Abstract
Fabry disease leads to renal, cardiac, and cerebrovascular manifestations. Phenotypic differences between classically and nonclassically affected patients are evident, but there are few data on the natural course of classical and nonclassical disease in men and women. To describe the natural course of Fabry disease stratified by sex and phenotype, we retrospectively assessed event-free survival from birth to the first clinical visit (before enzyme replacement therapy) in 499 adult patients (mean age 43 years old; 41% men; 57% with the classical phenotype) from three international centers of excellence. We classified patients by phenotype on the basis of characteristic symptoms and enzyme activity. Men and women with classical Fabry disease had higher event rate than did those with nonclassical disease (hazard ratio for men, 5.63, 95% confidence interval, 3.17 to 10.00; P<0.001; hazard ratio for women, 2.88, 95% confidence interval, 1.54 to 5.40; P<0.001). Furthermore, men with classical Fabry disease had lower eGFR, higher left ventricular mass, and higher plasma globotriaosylsphingosine concentrations than men with nonclassical Fabry disease or women with either phenotype (P<0.001). In conclusion, before treatment with enzyme replacement therapy, men with classical Fabry disease had a history of more events than men with nonclassical disease or women with either phenotype; women with classical Fabry disease were more likely to develop complications than women with nonclassical disease. These data may support the development of new guidelines for the monitoring and treatment of Fabry disease and studies on the effects of intervention in subgroups of patients.
Keywords: Fabry-s disease, alpha galactosidase A, natural history, natural disease course, phenotype
Fabry disease (FD; OMIM 301500) is a lysosomal storage disorder caused by deficiency of the enzyme α-galactosidase A (enzyme commission no. 3.2.1.22) due to mutations in the GLA gene located on the X chromosome (Xq22.1). Deficiency of α-galactosidase A leads to accumulation of glycosphingolipids, particularly globotriaosylceramide, in various cell types throughout the body.1,2 This accumulation of globotriaosylceramide can result in multisystem disease, mainly affecting the kidneys, heart, and nervous system.
The disease can be divided into a severe, classical phenotype, most often seen in men without residual enzyme activity, and a generally milder nonclassical phenotype. Patients with classical FD usually present with characteristic FD symptoms, such as neuropathic pain, cornea verticillata, and angiokeratoma. Long–term disease manifestations include hypertrophic cardiomyopathy, cardiac rhythm disturbances, progressive renal failure, and stroke.3 Nonclassical FD, also referred to as late-onset or atypical FD, is characterized by a more variable disease course, in which patients are generally less severely affected and disease manifestations may be limited to a single organ. Men with nonclassical disease typically have residual enzyme activity and lower levels of the deacetylated substrate (globotriaosylsphingosine [lysoGb3]).4 Patients with FD identified in screening studies of individuals with stroke, renal failure, or cardiomyopathy often have this more restricted phenotype.5 Despite the X–linked inheritance pattern, women often have signs and symptoms of FD, although they are, in general, less severely affected compared with men.6 It is hypothesized that skewed X inactivation underlies the variability of the phenotype in women.7–9
Treatment of FD consists of enzyme replacement therapy (ERT) and adjunctive treatment, including ACE inhibitors or angiotensin receptor blockers, antiplatelet drugs, and analgesics. Studies have shown that ERT can delay but not always prevent some of the clinical complications of the disease.10,11
The interpretation of long-term studies of ERT is hampered by insufficient information on the phenotypes of enrolled patients and the absence of a control group or reliable data on the natural course of the disease without ERT. Some studies conducted before the use of ERT showed that men with FD have a reduced life expectancy and an increased risk of developing complications, such as ESRD.12,13 These cohorts most likely consisted mainly of patients with a classical FD phenotype, because the wide spectrum of the disease was not fully recognized at that time. It is inappropriate, however, to study treatment effect in a combined classical and nonclassical FD cohort and subsequently, compare the results with natural course data in patients with classical FD. The limited availability of data on the disease course of untreated FD in patients prompted us to study the natural history of untreated FD in men and women with classical and nonclassical disease, which can be used for interpretation of studies on effectiveness of ERT as well as counseling of patients and their family members. Here, we present the results of a retrospective study in a large cohort of patients with FD recruited from three international centers of excellence in Germany, the United Kingdom, and The Netherlands.
