Abstract
Salt resistance/sensitivity refers specifically to the effect of dietary sodium chloride (salt) intake on BP. Increased dietary salt intake promotes an early and uniform expansion of extracellular fluid volume and increased cardiac output. To compensate for these hemodynamic changes and maintain constant BP in salt resistance, renal and peripheral vascular resistance falls and is associated with an increase in production of nitric oxide. In contrast, the decline in peripheral vascular resistance and the increase in nitric oxide are impaired or absent in salt sensitivity, promoting an increase in BP in these individuals. Endothelial dysfunction may pose a particularly significant risk factor in the development of salt sensitivity and subsequent hypertension. Vulnerable salt-sensitive populations may have in common underlying endothelial dysfunction due to genetic or environmental influences. These individuals may be very sensitive to the hemodynamic stress of increased effective blood volume, setting in motion untoward molecular and biochemical events that lead to overproduction of TGF-β, oxidative stress, and limited bioavailable nitric oxide. Finally, chronic high-salt ingestion produces endothelial dysfunction, even in salt-resistant subjects. Thus, the complex syndrome of salt sensitivity may be a function of the endothelium, which is integrally involved in the vascular responses to high salt intake.
Keywords: blood pressure, nitric oxide, TGF-beta, endothelium, hypertension
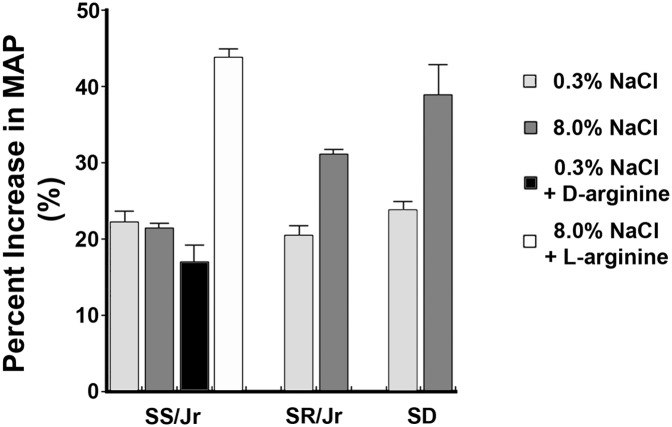
The year 2016 marks the 25th anniversary of the initial publication1 that explored the hypothesis that nitric oxide (NO) participates in the BP response to changes in dietary intake of sodium chloride (referred to as salt here). The research focused on Dahl/Rapp rats, which were derived from the Sprague–Dawley line. For more than a half-century, these interesting animals have been a source of inspiration in understanding salt-sensitive (SS) hypertension.2,3 The inbred Dahl/Rapp SS strain showed exquisite BP sensitivity to increased dietary salt intake, whereas the Dahl/Rapp salt-resistant (SR) rats were an inbred strain with BP that did not change with changes in dietary salt intake. The use of NG-monomethyl-l-arginine as an inhibitor of NO production showed that an increase in dietary salt intake increased NO in the SR strain and Sprague–Dawley rats but failed to do so in the SS rats (Figure 1). The subsequent explosion of investigations from multiple laboratories included confirmatory evidence of the role of NO in the BP response to dietary salt intake4,5 and the marked impairment in the ability of Dahl SS rats to respond to increased dietary salt with an increase in NO production.6–8
Figure 1.
High salt–induced production of NO is impaired in the Dahl/Rapp SS rat. Infusion of NG-monomethyl-l-arginine (L-NMMA) was used to determine the component of mean arterial pressure (MAP) that was dependent on NO production in young animals. Increased dietary salt intake increased the BP responses to L-NMMA in Dahl/Rapp SR and Sprague–Dawley rats but not in Dahl/Rapp SS rats. In these experiments, rats were fed for 2 weeks a diet that contained 0.3% NaCl, 8.0% NaCl, or 8.0% NaCl with either d-arginine as a control, because it cannot be used to produce NO, or l-arginine in the drinking water. SD, Sprague–Dawley. Modified from ref. 1, with permission.
