Abstract
Older patients with ESRD who receive a kidney transplant (KT) may develop post-KT dementia and Alzheimer's disease (AD) associated with their long-standing kidney disease and/or neurotoxic immunosuppressant agents. To investigate this possibility, we studied 40,918 older (aged ≥55 years) KT recipients (January 1, 1999 to December 31, 2011) linked to Medicare claims through the US Renal Data System. We estimated dementia and AD risk (cumulative incidence) and studied factors associated with these sequelae using competing risks models. We estimated the risk of death-censored graft loss and mortality after developing dementia or the AD subtype of dementia, separately, using adjusted Cox proportional hazards models. Older recipients had a 10-year dementia risk ranging from 5.1% for recipients aged 55–60 years to 17.0% for recipients aged ≥75 years; 10-year AD risk ranged from 1.0% to 6.7%, respectively. The strongest predictors for dementia and AD were older recipient age and pretransplant diabetes. The 10-year graft loss risk was 28.8% for those who did not develop dementia and 43.1% for those who did, and the corresponding mortality risks were 55.7% and 89.9%, respectively. Older recipients with dementia had a 1.52-fold (95% confidence interval, 1.39 to 1.68) increased risk of graft loss and a 2.38-fold (95% confidence interval, 2.26 to 2.49) increased risk of mortality. We observed similar results for AD. We conclude that older KT recipients have a high risk of post-KT dementia and AD, and these sequelae associate with a profound effect on patient and graft survival.
Keywords: dementia, kidney transplantation, epidemiology and outcomes
Kidney transplantation (KT) is a growing treatment option for older adults with ESRD; there has been a five-fold rise in the number of older deceased donor KT recipients since 1990.1 Access to KT is not only improving among older KT recipients, but older candidates who would have been considered marginal in years past are now receiving KT. Current recipients are not only older but also more likely to have lengthier pretransplant dialysis, have diabetes mellitus or hypertension, and receive marginal kidneys.1 Even with this changing landscape in transplantation for older adults, there have been improvements in graft loss and survival such that older recipients on average experienced 57% lower mortality and 65% lower graft loss over the past two decades.1 As older KT recipients live longer with a functioning graft, they are at risk of developing age-related conditions.
Dementia, the state of persistent and progressive cognitive impairment, is the leading cause of disability and dependence worldwide,2–4 and Alzheimer's disease (AD) is the most common subtype of dementia accounting for 50%–56% of dementia cases.5 As life expectancy increases, there is an exponential increase in both the prevalence and costs of dementia.4,6,7 By 2050, the annual number of incident cases of dementia and AD is projected to double.8
Dementia is a well recognized complication of ESRD9; among dialysis patients, development of dementia predicts poor outcomes including disability, hospitalization, and death.10–15 However, the risk and consequences of dementia or AD after KT are unclear. We hypothesized that older KT recipients are at elevated risk for dementia or AD due to their long-standing kidney disease as well as dependence on neurotoxic immunosuppressant agents. In fact, older adults are at the highest risk of the neurotoxic side effects of immunosuppression drugs,16 particularly the ubiquitous tacrolimus and steroids.17
As older KT recipients live longer with a functioning graft, it is important to better understand the post-transplant risk of dementia and AD. Therefore, the goals of this study were to (1) estimate the risk of incident all-cause dementia and the AD subtype of dementia in older KT recipients, (2) identify predictors of incident all-cause dementia and the AD subtype of dementia, and (3) compare the subsequent risk of graft loss and mortality between those who developed any dementia or the AD subtype of dementia and those who did not.
Results
Study Population
Among 40,918 older KT recipients, average age was 63.3 years, 37.5% were women, and 26.9% were black. After KT, 2312 had an International Statistical Classification of Diseases and Related Health Problems (ICD)-reported diagnosis of dementia and, of those who developed dementia, 570 (24.7%) had an ICD-reported diagnosis of the AD subtype of dementia (Table 1). The incidence rate was 17.9 per 1000 recipient-years for dementia and 4.0 per 1000 recipient-years for AD.
Table 1.
