Abstract
The vasopressin–cAMP–osmolality axis is abnormal in autosomal dominant polycystic kidney disease (ADPKD). In the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 Trial, a 3-year randomized, placebo-controlled trial in adults, the vasopressin V2 receptor antagonist tolvaptan slowed ADPKD progression in patients with preserved GFR. Here, we investigated the determinants of baseline urine osmolality (Uosm) and its value as a severity marker of ADPKD, the factors influencing the response to tolvaptan, and whether change in Uosm associated with key trial end points. At baseline, lower Uosm independently associated with female sex, presence of hypertension, lower eGFR, higher total kidney volume (TKV), and higher age. Tolvaptan consistently reduced Uosm by 200–300 mOsm/kg over 36 months. The Uosm response to tolvaptan depended on baseline eGFR and Uosm. Subjects with greater change in Uosm experienced a significant reduction in clinical progression events. Among subjects receiving tolvaptan, those with a greater suppression of Uosm had slower renal function decline. Assessment at follow-up, off medication, revealed a significant decrease in Uosm in both placebo and treated groups. Tolvaptan significantly increased plasma osmolality, which returned to baseline at follow-up. In conclusion, baseline Uosm in ADPKD reflects age, renal function, and TKV, and baseline Uosm, eGFR, and TKV influence the effect of tolvaptan on Uosm. The greatest renal benefit occurred in subjects achieving greater suppression of Uosm, that is, those with better eGFR at baseline. These results support the link between vasopressin V2 receptor signaling and ADPKD progression.
Keywords: polycystic kidney disease, vasopressin, cyclic AMP, collecting ducts, water transport
Autosomal dominant polycystic kidney disease (ADPKD) is the most frequent inherited nephropathy, characterized by the appearance and slow growth of fluid-filled cysts in the kidneys.1,2 The disease is caused by mutations in the PKD1 or PKD2 genes that encode the integral membrane proteins, polycystin-1 and polycystin-2. The latter form a functional complex localized in various cellular domains including the primary cilium. Mutations in PKD1/PKD2 alter intracellular calcium homeostasis and, in turn, attenuate cyclic nucleotide phosphodiesterase activity, while raising adenylate cyclase activity, resulting in elevated levels of intracellular cAMP.3–5 The chronically high intracellular cAMP levels are thought to act via calcium-mediated regulatory pathways, including protein kinase A and its downstream effectors, to induce cyst growth in ADPKD.6
On the basis of these observations, therapeutic interventions that lower intracellular cAMP levels might be expected to have significant benefit in ADPKD.7 The antidiuretic hormone arginine vasopressin (AVP) is a major inducer of cAMP production in the distal nephron via its interaction with the vasopressin V2 receptor (V2R) and, hence, has been a major focus of recent studies.8 In human ADPKD cyst epithelial cells, the V2R antagonist tolvaptan has been shown to inhibit cell proliferation and chloride-dependent fluid secretion.9 In rodent models, V2R antagonism inhibited disease development and either halted or caused regression of established disease.3,5 Finally, in the pivotal Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 Trial (TEMPO 3:4; ClinicalTrials.gov identifier: NCT00428948), long-term treatment with tolvaptan significantly decreased kidney growth and kidney pain, while slowing the decline in kidney function.10
Continuous cyst expansion in ADPKD physically displaces and obstructs renal tubules, blood vessels, and lymphatics, and promotes apoptosis, atrophy, and fibrosis of the renal parenchyma.11 Although this ultimately leads to ESRD in a majority of subjects, the first signs of renal function decline are not typically seen until the third to fourth decades of life, due to the slow growth rate of cysts and to the reflex hyperfiltration carried out by unaffected nephrons.11 However, the capacity of patients with ADPKD to concentrate urine is impaired early, even in pediatric patients with intact GFR.12 Importantly, the cyst burden is not a prerequisite for defective osmoregulation in ADPKD children,12,13 consistent with the observation that urinary concentrating defects precede renal cyst development in animal models of ADPKD.14,15 These combined results suggest that urine osmolality (Uosm), as a noninvasive marker of the urinary concentrating ability, may serve as a useful biomarker of ADPKD.