Results
Patients
The merged database contained data on 596 patients, of whom 13 were excluded because of insufficient data. Another 42 patients were excluded, because they harbored a neutral GLA variant (i.e., did not have FD). The remaining 541 patients were included in the analyses. Table 1 shows the characteristics of the adult patients at first visit (n=499). Pediatric patients (n=42) are discussed separately.
Table 1.
Characteristics at first visit of patients ≥18 years old
| Characteristics | Men | Women | ||
|---|---|---|---|---|
| Classical | Nonclassical | Classical | Nonclassical | |
| Patients | 138 (27.7%) | 66 (13.2%) | 147 (29.5%) | 148 (29.7%) |
| Age at first visit | 38.4 (10.8, 19–65) | 55.4 (13.1, 19–76) | 41.5 (13.5, 18–75) | 43.7 (15.0, 18–79) |
| Any event before first visit | 42 (30.4%) | 27 (40.9%) | 28 (19.0%) | 16 (10.8%) |
| Cardiac event before first visit | 16 (11.6%) | 19 (28.8%) | 11 (7.5%) | 9 (6.1%) |
| Arrhythmia-related event | 11 (8.0%) | 18 (27.3%) | 9 (6.1%) | 5 (3.4%) |
| Ischemia-related event | 5 (3.6%) | 1 (1.5%) | 2 (1.4%) | 4 (2.7%) |
| Renal event before first visit | 12 (8.7%) | 2 (3%) | 2 (1.4%) | 1 (0.7%) |
| Renal transplant | 0 | 0 | 1 (0.7%) | 1 (0.7%) |
| RRT | 9 (6.5%) | 1 (1.5%) | 0 | 0 |
| eGFR<15 ml/min per 1.73 m2 | 3 (2.2%) | 1 (1.5%) | 1 (0.7%) | 0 |
| Cerebral events before first visit | 15 (10.9%) | 6 (7%) | 16 (10.9%) | 6 (4.1%) |
| TIA | 10 (7.2%) | 3 (3.5%) | 9 (6.1%) | 5 (3.4%) |
| Stroke, symptoms >24 h | 5 (3.6%) | 3 (3.5%) | 7 (4.8%) | 1 (0.7%) |
| eGFR, ml/min per 1.73 m2 | 101 (7–139) | 83 (10–136) | 105 (10–145) | 95 (10–131) |
| eGFR<60 ml/min per 1.73 m2 | 38/131 (29.0%) | 18/65 (27.7%) | 8/142 (5.6%) | 14/147 (9.5%) |
| CKD stage A3 | 39/131 (33.3%) | 13/65 (23.6%) | 20/142 (14.9%) | 147/116 (13.8%) |
| LVM, g/m2.7 | 42 (21–140) | 56 (16–99) | 42 (21–140) | 36 (16–108) |
| RWT | 0.41 (0.24–1.45) | 0.47 (0.24–0.95) | 0.48 (0.21–0.93) | 0.42 (0.24–1.45) |
| Left ventricular hypertrophy | 66/105 (62.9%) | 33/49 (67.3%) | 59/132 (44.7%) | 40/128 (31.2%) |
| Concentric hypertrophy | 57/105 (54.3%) | 28/49 (57.1%) | 47/132 (35.6) | 32/128 (25.0%) |
| Concentric remodeling | 17/105 (16.2%) | 6/49 (12.2%) | 18/132 (13.6%) | 17/128 (13.3%) |
| LysoGb3, nmol/La | 111 (32–175) | 7.8 (1.2–47) | 9.3 (0.7–42) | 2.5 (0.4–20) |
Data are shown as mean (SD, range) or median (range) of continuous variables and number of patients (percentage) for discrete variables. Events represent the number of patients with one or more events before first visit. Arrhythmia-related event included atrial fibrillation, implantation of an implantable cardiac defibrillator or pacemaker, or admission for any rhythm disturbance. Ischemia-related event included myocardial infarction, coronary artery bypass graft surgery, or percutaneous transluminal angioplasty intervention. Left ventricular hypertrophy was defined as follows: LVM: men, ≥49 and women, ≥45. Concentric remodeling was defined as the absence of left ventricular hypertrophy and RWT>0.42. Concentric hypertrophy was defined as left ventricular hypertrophy and RWT>0.42. Upper reference limit lysoGb3 =0.6. TIA, transient ischemic attack.