Characteristics of older KT recipients by incident post-KT dementia and AD
| Characteristic | No Dementia n=38,606 | Dementia n=2312 | AD n=570 |
|---|---|---|---|
| Recipient factors | |||
| Age | 63.3 (5.8) | 65.8 (6.1) | 66.9 (5.8) |
| Women | 37.3 | 40.6 | 39.8 |
| Race | |||
| White | 52.8 | 53.9 | 53.2 |
| Black | 26.8 | 28.3 | 26.5 |
| Hispanic/Latino | 13.3 | 12.9 | 15.8 |
| Other/multiracial | 7.1 | 4.9 | 4.6 |
| Education | |||
| None | 0.8 | 1.1 | 1.1 |
| Grade school (0–8) | 8.2 | 8.8 | 9.8 |
| High school (9–12) or GED | 55.6 | 59.8 | 61.1 |
| College/technical school | 18.0 | 16.3 | 13.5 |
| Associate or bachelor degree | 11.4 | 8.4 | 9.7 |
| Postcollege graduate degree | 6.1 | 5.5 | 4.9 |
| Cause of ESRD | |||
| Glomerular diseases | 13.8 | 9.9 | 12.5 |
| Diabetes | 35.2 | 41.8 | 35.6 |
| Hypertension | 22.5 | 20.7 | 21.8 |
| Other causes | 28.5 | 27.6 | 30.2 |
| Body mass index | 27.9 (4.9) | 27.3 (4.7) | 26.8 (4.5) |
| Hypertension | 87.8 | 85.6 | 84.6 |
| Diabetes | 45.3 | 52.8 | 46.3 |
| Hepatitis C virus positive | 5.2 | 4.6 | 4.4 |
| Pre-emptive transplant | 4.1 | 4.1 | 4.0 |
| Years on dialysis | 3.1 (1.5–4.8) | 3.0 (1.4–4.6) | 3.1 (1.4–4.7) |
| Previous KT | 4.8 | 3.9 | 5.1 |
| Transplant factors | |||
| PRA | 16.7 (28.6) | 14.7 (26.3) | 14.9 (26.1) |
| ABO incompatible | 0.4 | 0.2 | 0 |
| 0 HLA mismatches | 9.2 | 10.9 | 9.3 |
| CIT>24 h | 30.2 | 33.7 | 35.3 |
| Donor factors | |||
| Age | 42.6 (15.6) | 42.4 (15.7) | 41.6 (15.8) |
| Women | 46.2 | 45.9 | 44.6 |
| Race/Ethnicity | |||
| White | 70.7 | 69.8 | 68.4 |
| Black | 13.0 | 14.5 | 14.0 |
| Hispanic/Latino | 12.8 | 12.4 | 13.7 |
| Other/multiracial | 3.5 | 3.3 | 3.9 |
| Donation after cardiac death | 6.3 | 4.3 | 3.3 |
| Expanded criteria donor | 22.5 | 23.2 | 21.9 |
| Hypertension | 25.8 | 25.6 | 24.6 |
| Diabetes | 6.2 | 5.9 | 4.4 |
| Hepatitis C virus positive | 2.0 | 2.2 | 2.3 |
Values are presented as percentages, median (interquartile range), or mean (SD). For time on dialysis median and interquartile range are presented. AD is a subtype of dementia; therefore, the AD cases are a subset of the dementia cases. Dementia and AD are ICD-reported diagnoses of dementia or AD. GED, General Educational Development test; PRA, panel reactive antibody; HLA, human leukocyte antigen; CIT, cold ischemia time.
Risk of Dementia and AD
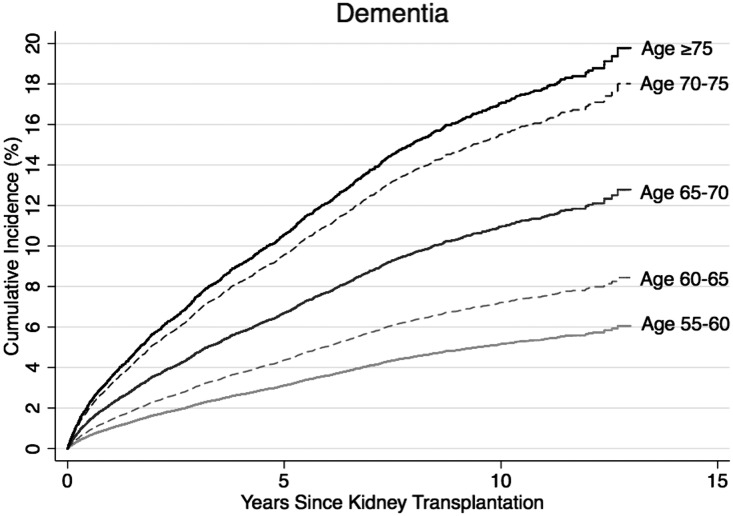
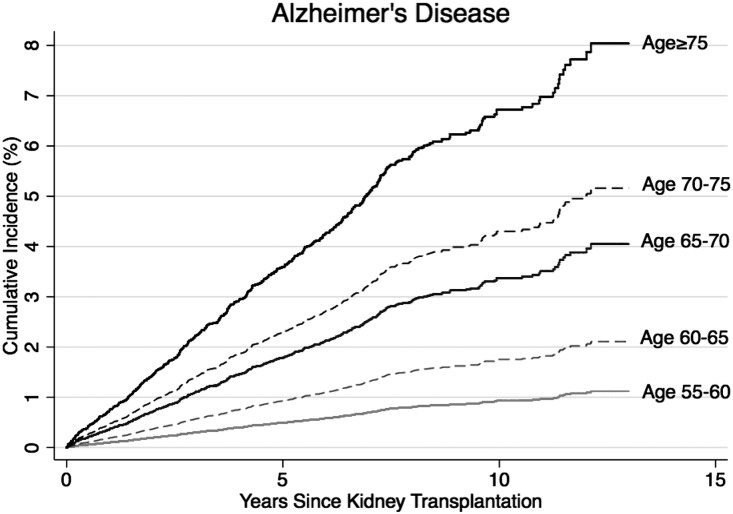
Under a competing risks framework, the 10-year risk of post-KT dementia was 5.1% for KT recipients aged 55–60, 7.2% for recipients aged 60–65, 11.0% for recipients aged 65–70, 15.6% for recipients aged 70–75, and 17.0% for recipients aged 75 and older (Figure 1). Similarly, the risk of AD increased with age at KT (Figure 2). The 10-year risk of AD was 1.0% for KT recipients aged 55–60 at KT, 1.8% for recipients aged 60–65, 3.5% for recipients aged 65–70, 4.2% for recipients aged 70–75, and 6.7% for recipients aged 75 and older.
Figure 1.
The risk of dementia by age at transplantation. The risk (cumulative incidence) was estimated for 40,918 older KT recipients using a competing risks survival analysis. Dementia represents the ICD-reported diagnoses of dementia.
Figure 2.
The risk of AD by age at transplantation. The risk (cumulative incidence) was estimated for 40,918 older KT recipients using a competing risks survival analysis. AD is a subtype of dementia; therefore, the AD cases are a subset of the dementia cases. AD represents the ICD-reported diagnoses of AD.