In this study, we took advantage of available measurements of osmoregulation parameters in a large ADPKD cohort to investigate (1) the determinants of fasting Uosm in ADPKD; (2) whether Uosm, as a marker of urine concentrating ability, may serve as a substitute of disease severity in ADPKD; (3) the factors influencing the response to tolvaptan; and (4) whether changes in Uosm during the TEMPO 3:4 trial associate with treatment outcomes.
Results
Subject Characteristics
The demographic characteristics of the entire TEMPO 3:4 ADPKD study population have been reported previously.10,16 In this post hoc analysis of fasting subjects, there were 1037 subjects of whom 82% white, 52% men, and 39±7 years of age, and a total kidney volume (TKV) of 1703±930 ml and an eGFR of 81±22 ml/min per 1.73 m2. Mean systolic and diastolic BP values were 127±13 and 82±10 mmHg, respectively. Concurrent medications at baseline included diuretics in 1.4% (n=15), lipid-lowering mediations in 13.2% (n=137), and antidepressants in 22.5% (n=233). The majority of subjects (72.2%, n=749) were taking angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers (Supplemental Table 1). These baseline characteristics were similar to the population in the TEMPO 3:4 cohort.10
Determinants of Fasting Morning Baseline Uosm
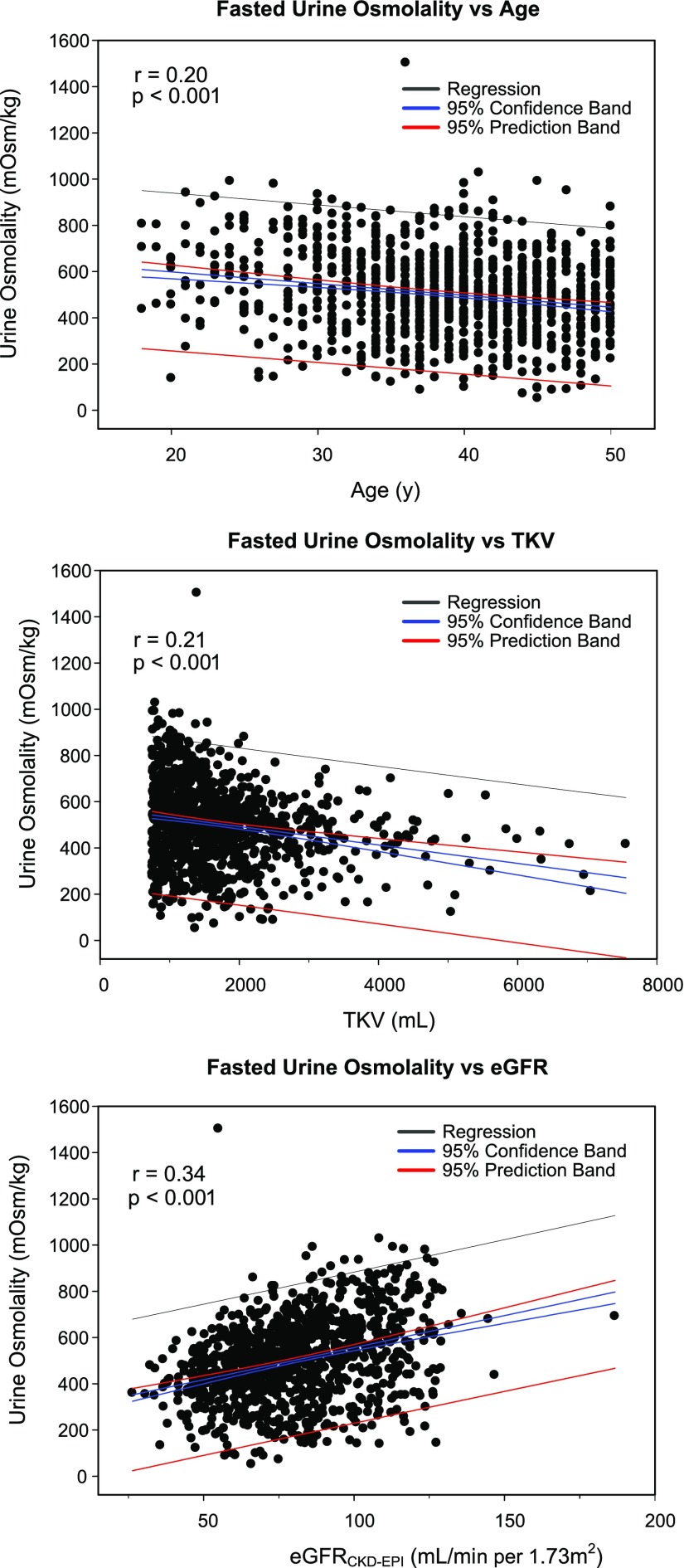
Average Uosm at baseline was 504±177 mOsm/kg with a normal distribution in the cohort (Supplemental Figure 1). Baseline Uosm correlated negatively with age (P<0.001, r2=0.041) and TKV (P<0.001, r2=0.044), and positively with eGFR (P<0.001, r2=0.117) (Figure 1). The Uosm/plasma osmolality (Posm) ratio, taken as an index of osmoregulation, showed similar correlations with age (P<0.001, r2=0.050), TKV (P<0.001, r2=0.041), and eGFR (P<0.001, r2=0.124) (Supplemental Figure 2). In a univariate analysis, ADPKD subjects in the lowest quartile (Q1) versus the highest quartile (Q4) of baseline Uosm were more likely to be women (60% versus 41%, P=0.002), less often white versus other races (79% versus 90%, P<0.001), more frequently hypertensive (84% versus 72%, P=0.001), older (40 versus 36 years, P<0.001), and shorter (171 versus 174 cm, P=0.005), with larger TKV (1762 versus 1335 ml, P<0.001), lower eGFR (75 versus 95 ml/min per 1.73 m2, P<0.001), and higher albumin-to-creatinine ratio (9.7 versus 4.9 mg/mmol, P<0.001), respectively (Table 1). In a multivariate regression analysis that included all individual significant variables (Table 2), sex, race, presence of hypertension, baseline TKV, and baseline eGFR significantly associated with baseline Uosm.
Figure 1.
Factors correlating with Uosm in the ADPKD population at baseline. Correlations of fasting baseline Uosm with age, TKV, and eGFR in the ADPKD population.
Table 1.
Baseline characteristics of the study population stratified according to quartiles of baseline fasting Uosm
| Parameter | Q1 (n=259) | Q2 (n=258) | Q3 (n=259) | Q4 (n=261) | P Value |
|---|---|---|---|---|---|
| Uosm, mOsm/kg, mean±SD | 280±77 | 449±34 | 558±35 | 729±101 | |
| Men, % | 40.9 | 54.7 | 51.4 | 59.8 | 0.002 |
| White, % | 79.2 | 74.8 | 84.6 | 89.7 | <0.001 |
| Hypertension, % | 84.2 | 87.2 | 79.5 | 72.4 | 0.001 |
| Medications, % | |||||
| Antihypertensives | 73.8 | 79.5 | 72.2 | 63.6 | 0.01 |
| Diuretics | 0.8 | 0.4 | 0.4 | 0.4 | 0.94 |
| Lipid-lowering | 16.2 | 14.0 | 13.1 | 9.6 | 0.15 |
| Antidepressants | 11.6 | 9.7 | 11.2 | 9.6 | 0.83 |
| Age, yr | 39.9 | 39.4 | 38.5 | 36.4 | <0.001 |
| Height, cm | 170.9 | 173.6 | 172.5 | 174.3 | 0.01 |
| Body weight, kg | 76.1 | 78.4 | 78.6 | 80.3 | 0.06 |
| TKV, ml | 1762 | 2013 | 1705 | 1335 | <0.001 |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | 75.4 | 73.5 | 81.2 | 94.9 | <0.001 |
| Creatinine, mg/dl | 1.095 | 1.141 | 1.043 | 0.928 | <0.001 |
| ACR, mg/mmol | 9.7 | 9.7 | 7.1 | 4.9 | <0.001 |
| BMI, kg/m2 | 25.9 | 25.8 | 26.3 | 26.3 | 0.19 |
Q1–Q4, quartiles 1–4; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ACR, albumin-to-creatinine ratio; BMI, body mass index.
Table 2.