LysoGb3 was not available for the N215S patient classified as having classical FD.
Clinical Events
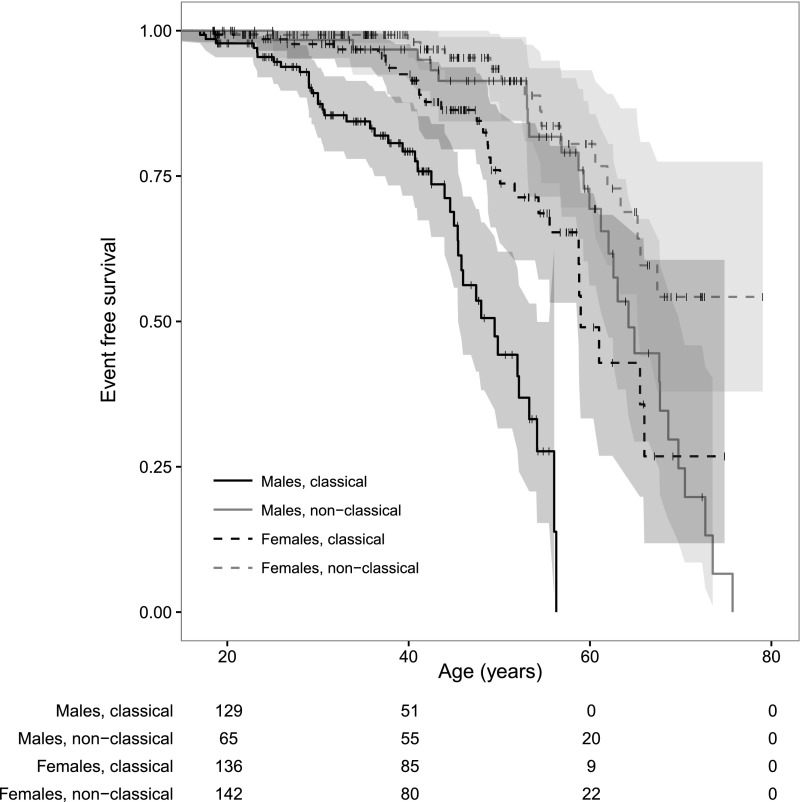
Table 1 shows the differences per group of the occurrence of cardiac, renal, and cerebral events. One hundred thirteen adult patients (22.5%) had experienced one or more events before their first visit to the referral center. Men with classical FD had the highest event rate, with a median event–free survival of 49.5 years (Figure 1 and Supplemental Figures A1–A3). The event rates of classically affected women and men with nonclassical FD showed overlap. Hazard ratios (HRs) per sex and phenotype are depicted in Table 2.
Figure 1.
Event-free survival (any event) stratified for sex and phenotype. Shaded areas represent the 95% CIs; + indicates censoring (i.e., first visit).
Table 2.
HRs of events
| Subgroups | Any Event | Cardiac Events | Cerebral Events | Renal Events |
|---|---|---|---|---|
| Men, classical versus nonclassical phenotype | 5.63 (3.17 to 10.00)a | 5.16 (2.22 to 12.04)a | 4.71 (1.74 to 12.76)b | 9.24 (1.73 to 49.45)b |
| Women, classical versus nonclassical phenotype | 2.88 (1.54 to 5.40)a | 2.34 (0.95 to 5.76) | 3.51 (1.36 to 9.06)b | 2.27 (0.21 to 25.13) |
| Classical phenotype, men versus women | 3.87 (2.32 to 6.55)a | 4.98 (2.13 to 11.62)a | 1.65 (0.81 to 3.39) | 9.07 (1.98 to 42.56)c |
| Nonclassical phenotype, men versus women | 1.98 (1.07 to 3.69)c | 2.26 (1.02 to 5.02)c | 1.23 (0.40 to 3.82) | 1.24 (0.20 to 25.72) |
HRs (95% CIs).
P<0.001.
P<0.01.
P<0.05.