For perspective, findings from the Framingham study suggest that the incidence of dementia is 1%–1.5% in adults aged 65 and 7.4%–7.6% in adults aged 75.18 Similarly, in this population-based cohort of older adults the incidence of AD is 0.6%–0.9% in adults aged 65 and 4.4%–5.4% in adults aged 75.18
Predictors of Dementia
Recipients who developed dementia were older at the time of KT (65.8 versus 63.3 years old; P<0.001), more likely to be women (40.6% versus 37.3%; P=0.001), more likely to have had diabetes at the time of KT (52.8% versus 45.3%; P<0.001), and less likely to have received a donation after cardiac death organ (4.3% versus 6.3%; P<0.001) (Table 1). For every 5-year increase in age at KT, the risk of post-KT dementia independently increased 1.5-fold (hazard ratio [HR], 1.50; 95% confidence interval [95% CI], 1.46 to 1.56) (Table 2). Additionally, recipients who were women (HR, 1.19; 95% CI, 1.09 to 1.29), black recipients (HR, 1.21; 95% CI, 1.09 to 1.33), and recipients with diabetes (HR, 1.64; 95% CI, 1.51 to 1.78) were at increased risk of developing dementia. The association between diabetes and dementia did not differ by age (P=0.45) or sex (P=0.51). Compared with recipients with Associate or Bachelor degrees, those with no education (HR, 1.66; 95% CI, 1.10 to 2.49), grade school education (HR, 1.24; 95% CI, 1.01 to 1.53), and a high school education (HR, 1.23; 95% CI, 1.06 to 1.44) were at increased risk of developing dementia. For every 5-year increase in time on dialysis the risk of dementia increased 1.1-fold (HR, 1.09; 95% CI, 1.02 to 1.16). The risk of dementia was decreasing with time since 1999, with a 0.91-fold (95% CI, 0.90 to 0.92) decreased risk of dementia per calendar year. Additionally, standard criteria deceased donor recipients were at 1.13-fold (95% CI, 1.01 to 1.27) increased risk of dementia. Patients with a calcineurin inhibitor–free maintenance immunosuppression regimen were at a decreased risk of dementia after adjusting for recipient, transplant, and donor factors in the competing risks model (HR, 0.83; 95% CI, 0.69 to 0.99). However, there was no association between steroid-free regimens and incident dementia (HR, 1.00; 95% CI, 0.89 to 1.12). None of the induction agents were associated with dementia.
Table 2.
Risk prediction models for incident post-KT dementia and AD among older (age ≥55) KT recipients
| Characteristic | Dementia HR (95% CI) | AD HR (95% CI) |
|---|---|---|
| Recipient factors | ||
| Age (per 5 yr) | 1.50 (1.46 to 1.56) | 1.69 (1.59 to 1.80) |
| Women | 1.19 (1.09 to 1.29) | — |
| Race/ethnicity | ||
| White | Reference | — |
| Black | 1.21 (1.09 to 1.33) | — |
| Hispanic/Latino | 1.03 (0.90 to 1.18) | — |
| Other/multiracial | 0.74 (0.61 to 0.91) | — |
| Body mass index, kg/m2 | ||
| <18.5 | 0.81 (0.47 to 1.40) | 0.48 (0.12 to 1.97) |
| 18.5–25 | 1.22 (1.09 to 1.36) | 1.32 (1.05 to 1.65) |
| 25–30 | 1.12 (1.01 to 1.24) | 1.11 (0.89 to 1.38) |
| ≥30 | Reference | Reference |
| Diabetes | 1.64 (1.51 to 1.78) | 1.32 (1.11 to 1.56) |
| Previous transplant | — | 1.31 (0.89 to 1.92) |
| Years on dialysis (per 5 yr) | 1.09 (1.02 to 1.16) | 1.09 (0.97 to 1.22) |
| Education | ||
| None | 1.66 (1.10 to 2.49) | 1.33 (0.57 to 3.13) |
| Grade school (0–8) | 1.24 (1.01 to 1.53) | 1.26 (0.87 to 1.83) |
| High school (9–12) or GED | 1.23 (1.06 to 1.44) | 1.15 (0.86 to 1.52) |
| Attended college/technical school | 1.18 (0.99 to 1.40) | 0.90 (0.63 to 1.27) |
| Associate or bachelor degree | Reference | Reference |
| Postcollege graduate degree | 1.17 (0.94 to 1.47) | 0.86 (0.54 to 1.35) |
| Transplant factors | ||
| ABO incompatible | 0.59 (0.24 to 1.42) | — |
| Year of KT (per 1 yr) | 0.91 (0.90 to 0.92) | 0.91 (0.89 to 0.93) |
| Donor factors | ||
| Donor type | ||
| Live | Reference | — |
| Deceased standard criteria | 1.13 (1.01 to 1.27) | — |
| ECD | 1.01 (0.87 to 1.18) | — |
| DCD | 1.06 (0.85 to 1.33) | — |
| Race/ethnicity | ||
| White | — | Reference |
| Black | — | 1.29 (1.01 to 1.64) |
| Hispanic/Latino | — | 1.22 (0.95 to 1.55) |
| Other/multiracial | — | 1.25 (0.81 to 1.91) |
| Hypertension | 1.04 (0.94 to 1.14) | 0.98 (0.81 to 1.19) |
| Diabetes | 1.03 (0.86 to 1.24) | — |
| HCV+ | 1.23 (0.93 to 1.63) | 1.33 (0.77 to 2.30) |
| c-statistic | 0.66 | 0.69 |
All HRs are from a single adjusted competing risks survival model for the dementia and AD outcomes, separately. AD is a subtype of dementia; therefore, the AD cases are a subset of the dementia cases. Dementia and AD are ICD-reported diagnoses of dementia or AD. —, not included in model; GED, General Educational Development test; ECD, expanded criteria donor; DCD, donation after cardiac death; HCV+, hepatitis C virus-positive.