Multivariate regression analysis investigating the effect of various parameters on baseline fasted Uosm
| Variable | Coefficient (Anti-Log) | SEM | P Value |
|---|---|---|---|
| Age, yr | 0.999 | 1.002 | 0.62 |
| Sex | 1.131 | 1.034 | 0.003 |
| Race (white versus nonwhite) | 1.068 | 1.034 | 0.048 |
| Hypertension | 0.901 | 1.048 | 0.03 |
| Log of baseline TKV, ml | 0.746 | 1.073 | <0.001 |
| Baseline eGFR (CKD-EPI), ml/min per 1.73 m2 | 1.004 | 1.001 | <0.001 |
| Albumin/creatinine ratio, mg/mmol | 0.999 | 1.001 | 0.13 |
Fasted Uosm is expressed in (log Uosm). Baseline eGFR, TKV, hypertension status, race, and sex significantly affect baseline Uosm. For continuous variables, such as baseline eGFR, when other factors are held constant, the baseline Uosm increases 1.004 times for every 1 ml/min per 1.73 m2 increase in baseline eGFR. For baseline TKV, for a multiplier change, the baseline Uosm will be changed by times the 0.746 power of the multiplier. For example, if the multiplier is equal to 2 (doubling of TKV), the baseline Uosm will be changed to the original Uosm times 2^0.746. For binary variables, such as race or hypertension status, when other factors are held constant, a white subject has a baseline Uosm 1.068 times a nonwhite subject; a subject with hypertension has a baseline Uosm 0.901 times a nonhypertension subject; and a male subject has a baseline Uosm 1.131 times a female subject. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ACR, albumin-to-creatinine ratio
Aquaresis and Osmoregulation in Response to Tolvaptan
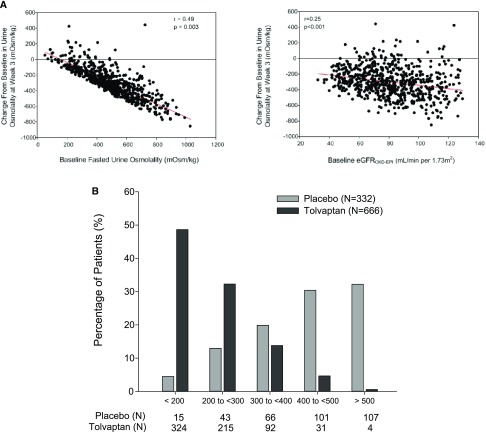
The initial response to tolvaptan was evaluated by assessing the change in Uosm from baseline to end of titration (EOT, corresponding to titration to the highest tolerable dose from day 1 to week 3 after each 1-week safety assessment) in 666 subjects.16 The most common side effects limiting the dose escalation were related to increased aquaresis (thirst, polyuria, pollakiuria, nocturia, and polydipsia), as reported previously.10 The initial decline in Uosm in response to tolvaptan correlated significantly and positively with baseline Uosm (P=0.003, r2=0.24) and eGFR (P<0.001, r2=0.060) (Figure 2A), and the average daily dose of tolvaptan at EOT (Table 3). Of note, although Japanese participants demonstrated a lower baseline Uosm before treatment, their response to tolvaptan was similar to that of non-Japanese subjects (data not shown). The majority of subjects receiving tolvaptan (n=539, 81%) achieved a Uosm<300 mOsm/kg, contrasting with only 17% (n=58) in the placebo group (Figure 2B).
Figure 2.
Influence of baseline characteristics on the response to tolvaptan and distribution of Uosm during the trial. (A) Effect of baseline Uosm and baseline eGFR on the magnitude of change in Uosm (EOT compared with baseline) induced by tolvaptan in 666 patients with ADPKD. (B) Distribution of average Uosm during the trial in the tolvaptan (n=666) and placebo (n=332) groups (percentage of patients for each threshold of Uosm as indicated).
Table 3.
Average Uosm in response to tolvaptan or placebo (EOT) and corresponding baseline eGFR
| Average Uosm at EOT, mOsm/kg | Treatment (N) | Average Dose of Tolvaptan at EOT, mg/d | Baseline eGFR, ml/min per 1.73m2 |
|---|---|---|---|
| <200 | Tolvaptan (324) | 104 | 77.1±20.4 |
| Placebo (15) | — | 84.3±21.9 | |
| 200–<300 | Tolvaptan (215) | 97 | 80.2±20.5 |
| Placebo (43) | — | 76.2±18.9 | |
| 300–<400 | Tolvaptan (92) | 92 | 89.3±21.5 |
| Placebo (66) | — | 74.9±23.0 | |
| 400–<500 | Tolvaptan (31) | 92 | 93.1±19.6 |
| Placebo (101) | — | 79.3±22.1 | |
| ≥500 | Tolvaptan (4) | 67 | 102±24.1 |
| Placebo (107) | — | 91.6±22.7 |
eGFR values are mean±SD. —, not dosed.
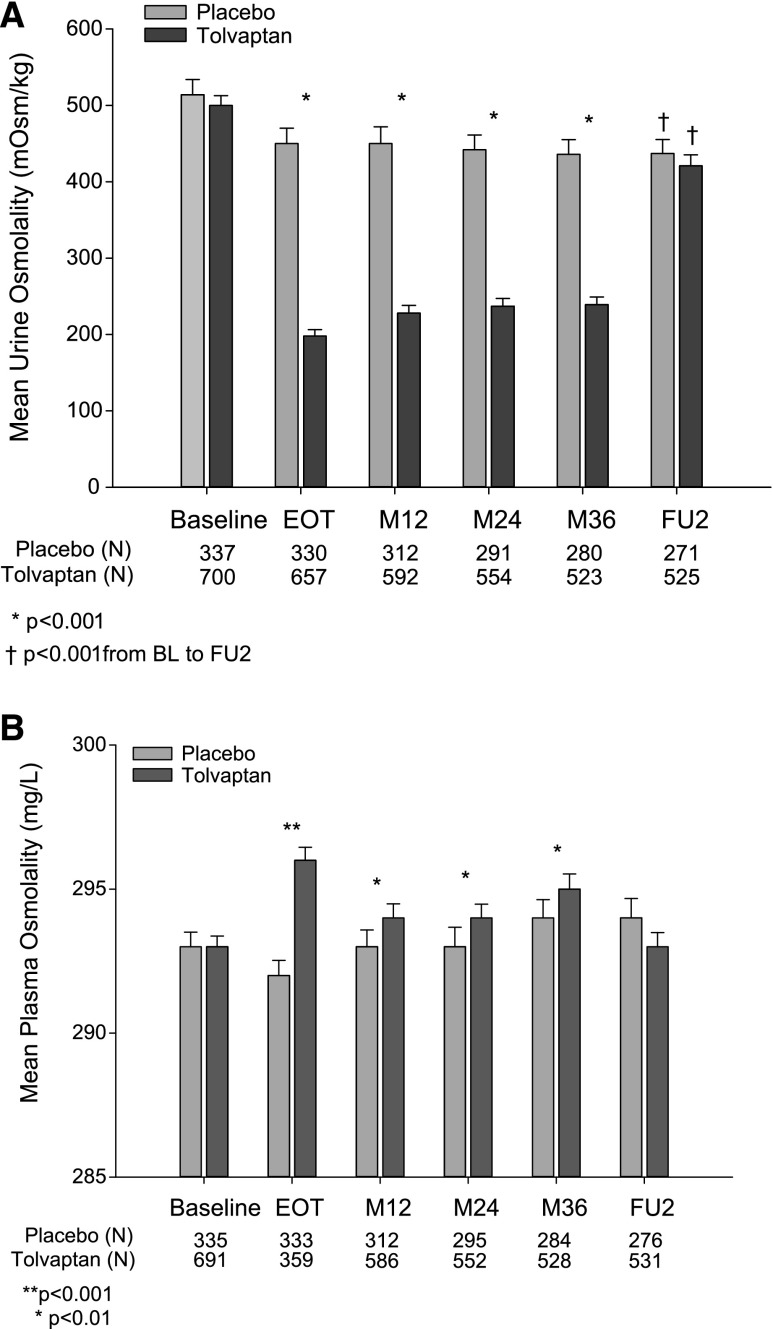
Treatment with tolvaptan resulted in a sustained and significant decrease in Uosm throughout the study, compared with baseline and to the concurrent placebo group (Figure 3A). There was a moderate but significant increase in Uosm in tolvaptan-treated subjects from EOT to month 36 (from 198±108 to 239±117 mOsm/kg, respectively, P<0.001). This may be explained in part due to a reduction in the average daily dose of tolvaptan from approximately 110 mg/d to approximately 97 mg/d from EOT to month 36, respectively (Supplemental Table 2). There was a significant decrease from baseline Uosm in both tolvaptan- and placebo-treated subjects that completed the study and provided a fasted urine sample at their follow-up visit (placebo, n=271: baseline 514±186 versus follow-up 437±155 mOsm/kg, P<0.001; tolvaptan, n=525: baseline 500±173 versus follow-up 421±167 mOsm/kg, P<0.001). There was no significant correlation between changes in Uosm from baseline to follow-up and the annual TKV growth rate over 3 years in the placebo group (correlation coefficient −0.01184, P=0.74). The Uosm/Posm ratios at follow-up were significantly lower than the baseline values in each arm (placebo, n=265: baseline 1.75±0.64 versus follow-up 1.49±0.53, P<0.001; tolvaptan, n=517: baseline 1.70±0.59 versus follow-up 1.44±0.57, P<0.001), the values being similar between groups. Of note, mean Uosm was similar at each time point in completers versus noncompleters of the study (Supplemental Figure 3).