Renal Function
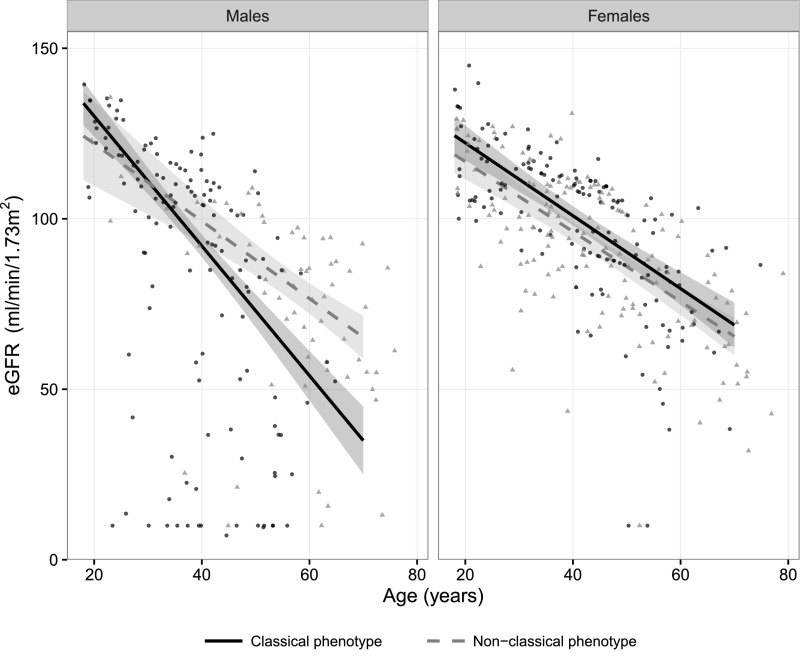
Data on eGFR were available for 485 (97%) of the adult patients. There was an association between the eGFR at first visit and age in men with classical FD (β=−1.90; 95% confidence interval [95% CI], −2.2 to −1.6; P<0.001). In the other subgroups, this relationship was also present, although less strong (βmen, nonclassical=−1.14; βwomen, classical=−1.07; βwomen, nonclassical=−1.03; all P<0.001) (Figure 2).
Figure 2.
Robust linear regression of eGFR. Shaded areas represent the 95% CIs for the fitted curves. Black dots represent patients with classical FD, and gray triangles represent patients with nonclassical FD.
Of 433 adult patients (85%), data were available on urinary excretion of protein and/or albumin. CKD stage A3 was present in 14%–33% of the patients with FD (Table 1). The odds ratio (OR) of having CKD stage A3 increased with age (OR, 1.04; 95% CI, 1.01 to 1.06; P<0.001). Men with classical FD had a higher risk of having CKD stage A3 than men with nonclassical FD (age-adjusted OR, 3.38; 95% CI, 1.6 to 8.2; P<0.01), whereas no differences were found between men with nonclassical FD and women with either classical or nonclassical FD. Inclusion of CKD stage A3 as a covariate in the previously mentioned linear regression model with eGFR as the dependent variable showed that CKD stage A3 was an important predictor of eGFR. This was most prominent in men with the classical phenotype: the eGFR in patients with CKD stage A3 was 27.1 ml/min per 1.73 m2 lower (95% CI, −37.5 to −16.7; P<0.001) at age 50 years old compared with that in patients without CKD stage A3. Details per sex and phenotype are in Supplemental Figures B1–B2.