Predictors of AD
Although most predictors of AD were similar to those identified for dementia, there were some notable differences in the strength of the associations (Table 2). For every 5-year increase in age at KT, the risk of AD increased 1.69-fold (95% CI, 1.59 to 1.80). Diabetic recipients were at 1.32-fold (95% CI, 1.11 to 1.56) increased risk of post-KT AD. The association between diabetes and AD did not differ by age (P=0.28) or sex (P=0.77). The risk of AD was decreasing over time since 1999, with a 0.91-fold (95% CI, 0.89 to 0.93) decreased risk of AD per calendar year. Induction and immunosuppression agents were not associated with incident AD in the competing risks model accounting for the recipient, transplant, and donor factors.
Death-Censored Graft Loss, Death with a Functioning Graft, and Mortality after Post-KT Dementia
The risk of death-censored graft loss, death with a functioning graft, and mortality was higher among older KT recipients who subsequently developed dementia (Table 3). The unadjusted 10-year risk of death-censored graft loss was 28.8% for those who did not develop post-KT dementia and 43.1% for those who did; the corresponding 10-year risks of death with a functioning graft were 46.2% and 86.7% and of mortality were 55.7% and 89.9%. Older KT recipients with dementia were at 1.52-fold (95% CI, 1.39 to 1.68) increased risk of death-censored graft loss, at 2.74-fold (95% CI, 2.59 to 2.89) increased risk of death with a functioning graft, and at 2.38-fold (95% CI, 2.26 to 2.49) risk of mortality, independent of other recipient, transplant, and donor factors (Table 4). Results were consistent in a sensitivity analysis in which we adjusted for all recipient, transplant, and donor factors (death-censored graft loss HR, 1.55; 95% CI, 1.41 to 1.71; death with functioning graft HR, 2.71; 95% CI, 2.57 to 2.86; mortality HR, 2.37; 95% CI, 2.26 to 2.49).
Table 3.
Risk of mortality and death-censored graft loss for KT recipients with dementia and AD
| Dementia and AD Status | Risk (%) | |||
|---|---|---|---|---|
| 1-yr | 3-yr | 5-yr | 10-yr | |
| Death-censored graft loss | ||||
| No dementia | 3.5 | 7.7 | 12.6 | 28.8 |
| Dementia | 4.0 | 11.1 | 21.0 | 43.1 |
| No AD | 3.5 | 7.8 | 12.8 | 29.3 |
| AD | 1.2 | 5.1 | 14.3 | 38.9 |
| Death with a functioning graft | ||||
| No dementia | 5.8 | 12.4 | 20.9 | 46.2 |
| Dementia | 19.6 | 43.2 | 60.9 | 86.7 |
| No AD | 6.0 | 13.0 | 21.9 | 48.1 |
| AD | 10.7 | 32.3 | 54.8 | 85.9 |
| Mortality | ||||
| No dementia | 7.4 | 16.0 | 26.3 | 55.7 |
| Dementia | 20.8 | 46.0 | 64.9 | 89.9 |
| No AD | 7.5 | 16.6 | 27.4 | 57.4 |
| AD | 11.8 | 34.7 | 58.6 | 88.6 |
The risks (cumulative incidences) are estimated using a Kaplan–Meier approach. AD is a subtype of dementia; therefore, the AD cases are a subset of the dementia cases. Dementia and AD are ICD-reported diagnoses of dementia or AD.
Table 4.
The impact of dementia and AD on death-censored graft loss and mortality stratified by age, race, sex, and diabetes status
| Effect Modifier | Death-Censored Graft Loss HR (95% CI) | Death with a Functioning Graft HR (95% CI) | Mortality HR (95% CI) |
|---|---|---|---|
| Dementia | |||
| Overall risk | 1.52 (1.39 to 1.68) | 2.74 (2.59 to 2.89) | 2.38 (2.26 to 2.49) |
| Age, yr | |||
| 55–65 | 1.55 (1.35 to 1.79) | 2.90 (2.67 to 3.16) | 2.51 (2.32 to 2.70) |
| ≥65 | 1.51 (1.32 to 1.72) | 2.78 (2.60 to 2.97) | 2.40 (2.25 to 2.55) |
| P for interaction | 0.74 | 0.43 | 0.37 |
| Race | |||
| Nonblack | 1.52 (1.34 to 1.71) | 2.82 (2.65 to 3.00) | 2.43 (2.30 to 2.57) |
| Black | 1.53 (1.31 to 1.79) | 2.48 (2.23 to 2.76) | 2.22 (2.02 to 2.42) |
| P for interaction | 0.92 | 0.04 | 0.08 |
| Sex | |||
| Men | 1.50 (1.33 to 1.70) | 2.66 (2.48 to 2.85) | 2.32 (2.18 to 2.47) |
| Women | 1.56 (1.34 to 1.81) | 2.86 (2.63 to 3.12) | 2.47 (2.29 to 2.66) |
| P for interaction | 0.71 | 0.17 | 0.21 |
| Diabetes | |||
| No | 1.36 (1.17 to 1.57) | 2.75 (2.55 to 2.97) | 2.37 (2.20 to 2.54) |
| Yes | 1.66 (1.46 to 1.88) | 2.69 (2.50 to 2.89) | 2.35 (1.13 to 1.94) |
| P for interaction | 0.04 | 0.66 | 0.89 |
| AD | |||
| Overall risk | 1.40 (1.15 to 1.70) | 2.49 (2.25 to 2.75) | 2.14 (1.95 to 2.36) |
| Age, yr | |||
| 55–65 | 1.94 (1.46 to 2.58) | 2.90 (2.41 to 3.48) | 2.52 (2.13 to 2.98) |
| ≥65 | 1.14 (0.86 to 1.49) | 2.50 (2.23 to 2.81) | 2.13 (1.90 to 2.38) |
| P for interaction | 0.01 | 0.19 | 0.10 |
| Race | |||
| Nonblack | 1.27 (0.99 to 1.63) | 2.47 (2.20 to 2.77) | 2.08 (1.87 to 2.32) |
| Black | 1.61 (1.18 to 2.21) | 2.60 (2.13 to 3.17) | 2.33 (1.96 to 2.78) |
| P for interaction | 0.24 | 0.66 | 0.24 |
| Sex | |||
| Men | 1.36 (1.05 to 1.76) | 2.52 (2.22 to 2.86) | 2.20 (1.95 to 2.47) |
| Women | 1.47 (1.09 to 1.99) | 2.48 (2.11 to 2.91) | 2.11 (1.82 to 2.45) |
| P for interaction | 0.71 | 0.87 | 0.68 |
| Diabetes | |||