Figure 3.
Evolution of Uosm and Posm during exposure to tolvaptan or placebo. (A) Uosm (fasting baseline and follow-up visits and nonfasting visits included). (B) Estimated Posm at the same time points. M12, Month 12; M24, Month 24; M36, Month 36; FU, Follow-up; BL, Baseline.
Changes in estimated Posm during the trial are shown in Figure 3B. Compared with the placebo group, exposure to tolvaptan induced a significant increase in Posm, which was highest at EOT and returned to baseline at follow up (Supplemental Table 3). No significant changes in Posm were seen in the placebo group.
Uosm and Clinical Outcomes
All subjects participating in the TEMPO 3:4 trial were instructed to increase their water intake and drink in advance of thirst. Evidence that this recommendation was followed is suggested by the significant decrease in Uosm from baseline to EOT in the placebo group (baseline, 514±186 versus EOT, 450±187 mOsm/kg, P<0.001). Because water intake and 24-hour-urine volumes were not obtained (to avoid unblinding of physicians to treatment arm), we analyzed the evolution of the Uosm/Posm ratio between baseline and EOT as an index of fluid intake.17 As shown in Supplemental Table 4, the tolvaptan group was characterized by a major fall in the Uosm/Posm ratio, reflecting a large decrease in Uosm and a significant increase in Posm at EOT. A significant decrease in the Uosm/Posm ratio, which primarily reflected a significant fall in Uosm, was observed in the placebo group—supporting a higher water intake.
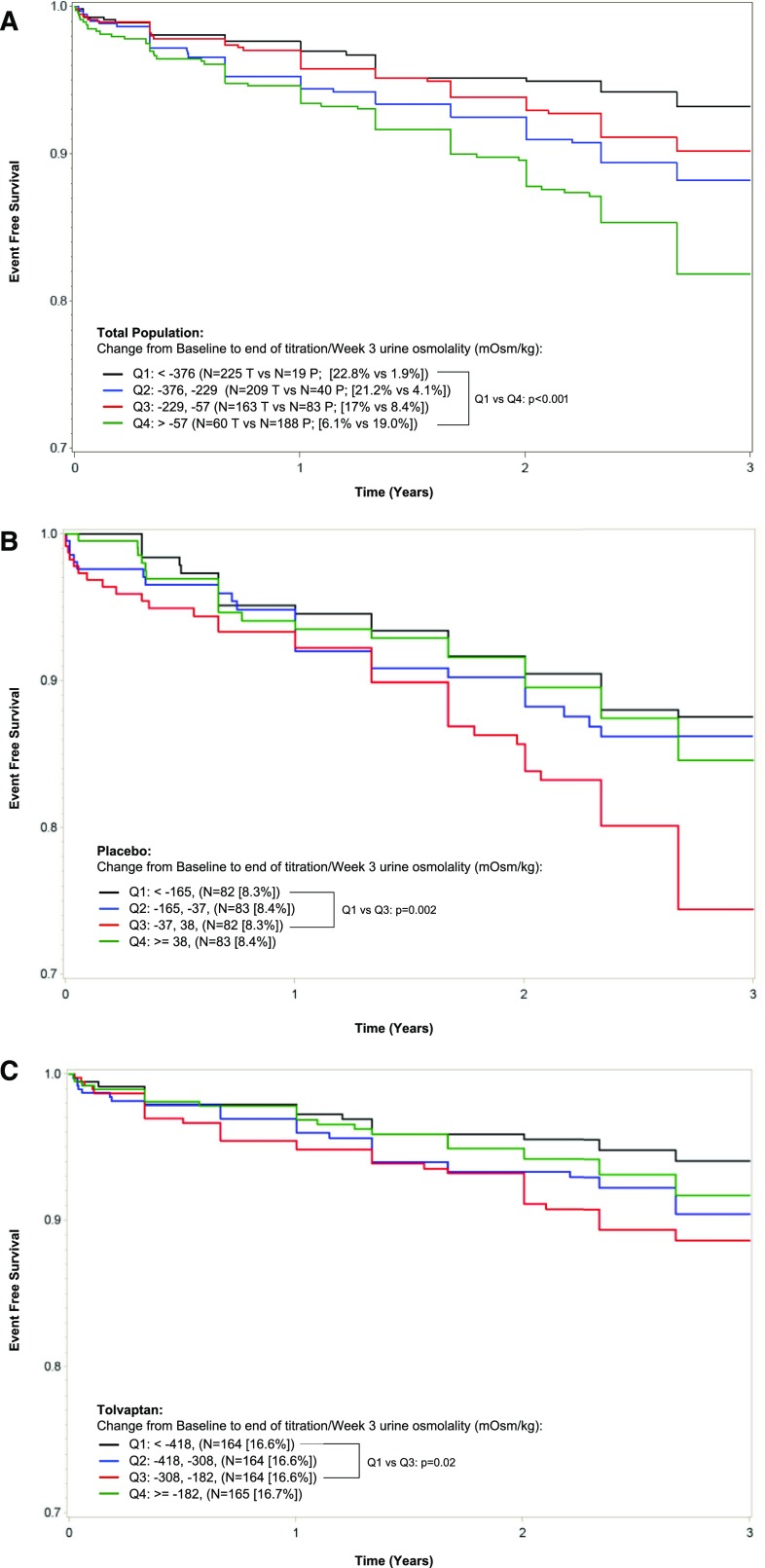
We next evaluated whether changes in Uosm from baseline to EOT were associated with clinical progression events of worsening renal function and kidney pain in the trial population (Figure 4). In the total population, subjects with a greater suppression in Uosm (Q1, negative change >−376 mOsm/kg) experienced a two-fold reduction in the occurrence of clinical progression events versus those with the smaller suppression (Q4, negative change <−57 mOsm/kg) (12.1% versus 23.3%, respectively, P<0.001), as confirmed by Kaplan–Meier analysis (Figure 4A). A similar trend (between Q1 and Q3) was observed for the placebo (Figure 4B) and tolvaptan (Figure 4C) subjects analyzed separately, although the fact that the vast majority of patients in the tolvaptan group had a major decrease in Uosm (ceiling effect) prevented a good separation.