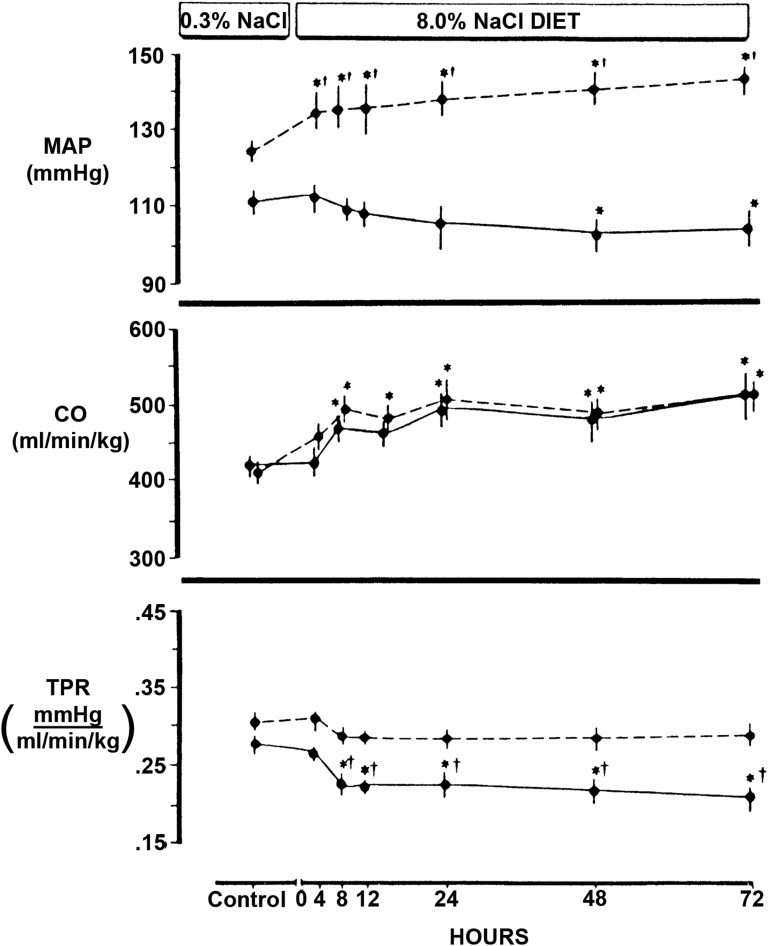
Despite these and other remarkable advances in vascular biology, the pathogenesis of SS hypertension has remained elusive, but animal and human studies have supported an underlying alteration in peripheral vascular resistance (PVR) that manifests in the setting of excess salt intake. In a series of carefully performed studies, Greene et al.9 clearly showed that increased dietary salt intake expanded blood volume and increased cardiac output (CO) in the Brookhaven strains of both Dahl SS and SR rats, but only in the SS rats did BP increase. By Ohm law, in response to increased salt intake, PVR, therefore, fell in SR but not SS rats (Figure 2). When blood volume expansion was prevented by use of a servocontrol system, BP of the SS rats did not increase over the 3-day study, which has been shown by the authors9 and us1 to be sufficient time for salt-induced increases in BP to develop in this strain. These 3-day studies also found that increases in plasma sodium concentration alone did not increase BP in this strain.9 In the servocontrolled experiments, normotensive Sprague–Dawley rats responded to increases in serum sodium concentration by lowering BP, whereas the SS rats did not, further suggesting an abnormal vascular response in SS rats.9
Figure 2.
The hemodynamic effects of dietary salt intake differed between Dahl SS (dashed lines) and SR (solid lines) rats. Despite similar increases in extracellular fluid volume and CO (middle panel), mean arterial pressure (MAP; top panel) increased only in the SS strain. TPR (bottom panel) fell within 8 hours of starting the high-salt (8.0% NaCl) diet in the SR strain but did not change in SS rats. *different than control; †differences between SS and SR. TPR, total peripheral resistance. Modified from ref. 9, with permission.
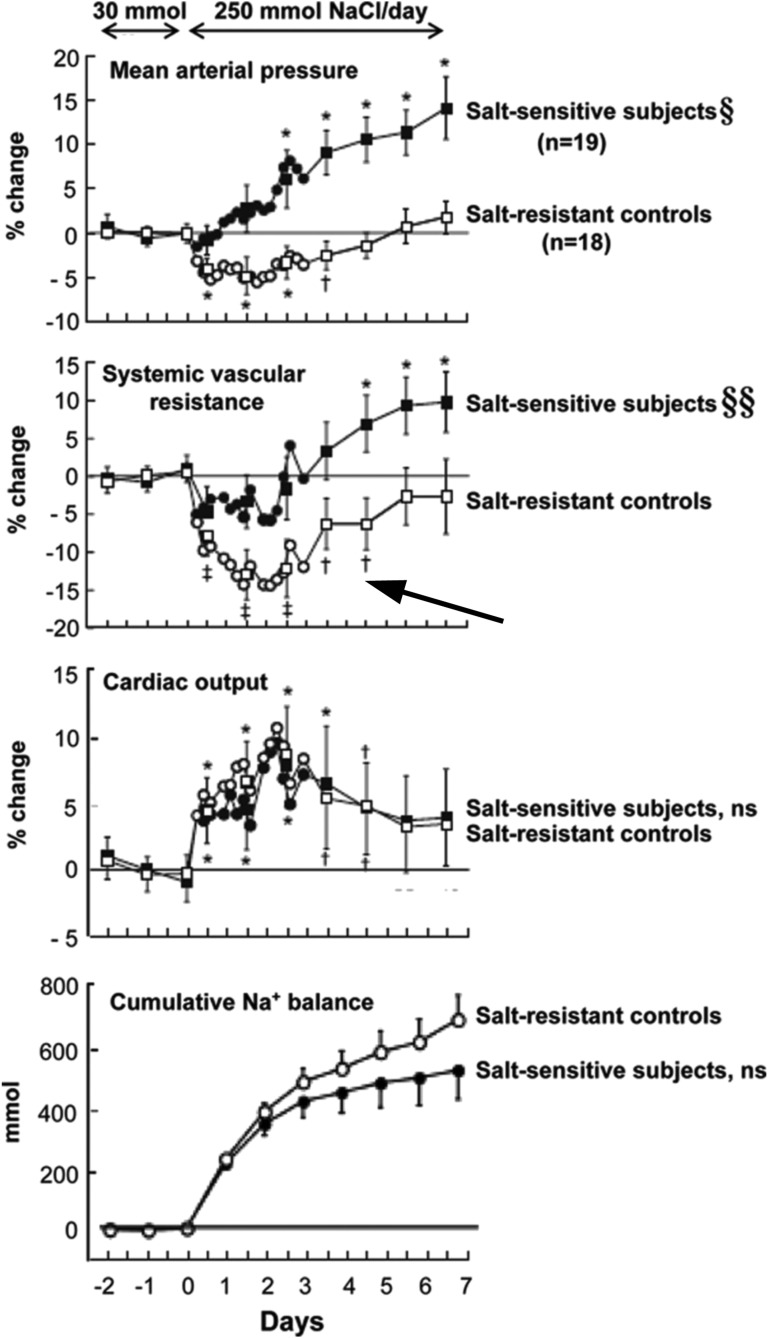
Studies with human volunteers have shown similar hemodynamic responses to dietary salt. Luft et al.10 showed that high salt intake increased CO and decreased PVR in normotensive men. Schmidlin et al.11 also observed the same findings in SR men, but in contrast, SS men volunteers did not show the anticipated decrease in PVR, and BP increased during high salt intake (270–281 mmol NaCl/d or estimated 6.2–6.5 g Na+/d) (Figure 3). In this study, the