endothelial cell markers in chronic uremia: Relationship with hemostatic defects and severity of renal failure. Thromb Res 88: 465–472, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Cetin O, Bekpinar S, Unlucerci Y, Turkmen A, Bayram C, Ulutin T: Hyperhomocysteinemia in chronic renal failure patients: Relation to tissue factor and platelet aggregation. Clin Nephrol 65: 97–102, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kario K, Matsuo T, Matsuo M, Koide M, Yamada T, Nakamura S, Sakata T, Kato H, Miyata T: Marked increase of activated factor VII in uremic patients. Thromb Haemost 73: 763–767, 1995 [PubMed] [Google Scholar]
- 19.Dubin R, Cushman M, Folsom AR, Fried LF, Palmas W, Peralta CA, Wassel C, Shlipak MG: Kidney function and multiple hemostatic markers: Cross sectional associations in the multi-ethnic study of atherosclerosis. BMC Nephrol 12: 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess K, Alzahrani SH, Mathai M, Schroeder V, Carter AM, Howell G, Koko T, Strachan MWJ, Price JF, Smith KA, Grant PJ, Ajjan RA: A novel mechanism for hypofibrinolysis in diabetes: The role of complement C3. Diabetologia 55: 1103–1113, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Farrington K, Kozarski R, Christopoulos C, Niespialowska-Steuden M, Moffat D, Gorog DA: Impaired thrombolysis: A novel cardiovascular risk factor in end-stage renal disease. Eur Heart J 34: 354–363, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Irish A: Cardiovascular disease, fibrinogen and the acute phase response: Associations with lipids and blood pressure in patients with chronic renal disease. Atherosclerosis 137: 133–139, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS; CRIC Study Investigators : Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7: 1938–1946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, Rahman M, Lash JP, Townsend RR, Ojo A, Roy-Chaudhury A, Go AS, Joffe M, He J, Balakrishnan VS, Kimmel PL, Kusek JW, Raj DS; CRIC Study Investigators : Inflammation and progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 11: 1546–1556, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane DA, Ireland H, Knight I, Wolff S, Kyle P, Curtis JR: The significance of fibrinogen derivatives in plasma in human renal failure. Br J Haematol 56: 251–260, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Goicoechea M, de Vinuesa SG, Gómez-Campderá F, Aragoncillo I, Verdalles U, Mosse A, Luño J: Serum fibrinogen levels are an independent predictor of mortality in patients with chronic kidney disease (CKD) stages 3 and 4. Kidney Int Suppl 111: S67–S70, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis 51: 212–223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Stancanelli B, Nicocia G, Buemi M: Fibrinogen, mortality and incident cardiovascular complications in end-stage renal failure. J Intern Med 254: 132–139, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Stack AG, Donigiewicz U, Abdalla AA, Weiland A, Casserly LF, Cronin CJ, Nguyen HT, Hannigan A: Plasma fibrinogen associates independently with total and cardiovascular mortality among subjects with normal and reduced kidney function in the general population. QJM 107: 701–713, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Shishehbor MH, Oliveira LP, Lauer MS, Sprecher DL, Wolski K, Cho L, Hoogwerf BJ, Hazen SL: Emerging cardiovascular risk factors that account for a significant portion of attributable mortality risk in chronic kidney disease. Am J Cardiol 101: 1741–1746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez M, Weisel JW, Ischiropoulos H: Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free Radic Biol Med 65: 411–418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW: Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol 20: 1354–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Parastatidis I, Thomson L, Burke A, Chernysh I, Nagaswami C, Visser J, Stamer S, Liebler DC, Koliakos G, Heijnen HF, Fitzgerald GA, Weisel JW, Ischiropoulos H: Fibrinogen beta-chain tyrosine nitration is a prothrombotic risk factor. J Biol Chem 283: 33846–33853, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolberg AS: Thrombin generation and fibrin clot structure. Blood Rev 21: 131–142, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Carter AM, Cymbalista CM, Spector TD, Grant PJ; EuroCLOT Investigators : Heritability of clot formation, morphology, and lysis: The EuroCLOT study. Arterioscler Thromb Vasc Biol 27: 2783–2789, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Ajjan R, Lim BC, Standeven KF, Harrand R, Dolling S, Phoenix F, Greaves R, Abou-Saleh RH, Connell S, Smith DA, Weisel JW, Grant PJ, Ariëns RA: Common variation in the C-terminal region of the fibrinogen beta-chain: Effects on fibrin structure, fibrinolysis and clot rigidity. Blood 111: 643–650, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rueth M, Lemke HDD, Preisinger C, Krieter D, Theelen W, Gajjala P, Devine E, Zidek W, Jankowski J, Jankowski V: Guanidinylations of albumin decreased binding capacity of hydrophobic metabolites. Acta Physiol (Oxf) 215: 13–23, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.