| No | 1.32 (0.99 to 1.75) | 2.87 (2.50 to 3.28) | 2.38 (2.10 to 2.71) |
| Yes | 1.48 (1.13 to 1.94) | 2.15 (1.86 to 2.49) | 1.93 (1.69 to 2.21) |
| P for interaction | 0.55 | 0.01 | 0.03 |
All models are adjusted for the recipient, transplant, and donor factors listed in Table 2. The HR are estimated using a Cox proportional hazards model with time-varying dementia and AD. AD is a subtype of dementia; therefore, the AD cases are a subset of the dementia cases. Dementia and AD are ICD-reported diagnoses of dementia or AD.
The association between dementia and death-censored graft loss differed by diabetes status (P for interaction=0.04) (Table 4). Older diabetic KT recipients who developed dementia were at 1.66-fold (95% CI, 1.46 to 1.88) increased risk of death-censored graft loss compared with those diabetic recipients who did not develop dementia. However, nondiabetic older KT recipients who developed dementia were at 1.36-fold (95% CI, 1.17 to 1.57) increased risk of graft loss. Additionally, the association between dementia and death with a functioning graft differed by race (P=0.04). Nonblack older KT recipients who developed post-KT dementia were at 2.82-fold (95% CI, 2.65 to 3.00) increased risk of death with a functioning graft compared with older nonblack recipients who did not. In contrast, older black KT recipients who developed dementia were at 2.48-fold (95% CI, 2.23 to 2.76) increased risk of death with a functioning graft.
Death-Censored Graft Loss, Death with a Functioning Graft, and Mortality after Post-KT AD
The unadjusted risk of death-censored graft loss was higher at 5 and 10 years post-KT among older KT recipient who subsequently developed AD (Table 3); the risk of death with a functioning graft and mortality was higher at all times. The 10-year risk of death-censored graft loss was 29.3% for those who did not develop post-KT dementia AD and 38.9% for those who did; the corresponding 10-year risks of death with a functioning graft were 48.1% and 85.9% and of mortality were 57.4% and 88.6%. Older KT recipients with AD were at 1.40-fold (95% CI, 1.15 to 1.70) increased risk of death-censored graft loss, at 2.49-fold (95% CI, 2.25 to 2.75) increased risk of death with a functioning graft, and at 2.14-fold (95% CI, 1.95 to 2.36) increased risk of mortality, independent of other recipient, transplant, and donor factors (Table 4). Results were consistent in a sensitivity analysis in which we adjusted for all recipient, transplant, and donor factors (death-censored graft loss HR, 1.44; 95% CI, 1.18 to 1.74; death with functioning graft HR, 2.55; 95% CI, 2.31 to 2.82; mortality HR, 2.21; 95% CI, 2.01 to 2.42).
The association between AD and death-censored graft loss differed by age (P for interaction=0.01) (Table 4). KT recipients aged 55–65 who developed AD were at 1.94-fold (95% CI, 1.46 to 2.58) increased risk of death-censored graft loss compared with those aged 55–65 who did not develop AD. However, KT recipients aged 65 and older who developed AD were not at risk of death-censored graft loss (HR, 1.14; 95% CI, 0.86 to 1.49). Additionally, the association between AD and death with a functioning graft as well as mortality differed by diabetes status (P=0.01, P=0.03). Nondiabetic older KT recipients with AD were at 2.87-fold (95% CI, 2.50 to 3.28) increased risk of death with a functioning graft as well as at 2.38-fold (95% CI, 2.10 to 2.71) increased risk of mortality compared with older nondiabetic recipients who did not develop AD. In contrast, older diabetic KT recipients with AD were at 2.15-fold (95% CI, 1.86 to 2.49) increased risk of death with a functioning graft and at 1.93-fold (95% CI, 1.69 to 2.21) increased risk of mortality.
Discussion
In this national study of 40,918 older KT recipients, the 10-year incidence of post-KT dementia ranged from 5% for KT recipients aged 55–60 to 17% for KT recipients aged 75 and older. Recipient, transplant, and donor factors were associated with an increased risk of dementia; importantly, maintenance immunosuppression regimens that were free of calcineurin inhibitors reduced the risk of incident post-KT dementia. For those recipients who subsequently developed dementia, the 10-year risk of death-censored graft loss was 43.1% and mortality was 86.7%. Older recipients who developed post-KT dementia were subsequently at 1.5-fold (95% CI, 1.39 to 1.68) increased risk of graft loss and 2.7-fold (95% CI, 2.59 to 2.89) increased risk of death with a functioning graft and 2.4-fold (95% CI, 2.26 to 2.49) increased risk of mortality; similar risks were observed for older KT recipients who developed post-KT AD. The effect of dementia on graft loss was stronger in diabetic recipients and the effect of dementia on death with a functioning graft was stronger in nonblack recipients.