increase in CO was related to increased stroke volume and not related to heart rate; persistently high salt intake actually promoted a decline in heart rate that reduced the stroke volume–induced elevation in CO.11 A clue to the underlying etiology of the defect in vasodilation in this particular population was the demonstration that high salt intake increased asymmetric dimethylarginine (ADMA) specifically in the SS volunteers. ADMA is a naturally occurring arginine analog that has an asymmetrically substituted dimethyl group on a guanidinium nitrogen. ADMA seemed to be equipotent to NG-monomethyl-l-arginine in inhibiting NO production in vitro,12 and as anticipated, infusion of ADMA increased PVR and BP in healthy volunteers.13 Schmidlin et al.11 also showed that plasma levels of ADMA observed on day 2 of the study predicted changes in PVR and mean arterial pressure.11 Because plasma levels of symmetric dimethylarginine did not change with salt intake in this SS population, the authors further suggested that dietary salt intake altered the function of dimethylarginine dimethylaminohydrolase, the enzyme known to metabolize ADMA.13 In a separate study, Hoffmann et al.14 observed an association between a gene polymorphism of nitric oxide synthase 3 (NOS3), reduced levels of NO metabolites, and larger BP lowering with dietary sodium restriction. These clinical observations comport well with the preclinical studies of Chen and Sanders,1,6 Chen et al.,1,6,7 and Greene et al.9 and suggest an important vasodilatory role for NO and particularly, the endothelium in determining the BP responses to increased dietary salt intake.
Figure 3.
The hemodynamic effects of chronic high salt intake differed between SS and SR volunteers. Despite similar increases in CO (row 3) and cumulative sodium balance (row 4), SS but not SR patients manifested salt-induced increases in mean arterial pressure (row 1). The SR volunteers showed rapid reductions in calculated systemic vascular resistance (SVR; row 2), whereas SVR did not decline and actually increased over time in the SS patients. It is worth noting that SVR also increased with continued high salt ingestion in SR patients (arrow). *P<0.01; †P<0.05; §§P<0.001, compared with period of low salt intake. ns, not significant. Modified from ref. 71, with permission.
