Abstract
Overexpression of the proximal tubular enzyme myo-inositol oxygenase (MIOX) induces oxidant stress in vitro. However, the relevance of MIOX to tubular pathobiology remains enigmatic. To investigate the role of MIOX in cisplatin-induced tubular AKI, we generated conditional MIOX-overexpressing transgenic (MIOX-TG) mice and MIOX-knockout (MIOX−/−) mice with tubule-specific MIOX overexpression or knockout, respectively. Compared with cisplatin-treated wild-type (WT) mice, cisplatin-treated MIOX-TG mice had even greater increases in urea, creatinine, and KIM-1 levels and more tubular injury and apoptosis, but these effects were attenuated in cisplatin-treated MIOX−/− mice. Similarly, MIOX-TG mice had the highest and MIOX−/− mice had the lowest renal levels of Bax, cleaved caspase-3, and NADPH oxidase-4 expression and reactive oxygen species (ROS) generation after cisplatin treatment. In vitro, cisplatin dose-dependently increased ROS generation in LLC-PK1 cells. Furthermore, MIOX overexpression in these cells accentuated cisplatin-induced ROS generation and perturbations in the ratio of GSH to oxidized GSH, whereas MIOX-siRNA or N-acetyl cysteine treatment attenuated these effects. Additionally, the cisplatin-induced enhancement of p53 activation, NF-κB binding to DNA, and NF-κB nuclear translocation in WT mice was exacerbated in MIOX-TG mice but absent in MIOX−/− mice. In vitro, MIOX-siRNA or NAC treatment reduced the dose-dependent increase in p53 expression induced by cisplatin. We also observed a remarkable influx of inflammatory cells and upregulation of cytokines in kidneys of cisplatin-treated MIOX-TG mice. Finally, analysis of genomic DNA in WT mice revealed cisplatin-induced hypomethylation of the MIOX promoter. These data suggest that MIOX overexpression exacerbates, whereas MIOX gene disruption protects against, cisplatin-induced AKI.
Keywords: oxidative stress, cisplatin nephrotoxicity, reactive oxygen species, renal injury, myo-inositol oxygenase
AKI is encountered in up to 30% of critically ill hospitalized patients, and it is quite often associated with acute tubular necrosis (ATN) leading to rapid deterioration of renal functions.1,2 There are a wide range of clinico-pathologic states that may be associated with ATN, and the latter can be classified under two broad categories, i.e., ischemic versus tubulo-toxic injury.3 The pathophysiologic mechanisms leading to AKI between these two categories may differ to a certain extent, and conceivably this may be one of the factors why different segments of the nephron are differentially affected, possibly involving different cellular and molecular mechanisms.4 For instance, although all of the three segments of proximal tubules are vulnerable to ischemic injury, the S3 segment is highly susceptible to tubulo-toxins by involving pathogenetic mechanisms that are restrictive to molecules expressed in this segment of the nephron.5 With respect to cellular specificity, past experiments have demonstrated that mice with selective ablation of p53 in proximal tubules are resistant to tubulo-toxins such as cisplatin, whereas p53 deletion in other segments of the nephron provided no protection against toxic injury.6 Intriguingly, repetitive tubulo-toxic assault can lead to both adaptive as well as maladaptive changes.7 Early adaptive changes could be beneficial in the form of cytoresistance, whereas late maladaptive changes may be detrimental; these include upregulation of inflammatory and profibrogenic cytokines along with oxidant stress, which ultimately could lead to tubulo-interstitial fibrosis, CKD, and renal failure.7–9
Myo-inositol oxygenase (MIOX), a proximal tubular enzyme, has been implicated in the pathogenesis of diabetic tubulopathy.10 It catabolizes myo-inositol, that is synthesized by isomerization and dephosphorylation of glucose 6-phospate, to D-glucuronic acid via the Glucuronate-Xylulose pathway (G-X pathway).11,12 It has osmotic-, carbohydrate-, and sterol- response elements in its promoter, and its expression is modulated by organic osmolytes, fatty acids, and obesity besides high glucose ambience or diabetes.13,14 In addition, the MIOX promoter region includes antioxidant and oxidant response elements and its transcription is regulated by a redox-sensitive response transcription factor, Nrf2.13 Moreover, MIOX overexpression in vitro accentuates the generation of reactive oxygen species (ROS), apparently derived both from mitochondrial and NADPH oxidase systems.15 The kidney is one of the major organs vulnerable to oxidant damage by ROS because of its high concentration of long-chain polyunsaturated fatty acids.16 Conceivably, the free radicals or ROS attack long-chain highly-unsaturated fatty acids and promote lipid peroxidation causing oxidative damage. In general, the ROS adversely affect homeostasis of the kidney in a variety of disease states, including diabetes mellitus, hypertension, obesity, ischemia, and ARF secondary to the administration of analgesics, antibiotics, and chemotherapeutic agents.16 Some of these states, e.g., secondary to the administration of therapeutic agents, are associated with AKI, which necessitates the development of various animal model systems to delineate the mechanisms that are relevant to the pathobiology of renal tubules.17
Cisplatin-induced nephropathy is a prototype toxic injury model which has been extensively utilized to investigate the mechanisms relevant to the pathogenesis of AKI.5 Cisplatin is taken up by proximal tubules, and inhibition of organic cation transporters with cimetidine decreases its cellular uptake. In 1view of the fact that cellular damage is confined to proximal tubules in toxin-induced nephropathy, and MIOX, a metabolic enzyme, is expressed in proximal tubules and its overexpression in vitro leads to the generation of ROS, studies were initiated to assess the effect of up- or downregulation of MIOX on the outcomes of cisplatin-induced nephropathy and associated signaling events in transgenic and knockout mice.
Results
Characterization of MIOX Transgenic and Null Mice
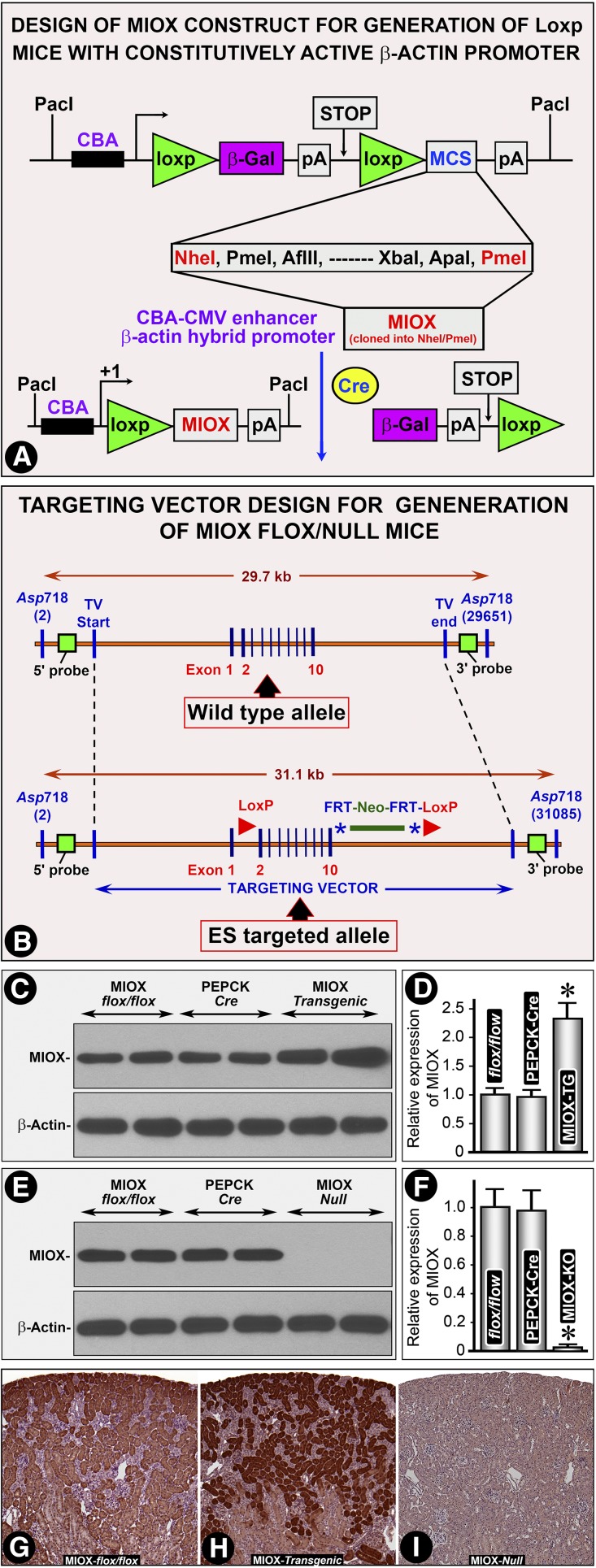
Western blot analyses revealed an increased expression (2–3-fold) of MIOX in kidneys of transgenic mice compared with the flox/flox or wild-type (WT) and PEPCK-Cre mice (Figure 1, C and D). No significant renal expression of MIOX was observed in MIOX-knockout (MIOX−/−) mice (Figure 1, E and F). By immuno-histochemistry a marked increase in MIOX expression was observed in renal proximal tubules in MIOX transgenic (MIOX-TG) mice (Figure 1, H versus G). No immuno-histochemical signal of MIOX was observed in MIOX−/− mice (Figure 1I). Both the mutant mice generated were proximal tubule–specific. Moreover, MIOX is a proximal tubule–specific enzyme. We did not observe any MIOX staining in any other renal compartment.
Figure 1.
Characterization of MIOX transgenic and Null mice. (A) Schematic drawing of the construct used for generation of MIOX flox/flox transgenic mice. MIOX gene was inserted after the second LoxP site. Its expression would be under the control of chicken–β-actin hybrid promoter. The flox/flox mice were generated and cross-bred with PEPCK-Cre mice to direct the expression primarily into proximal tubules. (B) Schematic drawing of the construct used to generate flox/Null mice. The strategy was to delete exons 2–10 of the MIOX gene in Null mice or flox/flox mice cross-bred with PEPCK-Cre mice. Western blots revealed an increased expression (2–3-fold) of MIOX in kidneys of transgenic mice (TG) compared with the flox/flox or WT and PEPCK-Cre mice (C and D). No significant renal expression of MIOX was observed in MIOX−/− (KO) mice (E and F). Immuno-histochemistry revealed an increased MIOX expression in renal proximal tubules of TG mice extending into the deeper cortex (H versus G). No expression of MIOX was observed in cortical tubules of MIOX−/− mice (I). *, P<0.01 compared with the flox/flox and PEPCK-Cre groups.