Cardiac Involvement
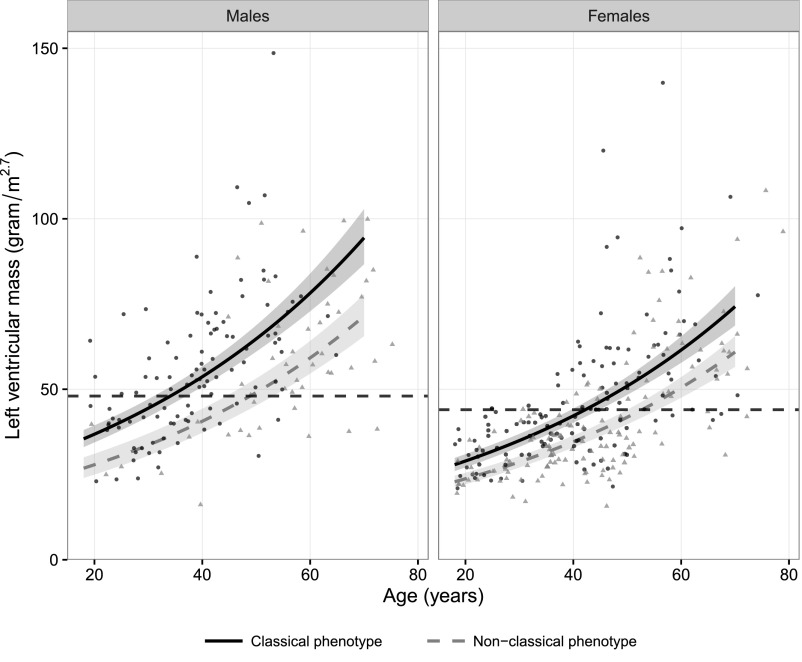
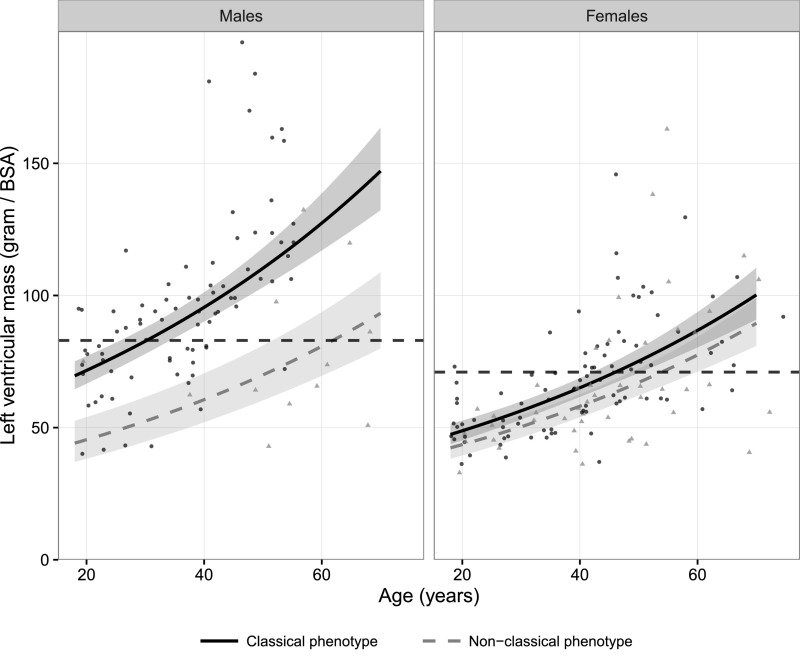
Left ventricular mass (LVM) measured by echocardiography and cardiac MRI was available in 414 (83%) and 225 (45%) adult patients, respectively. The log-transformed LVM was associated with age and phenotype (Figure 3, echocardiography, and Figure 4, cardiac MRI). The LVM measured by echocardiography in patients with classical FD was higher compared with the LVM measured by echocardiography in those with nonclassical FD (men: 32%; P<0.001; women: 22%; P<0.001). Men with classical FD had a 27% higher LVM compared with women with classical FD (P<0.001). Men with nonclassical FD and women with classical FD had comparable LVMs. The relative wall thickness (RWT) increased with age and was dependent of sex and phenotype (Supplemental Figures C1 and C2). The RWT was elevated in the majority (83%) of patients with an increased LVM (i.e., concentric hypertrophy). Concentric remodeling (RWT>0.42 but normal LVM) was predominantly observed in men with classical FD and older patients (Supplemental Figures C1 and C2).
Figure 3.
Log–linear regression curve of the LVM measured by echocardiography corrected for height (meters2.7). Shaded areas represent the 95% CIs for the fitted curves. The dashed horizontal lines represent the upper reference limits (men: 48 g/m2.7; women: 44 g/m2.7).43 Black dots represent patients with classical FD, and gray triangles represent patients with nonclassical FD.
Figure 4.
Log–linear regression curve of the LVM measured by cardiac MRI corrected for body surface area (BSA). Shaded areas represent the 95% CIs for the fitted curves. The solid black lines represent the adjusted (not including the papillary muscles) upper reference limits for men (83 g/m2), and the dashed black lines represent the upper reference limits for women (71 g/m2).49 Black dots represent patients with classical FD, and gray triangles represent patients with nonclassical FD.