Figure 4.
Change in Uosm from baseline to EOT and time to multiple ADPKD clinical progression events in the ADPKD population. Quartiles of changes in Uosm from baseline to EOT are presented for the whole population (A) and the placebo (B) and tolvaptan (C) groups, in relation to ADPKD clinical progression events defined as worsening renal function or pain. Q1–Q4, quartiles 1–4.
Because individuals in whom tolvaptan produced a larger fall in Uosm did better, presumably because they had a less advanced renal disease at baseline, we evaluated whether benefit continues to increase as achieved Uosm declines, or whether there is a Uosm level below which no further benefit is obtained. We analyzed the annual change in eGFR as a function of Uosm at the EOT phase (highest tolerable dose) in tolvaptan-treated subjects (Supplemental Table 5): there was no significant trend between these parameters, indicating that there is no additional benefit to decrease Uosm <250 mOsm/kg. The lack of benefit from reducing Uosm <250 mOsm/kg was also true for the composite secondary end point and for the renal pain events or renal function events. Instead, these events were more frequently observed in subjects with lower Uosm, probably reflecting the more severe disease at time of tolvaptan initiation (Supplemental Table 5).
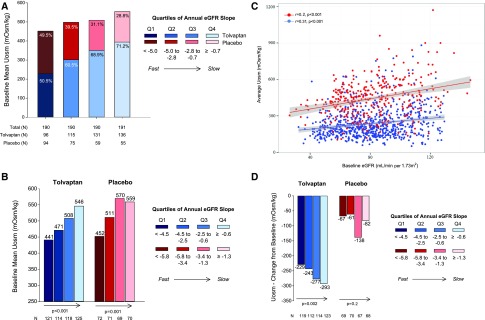
We next investigated the relationship between Uosm and eGFR in the context of tolvaptan treatment (Figure 5). The relationship between baseline Uosm and eGFR outcomes (quartiles of annual eGFR slope over 3 years) for the whole population and for each treatment arm are shown in Figure 5, A and B, respectively. A significant relationship between higher Uosm at baseline and a less negative slope of eGFR was observed in both treatment groups, supporting that ADPKD subjects with higher baseline Uosm experience slower renal function decline. In the placebo group, baseline Uosm significantly predicted the annual change in eGFR (P=0.002), with persistence of a trend (P=0.06) after adjustment for baseline eGFR and TKV.
Figure 5.
Relationship between Uosm and eGFR outcome during the trial. (A) Relationship between mean baseline Uosm and quartiles of annual eGFR slope over 3 years in the total population. The proportion of tolvaptan (blue shaded boxes) and placebo (red shaded boxes) for each quartile are represented as a stacked bar chart with decreasing eGFR slope from dark to light (<−4.5 to ≥−0.7 ml/min per 1.73 m2). Slower progression is reflected by a progressively higher Uosm at baseline and a higher proportion of treatment with tolvaptan. (B) Relationship between mean baseline Uosm and quartiles of annual eGFR slope over 3 years in tolvaptan (blue shaded boxes; darkest to lightest, <−4.5 to ≥−0.6 ml/min per 1.73 m2) and placebo groups (red shaded boxes; darkest to lightest, <−5.8 to ≥−1.3 ml/min per 1.73 m2). Arrows indicate the significant trend between increased Uosm at baseline and slower rate of eGFR decrease as tested by ANOVA. Slower progression is reflected by a progressively higher Uosm at baseline in both groups. (C) Correlation between baseline eGFR and average Uosm during the trial (blue symbols, tolvaptan; red symbols, placebo). (D) Relationship between change in Uosm (EOT compared with baseline) and quartiles of annual eGFR slopes over 3 years in tolvaptan (blue shaded boxes; darkest to lightest, <−4.5 to >−0.6 ml/min per 1.73 m2) and placebo groups (red shaded boxes; darkest to lightest, <−5.8 to >−1.3 ml/min per 1.73 m2). Arrows indicate a significant trend between change in Uosm and slower rate of eGFR decrease in the tolvaptan group, as tested by ANOVA. Slower progression is reflected by a higher initial response to Uosm in the tolvaptan but not in the placebo group. Q1–Q4, quartiles 1–4.
Average Uosm achieved during the trial correlated positively with baseline eGFR in placebo and tolvaptan groups with, as expected from the action of tolvaptan, a clear separation of Uosm levels between the two groups (Figure 5C). The relationship between Uosm suppression (i.e., change in Uosm from baseline to EOT) and renal function decline is shown in Figure 5D. In the tolvaptan group, subjects with higher change in Uosm at EOT showed a slower renal function decline over the 3 years. This association was not observed in the placebo group. When considering the annualized eGFR decline in both groups (Supplemental Table 6), slow progressors experienced a greater reduction in Uosm compared with fast progressors in the tolvaptan group whereas no significant difference was observed in the placebo group, characterized by a much smaller level of Uosm suppression. A sensitivity analysis verified that the baseline eGFR was a key determinant of both change in Uosm and eGFR slope in both tolvaptan and placebo subjects (Supplemental Figure 4).
Discussion
Multiple lines of evidence have implicated AVP and cAMP in the pathogenesis and progression of ADPKD.18 Our study provides the first detailed assessment of Uosm, a parameter that directly reflects the action of AVP in the kidney, at baseline and after long-term administration of tolvaptan in a large, multi-center cohort of ADPKD subjects with preserved renal function. At baseline, Uosm in ADPKD subjects correlated negatively with age and TKV and positively with eGFR. By multivariate analysis, Uosm is affected by sex, presence of hypertension, TKV, and eGFR. Treatment with tolvaptan reduced Uosm by 200–250 mOsm/kg with no loss of effect over 36 months. The initial response to tolvaptan was influenced by baseline eGFR and Uosm. In both treatment groups, subjects with higher Uosm at baseline experienced slower renal function decline over the 3 years. Subjects with a greater suppression in Uosm—and thus a better eGFR at baseline—experienced a significant reduction in the occurrence of clinical progression events. At follow-up, there was a significant decrease in Uosm over the 3 years in both placebo and treated groups, reflecting progression of ADPKD and/or modified drinking behavior.