MIOX Gene Disruption Ameliorates Cisplatin-Induced AKI
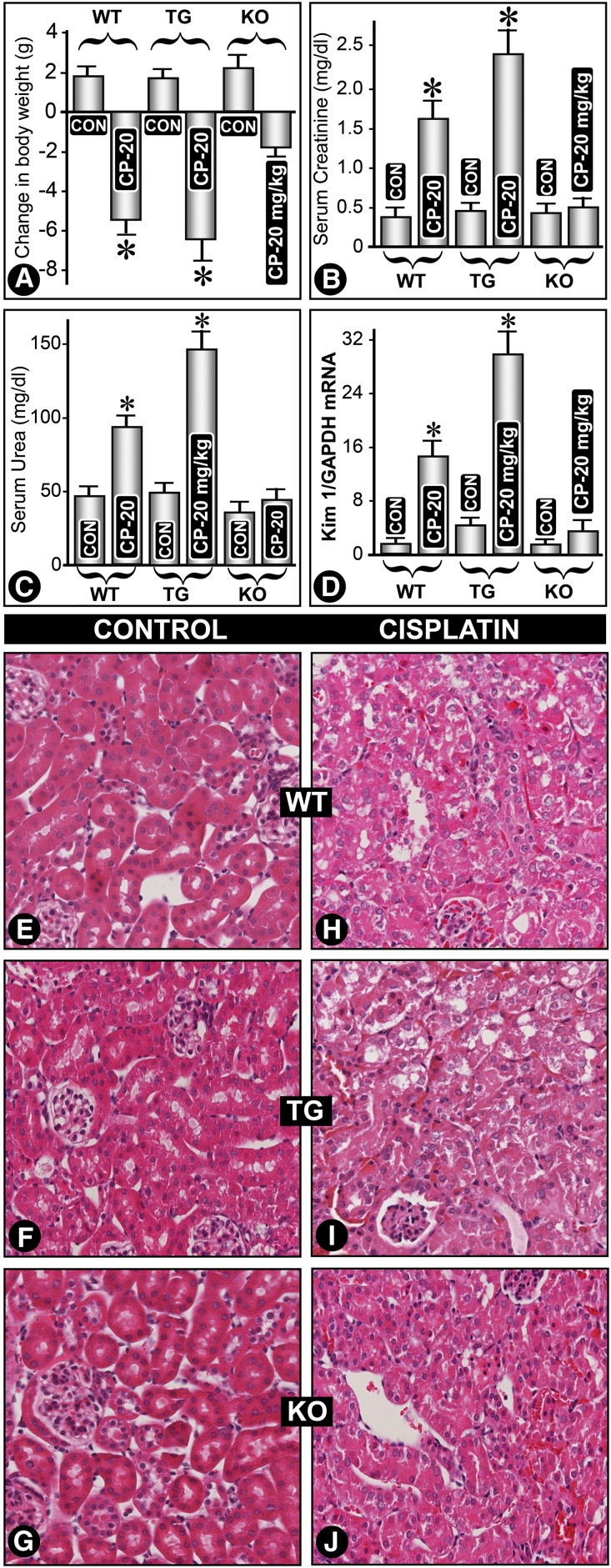
Cisplatin (20 mg/kg) induced significant weight loss in MIOX-TG and WT mice, whereas MIOX−/− mice had minimal body weight loss (Figure 2A). Maximal renal functional changes were seen in cisplatin-treated MIOX-TG mice. Both serum creatinine and urea were markedly elevated in MIOX-TG mice (Figure 2, B and C). A minimal increase in creatinine or urea levels was seen in MIOX−/− mice. A significant increase in expression of Kim-1 mRNA, a marker of AKI,18 was observed in kidneys of cisplatin-treated MIOX-TG mice (Figure 2D). WT mice showed cytolysis of tubular cells with loss of brush border (Figure 2, H versus E). These changes were accentuated in MIOX-TG mice (Figure 2, I versus F). Interestingly, the kidneys of cisplatin-treated MIOX−/− mice had mild cytolytic changes compared with WT or TG strains (Figure 2, J versus G).
Figure 2.
MIOX overexpression exacerbates cisplatin-induced AKI, whereas its gene disruption ameliorates AKI. Cisplatin treatment (20 mg/kg) led to a significant weight loss in MIOX transgenic (TG) and WT mice, whereas MIOX−/− (KO) mice had a lesser degree of body weight loss (A). Cisplatin administration also led to a marked elevation of both serum creatinine and urea levels in TG mice compared with WT and KO mice (B and C). The KO mice had minimal increase in creatinine and urea levels. A remarkable increase in Kim-1 mRNA expression was observed in kidneys of MIOX-TG mice compared with WT or KO mice (D). Kidney sections of WT mice that had undergone cisplatin treatment showed cytolysis of tubular cells with detachment from the underlying basal lamina and loss of brush border (H versus E). These changes were accentuated in MIOX-TG mice receiving cisplatin, and a marked vacuolization of tubular cells was observed (I versus F). The glomeruli had collapse of the capillary loops. The kidneys of MIOX−/− KO mice had a relatively lesser degree of cellular damage compared with the WT or MIOX-TG strains of mice (J versus G).
MIOX Gene Disruption Inhibits, Whereas Its Overexpression Accentuates, Renal Tubular Cell Apoptosis in Cisplatin-Induced AKI
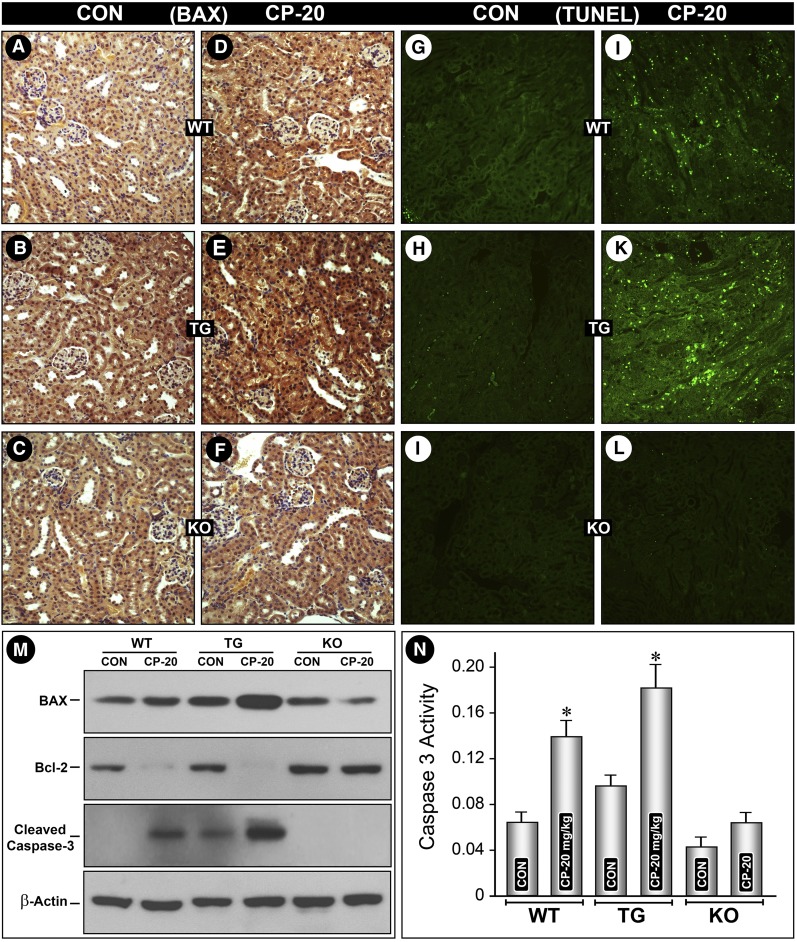
By immuno-histochemistry and immunoblot analyses an increased Bax expression was seen in tubules of cisplatin-treated WT mice (Figures 3, D versus A, and M). It was markedly increased in MIOX-TG mice (Figure 3, E versus B, and M). No significant increase was observed in MIOX−/− mice (Figure 3, F versus C, and M). On the other hand, Bcl-2 expression remarkably decreased in both WT and MIOX-TG mice, whereas it remained unchanged in MIOX−/− mice (Figure 3M). The status of another executioner of the apoptosis pathway, known as caspase-3, was investigated. It is activated after cleavage and catalyzes specific cleavage of multiple cellular proteins and thus activates apoptosis.19 An increased expression of cleaved caspase-3 was observed in kidneys of cisplatin-treated WT mice (Figure 3M). This increase was accentuated in MIOX-TG mice. No expression of cleaved caspase-3 was observed in MIOX−/− mice (Figure 3M). An increase in caspase-3 activity in kidneys of cisplatin-treated WT mice was observed (Figure 3N), but it was accentuated in MIOX-TG mice. A mild increase in its activity was observed in cisplatin-treated MIOX−/− mice (Figure 3N). Using the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) procedure, a notable degree of apoptosis was observed in tubules of cisplatin-treated WT mice (Figure 3, J versus G). Whereas a fulminant apoptosis was observed in cisplatin-treated MIOX-TG mice (Figure 3, K versus H), no TUNEL-positive nuclei were seen in MIOX−/− mice (Figure 3, I and L).
Figure 3.
MIOX gene disruption inhibits, whereas its overexpression accentuates, renal tubular cell apoptosis. Expression of proapoptogenic Bax was increased after cisplatin treatment in kidney tubules of WT mice (D versus A, and M). It was highly accentuated in MIOX-TG mice (E versus B, and M). No increase was observed in MIOX-KO mice (F versus C, and M). Intriguingly, at times a mild decrease in BAX expression was noted in KO mice (M), whereas antiapoptogenic Bcl-2 expression drastically decreased in both the WT and MIOX-TG mice, and it was unchanged in KO mice (M). An increased expression of cleaved caspase-3 was observed in kidneys of WT mice after cisplatin treatment (M), which was accentuated in MIOX-TG mice. No expression of cleaved caspase-3 was observed in treated or untreated MIOX-KO mice (M). After cisplatin treatment, a marked increase in caspase-3 activity was observed in MIOX-TG mice, whereas a moderate increase was also observed in WT mice (N). No significant increase in caspase-3 was observed in kidneys of KO mice. Along these lines, a fulminant degree of apoptosis was observed in kidneys of MIOX-TG mice (K versus H), although a mild-to-moderate degree of apoptosis was also observed in WT mice (J versus G), and no apoptosis was detected in MIOX-KO mice (I and L).