Dietary salt and potassium intake have profound effects on endothelial function. Multiple studies in rats have observed that macrovascular endothelial function was altered independently of the serum sodium concentration and before the development of hypertension.15–28 The cell signaling pathways activated by dietary salt are reminiscent of endothelial cell mechanoreceptor signaling, likely directly related to the expansion of blood volume during high salt intake. Early studies also focused on TGF-β, in part because Ohno et al.29 initially showed that induction of fluid shear stress opened a potassium channel on endothelial cells in culture and thereby, provoked the production of TGF-β. Indeed, high salt intake increased the vascular and renal production of active TGF-β in vivo through a complex signaling mechanism that was initiated by opening of a potassium channel.15–17,20,22–26 Yu et al.30 confirmed the effect of high salt intake on TGF-β production and further showed the associated functional consequences of kidney and cardiac fibrosis in normotensive as well as hypertensive rats. TGF-β also figures prominently in the pathogenesis of end organ damage in the Dahl SS rat.31 For Dahl SS rats, salt-induced increases in CO along with significant increases in both systolic and diastolic pressures should have promoted even higher shear forces on the endothelium. The selective failure of the endothelium to respond with a concomitant increase in NO production, therefore, was a unique feature of these animals.21
These studies identified a paradigm that featured not only an intrinsic role of the endothelium in the vascular regulation of TGF-β but also, an autacoid function for TGF-β in endothelial function during changes in dietary salt and potassium intake.15,21,22,24,26 Active TGF-β produced in the setting of high salt intake increased endothelial NOS (NOS3).15,17,21,22,24,26 A direct relationship existed between active TGF-β1 and NO metabolites in both SS and SR rats, but key to the underlying pathophysiology was the finding that production of TGF-β1 relative to NO was increased in prehypertensive SS rats and further exaggerated with the increase in dietary salt intake21 (Figure 4). These findings were further clarified by the identification of response elements for TGF-β in the promoter region of NOS3 and the observation that addition of active TGF-β increased NOS3 expression, which was associated with an increase in production of NO, in bovine aortic endothelial cells.32 Interestingly, NO also provided a negative feedback of TGF-β signaling potentially by accelerating the degradation of activated Smad2 in endothelium33 and activating dynamin-2 to decrease surface expression of TGF-β type 1 receptor on vascular smooth muscle cells.28 The role of potassium is interesting, because unlike sodium, the effect seemed to depend on the concentration of potassium and was not due to induction of mechanotransduction signaling.25 Increases in potassium mitigated the salt-mediated production of TGF-β in vivo24 and in vitro.25 An integral role for this pleiotropic growth factor in endothelial function and BP regulation has been observed in mice lacking essential components that regulate this pathway. Increased activity of TGF-β accounted for the development of hypertension in mice lacking elastin microfibril interfacer 1, which regulates TGF-β in the vasculature.34 Mice haploinsufficient for endoglin, which is constitutively expressed on endothelial cells and facilitates TGF-β–mediated signaling through ALK5, showed marked impairment in NOS3 function.35,36 Transgenic mice that overexpressed S-endoglin, an alternative splice form of endoglin that preferentially promoted endothelial TGF-β/ALK5 signaling, were hypertensive.37 The combined findings suggested that a dynamic balance between NO and TGF-β serves to regulate the BP, especially during increased salt intake, and that perturbation of this interaction may result in hypertension.
Figure 4.
Production of active TGF-β1 and NO metabolites (NOx) by aortic ring preparations from both Dahl/Rapp SS (black squares) and Dahl/Rapp SR (white circles) rats were tightly coordinated. At every level of NOx production, however, the relative amount of vascular TGF-β1 production was increased in SS rats compared with SR rats. Modified from ref. 21, with permission.
Endothelial dysfunction (ED) has been shown to be predictive of subsequent cardiovascular events and death.38–40 Recent studies clearly showed that continuous administration of a high salt (300–350 mmol Na+/d or 6.9–8.0 g Na+/d) diet for 1 week impaired forearm endothelium-dependent vasodilation41 and cutaneous microvascular function42 independent of BP and serum sodium concentration. High salt intake has been shown to generate oxidative stress, reducing bioavailable NO and endothelium-dependent dilation in humans,42 mice,43 and rats.44,45 In normotensive Sprague–Dawley rats, a diet high in salt content stimulated an ALK5-dependent increase in endothelial NADPH oxidase-445 and a generation of H2O2 (P.W. Sanders, unpublished observations), which is the principal product of this enzyme.46 These data were consistent with those in the work by Thannickal et al.,47 