Our observed incidence of post-KT dementia of 11.0% in those transplanted at ages 65–70, rising to 17.0% for those >75, is higher than the findings from the Framingham study of a 1%–1.5% incidence in adults aged 65 and a 7.4%–7.6% incidence in adults aged 75.18 Similarly, our observed incidence of post-KT AD of 3.5% in those transplanted at ages 65–70, rising to 6.7% for those >75, is higher than the findings from the Framingham study of a 0.6%–0.9% incidence in adults aged 65 and a 4.4%–5.4% incidence in adults aged 75.18 Although both studies used a competing risks survival model, the estimates from the Framingham study included both diagnosed and undiagnosed cases. These differences in defining dementia and AD cases are not trivial, given that a previous validation study suggests that only half of those who would meet diagnostic criteria for dementia receive a physician diagnosis of dementia.19 With this estimated rate of under-diagnosis in mind, the risk of dementia and AD after KT compared with the general older adult population is even more substantial than comparing incidence rates would suggest.
We were unable to compare the risk of dementia and AD among older KT recipients to that among older dialysis patients because, to our knowledge, there are no studies of incidence of ICD-defined dementia or AD in the dialysis population, let alone studies which accounted for competing risks to estimate the risks of these outcomes. Previous studies of dialysis patients have focused on the prevalence of dementia.14,20,21
We have extended the study of dementia and AD to the novel population of KT recipients and confirmed that, like in older adults8,22–27 and patients of all ages with ESRD on dialysis,13,28 the risk of dementia and AD increases with age and is greater among women and nonwhite patients as well as those with lower education and those with diabetes. Importantly, we have shown that among KT recipients age is a strong risk factor for dementia and AD, but age alone is not sufficient. Dementia is not a part of normal healthy aging and many older KT recipients will never develop these cognitive outcomes. We have also extended the previous findings of a declining incidence of dementia in more recent cohorts of older adults to the novel population of older KT recipients.29 Finally, we identified calcineurin inhibitor–free maintenance immunosuppression regimens, one of the few modifiable risk factors, as being associated with a decreased risk of incident dementia.
Similar to findings from studies of older adults30–32 and patients undergoing dialysis,13 we found that there is a great burden of mortality among those who develop dementia or AD. Among ESRD patients of all ages who had a predialysis diagnosis of dementia, there was a 1.87-fold increased risk of postdialysis mortality,13 which is a substantially weaker association than we observed for incident dementia and post-KT mortality. We have also extended these findings to include an association between dementia, AD, and graft loss as well as death with a functioning graft. The increased risk of graft loss for those with dementia may result from a decline in the ability to perform self-care tasks including managing medication such as immunosuppression. Mortality can result among those with dementia as the result of lacking the ability to perform self-care and also from inadequate nutrition, the inability to chew and swallow, personal safety issues, the inability to communicate new symptoms, and infections, particularly pneumonia.33,34
This study has a few notable limitations. In order to identify incident cases of dementia, we had to limit our population to Medicare-primary KT recipients, a criterion which could differentially affect those under 65 and, thus, the generalizability. However, given that all ESRD patients requiring dialysis therapy are eligible for Medicare, this is a common inclusion criterion in studies of ESRD patients.35–40 Additionally, this national registry does not capture important metrics of aging, like frailty and cognitive function, which may be predictors of incident dementia after KT. Nor does this national registry capture treatment for dementia and AD, information on a patient’s social environment, or cause of death-censored graft loss or mortality. Also, the distinction between the general category of “dementia” versus the specific diagnosis of “AD” is challenging in real-world clinical settings reflected in Medicare claims data, and thus it is likely that the diagnosis of AD is an underestimate. AD cannot be truly diagnosed without a postmortem pathologic confirmation and it likely that the claims-based diagnosis of AD more likely reflects a doctor’s suspected diagnosis. Finally, our study was based on observational data and, thus, it remains unclear whether preventing dementia or AD would improve the rates of graft loss and mortality among older KT recipients.
In conclusion, the risk of dementia and AD among older KT recipients is substantially greater than the previously published risks of dementia and AD among older adults in general. A number of recipient, transplant, and donor factors can help predict post-KT dementia and AD. Importantly, older KT recipients who are diagnosed with dementia and AD are at increased risk of subsequent graft loss and at more than twice the risk of death with a functioning graft and mortality. The risk of dementia and AD should be weighed against the burden of dialysis and not adversely affect the decision to transplant otherwise acceptable older KT candidates. There is the need for greater awareness of the risk for dementia and AD in older patients undergoing KT.
Concise Methods
Study Population
We studied 40,918 older (aged ≥55) kidney-only transplant recipients between January 1, 1999 and December 31, 2011, as reported to the Organ Procurement and Transplantation Network (OPTN) and linked to Medicare claims by the US Renal Data System (USRDS). To allow for appropriate longitudinal follow-up, the population was limited to those recipients with Medicare as their continuous primary insurer post-KT. Donor, recipient, and transplant factors were gleaned from the Scientific Registry of Transplant Recipients (SRTR). SRTR is a national registry that includes data on all donors, waitlisted candidates, and transplant recipients in the United States submitted by the member of the OPTN. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
As is standard with SRTR and USRDS data, mortality and graft loss were augmented through linkage with the Social Security Death Master File, data from the Centers for Medicare and Medicaid Services (CMS), and waitlist data. Graft loss was defined as irreversible graft failure signified by return to long-term dialysis (ascertained from CMS), listing for KT (ascertained from SRTR), or retransplantation (ascertained from SRTR). Death with a functioning graft was defined as mortality without prior graft loss.