MIOX Accentuates Cisplatin-Induced ROS Generation
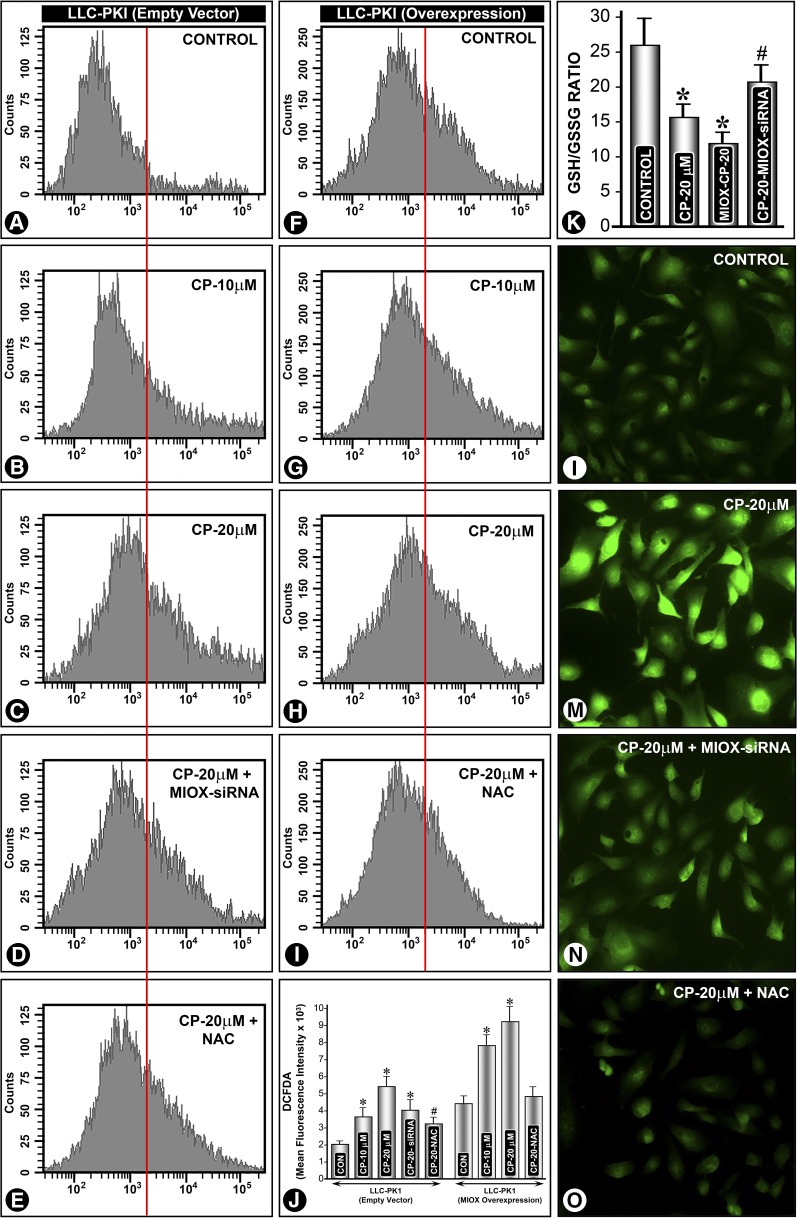
Generation of ROS is one of the major mechanism(s) responsible for cisplatin-induced AKI.20 The status of ROS was investigated because it is known that MIOX overexpression accentuates ROS generation.15 Flow cytometric analyses revealed a dose-dependent increase in ROS levels in cells transfected with empty vector after cisplatin (20 µM) treatment (Figure 4, A–C, and J), whereas MIOX-overexpressing cells exhibited a remarkable further increase in ROS levels after cisplatin treatment (Figure 4, F–H, and J). After MIOX-siRNA or anti-oxidant NAC treatment the cisplatin-induced increase in ROS was reduced (Figure 4, D and E, I and J). Fluorescent microscopy of cells subjected to various treatments confirmed the observations of flow cytometry (Figure 4, L–O). A notable increase in intensity of staining was observed in cisplatin-treated cells (Figure 4M), and MIOX siRNA and NAC treatments reduced the intensity of fluorescence, suggesting that cisplatin-induced ROS generation is mediated by MIOX (Figure 4, N and O). Likewise, a decrease in the ratio of reduced GSH/oxidized glutathione (GSSG) was observed after cisplatin treatment (Figure 4K). A further decrease in the GSH/GSSG ratio was observed in MIOX-overexpressing cells. Treatment of MIOX siRNA normalized the cisplatin-induced decrease in the GSH/GSSG ratio (Figure 4K).
Figure 4.
MIOX accentuates cisplatin-induced ROS generation in vitro. LLC-PK1 cells transfected with empty vector and MIOX pcDNA (overexpressing proximal tubular cell line) were treated with cisplatin (20 μM), and ROS generation was assessed by CM-H2DCF-DA staining and flow cytometric analyses. The analyses revealed a dose-dependent increase in ROS levels in cells transfected with empty vector (A–C, and J). MIOX-overexpressing cells had higher ROS levels after cisplatin treatment (F–H, and J). After MIOX-siRNA or antioxidant NAC treatment the cisplatin-induced increase in ROS was notably reduced (D, E, I, and J). Fluorescent microscopy of cells confirmed the observations made by flow cytometric analyses (L–O). Cellular redox was quantified by assessing the levels of GSSG and reduced GSH (K). A decrease in the ratio of GSH/GSSG was seen after cisplatin treatment, which was further reduced in MIOX-overexpressing cells. MIOX siRNA treatment largely normalized the GSH/GSSG ratio (K). *, P<0.01 compared with the control group; #, P<0.05 compared with the control group.
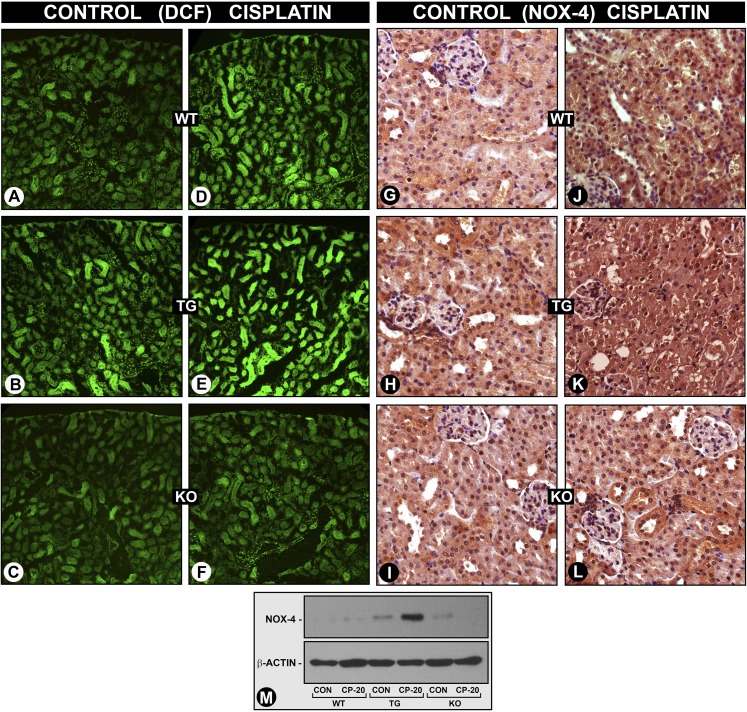
The ROS generation was also assessed in vivo by staining renal tissues with CM-H2DCFDA. An accentuated ROS generation in cisplatin-treated MIOX-TG mice, compared with control WT mice, was observed (Figures 5, E versus D). However, MIOX−/− mice showed no induction of ROS generation (Figure 5, F versus C). A relatively higher signal of ROS generation, compared with WT mice, was also detected in untreated MIOX-TG mice (Figures 5, B versus A). During a series of reactions in the G-X pathway there were perturbations in NADPH/NADP+ and NAD+/NADH ratios, and as a result there was increased generation of ROS and NOX-4 expression in diabetic and obesity states.13–15 Similarly, a mild increase in NOX-4 expression was observed in cisplatin-treated WT mice (Figures 5, J versus G, and M), and it was accentuated in MIOX-TG mice (Figure 5, K versus H, and M). No significant increase in NOX-4 expression was observed in MIOX−/− mice (Figure 5, L versus I, and M).
Figure 5.
MIOX accentuates cisplatin-induced ROS generation and NOX-4 expression in vivo. The ROS generation was assessed by staining renal tissues of mice with CM-H2DCFDA (DCFA) after cisplatin treatment. A mild increase in DCFA staining was observed in WT type mice (D versus A), which was accentuated in MIOX-TG mice (E versus B). The MIOX-KO mice showed no induction of ROS generation after cisplatin treatment (F versus C). Compared with WT mice, a relatively higher degree of signal of DCFA staining was observed in TG mice that did not receive the cisplatin treatment (B versus A). Likewise, NOX-4 expression was markedly increased in MIOX-TG mice compared with WT mice (J versus G, K versus H, and M). No significant increase in the expression was observed in MIOX-KO mice (L versus I, and M).
Modulation of ROS-Dependent p53 Induction in Cisplatin-Induced AKI
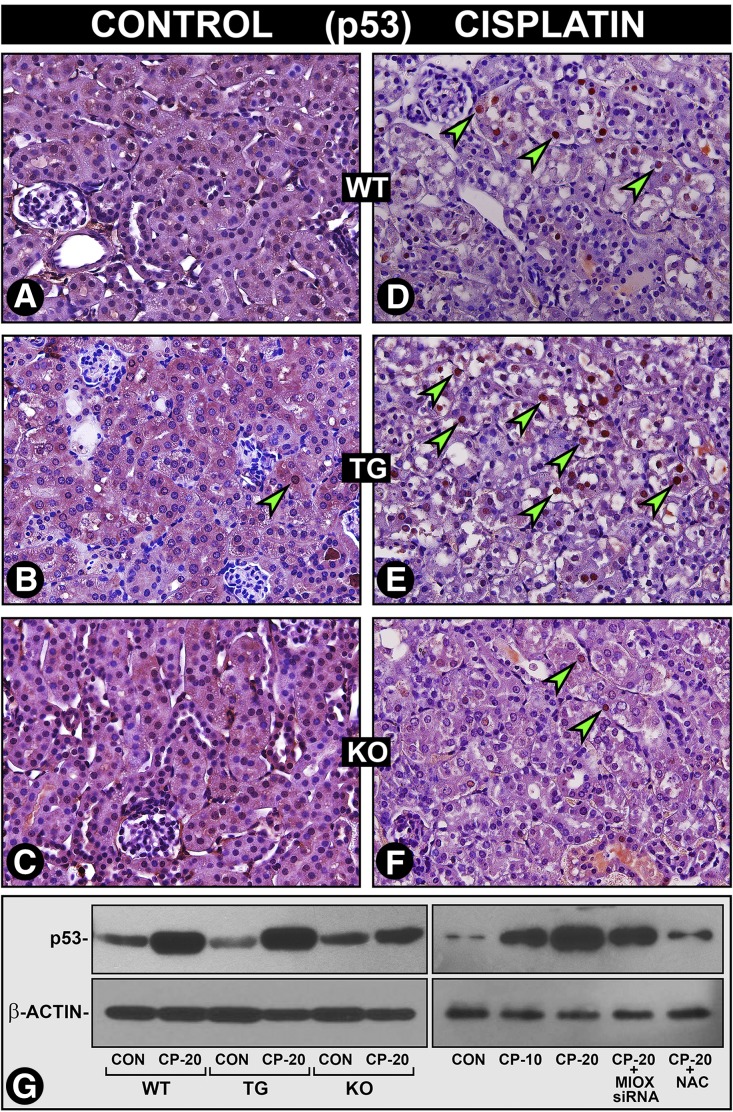
Cisplatin induces p53 expression in tubular cells via ROS generation resulting in apoptosis.5,20,21 Because MIOX facilitates ROS generation, which in turn could modulate the induction of p53, we evaluated the expression of p53 in WT, MIOX-TG, and MIOX−/− mice and cultured cells. In cisplatin-treated WT mice a notable increase in the nuclear expression of p53 in renal tubules was observed (Figure 6, D versus A, arrowheads and G), and it was highly accentuated in MIOX-TG mice (Figure 6, E versus B, arrowheads and G). A very mild increase in nuclear p53 expression was observed in cisplatin-treated MIOX−/− mice (Figure 6, F versus C, and G). Likewise, cisplatin-treated HK-2 cells revealed a dose-dependent increase in the expression of p53 and it was notably reduced by NAC or MIOX-siRNA treatment (Figure 6G, right panel), suggesting that p53 activation is related to ROS generation partly mediated via upregulation of MIOX.