which showed that TGF-β1 increased production of H2O2 and reduced cellular glutathione stores in endothelial cells in culture. Boulden et al.48 also showed that H2O2 promoted ED by decreasing tetrahydrobiopterin and bioavailable NO. Cowley et al.49 recently showed that Dahl SS rats lacking NADPH oxidase-4 exhibited reductions in dietary salt–induced hypertension and associated kidney injury, indicating an important effect of this enzyme on BP regulation in these animals. Although TGF-β facilitated NOS3 expression and NO production during high salt intake, other effects, which included TGF-β–dependent NADPH oxidase-4 expression, can mitigate this effect chronically and ultimately produced ED and subsequent increases in BP in Sprague–Dawley rats, which are typically resistant to the effects of salt on BP. These effects were mediated through TGF-β–dependent ALK5 signaling.45 Meneely and Ball50 showed that continued high salt intake promoted hypertension, arteriolosclerosis, and a dose-dependent reduction in lifespan in Sprague–Dawley rats. Finally, recent work reviewed elsewhere51 has suggested that excess salt intake induced changes in immune function sufficient to exaggerate sympathetic activity, T lymphocyte responses, and dendritic cell–mediated lymphangiogenesis and extravascular storage of sodium in the interstitium. These effects may also alter endothelial function through direct or indirect interactions, such as reduction in effective blood volume through the expansion of vascular capacity,52 elaboration of cytokines/chemokines, or perhaps, a direct effect of sodium storage in promoting vascular dysfunction.53
In a recent review, Hall54 considered the important role of the kidney in salt-induced hypertension. All of the currently identified monogenic forms of human hypertension directly involved renal sodium handling, although genome-wide association studies have found other potentially relevant genetic variants not obviously involved in kidney function55 as well as NOS3.56 In addition to regulation of PVR, two excellent reviews have discussed the effects of NO on renal epithelial cell regulation of salt and water balance57 and renal hemodynamics58 and the relationship with arterial pressure. Roman59 showed a rightward shift in the pressure-natriuresis curve in prehypertensive Dahl SS rats; this defect in pressure-natriuresis was repaired by chronic administration of l-arginine.60 For Dahl SS rats, therefore, the defect in arterial vasodilation blunted the expected normal salt-induced vasodilatory responses in both extrarenal and renal vasculature. During high salt intake, patients who had SS hypertension also showed impaired renal vasodilation with reduction in renal blood flow rates compared with SR volunteers.61,62 Accordingly, genetic or acquired defects in the extrarenal and renal vasodilatory responses to high salt intake as well as renal epithelial sodium handling may promote SS hypertension through involvement of not only the peripheral vasculature but also, the kidney.
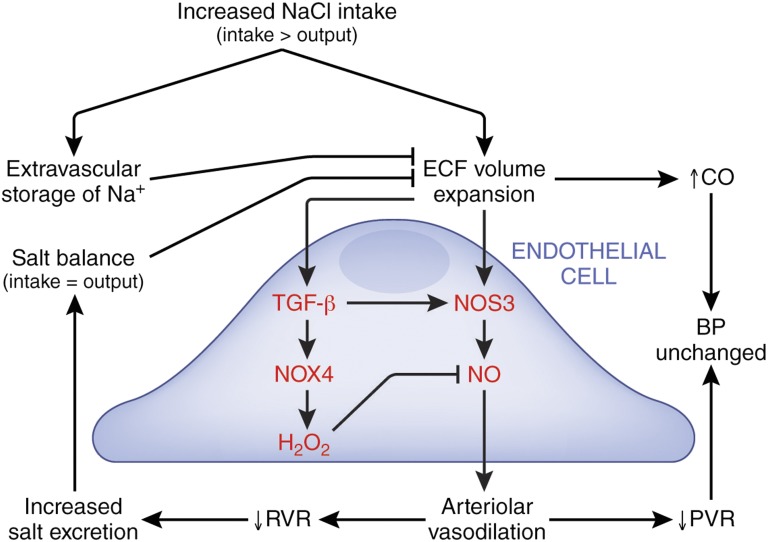
The conclusion that BP is determined by two factors—CO and PVR—is important, but the underlying biology that dictates the hemodynamic responses to high salt intake is complex and may include an increase in adrenergic drive, such that an increase in heart rate drives the augmentation in CO rather than purely an increase in heart stroke volume (Figure 5). Both preclinical and clinical data supported the hypothesis that altered responses of the vasculature to a high-salt diet seemed necessary to generate SS hypertension. The demonstration of direct effects of dietary salt on endothelium of normotensive rats provided one explanation for acquired ED and a subsequent loss of vasodilation, which was also observed in both SS and SR volunteers when a higher salt intake (estimated 6.2–6.5 g Na+/d) was maintained for 7 days (Figure 3).11 For rodents, the local production of TGF-β in response to increased salt intake plays a critical role in this process.45
Figure 5.
Endothelial responses to high salt intake affect arteriolar vasodilation and BP. Endothelium-specific responses are shown in red. Increased salt intake expands extracellular fluid (ECF) volume and increases CO. In response, normally functioning endothelium increases NO production to promote arteriolar vasodilation, which reduces PVR, and the net effect is unchanged BP. A decrease in renal vascular resistance (RVR) facilitates natriuresis and subsequent return to salt balance. The increase in NO production is facilitated by production and activation of TGF-β. However, TGF-β–dependent ALK5 signaling also increases endothelial NADPH oxidase-4 (NOX4),45 an enzyme that produces hydrogen peroxide, which limits NO bioavailability and ultimately promotes increased BP.48 As reviewed in detail by Laffer et al.,53 the recently described extravascular storage of sodium may mitigate the hemodynamic effects of higher salt intake by increasing effective vascular capacity and limiting expansion of ECF volume. This paradigm partially shows the complexity of the pathophysiology of high salt intake and potentially how disruption of any of the pathways may result in salt sensitivity.