Defective urinary concentration is one of the first manifestations of ADPKD.6 Early studies on small cohorts showed that the defect was more evident in subjects with large kidneys.19 A peripheral resistance to vasopressin, potentially explained by cystic lesions affecting the interstitial osmotic gradient, has been suggested.12,13 We evaluated the determinants of fasting Uosm at baseline: the opposite correlations between Uosm and TKV and eGFR, as well as the multivariate analysis, support the influence of kidney structure and function on urinary-concentrating ability in ADPKD. Most complications in ADPKD depend on the number, size, and location of kidney cysts. Larger kidneys predict functional decline,20 and they are associated with lower Uosm in this cohort. The effect of age on Uosm is also expected: in addition to disease progression, multiple abnormalities in the central release of AVP and its renal effects have been described in association with aging.21 Those changes may include a progressive decrease in the V2R expression/function in the distal nephron.22 It should be pointed out that the strength of the associations between fasting Uosm and various parameters at baseline was relatively modest, being valuable owing to the large number of subjects studied. These data nevertheless support the value of fasting Uosm as a noninvasive, integrative marker of the state of kidney disease in ADPKD.
By univariate analysis, ADPKD subjects in the lowest Uosm quartile were more likely to be women, nonwhite, hypertensive, older, shorter, and have larger kidneys and lower eGFR. Among these factors, only female sex, presence of hypertension, TKV, and baseline eGFR were independently predictive of Uosm. If hypertension and eGFR are known to reflect the disease progression in ADPKD, the effect of sex on Uosm in our cohort should be emphasized. It is generally estimated that ADPKD is less progressive in women than in men.2 Furthermore, women have a better response to AVP or its analog desmopressin, reflecting possible higher levels of V2R (X-linked) expression than in men.23,24 Taken together, these effects should rather be reflected by higher Uosm in women. Conversely, physiologic studies have found greater 24-hour urinary AVP excretion,25 and higher plasma AVP levels, in men compared with women.26 Preclinical studies in conscious rats have shown that, after 24 hours of water deprivation, urine flow was significantly higher and Uosm lower in females than in males.27 In addition, women may be more prone to abundant water drinking, a practice that is currently advised in most ADPKD clinics.28
This study allowed for the analysis of factors that influence the initial response to tolvaptan in ADPKD subjects. At EOT, 81% of the subjects had a Uosm<300 mOsm/kg, a proportion similar to that reported in a preliminary study.29 It is important to note that the initial aquaretic response is closely related to the state of disease, as indicated by eGFR and Uosm, as well as the dose of tolvaptan. Previous studies in non-ADPKD subjects have shown that impaired renal function (24-hour creatinine clearance <60 ml/min) attenuated the aquaretic response to administration of a single oral dose of tolvaptan, despite higher exposure to the drug.30 A significantly lower response in terms of urine volume was also observed when comparing ADPKD patients with eGFR>60 ml/min per 1.73 m2 and those with eGFR<30 ml/min per 1.73 m2.31 Conversely, there was not influence of ethnicity because a similar response was observed in Japanese versus white subjects (14% versus 86% in both placebo and tolvaptan groups, respectively), despite distinct NaCl intake at baseline.
The longitudinal assessment of Uosm over >3 years revealed that treatment with tolvaptan reduced Uosm by 250–300 mOsm/kg (versus baseline) with no loss of effect over time. The slight but significant increase in Uosm observed over time in the tolvaptan group probably reflected at least in part a slight decrease in the average drug dose during the trial. The changes in Uosm explain why almost 80% of ADPKD subjects who received tolvaptan (752 of 961, 78%) reported adverse events related to increased aquaresis (i.e., polyuria, pollakiuria, polydipsia, nocturia, and thirst). The aquaretic effect of tolvaptan was reflected by significant changes in estimated Posm, with a larger increase (average +2.7 mOsm/kg) observed at EOT. The changes in Uosm and Posm in the tolvaptan-treated subjects were fully reversible at the follow-up visit (washout). The significant decrease in Uosm and Uosm/Posm ratio at follow-up, which was similar in the tolvaptan and placebo groups, could reflect the composite effect of the progression of ADPKD as well as changes in the drinking behavior. Of note, there was no significant correlation between changes in Uosm from baseline to follow-up and the annual TKV growth rate in the placebo group.
Our results support the use of Uosm as guidance to tolvaptan treatment—a measure that has been adopted by a regulatory agency (Health Canada). The latter states that fasting morning Uosm provides the best guidance for dosing decisions to ensure target Uosm (e.g., ideally <300 mOsm/kg) is achieved to ensure complete AVP suppression.32 We also show that there is no additional benefit to decrease Uosm <250 mOsm/kg. Should it be recommended to stop tolvaptan if Uosm does not decrease sufficiently? As mentioned above, 81% of subjects receiving tolvaptan achieved a Uosm<300 mOsm/kg at EOT. In very rare cases, an activating mutation in the V2R could explain a patient’s resistance to tolvaptan treatment.33 We would anticipate in a subject with this type of activating mutation that the treatment with tolvaptan would fail to have the expected action on Uosm or on improved disease progression. Given the rarity of this condition, a lack of sufficient change in Uosm should not be used as a sole reason for drug discontinuation before excluding the more likely possibility of poor patient compliance or inadequate dose which may be limited by tolerability.
The protocol of the TEMPO 3:4 trial required standardization of background clinical care including management of BP and restricted use of diuretics, as well as dietary and standard fluid ingestion recommendations. In particular, all subjects were asked to drink enough water to prevent thirst throughout the daytime period and an additional one to two cups of water before bedtime.16 The initial suppression of Uosm (either due to increased water intake or treatment with tolvaptan) is negatively associated with the occurrence of clinical progression events in the whole cohort. In subjects receiving tolvaptan, there was a strong, inverse relationship between the magnitude of the aquaretic response and the loss of renal function during the study. By contrast, this effect was not observed in the placebo group. When interpreting these data, one should take into account the distinct magnitude (average decrease in Uosm at EOT: −65 versus −300 in placebo versus tolvaptan, respectively) and mechanism (primary water intake versus V2R blockade causing a ceiling effect, respectively) of aquaresis in these two groups. The response to V2R, as reflected by Uosm, is indeed fundamentally dependent on the renal function as evidenced by the multivariate analysis for baseline parameters (Table 2).