Figure 6.
MIOX accentuates cisplatin-induced ROS-dependent p53 induction in vivo and in vitro. A moderate increase in the nuclear expression of p53 was observed in WT mice after cisplatin treatment (D versus A, arrowheads, and G, left). The p53 nuclear expression was markedly accentuated in MIOX-TG mice (E versus B, arrowheads, and G, left). A very mild increase in the p53 nuclear expression was observed in MIOX-KO mice (F versus C, and G, left). After cisplatin treatment a dose-dependent increase in p53 expression was observed in HK-2 cells, and it was reduced after MIOX-siRNA or antioxidant NAC treatment (G, right).
MIOX Overexpression Promotes NF-κB Activation While Dampened by Its Gene Disruption in Cisplatin-Induced AKI
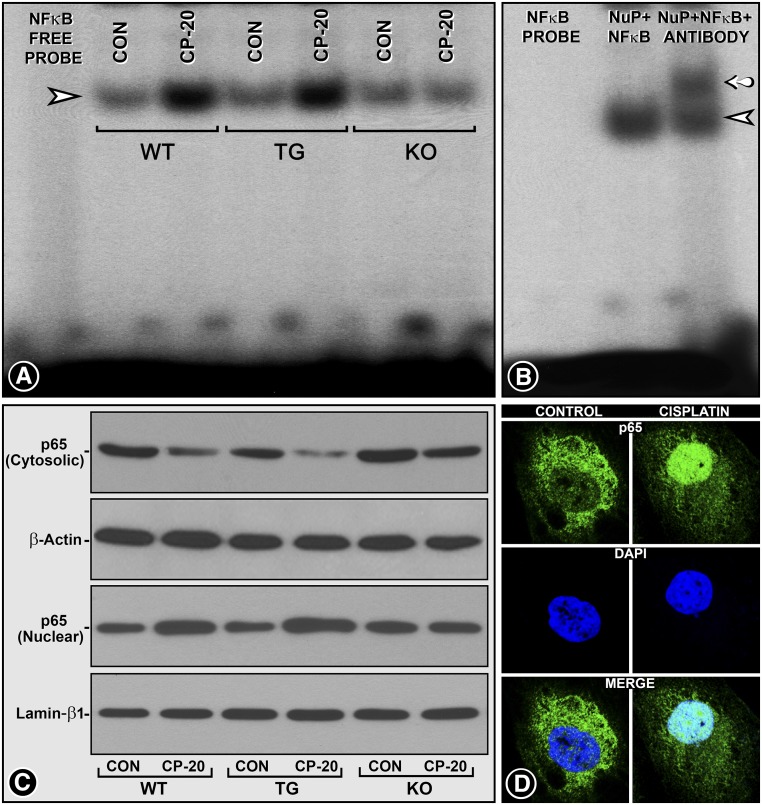
Activation of p53 by NF-κB is one of the major mechanism(s) in the pathogenesis of folate-induced AKI.22 Conceivably, NF-κB binds to the p53 promoter and modulates various pathobiologic processes; on the other hand p53 is regarded as the regulator of NF-κB repression.22–25 This suggests an intricate crosstalk between p53 and NF-κB.22 In view of the interconnectivity of these various signaling events, we investigated the pathobiology of NF-κB among three strains of MIOX mice. Electrophoretic mobility shift assays (EMSA) demonstrated an increased binding of NF-κB–specific probe in cisplatin-treated WT mice (Figure 7A, lane 3, arrowhead). The binding was accentuated in cisplatin-treated MIOX-TG mice (Figure 7A, lane 5). No significant increase in NF-κB binding was observed in MIOX−/− mice (Figure 7A, lane 7). The specificity of the NF-κB probe binding was confirmed by incubating DNA-protein complexes of renal extracts from WT cisplatin-treated mice with antibody against the p65 subunit of NF-κB. Another high molecular band indicating supershift was observed after incubation with the antibody (Figure 7B, lane 3, arrow). Western blot analyses revealed a translocation of p65 into the nucleus from cytoplasm in cisplatin-treated WT mice, and it was accentuated in MIOX-TG mice (Figure 7C, lanes 2 and 4). No significant p65 translocation was observed in MIOX−/− mice (Figure 7C, lanes 5 and 6). Cisplatin-treated HK-2 cells also showed cytoplasm-to-nuclear translocation of the p65, thus establishing the role of NF-κB p65 in AKI (Figure 7D).
Figure 7.
MIOX overexpression promotes NF-κB activation while dampened by its gene disruption in AKI. EMSA revealed an increased binding of the NF-κB–specific probe with the nuclear extracts isolated from kidneys of WT mice treated with cisplatin (A, lane 3, arrowhead). The binding was further accentuated in MIOX-TG mice, as gauged by the intensity of the band (A, lane 5). Minimal binding of NF-κB probe was observed in MIOX-KO mice receiving cisplatin (A, lane 7). The specificity of the NF-κB probe binding was confirmed by incubating renal nuclear extracts of WT mice treated with anti-p65 antibody before EMSA. An additional high molecular band indicating supershift was observed after incubation with antibody against the p65 subunit of NF-κB (B, lane 3, arrow). Subcellular compartmentalization of the transcriptional subunit of NF-κB p65 after cisplatin treatment demonstrated the activation of NF-κB. Western blot analyses revealed a translocation of p65 from the cytoplasm into the nucleus after cisplatin administration in WT mice (C, lane 2), and the translocation was further accentuated in MIOX-TG mice, whereas no significant translocation was observed in MIOX-KO mice after cisplatin treatment (C, lanes 4 and 6). The β-actin and lamin–β-1 served as controls. The cisplatin-induced p65 translocation was confirmed by in vitro experiments in HK-2 cells treated with cisplatin (D).
MIOX Modulates the Expression of Inflammatory Cytokines in Cisplatin-Induced AKI
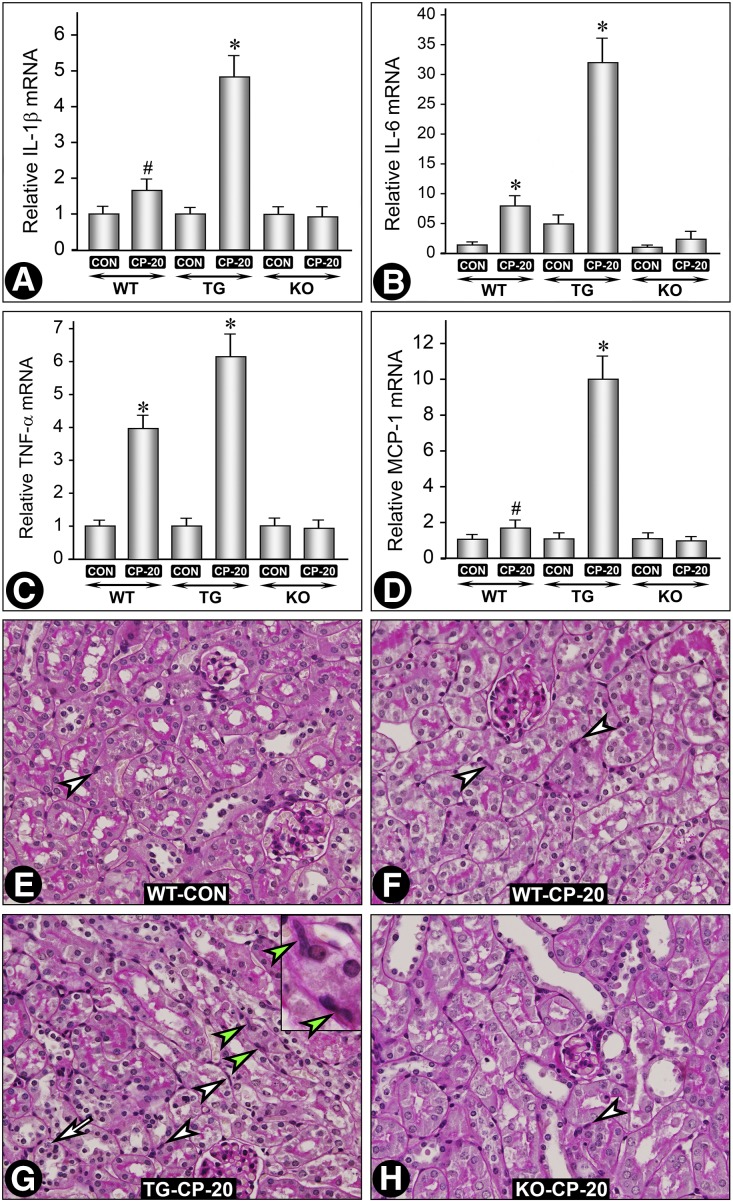
NF-κB plays a role in inflammation via induction of proinflammatory cytokines.26,27 Because they are implicated in the pathogenesis of AKI, we investigated the expression of various cytokines, e.g., IL-1β, IL-6, TNF-α, and MCP-1, in different strains of mice (Figures 8, A –D). Overall, the changes in their expression were similar. A remarkable increase in expression of all of the cytokines was observed in cisplatin-treated MIOX-TG mice, although a moderate increase in TNF-α expression was observed in cisplatin-treated WT mice (Figure 8C). The source of these cytokines in AKI could be from the tubular or resident dendritic cells or cells influxed into the tubulo-interstitium.5,28,29 Keeping in view these possibilities we assessed if there was a differential increase in infiltrating cells among various strains of mice. Normally, very few resident inflammatory cells were seen in the interstitium (Figure 8E, arrowhead). After cisplatin treatment, a mild-to-moderate increase in the influxed inflammatory cells was observed (Figure 8, F–H, arrowheads). A remarkable increase was seen in cisplatin-treated MIOX-TG mice (Figure 8G, arrowheads). The inset shows the morphology of infiltrating inflammatory cells (green arrowheads). A few tubular cells undergoing apoptosis were also seen in MIOX-TG mice (Figure 8G, arrow).
Figure 8.
MIOX modulates the expression of inflammatory cytokines in cisplatin-induced AKI. A remarkable increase in the expression of IL-1β, IL-6, TNF-α, and MCP-1 was observed in MIOX-TG mice receiving cisplatin compared with WT or MIOX-KO mice or untreated control mice (A–D), although a moderate increase of TNF-α was observed in WT mice after cisplatin treatment (C). Examination of kidneys revealed a remarkable increase of interstitial inflammatory cells in the MIOX-TG mice receiving cisplatin (G, arrowheads). The inset shows the morphologic nature of the infiltrating inflammatory cells (green arrowheads). A mild increase was also observed in cisplatin-treated WT mice (F, arrowheads). The MIOX-KO mice had no significant increase of inflammatory cells compared with the untreated WT control (H versus E, arrowheads). A few foci of apoptosis were also seen in MIOX-TG mice (G, arrow). *, P<0.01 compared with the control group; #, P<0.05 compared with the control group.