The heritability of the BP response to high salt intake has been estimated to be 0.49–0.51,63 and therefore, the remaining one half is related to environment or other factors. For example, aging, obesity, diabetes mellitus, and CKD generally are acquired risk factors that associate with salt sensitivity,51,64–66 but also, they are known to promote ED.64,67,68 The risk of chronic high salt intake may be particularly important in these vulnerable SS populations, where there is little reserve in normal homeostatic balance of NO production. Thus, the hemodynamic stress of increased effective blood volume could set in motion untoward endothelial molecular/biochemical events that reduce the ability of the vasculature to vasodilate in response to higher salt intake (Figure 5). Regardless, even in SR volunteers, continuous high salt intake (300–350 mmol Na+/d or 6.9–8.0 g Na+/d) for 1 week was sufficient to produce ED.41,42 These findings correlated with data showing that, compared with a reference group that had an estimated sodium excretion rate of 4–5.99 g Na+/d, the group excreting ≥7 g Na+/d had an increased risk of composite outcome of death and major cardiovascular events.69 If participants with cardiovascular events in the first 2 years of the study were excluded, the association of higher sodium excretion rates (6–6.99 g Na+/d) with the composite outcome was also significant. Participants with hypertension at baseline were particularly sensitive to the cardiovascular effects of higher salt intake.69 Another important environmental factor was dietary potassium content, which seemed to modify the effects of salt on BP.70 O’Donnell et al.69 postulated that increased potassium intake reduced the risk of death and cardiovascular disease through effects on BP or perhaps, is simply a marker of healthy dietary patterns that are rich in potassium (e.g., high consumption of fruit and vegetables). Alternatively, our studies have uncovered a novel mitigating influence of potassium on dietary salt–induced effects on endothelial function.24,25 The data showed that salt sensitivity is not a single disease but an entity that is superimposed on a constellation of disorders.3 We posit that a central feature may be the delicate balance of endothelial homeostatic function in response to extracellular fluid volume expansion (Figure 5). Multiple inherited and acquired disorders of the endothelium or perhaps, chronic ingestion of a high-salt diet alone or in combination with low potassium intake disrupt the endothelial responses to high salt intake and promote the complex syndrome of salt sensitivity. Certainly, more work is needed to understand the underlying pathobiology of acute and chronic effects of high salt itself on the vasculature in the context of SS hypertension, and also, we must raise the question, especially in the free salt Western diet, of the possibility of inducing ED in the otherwise non-SS population.
Disclosures
None.
Acknowledgments
Office of Research and Development, Medical Research Service, Department of Veterans Affairs grant 1 IP1 BX001595, National Institutes of Health George M. O’Brien Kidney and Urological Research Centers Program grant P30 DK079337, American Heart Association grant 15SDG25760063, University of Alabama at Birmingham School of Medicine AMC21 Multi‐PI Grant, and Anderson Innovation awards supported the authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Chen PY, Sanders PW: L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest 88: 1559–1567, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zicha J, Dobešová Z, Vokurková M, Rauchová H, Hojná S, Kadlecová M, Behuliak M, Vaněčková I, Kuneš J: Age-dependent salt hypertension in Dahl rats: Fifty years of research. Physiol Res 61[Suppl 1]: S35–S87, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; Stroke Council : Salt sensitivity of blood pressure: A scientific statement from the American Heart Association. Hypertension 68: e7–e46, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Shultz PJ, Tolins JP: Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest 91: 642–650, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolins JP, Shultz PJ: Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int 46: 230–236, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Chen PY, Sanders PW: Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension 22: 812–818, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Chen PY, St. John PL, Kirk KA, Abrahamson DR, Sanders PW: Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary L-arginine supplementation. Lab Invest 68: 174–184, 1993 [PubMed] [Google Scholar]
- 8.Barton M, Vos I, Shaw S, Boer P, D’Uscio LV, Gröne HJ, Rabelink TJ, Lattmann T, Moreau P, Lüscher TF: Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: Mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and Glomerulosclerosis. J Am Soc Nephrol 11: 835–845, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Greene AS, Yu ZY, Roman RJ, Cowley AW Jr.: Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol 258: H508–H514, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Luft FC, Rankin LI, Bloch R, Weyman AE, Willis LR, Murray RH, Grim CE, Weinberger MH: Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation 60: 697–706, 1979 [DOI] [PubMed] [Google Scholar]