ICD-Reported Diagnosed Dementia and AD
Similar to other studies,41–45 we identified incident dementia (331.0, 331.1, 331.2, 331.7, 290.0, 290.1, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.1, 294.8, 797) and AD (331.0) using ICD-9 codes. A previous publication has reported the sensitivity and specificity of these Medicare claims as 0.85 and 0.89, respectively, for dementia and 0.64 and 0.95, respectively, for AD.41 We considered an ICD-reported diagnosis of the AD subtype of dementia, separate from an ICD-reported diagnosis of all-cause dementia, such that a recipient could have a diagnosis of non-AD dementia and then a subsequent AD dementia or vice versa. Although dementia is generally considered an absolute contraindication for transplantation, we made sure to exclude all older KT recipients with a claim for dementia before KT; only 227 (0.6%) were excluded (final sample n=40,918).
Predictors of Dementia and AD
We included recipient factors (sex, age, race, education level, body mass index, hepatitis C virus status, cause of ESRD, history of hypertension, history of diabetes, number of years on dialysis, previous KT, and peak panel reactive antibody), transplant (year of KT, pre-emptive KT, number of human leukocyte antigen mismatches, ABO incompatibility, and cold ischemia time), and donor factors (age, race, hypertension, diabetes, hepatitis C virus status, donation after cardiac death, and expanded criteria donor) as potential predictors. Induction agent (ATG, IL-2 receptor antagonists, alemtuzumab, OKT3, or no induction) and maintenance immunosuppression (steroid, tacrolimus, cyclosporine, mycophenolate mofetil, mTOR, and azathioprine) were ascertained from SRTR and also considered as predictors of dementia and AD.
Prediction Model for Post-KT Dementia and AD
We used survival analysis to estimate the incidence rate per year and Cox proportional hazard regression models to identify recipient, donor, and transplant factors that predicted incident dementia, censoring for end of follow-up (31 December, 2011), end of Medicare coverage, mortality, or graft loss. The final multivariate model of recipient, donor, and transplant factors was selected based on optimal parsimony by minimizing the Akaike Information Criterion. We then calculated the Harrell C-statistic for the model to predict post-KT dementia. However, because both death and graft loss represent competing risks for dementia, we estimated the associations between the recipient, donor, and transplant factors and dementia using a competing risks survival regression based on the Fine and Gray proportional subhazards model.46 We tested for effect modification of the association between diabetes and dementia by age and sex. Using this method, we estimated and plotted the cumulative incidence function, i.e., the risk of being diagnosed with dementia by a given time. We then tested whether type of induction and immunosuppression regimen was associated with dementia in the competing risks survival model. We used a similar approach to identify predictors of AD.
Death-Censored Graft Loss, Death with a Functioning Graft, and Mortality after Dementia and AD
We then estimated the HR for death-censored graft loss, death with a functioning graft, and mortality associated with developing dementia or AD using a Cox proportional hazard regression model. We considered dementia as time-varying exposure; in other words, older KT recipients contributed person-time to the nondementia group until the diagnosis of dementia, and then they contributed person-time to the dementia group until graft loss, death with a functioning graft, mortality, or administrative censoring (February 28, 2016). When death with a functioning graft was the outcome we censored at graft loss but did not do so when mortality was the outcome. We used a parallel approach to study the effect of AD.
Statistical Analyses
All analyses were performed using Stata 14.0. The Johns Hopkins Institutional Review Board approved the cohort study and the use of SRTR data. This work was conducted with adherence to the Declaration of Istanbul.
This study was deemed exempt from consent by Johns Hopkins.
Disclosures
None.
Acknowledgments
This study was supported by National Institutes of Health grant R01AG042504 (Principal Investigator: to D.L.S.) and K24DK101828 (Principal Investigator: to D.L.S.). M.A.M.-D. was supported by the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant and Johns Hopkins University Claude D. Pepper Older Americans Independence Center, the National Institute on Aging (P30-AG021334), and K01AG043501 from the National Institute on Aging. C.H.B. was supported by the International Anesthesia Research Society, the Johns Hopkins Clinician Scientist Award, and the Research Career Development Core of the Johns Hopkins Pepper Older Americans Independence Center, NIA P30AG021334.