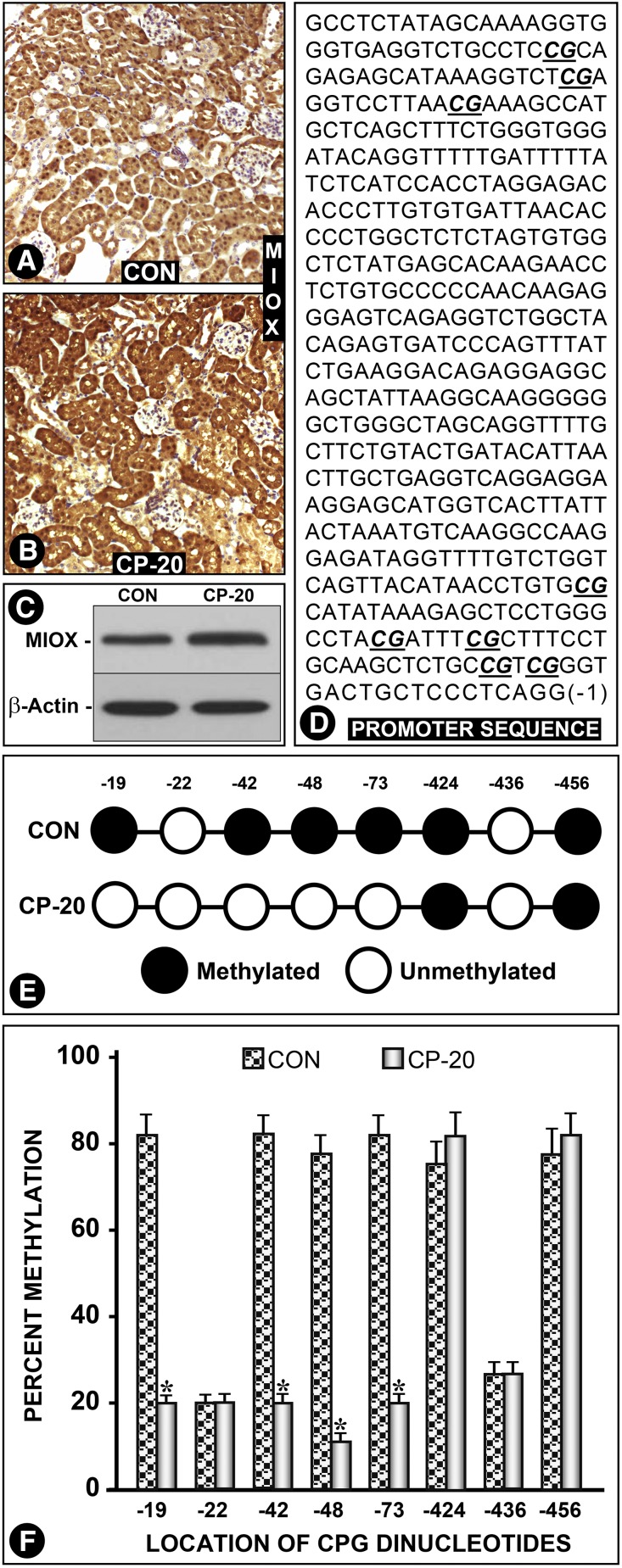
Cisplatin Increases MIOX Expression via Demethylation of Its Promoter
Here, we addressed the question of whether cisplatin can increase the expression of MIOX and if as a result there is an excessive ROS generation, which would further accentuate renal injury. The answer seems to be affirmative because cisplatin administration caused an increased MIOX expression (Figure 9, A–C). The increase was remarkable and was confined to tubules (Figure 9B). Next, mechanism(s) that induced the increased MIOX expression were explored. Genomic DNA samples were isolated from kidneys of WT and cisplatin-treated mice. They were subjected to bisulfite treatment, followed by PCR and nucleotide sequencing to assess the efficiency of conversion (cytosine to thymine) of CpG dinucleotides. Their nucleotide analyses revealed that the MIOX promoter is devoid of CpG islands but it has eight CpG dinucleotides at −19, −22, −42, −48, −73, −424, −436, and −456 bp sites. In WT control mice CpG at −22 and −436 bp were unmethylated, whereas six others were methylated (Figure 9, D and E). The samples from cisplatin-treated mice had all of the CpG dinucleotides unmethylated, except for −424 and −456. Overall, the efficiency of conversion of C to T nucleotide was >95%. The analyses of percentage of methylation of individual dinucleotide residues between control and cisplatin-treated mice were plotted as bar graphs, which suggested that cisplatin-induced hypomethylation is most likely responsible for the MIOX upregulation in AKI (Figures 9F).
Figure 9.
Cisplatin treatment per se upregulates MIOX via demethylation of its promoter. WT mice treated with cisplatin had an increased renal expression of MIOX, and it was confined to the tubules (A–C). No significant MIOX expression was seen in glomeruli. Genomic DNA was isolated from kidneys of WT and cisplatin-treated mice and subjected to bisulfite treatment. Promoter fragments were isolated by PCR, cloned into pGEM-T plasmid, and transformed in DH5α cells. Ten clones from each variable were selected and subjected to nucleotide sequencing to assess the efficiency of conversion (cytosine to thymine) of CpG dinucleotides. Nucleotide analyses revealed eight CpG dinucleotides at −19, −22, −42, −48, −73, −424, −436 and −456 bp sites. In WT, untreated control mice samples, CpG at −22 and −436 bp were found to be unmethylated, whereas six others were methylated (D and E). The samples from cisplatin-treated mice were found to have all of the CpG dinucleotides unmethylated, except for −424 and −456. Overall, the efficiency of conversion of C to T nucleotide was >95%. The percentages of methylation of individual dinucleotide residues are presented as bar graphs, which indicate that cisplatin induces hypomethylation that is likely responsible for the MIOX upregulation (F).
Discussion
Several years ago, MIOX was identified in proximal tubules of newborn mice.30 Since then its relevance in the pathogenesis of diabetic nephropathy has been described in several publications.10,13–15 These studies highlight its biochemical characteristics pertaining to transcriptional, translational, and post-translational events that modulate MIOX expression. However, its relevance to the pathobiology of tubules remains enigmatic, and that warranted generation of mice with its genetic overexpression or deletion. We employed Cre/loxP technology to direct its overexpression in renal tubules by cross-breeding floxed mice with PEPCK-Cre mice. These mice express Cre recombinase gene, and the latter is under the control of mutated PEPCK promoter and thus would express MIOX mainly in proximal tubules.31 Incidentally, the PEPCK Cre transgene is expressed on the X chromosome, and therefore male mice exhibit greater penetrance than females. In view of this, we selected male mice for our studies. Nevertheless, both male and female mice exhibited identical phenotypes, i.e., MIOX overexpression in proximal tubules of MIOX-TG mice and no expression in MIOX−/− mice (Figure 1).
Using these mutant mice we explored additional functionalities of MIOX in the context of pathobiology of proximal tubules. The latter are adversely affected in various disease states that result in AKI. A prototype of AKI is cisplatin-induced nephrotoxicity, where proximal tubules bear the brunt of injury, possibly due to their uptake of cisplatin.32 In this scenario, in which proximal tubules are targeted and where MIOX is expressed, it would indicate that the cisplatin-induced AKI is an appropriate model to delineate the pathogenetic role of MIOX in renal injury. Numerous reports have documented the cellular toxicity in proximal tubules accompanied with compromise in renal functions.4,5,32 Likewise, we observed vacuolization and necrosis of tubular cells accompanied with a rise in serum urea and creatinine, and loss of body weight in C57BL/6J mice (Figure 2). These changes were highly accentuated in MIOX-TG mice. Interestingly, the MIOX−/− mice had a much lesser degree of cisplatin-induced tubular toxicity and minimal elevation of serum creatinine. An affirmation of these results was derived by assessing the status of KIM-1, a marker of tubular injury.18 A remarkable elevation of KIM-1 levels was observed in MIOX-TG mice, whereas there was a modest increase in control mice and a minimal rise in MIOX−/− mice. Besides necrosis, cellular apoptosis has been well described in cisplatin nephrotoxicity, which apparently relates to the activation of proapoptotic protein Bax and caspases while diminishing the expression/activity of antiapoptotic Bcl-2.20,21,33,34 Interestingly, apoptosis, Bax activation, and expression of cleaved caspase-3 and its activity were markedly accentuated in MIOX-TG mice compared with control animals (Figure 3). Notably, the MIOX−/− mice had no apoptosis or Bax activation or expression of cleaved caspase-3, and only a marginal increase in the caspase-3 activity, whereas a marked downregulation of antiapoptotic protein Bcl-2 was observed in MIOX-TG after cisplatin treatment. Taken together, these data indicate that MIOX overexpression accentuates AKI, whereas its gene disruption is protective for kidney homeostasis.
The next question that was addressed was how MIOX accentuates AKI. Besides necrosis/apoptosis, oxidant stress has been regarded as one of the factors for induction of nephrotoxicity.16 Previous studies indicate that MIOX promoter includes oxidant response elements, and its overexpression in vitro accentuates generation of ROS.10,14 The current data regarding accentuated expression, as well as activity, of caspase-3 points toward mitochondria as the source of excessive generation of ROS in MIOX-TG mice. In this regard, MIOX overexpression in LLC-PK1 cells has been reported to cause exaggerated mitochondrial dysfunctions, such as cytochrome C release, DNA fragmentation, and mitochondrial fission, under high glucose ambience.14 However, in cisplatin-induced nephropathy the increased ROS generation in MIOX-overexpressing cells could be also related to the pathobiology of the NADPH oxidase system. In this regard, perturbations in NAD+/NADH ratios have been described in the MIOX-initiated G-X pathway.14 In support of the latter contention our in vitro data indicate a perturbed GSH (oxidized)/GSSG (reduced) ratio after cisplatin treatment, which was largely normalized by the concomitant administration of MIOX-siRNA (Figure 4). Likewise, MIOX-siRNA treatment of LLC-PK1 cells markedly reduced the generation of ROS after cisplatin exposure, as assessed by FACS analyses and immunofluorescence microscopy after DCFDA staining (Figure 4). The production of ROS was markedly accentuated in the renal tubular compartment of MIOX-TG mice, whereas MIOX−/− mice had minimal generation of ROS, even after cisplatin treatment (Figure 5). Further support for the involvement of NADPH oxidase in the current model comes from expression studies on the induction of tubular–NOX-4. A prominent expression of NOX-4 was observed after cisplatin treatment in MIOX-TG mice, whereas it was barely detectable in WT and MIOX−/− mice (Figure 5). Taking these data together, one can conclude that MIOX accentuates cisplatin-induced AKI, most likely via boosting ROS generation.
Besides ROS and apoptosis, various interlinked p53-associated pathways and molecules are also involved in the pathogenesis of cisplatin toxicity. They may include p53 upregulated modulator of apoptosis (PUMA), histone deacetylase inhibitors, taurine transporter (TauT) gene, and Sirt1.20,21,35–37 The induction of PUMA is dependent on the activation of p53 because inhibition of p53 with pifithrin or its genetic ablation reduces apoptosis.21 Interestingly, N-acetyl-cyteine (NAC) or dimethylthiourea administration reduces hydroxyl radical generation, i.e., oxidant stress, p53 activation, and suppression of PUMA-α and apoptosis.20 Along these lines the TauT transgenic mice exhibit attenuation of cisplatin-induced apoptosis by dampening the activation of p53.36 Similarly, Sirt1, an NAD-dependent deacetylase, has been reported to reduce ROS-induced apoptosis most likely via deacetylation of p53, thereby reducing its transcriptional activation.38 On the other hand, histone deacetylase inhibitor trichostatin A was administered along with cisplatin, and although it enhances tumor cell death it limits cisplatin-induced toxicity or apoptosis in the kidney, probably by increasing phosphorylation of cAMP-response element binding protein and reversing cAMP-response element binding protein–mediated gene repression.39 In line with these literature data we observed increased p53 expression after cisplatin administration, especially in MIOX-TG mice having accentuated ROS generation (Figure 6). The question of whether MIOX-generated ROS induces p53 expression was assessed by in vitro experiments. The cisplatin-treated cells had increased expression of p53, which was abrogated with MIOX-siRNA or NAC treatment, suggesting that MIOX accentuates p53-mediated injury via excessive ROS generation (Figure 6).