The analyses presented here allow us to address two additional clinical issues. The first is whether solely drinking (without use of tolvaptan) is sufficient to decrease the progression of ADPKD. If experimental data suggest that increasing water intake may slow down the progression of ADPKD,34 data from randomized trials are lacking. As mentioned above, the fact that all participants were asked to drink abundantly during the trial may have led to an underestimation of the beneficial effects of tolvaptan. The kidney growth rate in TEMPO 3:4 was indeed lower in the placebo group than in the control groups of previous ADPKD trials (6.8% versus 11.8% per year, respectively10). Conversely, high urine volume and low Uosm have been shown to be independent risk factors for faster GFR decline in a retrospective analysis of the Modification of Diet in Renal Disease study, including 139 patients with ADPKD.35 More recently, Higashihara et al. reported that high water intake enhanced disease progression in a cohort of 18 patients with ADPKD, compared with 16 control patients (free water intake).36 These results are challenging to interpret as the high water and standard water groups were not randomized but were rather left to the choice of the participating subject. A specifically designed clinical trial would be necessary to determine the effectiveness of chronic, high water intake on disease progression and how the effect would compare with tolvaptan. Because of the reactivity of vasopressin release and its short half-life, a sustained water intake would be required to achieve prolonged inhibition of the V2R signaling. In a small pilot study, Wang et al. demonstrated that 5 of 8 subjects were able to decrease their mean 24-hour Uosm <285 mOsm/kg with increased water intake for 1 week.37 It would be more difficult to sufficiently suppress vasopressin during prolonged periods of time with water alone, as the volume and frequency of water intake is highest during waking hours.38 It should also be pointed out that factors including salt and/or protein depletion potentially limit water intake in ADPKD, with development of hyponatremia being a concern.34
The second issue is the prognostic value of fasting Uosm at baseline to predict disease progression in ADPKD subjects. For the specific population studied here, high baseline fasting Uosm is a good predictor for eGFR over 3 years, both in placebo and tolvaptan groups (Figure 5, A and B). In view of our multivariate analysis, which clearly shows the integrative nature of Uosm, the prognostic value of fasting Uosm cannot be untangled from TKV and eGFR. Predictive scores on the basis of TKV and genetic information remain limited by cost, availability, and expertise required to obtain such parameters.39 Determination of Uosm is noninvasive, affordable, convenient, and of recognized value as an integrative marker of renal function and of disorders of osmoregulation.6,40 Future studies are necessary to test whether Uosm could improve or complete the existing scores to predict renal outcome in patients with ADPKD.
Our study combines the advantages of the first, centralized assessment of Uosm in a large, multicentric, and multiethnic study. The parameters were obtained at baseline and during a 36-month intervention with tolvaptan or placebo, and they were correlated with reliable indicators of renal function and disease progression (TKV measured by magnetic resonance imaging). Limitations of this study include the fact that subjects were asked to drink abundantly during the trial, which may influence the natural evolution of the disease in the control group; the fact that most subjects had hypertension at baseline, with associated antihypertensive and other medications; and the fact that the baseline and follow-up samples were obtained in the fasting state, whereas intermediate samples during the study were nonfasting. These different conditions may have an effect on the variations presented in the study—in particular between baseline and EOT—because water intake and alimentation are well known to influence Uosm41; and finally, ADPKD per se is associated with defects in AVP release/action, which could affect the interpretation of Uosm.6,34 Variations in copeptin, a surrogate marker of AVP that is inversely associated with eGFR and positively with Uosm and simple cysts in the general population,42 should also be analyzed in the context of the TEMPO 3:4 trial.
In summary, these results provide the first longitudinal analysis of osmoregulation parameters in a large cohort of ADPKD subjects. They show the determinants of baseline Uosm, demonstrate the effectiveness of tolvaptan over 36 months, and decipher the factors associated with the response to the drug. In both treatment groups, subjects with a greater suppression in Uosm—and thus a better eGFR at baseline—experienced a significant reduction in the occurrence of clinical progression events. Altogether, these analyses support the link between vasopressin V2R signaling and renal disease progression in ADPKD.
Concise Methods
Study Design
This study was a prespecified and post hoc, exploratory subanalysis of the TEMPO 3:4 study (12), a phase 3, multicenter, double-blind, placebo-controlled, 3-year trial assessing the efficacy and safety of tolvaptan in subjects with ADPKD.16 ADPKD subjects with a TKV≥750 ml measured by magnetic resonance imaging, a creatinine clearance ≥60 ml per minute as estimated by the Cockcroft–Gault formula, and between the ages of 18 and 50 years participated. A total of 1445 eligible subjects were randomly assigned in a 2:1 ratio to receive either tolvaptan (n=961) or matching placebo (n=484). After randomization, subjects received two doses of study drug per day for 3 years, initiated with a 3-week titration period. During the first week of the titration period, the active arm received tolvaptan at a dose of 45 mg in the morning and 15 mg in the afternoon. These doses were increased to 60 mg/30 mg and 90 mg/30 mg in weekly intervals on the basis of subject-reported tolerability. The maximal dose at week 3 (EOT) was defined as the highest tolerable dose, mostly related to increased aquaresis (thirst, polyuria, pollakiuria, nocturia, and polydipsia) events.10,16
After the titration phase of the study, subjects were able to up- or down-titrate on the basis of tolerability throughout the remaining study period. To offset the water loss due to the aquaretic effect of tolvaptan, diuretics were avoided and water intake was encouraged in advance of thirst in the study population.
Assessments
Strictly fasting morning (ideally, second void) spot urine samples were collected before drug administration to determine trough Uosm at randomization (baseline) and at follow-up which occurred 14–42 days after the last dose of study medication. Nonfasting morning spot urine samples were collected before the morning dose at the end of the titration period (EOT, 3 weeks) and at months 12, 24, and 36. Only subjects that indicated their baseline sample was a fasting urine were included in the analysis (tolvaptan 700 of 899 [78%], placebo 337 of 472 [71%]). Uosm was centrally measured (freezing point depression osmometry) using an Advanced Instruments Osmometer Model 220 (Advanced Instruments Inc, Norwood, MA). The system has an intra-assay and interassay precision (coefficient of variation) of 0.8% and 0.25%, respectively. Other assessments, including plasma parameters, TKV, and eGFR (Chronic Kidney Disease Epidemiology Collaboration), were performed as described previously.10,16 Posm was calculated using the following formula: Posm = 2 × Sodium + (Glucose/18) + (Blood urea nitrogen/2.8). Blood and urine samples were obtained on the same day per protocol. The composite secondary end point was the time to investigator-assessed clinical progression, defined as worsening kidney function (a 25% reduction in the reciprocal of the serum creatinine level from the value at the end of the dose-adjustment period); clinically significant kidney pain necessitating medical leave, pharmacologic treatment (narcotic or last-resort analgesic agents), or invasive intervention; worsening hypertension (changes in BP category, as defined in the protocol, or worsening of hypertension requiring an increase in hypertensive treatment); and worsening albuminuria (according to sex-specified categories as defined in the protocol).10
Statistical Analyses
Only fasted subjects at baseline were included (n=1037) in these analyses. The annual eGFR change was calculated on the basis of: (month 36 − EOT/Week 3)/3, and distributed into quartiles. The treatment effect was evaluated by analysis of covariance with treatment groups treated categorically and baseline values treated as a covariate. Subjects with missing average Uosm data were omitted. Trend tests were conducted by ANOVA, analysis of covariance, or Cochran–Armitage analyses, as appropriate. P values for comparing binary baseline characteristics were calculated by Fisher exact test, whereas P values for comparing continuous baseline characteristics were generated by Wilcoxon test. Comparison between visits was performed by Wilcoxon signed ranked test.