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and should in no way be seen as an official policy of or interpretation by the SRTR or the United States government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL: Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc 62: 2235–2242, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, Jacob KS, Jotheeswaran AT, Rodriguez JJ, Pichardo GR, Rodriguez MC, Salas A, Sosa AL, Williams J, Zuniga T, Prince M: Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: A 10/66 Dementia Research Group population-based survey. Lancet 374: 1821–1830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sousa RM, Ferri CP, Acosta D, Guerra M, Huang Y, Jacob K, Jotheeswaran A, Hernandez MA, Liu Z, Pichardo GR, Rodriguez JJ, Salas A, Sosa AL, Williams J, Zuniga T, Prince M: The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: A 10/66 Dementia Research Group population-based survey. BMC Geriatr 10: 53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harwood RH, Sayer AA, Hirschfeld M: Current and future worldwide prevalence of dependency, its relationship to total population, and dependency ratios. Bull World Health Organ 82: 251–258, 2004 [PMC free article] [PubMed] [Google Scholar]
- 5.Querfurth HW, LaFerla FM: Alzheimer’s disease. N Engl J Med 362: 329–344, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Hurd MD, Martorell P, Langa K: Future monetary costs of dementia in the United States under alternative dementia prevalence scenarios. J Popul Ageing 8: 101–112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM: Monetary costs of dementia in the United States. N Engl J Med 368: 1326–1334, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebert LE, Beckett LA, Scherr PA, Evans DA: Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord 15: 169–173, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Kurella Tamura M, Yaffe K: Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int 79: 14–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drew DA, Weiner DE, Tighiouart H, Scott T, Lou K, Kantor A, Fan L, Strom JA, Singh AK, Sarnak MJ: Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis 65: 303–311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Qiu Y, Li S, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: Excerpts from the United States renal data system 2006 annual data report. Am J Kidney Dis 49: A6–A7, S1–S296, 2007 [DOI] [PubMed]
- 12.Cohen LM, Ruthazer R, Moss AH, Germain MJ: Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 5: 72–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakowski DA, Caillard S, Agodoa LY, Abbott KC: Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol 1: 1000–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ: Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis 30: 41–49, 1997 [DOI] [PubMed] [Google Scholar]
- 16.DiMartini A, Fontes P, Dew MA, Lotrich FE, de Vera M: Age, model for end-stage liver disease score, and organ functioning predict posttransplant tacrolimus neurotoxicity. Liver Transpl 14: 815–822, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattarozzi K, Cretella L, Guarino M, Stracciari A: Minimal hepatic encephalopathy: Follow-up 10 years after successful liver transplantation. Transplantation 93: 639–643, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA: The lifetime risk of stroke: Estimates from the Framingham Study. Stroke 37: 345–350, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN; U.S. Preventive Services Task Force : Screening for dementia in primary care: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 138: 927–937, 2003 [DOI] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, Tan J, Salter ML, Gross A, Meoni LA, Jaar BG, Kao WH, Parekh RS, Segev DL, Sozio SM: Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol 10: 2181–2189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Lone E, Connors M, Masson P, Wu S, Kelly PJ, Gillespie D, Parker D, Whiteley W, Strippoli GF, Palmer SC, Craig JC, Webster AC: Cognition in people with end-stage kidney disease treated with hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 67: 925–935, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, Jones B, Lyketsos C, Dulberg C: Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 52: 195–204, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB: Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol 59: 1737–1746, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, Hebert LE, Aggarwal N, Beckett LA, Joglekar R, Berry-Kravis E, Schneider J: Incidence of Alzheimer disease in a biracial urban community: Relation to apolipoprotein E allele status. Arch Neurol 60: 185–189, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R: Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271: 1004–1010, 1994 [PubMed] [Google Scholar]
- 26.Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, Pilgrim DM, Taylor JO: Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol 54: 1399–1405, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P: Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol 5: 64–74, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, Chertow GM; Frequent Hemodialysis Network Trial Group : Prevalence and correlates of cognitive impairment in hemodialysis patients: The frequent hemodialysis network trials. Clin J Am Soc Nephrol 5: 1429–1438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S: Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 374: 523–532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ: Lethality of Alzheimer disease and its impact on nursing home placement. Alzheimer Dis Assoc Disord 24: 90–95, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Weuve J, Hebert LE, Scherr PA, Evans DA: Deaths in the United States among persons with Alzheimer’s disease (2010-2050). Alzheimer Dement 10: e40–e46, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA: Contribution of Alzheimer disease to mortality in the United States. Neurology 82: 1045–1050, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns A, Jacoby R, Luthert P, Levy R: Cause of death in Alzheimer’s disease. Age Ageing 19: 341–344, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Brunnström HR, Englund EM: Cause of death in patients with dementia disorders. Eur J Neurol 16: 488–492, 2009 [DOI] [PubMed] [Google Scholar]
- 35.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL: Early hospital readmission after kidney transplantation: Patient and center-level associations. Am J Transplant 12: 3283–3288, 2012 [DOI] [PubMed] [Google Scholar]
- 36.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL: Sequelae of early hospital readmission after kidney transplantation. Am J Transplant 14: 397–403, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobbels F, Skeans MA, Snyder JJ, Tuomari AV, Maclean JR, Kasiske BL: Depressive disorder in renal transplantation: An analysis of medicare claims. Am J Kidney Dis 51: 819–828, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Snyder JJ, Israni AK, Peng Y, Zhang L, Simon TA, Kasiske BL: Rates of first infection following kidney transplant in the United States. Kidney Int 75: 317–326, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Weinhandl ED, Snyder JJ, Israni AK, Kasiske BL: Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplantat 9: 506–516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grams ME, McAdams Demarco MA, Kucirka LM, Segev DL: Recipient age and time spent hospitalized in the year before and after kidney transplantation. Transplantation 94: 750–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL: The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. J Alzheimers Dis 17: 807–815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerra C, Linde-Zwirble WT, Wunsch H: Risk factors for dementia after critical illness in elderly medicare beneficiaries. Crit Care 16: R233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C, Gao S, Hendrie HC, Kesterson J, Campbell NL, Shekhar A, Callahan CM: Antidepressant use in the elderly is associated with an increased risk of dementia. Alzheimer Dis Assoc Disord 30: 99–104, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfgram DF, Szabo A, Murray AM, Whittle J: Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int 35: 189–198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutcher SK, Rattinger GB, Langenberg P, Chhabra PT, Liu X, Rosenberg PB, Leoutsakos JM, Simoni-Wastila L, Walker LD, Franey CS, Zuckerman IH: Effect of medications on physical function and cognition in nursing home residents with dementia. J Am Geriatr Soc 62: 1046–1055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]