Another pathobiologic process that plays a noteworthy role germane to cisplatin-induced AKI relates to inflammation.28 In this realm there may be activation of transcription factors, generation of cytokines, and influx of inflammatory cells. One of the important transcription factors that is induced after oxidant stress includes NF-κB.40 It modulates diverse biologic processes, including inflammatory responses, while at the same time modulating p53 expression.22 Interestingly, recent data also suggest that p53 itself can regulate NF-κB repression.25 This would suggest an intricate reciprocal crosstalk between these two molecules during an inflammatory response in AKI.22 The role of NF-κB has been ascribed to diverse diseases associated with renal inflammation.27 Also, its role in the folic acid–induced toxic injury model is well known, where folic acid administration was shown to concomitantly upregulate expression of p53, NF-κB, and its active transcriptional subunit, relA/p65.22 Administration of pyrrolidine dithio-carbamate ammonium, an NF-κB inhibitor, reduced the upregulated expression of all of these molecules along with improvement in renal functions. Along these lines, we investigated the role of NF-κB in cisplatin-induced injury in MIOX-TG and MIOX−/− mice. By EMSA, an increased NF-κB binding was seen in cisplatin-treated WT mice, and it was accentuated in MIOX-TG, although no significant binding was seen in MIOX−/− mice (Figure 7). The role of NF-κB in cisplatin-induced nephropathy was further supported by accentuated translocation of p65 into the nuclear compartment in renal cells of MIOX-TG mice. The downstream of NF-κB activation would include upregulation of various inflammatory cytokines, including TNF-α.41 In fact, TNF-α generation after the activation of NF-κB in cisplatin-induced nephropathy is well described.42 In addition, there is an increased expression of other cytokines, including IL-1β, MCP-1, and RANTES. We also observed an upregulation of inflammatory cytokines after cisplatin administration, especially in MIOX-TG mice (Figure 8). The concomitant rise of different cytokines may suggest that they synergistically stimulate one another’s production in MIOX-TG mice that are endowed with increased generation of ROS in their kidneys. Some of these cytokines, e.g., MCP-1, are chemotactic for a variety of inflammatory cells, including neutrophils, monocytes, and lymphocytes.42 In concurrence with the production of cytokines an accentuated influx of inflammatory cells into renal parenchyma was observed in MIOX-TG mice (Figure 8). No significant influx was observed in MIOX−/− mice, suggesting that increased MIOX expression leads to an activation of various inflammatory responses.
Several publications indicate that in a variety of pathobiologic states the increased expression of MIOX seems to be the result of its transcriptional modulation, where conceivably various transcription factors bind to specific consensus sequences localized within its promoter.10,13–15,43 Therefore, the question that warrants an answer is, whether there is an increased expression of MIOX after cisplatin treatment, and if so, what are the mechanism(s) responsible for such an upregulation? Indeed, there was increased expression of MIOX after cisplatin treatment (Figure 9). Because there are no known transcription factors which could bind to the MIOX promoter and induce its upregulation in cisplatin toxicity, we therefore proceeded to search for an alternative mechanism. One such mechanism that we explored pertains to the epigenetic regulation of MIOX, i.e., methylation/demethylation of its promoter. Epigenetic modification of various genes has been implicated in various renal diseases with consequential altered expression.44 In this regard, hypomethylation/demethylation has been reported in AKI induced by ischemia reperfusion or contact freezing of the kidney surface.45–47 Although a multitude of mechanisms have been described in cisplatin-induced AKI, the studies relating to epigenetic modifications are sparsely reported.5,32,48 In line with the observations made in the ischemia reperfusion injury model, we observed a marked demethylation of the CpG dinucleotides in the MIOX promoter (Figure 9), which could explain its increased expression and ultimately exacerbation of cisplatin-induced injury in MIOX-TG mice.
In summary, this investigation highlights the worsening of AKI in cisplatin-induced injury via a multitude of mechanisms in states of overexpression of MIOX, although its gene disruption seems to ameliorate toxic renal proximal tubular injury.
Concise Methods
Reagents
Reagents were purchased from the following vendors. Sigma-Aldrich: cisplatin (# P4394), anti–β-actin antibody (# A5441), Caspase-3 assay kit, Colorimetric (# CASP-3C), poly-(deoxyinosinic-deoxycytidylic) acid sodium salt (# P4929), NAC (# A7250); BioAssay Systems: QuantiChrom creatinine assay kit (# DICT-500) and QuantiChrom urea assay kit (# DIUR-500); Life Technologies: Power SYBR Green PCR Master Mix (# 4367659), Chloromethyl derivative of 2′, 7′-dichlorofluorescein diacetate (CM-H2DCF-DA, # C6827), 4′-6-diamidino-2-phenylindole (DAPI, # D1306), and TRIzol Reagent (# 15596026); Cell Signaling Technology: anti–cleaved caspase-3 antibody (# D175), anti-p53 (1C12) antibody (# 2524), and anti-p65 antibody (# D14E12); Abcam: anti–lamin B1(ab16048); Enzo Life Sciences: DNA methylation gold kit (# D5005); Santa Cruz Biotechnology: anti-Bax antibody (# SC-526), anti–NOX-4 antibody (# SC-21860), anti-p53 antibody (# SC-6243); Roche Diagnostic: In Situ Cell Death Detection Kit, Fluorescein (# 11684795910); Cayman Chemicals: GSH assay kit (# 703002); Promega Corporation: NF-κB EMSA oligo (# E329A) and pGEM-T plasmid vector; Perkin Elmer: ATP γ-32p (# BLU002A); and Thermo Scientific: polynucleotide kinase (EK0032). OriGene Technologies: MIOX siRNA (# SR310776). Anti-MIOX polyclonal antibody was prepared in our laboratory using recombinant mouse MIOX as the immunogen.10
Generation of MIOX Transgenic and Null Mice
The transgenic CBA-flox mice were generated using the pCMV flox vector (a generous gift from Dr. Holzman).49 The CMV promoter was replaced with the cytomegalovirus–chicken β-actin hybrid promoter which contains the cytomegalovirus immediate early enhancer linked to chicken β-actin promoter and a chimeric intron of β-globin and immunoglobin genes.50,51 This hybrid promoter demonstrates enhanced activity in vivo and was used to drive the expression of a flanked β-gal-stop-codon cassette.52 The MIOX cDNA was inserted downstream of the β-gal-stop-codon cassette at the NheI and PmeI sites (Figure 1A). This construct enables the expression of the β-gal gene (as verified by X-gal staining in LLC-PK1 cells), but it does not express the MIOX gene. After recombination of the Loxp sites in the presence of Cre recombinase, the β-gal gene is deleted and MIOX is expressed under the regulation of the β-actin hybrid promoter. The CBA-flox construct was released from the plasmid vector by digestion with PacI and microinjected into the pronucleus of fertilized ova from C57BL/6 F1 mice. The litter was screened for the presence of transgene using β-gal–specific primers as previously described.52 The founders were cross-bred with PEPCK-Cre C57BL/6J mice (a generous gift from Dr. Volker Hasse)31 to direct expression primarily into the proximal tubules. The mice were then screened for the MIOX transgene using primers encompassing the NheI and PmeI restriction sites. Their kidneys were tested for the extent of MIOX expression by Western blot analyses and immuno-histochemical techniques.
MIOX null mice were generated at Cell Molecular Technology Inc., Phillipsburg, New Jersey. The design of the targeting vector for generation of MIOX knockout mice is included in Figure 1B. Briefly, the targeting vector was designed to generate both Null and Flox mice lines. A 26 kb BAC subclone was cloned by applying Gene Bridges technology.53 A 5′ LoxP site was placed in intron 1 at the AfIII site and a FRT-Neo-FRT-3′ LoxP cassette at the AatII site after exon 10. In doing so, Cre-recombinase would excise exons 2–10 along with the Neo cassette leaving behind a single LoxP site to generate Null mice lines; whereas FLP recombinase would excise only the Neo cassette leaving exons 2–10 and Loxp sites intact to generate the conditional (Flox) line in embryonic stem cell (ES) recombinants. The targeting vector was electroporated into 129/SVEV ES cells. The ES recombinant clones were picked and tested by PCR to make sure the excisions had taken place. After injection of ES recombinants into blastocysts and subsequent implantation, chimeras were generated and mouse lines expanded. The Flox mouse lines were also cross-bred with PEPCK-Cre mice to selectively delete the MIOX gene from the proximal tubules. Both the mouse lines were backcrossed with C57BL/6J for seven generations to generate mouse lines with a homogenous background. Their kidneys were harvested to confirm deletion of MIOX genes by Western blot analyses and immuno-histochemical techniques.14,15
Design of In Vivo Experiments
For AKI experiments, 8-week-old male WT mice and sex- and age-matched MIOX−/− (KO) and MIOX-overexpressing transgenic (MIOX-TG) mice were utilized. Initially, various dosages (5–25 mg/kg body wt) of cisplatin (single intraperitoneal injection) were tested for tolerance among these three strains of mice. A dose of 20 mg/kg (1 mg/ml solution in sterile normal saline) was tolerated by all three of the strains without having significant mortality, and this dose was chosen for all of the subsequent experiments (n=6). The control mice were injected with normal saline only. Mice were euthanized 3 days after the cisplatin treatment. Blood samples were collected for measuring levels of serum creatinine and urea using QuantiChrom Creatinine and QuantiChrom Urea assay kits (BioAssay Systems). Their kidneys were utilized for various morphologic, biochemical, and molecular biology studies. All animal procedures used in this study were approved by the Animal Care and Use committee of Northwestern University.
Cell Culture Studies
HK-2 cells and LLC-PK1 cells were purchased from ATCC (Manassas, VA) and used in this study. The HK-2 cells were grown in low glucose DMEM medium supplemented with 5% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. LLC-PK1 cells were grown in low glucose M199 medium supplemented with 5% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells in quadruplicate plates were maintained in a humidified atmosphere of 5% CO2, 95% air, at 37°C. In addition, MIOX-overexpressing LLC-PK1 cells were also used in some of the experiments. The generation of overexpressing cell lines has been detailed in previous publications.15 Approximately 1 × 106 cells were seeded per well in six-well collagen-coated plates and allowed to adhere to the culture plates overnight. The cells were then treated with various concentrations of cisplatin (5–20 μM) for 12–24 hours, and then processed for various morphologic and biochemical studies. In addition, MIOX siRNA (50 nM) and ROS scavenger (NAC, 10 mM) studies were also carried out. The LLCPK1 cells were transfected with siRNA against MIOX14 or scramble (serve as a control) using Lipofectamine 2000 reagent (Invitrogen) in the presence of 20 μM of cisplatin. Forty-eight hours later the ROS generation, GSH/GSGG ratios, and p53 expression were assessed in the cells.