- 11.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC Jr.: Salt sensitivity in blacks: Evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension 58: 380–385, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Vallance P, Leone A, Calver A, Collier J, Moncada S: Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol 20[Suppl 12]: S60–S62, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P: Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23: 1455–1459, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann IS, Tavares-Mordwinkin R, Castejon AM, Alfieri AB, Cubeddu LX: Endothelial nitric oxide synthase polymorphism, nitric oxide production, salt sensitivity and cardiovascular risk factors in Hispanics. J Hum Hypertens 19: 233–240, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Ying WZ, Sanders PW: Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-beta1. Am J Physiol 275: F18–F24, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Ying WZ, Sanders PW: Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol 274: F635–F641, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Ying WZ, Sanders PW: Dietary salt increases endothelial nitric oxide synthase and TGF-beta1 in rat aortic endothelium. Am J Physiol 277: H1293–H1298, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Sanders PW, Gibbs CL, Akhi KM, MacMillan-Crow LA, Zinn KR, Chen YF, Young CJ, Thompson JA: Increased dietary salt accelerates chronic allograft nephropathy in rats. Kidney Int 59: 1149–1157, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Ying WZ, Sanders PW: Dietary salt intake activates MAP kinases in the rat kidney. FASEB J 16: 1683–1684, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Ying W-Z, Sanders PW: Increased dietary salt activates rat aortic endothelium. Hypertension 39: 239–244, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ying WZ, Sanders PW: The interrelationship between TGF-beta1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol 285: F902–F908, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ying WZ, Aaron K, Sanders PW: Mechanism of dietary salt-mediated increase in intravascular production of TGF-beta1. Am J Physiol Renal Physiol 295: F406–F414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying WZ, Aaron K, Sanders PW: Dietary salt activates an endothelial proline-rich tyrosine kinase 2/c-Src/phosphatidylinositol 3-kinase complex to promote endothelial nitric oxide synthase phosphorylation. Hypertension 52: 1134–1141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying WZ, Aaron K, Wang PX, Sanders PW: Potassium inhibits dietary salt-induced transforming growth factor-beta production. Hypertension 54: 1159–1163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying WZ, Aaron KJ, Sanders PW: Effect of aging and dietary salt and potassium intake on endothelial PTEN (Phosphatase and tensin homolog on chromosome 10) function. PLoS One 7: e48715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying WZ, Aaron KJ, Sanders PW: Transforming growth factor-β regulates endothelial function during high salt intake in rats. Hypertension 62: 951–956, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying WZ, Aaron KJ, Sanders PW: Sodium and potassium regulate endothelial phospholipase C-γ and Bmx. Am J Physiol Renal Physiol 307: F58–F63, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovater MB, Ying WZ, Agarwal A, Sanders PW: Nitric oxide and carbon monoxide antagonize TGF-β through ligand-independent internalization of TβR1/ALK5. Am J Physiol Renal Physiol 307: F727–F735, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno M, Cooke JP, Dzau VJ, Gibbons GH: Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest 95: 1363–1369, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu HCM, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI: Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 98: 2621–2628, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Murphy SR, Dahly-Vernon AJ, Dunn KM, Chen CC, Ledbetter SR, Williams JM, Roman RJ: Renoprotective effects of anti-TGF-β antibody and antihypertensive therapies in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 303: R57–R69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue N, Venema RC, Sayegh HS, Ohara Y, Murphy TJ, Harrison DG: Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-β 1. Arterioscler Thromb Vasc Biol 15: 1255–1261, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Saura M, Zaragoza C, Herranz B, Griera M, Diez-Marqués L, Rodriguez-Puyol D, Rodriguez-Puyol M: Nitric oxide regulates transforming growth factor-beta signaling in endothelial cells. Circ Res 97: 1115–1123, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM: Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 124: 929–942, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Santibanez JF, Letamendia A, Perez-Barriocanal F, Silvestri C, Saura M, Vary CP, Lopez-Novoa JM, Attisano L, Bernabeu C: Endoglin increases eNOS expression by modulating Smad2 protein levels and Smad2-dependent TGF-beta signaling. J Cell Physiol 210: 456–468, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Jerkic M, Rivas-Elena JV, Prieto M, Carrón R, Sanz-Rodríguez F, Pérez-Barriocanal F, Rodríguez-Barbero A, Bernabéu C, López-Novoa JM: Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J 18: 609–611, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Blanco FJ, Grande MT, Langa C, Oujo B, Velasco S, Rodriguez-Barbero A, Perez-Gomez E, Quintanilla M, López-Novoa JM, Bernabeu C: S-endoglin expression is induced in senescent endothelial cells and contributes to vascular pathology. Circ Res 103: 1383–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Muiesan ML, Salvetti M, Paini A, Monteduro C, Galbassini G, Poisa P, Porteri E, Agabiti-Rosei C, Paderno V, Belotti E, Rizzoni D, Castellano M, Agabiti-Rosei E: Prognostic role of flow-mediated dilatation of the brachial artery in hypertensive patients. J Hypertens 26: 1612–1618, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM: Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Bruno RM, Bianchini E, Faita F, Taddei S, Ghiadoni L: Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovasc Ultrasound 12: 34, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG: High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB: Dietary sodium loading impairs microvascular function independent of blood pressure in humans: Role of oxidative stress. J Physiol 590: 