Disclosures
O.D., A.B.C., R.T.G., E.H., R.D.P., V.E.T., J.O., and F.S.C. are members of the steering committee of the TEMPO 3:4 trial. V.E.T., O.D., A.B.C., R.T.G., and R.D.P. have received research funding from Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ); E.H. has received research funding and consultancy fees from Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan); J.O., J.D.B., W.Z., and F.S.C. are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ).
Supplementary Material
Acknowledgments
We thank Jennifer Lee for validating the statistical analyses in this manuscript and Lise Bankir for fruitful discussions.
O.D. acknowledges the support of TranCYST – Marie Curie Initial Training Network on Polycystic Kidney Disease (EU-FP7 funded project, Grant Agreement number 317246).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016040448/-/DCSupplemental.
References
- 1.Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Gattone VH 2nd, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Nagao S, Kasahara M, Takahashi H, Grantham JJ: Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis 30: 703–709, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Devuyst O, Torres VE: Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens 22: 459–470, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Ong AC, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases : Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet 385: 1993–2002, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Rinschen MM, Schermer B, Benzing T: Vasopressin-2 receptor signaling and autosomal dominant polycystic kidney disease: From bench to bedside and back again. J Am Soc Nephrol 25: 1140–1147, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP: Tolvaptan inhibits ERK-dependent cell proliferation, Cl⁻ secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol 301: F1005–F1013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 11.Grantham JJ, Mulamalla S, Swenson-Fields KI: Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Ho TA, Godefroid N, Gruzon D, Haymann JP, Maréchal C, Wang X, Serra A, Pirson Y, Devuyst O: Autosomal dominant polycystic kidney disease is associated with central and nephrogenic defects in osmoregulation. Kidney Int 82: 1121–1129, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Zittema D, Boertien WE, van Beek AP, Dullaart RP, Franssen CF, de Jong PE, Meijer E, Gansevoort RT: Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol 7: 906–913, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Carone FA, Ozono S, Samma S, Kanwar YS, Oyasu R: Renal functional changes in experimental cystic disease are tubular in origin. Kidney Int 33: 8–13, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Gattone VH 2nd, Maser RL, Tian C, Rosenberg JM, Branden MG: Developmental expression of urine concentration-associated genes and their altered expression in murine infantile-type polycystic kidney disease. Dev Genet 24: 309–318, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang JJ, Czerwiec FS: Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3-4 Study. Am J Kidney Dis 57: 692–699, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Perrier E, Demazières A, Girard N, Pross N, Osbild D, Metzger D, Guelinckx I, Klein A: Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur J Appl Physiol 113: 2143–2151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bankir L, Bouby N, Ritz E: Vasopressin: A novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 9: 223–239, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, Schrier RW: The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35: 675–680, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowen LE, Hodak SP, Verbalis JG: Age-associated abnormalities of water homeostasis. Endocrinol Metab Clin North Am 42: 349–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juul KV, Bichet DG, Nielsen S, Nørgaard JP: The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol 306: F931–F940, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Juul KV, Klein BM, Sandström R, Erichsen L, Nørgaard JP: Gender difference in antidiuretic response to desmopressin. Am J Physiol Renal Physiol 300: F1116–F1122, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Juul KV, Klein BM, Nørgaard JP: Long-term durability of the response to desmopressin in female and male nocturia patients. Neurourol Urodyn 32: 363–370, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Crofton JT, Dustan H, Share L, Brooks DP: Vasopressin secretion in normotensive black and white men and women on normal and low sodium diets. J Endocrinol 108: 191–199, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Stachenfeld NS, Mack GW, Takamata A, DiPietro L, Nadel ER: Thirst and fluid regulatory responses to hypertonicity in older adults. Am J Physiol 271: R757–R765, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Wang YX, Crofton JT, Miller J, Sigman CJ, Liu H, Huber JM, Brooks DP, Share L: Sex difference in urinary concentrating ability of rats with water deprivation. Am J Physiol 270: R550–R555, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC; Conference Participants : Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 88: 17–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, Horie S, Nutahara K, Ouyang J, Krasa HB, Czerwiec FS; TEMPOFormula and 156-05-002 Study Investigators : Tolvaptan in autosomal dominant polycystic kidney disease: Three years’ experience. Clin J Am Soc Nephrol 6: 2499–2507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoaf SE, Bricmont P, Mallikaarjun S: Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int 85: 953–961, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boertien WE, Meijer E, de Jong PE, Bakker SJ, Czerwiec FS, Struck J, Oberdhan D, Shoaf SE, Krasa HB, Gansevoort RT: Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int 84: 1278–1286, 2013 [DOI] [PubMed] [Google Scholar]
- 32.JINARC Health Canada Product Monograph: Available at http://otsukacanada.com/docs/default-source/default-document-library/jinarc-pm-en-23feb2015_final.pdf?sfvrsn=2. Accessed August 25, 2016
- 33.Decaux G, Vandergheynst F, Bouko Y, Parma J, Vassart G, Vilain C: Nephrogenic syndrome of inappropriate antidiuresis in adults: High phenotypic variability in men and women from a large pedigree. J Am Soc Nephrol 18: 606–612, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Torres VE, Bankir L, Grantham JJ: A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol 4: 1140–1150, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Hebert LA, Greene T, Levey A, Falkenhain ME, Klahr S: High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis 41: 962–971, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Higashihara E, Nutahara K, Tanbo M, Hara H, Miyazaki I, Kobayashi K, Nitatori T: Does increased water intake prevent disease progression in autosomal dominant polycystic kidney disease? Nephrol Dial Transplant 29: 1710–1719, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang CJ, Creed C, Winklhofer FT, Grantham JJ: Water prescription in autosomal dominant polycystic kidney disease: A pilot study. Clin J Am Soc Nephrol 6: 192–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magpantay L, Ziai F, Oberbauer R, Haas M: The effect of fluid intake on chronic kidney transplant failure: A pilot study. J Ren Nutr 21: 499–505, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Cornec-Le Gall E, Le Meur Y: Polycystic kidney disease: Kidney volume--a crystal ball for ADPKD prognosis? Nat Rev Nephrol 10: 485–486, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Decaux G, Musch W: Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol 3: 1175–1184, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Hadj-Aïssa A, Bankir L, Fraysse M, Bichet DG, Laville M, Zech P, Pozet N: Influence of the level of hydration on the renal response to a protein meal. Kidney Int 42: 1207–1216, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Ponte B, Pruijm M, Ackermann D, Vuistiner P, Guessous I, Ehret G, Alwan H, Youhanna S, Paccaud F, Mohaupt M, Péchère-Bertschi A, Vogt B, Burnier M, Martin PY, Devuyst O, Bochud M: Copeptin is associated with kidney length, renal function, and prevalence of simple cysts in a population-based study. J Am Soc Nephrol 26: 1415–1425, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.