Renal Tissues’ Histologic and Immuno-Histochemical Studies
Kidneys from control (WT) and experimental (TG/KO) mice were harvested, and 3–4 mm thick slices were prepared. They were fixed in 10% buffered formalin, dehydrated by graded concentrations (50%–100%) of ethanols, and then transferred into xylene and embedded in paraffin. For immuno-histochemistry, 4 μM thick sections were prepared and mounted on glass slides. The tissue sections were air-dried overnight. They were then deparaffinized, hydrated, and then processed for antigen retrieval. After antigen retrieval, endogenous peroxidase activity in the tissues was quenched by immersing glass slides with tissue sections in 3% H2O2 for 3 minutes. Tissue sections were immersed in 3% BSA for 1 hour and incubated in a primary antibody overnight at 4°C. After washing with PBS the sections were incubated with horse radish peroxidase (HRP)–conjugated anti-rabbit IgG, immPRESS (Vector Laboratories), for 1 hour. The sections were subjected to another PBS wash and then incubated with HRP substrate diaminobenzadine for color development. Sections were rewashed and counterstained with hematoxylin to delineate the cellular details. The sections were then dehydrated in a graded series of ethanols, treated with xylene, and coverslip-mounted after immersion in a drop of mounting media (Vector Labs). Slides were then viewed and photographed.
Immunoblotting Analyses of Relevant Proteins Expressed in Kidney Tissues
Kidney tissues from control and cisplatin-treated WT, MIOX-TG, and MIOX−/− mice were harvested and lysed in RIPA buffer. Supernatants were collected and protein concentration was measured using Bradford reagent. Equal amounts of protein for each sample were mixed with 5× SDS sample buffer and boiled for 5 minutes. The proteins in the lysates were fractionated by SDS-PAGE and electro-blotted onto PVDF membranes. The blots were then probed with various primary antibodies, followed by incubation with secondary antibodies conjugated with HRP. Autoradiograms were developed for detection of the bands using an Enhanced ChemiLuminence kit (Thermoscientific). Equal loading of the samples was confirmed by probing the immunoblots with mouse β-actin or lamin-β1 antibodies.15
TUNEL Staining Studies in Kidney Tissues
The extent of apoptosis in kidney tissues was determined by established TUNEL procedures.14 Deparaffinized kidney sections were hydrated and then digested with Proteinase K (240 unit/ml; Promega, Madison) for 30 minutes at 22°C. After washing tissue sections with PBS, they were incubated with TUNEL reagent for enzymatic labeling of the nicked DNA with a fluorescent nucleotide probe (Roche Applied Science).
Assessment of Caspase-3 Activity in Kidney Tissues
The assay was performed using a Colorimetric caspase-3 assay kit (Sigma), as previously described.15 Kidney tissues from WT, MIOX-TG, and MIOX−/− mice were harvested. Their cortices were dissected and homogenized in a lysis solution provided in the kit. The tissue lysates were then centrifuged at 16,000 × g for 10 minutes at 4°C. Supernatants were transferred into a new tube and protein concentration measured by using the Biorad Bradford Reagent. After adjusting the protein concentration to 5 mg/ml the caspase-3 activity was determined. Each sample was assayed in duplicate in a 96-well plate in 200 μl reaction volume. The reaction mixture included 10 μl of lysate containing 50 μg of protein per sample, 170 μl of 1× assay buffer, and 20 μl of caspase-3 substrate. Wells were then covered with adhesive film and incubated at 37°C for 3 hours. The sample absorbance was read at 405 nm.
Assessment of ROS in Tissues and Cells
For determination of ROS in kidneys, tissues from both control and cisplatin-treated WT, MIOX-TG, and MIOX−/− mice were embedded in OCT compound and processed for frozen sectioning. Eight-micrometer-thick frozen sections of kidney were prepared, transferred onto glass slides, and air dried for 5 minutes. The slides were then rinsed with PBS to remove the OCT compound from tissue sections. Tissue sections were then stained with CM-H2DCFDA for 15 minutes at 37°C in the dark and visualized using a fluorescent microscope equipped with UV illumination.
ROS levels were determined in cisplatin-treated cells by CM-H2DCFDA staining using flow cytometry and fluorescent microscopy.14,15 For flow cytometry, cells transfected with empty vector and MIOX-overexpressing LLCPK1 cells were seeded onto six-well plates and treated overnight with different concentrations of cisplatin, as indicated above. Cells were then washed in PBS and detached from the plate using BD Accutase cell detachment solution. Cells were then stained with of CM-H2DCFDA (5 μM) for 15 minutes at 37°C in dark. After washing twice with PBS the cells were resuspended in 300 μl of PBS and processed for acquisition of fluorescence with a flow cytometer. After which, mean fluorescent intensity of the CM-H2DCFDA was measured using standard operational procedures of flow cytometry and employing FACSDiva software (Becton Dickinson). For fluorescence microscopy, HK-2 cells were seeded onto coverslips and treated with different concentrations of cisplatin (10 and 20 μM) for 12 hours. Cells were then washed and stained with 5 μM of CM-H2DCFDA for 15 minutes at 37°C in the dark. After a brief rewash with PBS the coverslips with attached cells were inverted and mounted on the glass slides after placing a drop of DAKO mounting medium. The cells were then examined with a UV microscope.
Determination of GSH/GSSG Ratio in Cultured Cells
This was measured using GSH assay kit (Caymen Chemicals) using the manufacturer’s instructions, as previously described.15 Both empty vector control LLCPK1 cells and MIOX-overexpressing LLCPK1 cells were used in this study. Cells that had undergone various treatments were collected and sonicated in 500 μl of MES buffer. Lysed cells were then centrifuged to collect supernatant. Protein concentration in supernatant (#1) was measured using the Biorad Bradford Reagent. The supernatants were then deproteinated by using equal volumes of freshly prepared 10% metaphosphoric acid. The precipitates were allowed to settle and supernatants (#2) were utilized for GSH measurement. Fifty microliters of triethanolamine reagent was added to each 1 ml sample of the supernatants (#2) and mixed by immediate vortexing. Fifty microliters of mixture was used for determination of GSH. For GSSG determination, 10 μl of 1 M 2-vinylpyridine was added to 1 ml of triethanolamine reagent mixture, as prepared above. The mixture was vortexed and incubated at 22°C for 1 hour. Fifty microliters of this mixture was added to the 150 μl of assay solution provided in the kit and gently shaken in the dark on an orbital shaker for 45 minutes to achieve full color development. Spectrophotometer readings were made from four experiments at 415 nm, and concentrations of GSH and GSSG were measured using a standard curve.
Preparation of Cytosolic and Nuclear Extracts from Kidney Tissues for EMSA
Kidney tissues harvested from control and cisplatin-treated WT, MIOX-TG, and MIOX−/− mice were first homogenized in cytoplasmic extraction buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, and 0.1 mM EGTA) and incubated for 15 minutes on ice. Then, 12.5 μl of 10% NP-40 per 400 μl of lysis buffer was added and vortexed vigorously for 10 seconds to lyse the cells. The lysate was then centrifuged for 1 minute, and the supernatant was collected and designated as the cytosolic extract. Pellets were then processed for nuclear extract preparation. They were incubated in nuclear extraction buffer (20 mM HEPES, 400 mM NaCl, 1 mM EDTA, and 1 mM EGTA) for 30 minutes on ice with intermittent vigorous vortexing to lyse nuclei. Nuclear extract was collected as supernatant after 5 minutes of centrifugation, and protein concentration in various samples was measured. The nuclear extracts prepared above from various kidney tissues were utilized for EMSA to assess the NF-κB activation and its nuclear translocation. Ten micrograms of nuclear extract from each sample was incubated with 32P-labeled double stranded NF-κB probe (Promega) for 15 minutes at 37°C. The DNA-protein complex formed was subjected to 6.6% native PAGE. Gels were dried and exposed to x-ray film and autoradiograms prepared. Specificity of binding of NF-κB oligo probe with NF-κB protein was assessed by performing a supershift assay. Nuclear extract was prepared from cisplatin-treated WT mice kidneys. It was incubated with antibody against the p65 subunit of NF-κB for 15 minutes at 22°C before incubation with labeled NF-κB probe. The shift in the DNA-protein-antibody complex migration in the gel was visualized after autoradiography.
Cellular Translocation of NF-κB
To assess the translocation of NF-κB from cytoplasm to the nucleus in HK-2 cells, immunofluorescence studies were performed. Briefly, 5 × 105 cells were seeded onto coverslips in a six-well plate and allowed to attach overnight. Cells were then treated with 20 μM of cisplatin for 12 hours. After treatment, the cells were fixed with 4% paraformaldehyde for 5 minutes in PBS and washed three times with PBS. They were then permeabilized with ice cold methanol for 10 minutes at −20°C. Cells were rewashed twice with PBS and incubated in a blocking solution (1× PBS/1% BSA/0.3% Triton X-100) for 1 hour. After blocking, fixed cells were incubated with anti-p65 antibody diluted in blocking solution for 2 hours at 22°C. Cells were then washed three times with PBS and incubated with FITC-conjugated secondary antibody for 2 hours in the dark. Cells were then rewashed with PBS, and nuclei were stained with 0.5 μg/ml of DAPI for 5 minutes. After another PBS wash, a drop of DAKO fluorescent mounting media was placed on the coverslips, and they were then inverted and mounted onto the glass slides. The images were captured using a Nikon A1R Spectral Confocal microscope.
Assessment of Tissue Expression of Inflammatory Cytokines and Kidney Injury Biomarkers by Real-Time PCR
Total RNA was isolated from kidneys using TRIzol Reagent (Invitrogen) and cDNA was synthesized using Go Script reverse transcription system (Promega). The mRNA levels of various genes were quantified using Step One Plus System Real Time PCR (Applied Biosystems). A 20 μl total reaction mix included 100 ng of cDNA, 50 nmol/L forward/reverse primers, and 1× Fast SYBR Green Master Mix (Life Techologies). GAPDH was used as an internal control and the amount of mRNA was calculated by comparative CT method. PCR reactions were run in quadruplicate. Primers used were, Kim 1: Forward, 5′-GGAAGTAAAGGGGGTAGTGGG-3′, Reverse, 5′-AAGCAGAAGATGGGCATTGC-3′; IL-1β: Forward, 5′-ACCTGTCCTGTGTAATGAAAGACG-3′, Reverse, 5′-TGGGTATTGCTTGGG ATCC-3′; MCP-1: Forward, 5′-CCCAATGAGTAGGCTGGAGAG-3′, Reverse, 5′-TGGTTG AAAAGGTAGTGGATG-3′; TNF-α: Forward, 5′-AGAAGAGGCACTCCCCCAAAAG-3′, Reverse, 5′-TTCAGTAGACAGAAGAGCGTGGTG-3′; GAPDH: Forward, 5′-GAATAC GGCTACAGCAACAGG 3′, Reverse, 5′-GGTCTGGGATGGAAATTGTG-3′; and IL-6: Forward, 5′-CCGGAGAGGAGACTTCACAG-3′, Reverse, 5′-CAGAATTGCCATTGCACAAC-3′.