5519–5528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nurkiewicz TR, Boegehold MA: High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Raffai G, Durand MJ, Lombard JH: Acute and chronic angiotensin-(1-7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am J Physiol Heart Circ Physiol 301: H1341–H1352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng W, Ying WZ, Aaron KJ, Sanders PW: Transforming growth factor-β mediates endothelial dysfunction in rats during high salt intake. Am J Physiol Renal Physiol 309: F1018–F1025, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD: Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry 53: 5111–5120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thannickal VJ, Hassoun PM, White AC, Fanburg BL: Enhanced rate of H2O2 release from bovine pulmonary artery endothelial cells induced by TGF-beta 1. Am J Physiol 265: L622–L626, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC Jr.: Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med 41: 810–817, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cowley AW Jr., Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A: Evidence of the importance of Nox4 in production of hypertension in Dahl salt-sensitive rats. Hypertension 67: 440–450, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meneely GR, Ball CO: Experimental epidemiology of chronic sodium chloride toxicity and the protective effect of potassium chloride. Am J Med 25: 713–725, 1958 [DOI] [PubMed] [Google Scholar]
- 51.Oh YS, Appel LJ, Galis ZS, Hafler DA, He J, Hernandez AL, Joe B, Karumanchi SA, Maric-Bilkan C, Mattson D, Mehta NN, Randolph G, Ryan M, Sandberg K, Titze J, Tolunay E, Toney GM, Harrison DG: National Heart, Lung, and Blood Institute Working Group Report on salt in human health and sickness: Building on the current scientific evidence. Hypertension 68: 281–288, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J: Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15: 545–552, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Laffer CL, Scott RC 3rd, Titze JM, Luft FC, Elijovich F: Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension 68: 195–203, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall JE: Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation 133: 894–906, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padmanabhan S, Caulfield M, Dominiczak AF: Genetic and molecular aspects of hypertension. Circ Res 116: 937–959, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, Arima H, Hoggart C, Tichet J, Nikitin YP, Conti C, Seidlerova J, Tikhonoff V, Stolarz-Skrzypek K, Johnson T, Devos N, Zagato L, Guarrera S, Zaninello R, Calabria A, Stancanelli B, Troffa C, Thijs L, Rizzi F, Simonova G, Lupoli S, Argiolas G, Braga D, D’Alessio MC, Ortu MF, Ricceri F, Mercurio M, Descombes P, Marconi M, Chalmers J, Harrap S, Filipovsky J, Bochud M, Iacoviello L, Ellis J, Stanton AV, Laan M, Padmanabhan S, Dominiczak AF, Samani NJ, Melander O, Jeunemaitre X, Manunta P, Shabo A, Vineis P, Cappuccio FP, Caulfield MJ, Matullo G, Rivolta C, Munroe PB, Barlassina C, Staessen JA, Beckmann JS, Cusi D: Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension 59: 248–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou AP, Cowley AW Jr.: Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep 1: 178–186, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Carlström M, Wilcox CS, Arendshorst WJ: Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roman RJ: Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol 251: F57–F65, 1986 [DOI] [PubMed] [Google Scholar]
- 60.Patel A, Layne S, Watts D, Kirchner KA: L-arginine administration normalizes pressure natriuresis in hypertensive Dahl rats. Hypertension 22: 863–869, 1993 [DOI] [PubMed] [Google Scholar]
- 61.Campese VM, Parise M, Karubian F, Bigazzi R: Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 18: 805–812, 1991 [DOI] [PubMed] [Google Scholar]
- 62.Schmidlin O, Forman A, Tanaka M, Sebastian A, Morris RC Jr.: NaCl-induced renal vasoconstriction in salt-sensitive African Americans: Antipressor and hemodynamic effects of potassium bicarbonate. Hypertension 33: 633–639, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Gu D, Rice T, Wang S, Yang W, Gu C, Chen CS, Hixson JE, Jaquish CE, Yao ZJ, Liu DP, Rao DC, He J: Heritability of blood pressure responses to dietary sodium and potassium intake in a Chinese population. Hypertension 50: 116–122, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savica V, Bellinghieri G, Kopple JD: The effect of nutrition on blood pressure. Annu Rev Nutr 30: 365–401, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann IS, Alfieri AB, Cubeddu LX: Effects of lifestyle changes and metformin on salt sensitivity and nitric oxide metabolism in obese salt-sensitive Hispanics. J Hum Hypertens 21: 571–578, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Richardson SI, Freedman BI, Ellison DH, Rodriguez CJ: Salt sensitivity: A review with a focus on non-Hispanic blacks and Hispanics. J Am Soc Hypertens 7: 170–179, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanhoutte PM, Zhao Y, Xu A, Leung SW: Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res 119: 375–396, 2016 [DOI] [PubMed] [Google Scholar]
- 68.Martens CR, Edwards DG: Peripheral vascular dysfunction in chronic kidney disease. Cardiol Res Pract 2011: 267257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S; PURE Investigators : Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 371: 612–623, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S; PURE Investigators : Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371: 601–611, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Morris RC Jr, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW: Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation 133: 881–893, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]