Analysis of MIOX Promoter Methylation/Demethylation in Kidney Tissues after Cisplatin Treatment
Kidneys of mice treated with cisplatin were harvested for isolation of DNA. Their cortices were dissected, and an aliquot of cortex (20 mg) was homogenized in 550 μl of lysis buffer (50 mM Tris-HCI, 10 mM EDTA, 100 Mm NaCl, 1% SDS) containing RNAase1 (1 μg/μl). The homogenate was kept at 55°C for 2 hours to achieve complete solubilization of cellular proteins. After a phenol-choloroform extraction the DNA was precipitated with the addition of isopropanol at 4°C. The precipitated DNA was pelleted by a centrifugation at 10,000 × g for 5 minutes. The DNA pellet was washed with 70% ethanol, air-dried, and dissolved in nuclease-free water. The isolated DNA (2 μg) was subjected to bisulphite modification, using EZ DNA methylation Gold Kit (Zymo Res. Irvine, CA), and by following the methodology originally described by Frommer et al.54 In doing so, the sodium bisulphite in an acidic environment will convert all cytosine residues in single stranded DNA to uracil, excluding the methylated cytosine. The bisulfite-treated DNA was loaded into spin columns provided in the kit and eluted with 20 μl of elution buffer. This purified and bisulfite-treated DNA was used to determine the frequency of methylation of CpG islands within the MIOX promoter, using bisulfite-specific PCR primers. These primers were devoid of CpG sites. For amplification of the mouse MIOX promoter, 2 μl of treated DNA was amplified in a reaction mixture containing: 4 μM primers (Forward- 5′-GTTTTTATAGTAAAAGGTGGG-3′ Reverse- 5′-CCTAAAAAAACAAACACC-3′), 1× PCR buffer, 0.4 mM dNTP mix, 1 U Taq polymerase (1 U/μl), and the final volume of the reaction mixture was adjusted to 25 μl with nuclease-free water. Amplification conditions were as follows: 94°C for 5 minutes, 39 cycles at 94°C for 30 seconds, 55°C for 45 seconds, 70°C 2 minutes, and final extension at 70°C for 7 minutes. Amplified PCR products were subjected to 1.5% agarose gel electrophoresis, and the relevant band was eluted using QIA quick gel extraction kit (Qiagen). The PCR product was then cloned into pGEM-T Vector System I. After transformation in the DH5α strain of E.coli, the transfectants were plated on ampicillin agar plates. Ten positive colonies of each sample were amplified for preparation and purification of plasmid DNA containing the MIOX promoter. For sequencing of plasmid DNA, T7 promoter and SP6 promoter primers were used.
Statistical Analyses
Results were expressed as mean±SD after statistical analyses. t test was used to compare the data between groups. A P value of <0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
We are thankful to Dr. Anupam Agarwal, Dr. Subhashini Bolisetty, Dr. Volker Hasse, and Dr. Larry Holzman for providing the plasmid constructs and PEPCK mice.
Supported by National Institutes of Health grants DK60635, DK78314, and T32 DK007139.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 2.de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F: Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26: 915–921, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Racusen L, Kashgarian M: Ischemic and toxic acute tubular injury and other ischemic renal injury. In: Pathology of the Kidney, Vol. II, 6th Ed., edited by Jennette JC, Olson JL, Schwartz MM, Silva FG, Philadelphia, Lippincott-Williams & Wilkins, 2007, pp 1139–1198 [Google Scholar]
- 4.Agarwal A, Dong Z, Harris R, Murray P, Parikh SM, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Working Group : Cellular and molecular mechanisms of AKI. J Am Soc Nephrol 27: 1288–1299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozkok A, Edelstein CL: Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int 2014: 967826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z: Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 25: 2278–2289, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zager RA: ‘Biologic memory’ in response to acute kidney injury: Cytoresistance, toll-like receptor hyper-responsiveness and the onset of progressive renal disease. Nephrol Dial Transplant 28: 1985–1993, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath KA, Croatt AJ, Haggard JJ, Grande JP: Renal response to repetitive exposure to heme proteins: Chronic injury induced by an acute insult. Kidney Int 57: 2423–2433, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bydash JR, Ishani A: Acute kidney injury and chronic kidney disease: A work in progress. Clin J Am Soc Nephrol 6: 2555–2557, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Nayak B, Xie P, Akagi S, Yang Q, Sun L, Wada J, Thakur A, Danesh FR, Chugh SS, Kanwar YS: Modulation of renal-specific oxidoreductase/myo-inositol oxygenase by high-glucose ambience. Proc Natl Acad Sci USA 102: 17952–17957, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charalampous FC, Lyras C: Biochemical studies on inositol. IV. Conversion of inositol to glucuronic acid by rat kidney extracts. J Biol Chem 228: 1–13, 1957 [PubMed] [Google Scholar]
- 12.Charalampous FC: Biochemical studies on inositol. V. Purification and properties of the enzyme that cleaves inositol to D-glucuronic acid. J Biol Chem 234: 220–227, 1959 [PubMed] [Google Scholar]
- 13.Nayak B, Kondeti VK, Xie P, Lin S, Viswakarma N, Raparia K, Kanwar YS: Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J Biol Chem 286: 27594–27611, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tominaga T, Dutta RK, Joladarashi D, Doi T, Reddy JK, Kanwar YS: Transcriptional and translational modulation of myo-inositol oxygenase (MIOX) by fatty acids: Implications in renal tubular injury induced in obesity and diabetes. J Biol Chem 291: 1348–1367, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Dutta RK, Xie P, Kanwar YS: myo-Inositol oxygenase overexpression accentuates generation of reactive oxygen species and exacerbates cellular injury following high glucose ambience: A NEW MECHANISM RELEVANT TO THE PATHOGENESIS OF DIABETIC NEPHROPATHY. J Biol Chem 291: 5688–5707, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Ozbek E: Induction of oxidative stress in kidney. Int J Nephrol 2012: 465897, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, Dhawan R: Animal models of acute renal failure. Pharmacol Rep 64: 31–44, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Gross A, McDonnell JM, Korsmeyer SJ: BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Jiang M, Wei Q, Pabla N, Dong G, Wang CY, Yang T, Smith SB, Dong Z: Effects of hydroxyl radical scavenging on cisplatin-induced p53 activation, tubular cell apoptosis and nephrotoxicity. Biochem Pharmacol 73: 1499–1510, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z: Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Singla SK, Puri V, Puri S: The restrained expression of NF-kB in renal tissue ameliorates folic acid induced acute kidney injury in mice. PLoS One 10: e115947, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Lozano G: NF-κB activation of p53. A potential mechanism for suppressing cell growth in response to stress. J Biol Chem 269: 20067–20074, 1994 [PubMed] [Google Scholar]
- 24.Ryan KM, Ernst MK, Rice NR, Vousden KH: Role of NF-κB in p53-mediated programmed cell death. Nature 404: 892–897, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Murphy SH, Suzuki K, Downes M, Welch GL, De Jesus P, Miraglia LJ, Orth AP, Chanda SK, Evans RM, Verma IM: Tumor suppressor protein (p)53, is a regulator of NF-κB repression by the glucocorticoid receptor. Proc Natl Acad Sci USA 108: 17117–17122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guijarro C, Egido J: Transcription factor-κB (NF-κB) and renal disease. Kidney Int 59: 415–424, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: NF-κB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Akcay A, Nguyen Q, Edelstein CL: Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009: 137072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative Consensus XIII Work Group : Inflammation in AKI: Current understanding, key questions and knowledge gaps. J Am Soc Nephrol 27: 371–379, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Dixit B, Wada J, Tian Y, Wallner EI, Srivastva SK, Kanwar YS: Identification of a renal-specific oxido-reductase in newborn diabetic mice. Proc Natl Acad Sci USA 97: 9896–9901, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Cummings BS, Schnellmann RG: Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302: 8–17, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Kaushal V, Haun RS, Seth R, Shah SV, Kaushal GP: Transcriptional activation of caspase-6 and -7 genes by cisplatin-induced p53 and its functional significance in cisplatin nephrotoxicity. Cell Death Differ 15: 530–544, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Sridevi P, Nhiayi MK, Wang JY: Genetic disruption of Abl nuclear import reduces renal apoptosis in a mouse model of cisplatin-induced nephrotoxicity. Cell Death Differ 20: 953–962, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Yue J, Chesney RW: Functional TauT protects against acute kidney injury. J Am Soc Nephrol 20: 1323–1332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M, Skorecki K, Hayashi K, Itoh H: Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 285: 13045–13056, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA: hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Arany I, Herbert J, Herbert Z, Safirstein RL: Restoration of CREB function ameliorates cisplatin cytotoxicity in renal tubular cells. Am J Physiol Renal Physiol 294: F577–F581, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT: Role of oxidants in NF-κB activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol 165: 1013–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M: Canonical pathway of nuclear factor κB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA 101: 5634–5639, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramesh G, Reeves WB: TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prabhu KS, Arner RJ, Vunta H, Reddy CC: Up-regulation of human myo-inositol oxygenase by hyperosmotic stress in renal proximal tubular epithelial cells. J Biol Chem 280: 19895–19901, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Susztak K: Understanding the epigenetic syntax for the genetic alphabet in the kidney. J Am Soc Nephrol 25: 10–17, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratt JR, Parker MD, Affleck LJ, Corps C, Hostert L, Michalak E, Lodge JP: Ischemic epigenetics and the transplanted kidney. Transplant Proc 38: 3344–3346, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Huang N, Tan L, Xue Z, Cang J, Wang H: Reduction of DNA hydroxymethylation in the mouse kidney insulted by ischemia reperfusion. Biochem Biophys Res Commun 422: 697–702, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Endo K, Kito N, Fukushima Y, Weng H, Iwai N: A novel biomarker for acute kidney injury using TaqMan-based unmethylated DNA-specific polymerase chain reaction. Biomed Res 35: 207–213, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moeller MJ, Soofi A, Sanden S, Floege J, Kriz W, Holzman LB: An efficient system for tissue-specific overexpression of transgenes in podocytes in vivo. Am J Physiol Renal Physiol 289: F481–F488, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Xu ZL, Mizuguchi H, Ishii-Watabe A, Uchida E, Mayumi T, Hayakawa T: Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene 272: 149–156, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Agarwal A, Glushakova OY, Jorgensen MS, Salgar SK, Poirier A, Flotte TR, Croker BP, Madsen KM, Atkinson MA, Hauswirth WW, Berns KI, Tisher CC: Gene delivery in renal tubular epithelial cells using recombinant adeno-associated viral vectors. J Am Soc Nephrol 14: 947–958, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Hull TD, Bolisetty S, DeAlmeida AC, Litovsky SH, Prabhu SD, Agarwal A, George JF: Heme oxygenase-1 expression protects the heart from acute injury caused by inducible Cre recombinase. Lab Invest 93: 868–879, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Testa G, Vintersten K, Zhang Y, Benes V, Muyrers JP, Stewart AF: BAC engineering for the generation of ES cell-targeting constructs and mouse transgenes. Methods Mol Biol 256: 123–139, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL: A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA 89: 1827–1831, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]