Information on late gadolineum enhancement (LGE) was available for 208 (92%) of the patients who had MRI performed. LGE was present in 30.3% of the cardiac MRIs. The older the patient, the higher the risk of LGE (OR, 1.16; 95% CI, 1.11 to 1.21; P<0.001). Also, men with classical FD had an increased risk of having LGE compared with men with the nonclassical phenotype (age-adjusted OR, 7.11; 95% CI, 1.32 to 42.77; P<0.05) and women with classical FD (age-adjusted OR, 2.91; 95% CI, 1.22 to 7.29; P<0.05). The odds of having LGE did not differ between men with nonclassical FD and women with either classical or nonclassical FD.
In contrast to men, in whom left ventricular hypertrophy on cardiac MRI was a strong predictor for the presence of LGE (age-adjusted OR, 8.63; 95% CI, 2.43 to 36.01; P<0.01), the risk of having LGE was not associated with left ventricular hypertrophy in women (age-adjusted OR, 1.26; 95% CI, 0.44 to 3.53; P=0.66) (Supplemental Figures D1 and D2).
Patients with the nonclassical phenotype were under-represented in the cardiac MRI data, because no cardiac MRI data were available for the majority of patients with the N215S mutation, which most often results in nonclassical FD.
Cerebrovascular Manifestations
Information on the presence or absence of white matter lesions (WMLs) was available for 283 adult patients (57%). WMLs were present at first visit in 45% of the patients, and the odds of having WMLs on cerebral MRI at first visit increased with age (OR, 1.12; 95% CI, 1.09 to 1.15; P<0.001). Men with classical FD were more likely to have WMLs than men with nonclassical FD (age-adjusted OR, 8.72; 95% CI, 2.55 to 27.32; P<0.001), and there was a trend toward a higher risk compared with women with classical FD (OR, 2.19; 95% CI, 0.96 to 5.12; P=0.06). Women with classical FD had a higher risk of WMLs compared with women with nonclassical FD (OR, 2.11; 95% CI, 1.07 to 4.23; P<0.05). There was no difference between men and women with nonclassical FD.
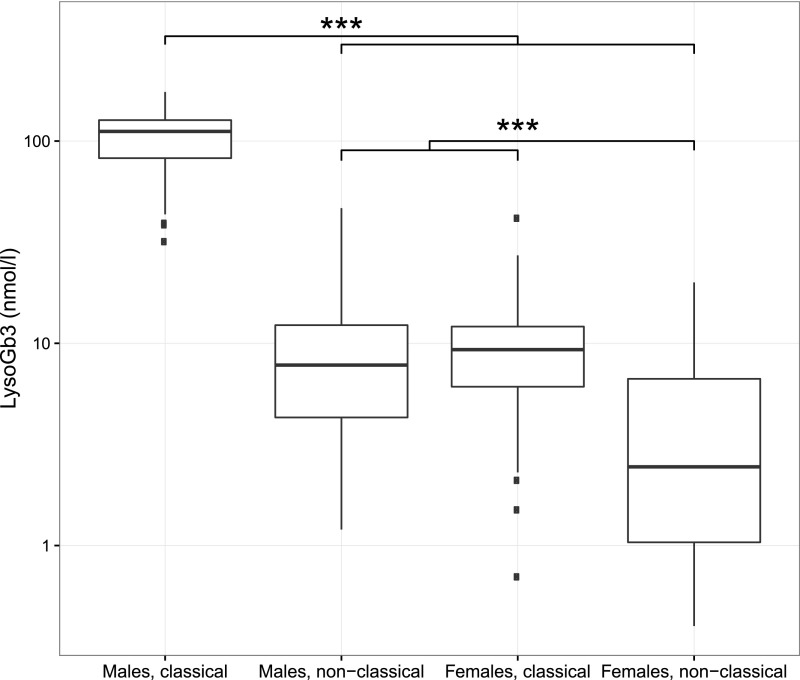
LysoGb3
Samples for analysis of plasma lysoGb3 concentration were available for 351 adult patients (70%). LysoGb3 concentrations differed between all groups (P<0.001), except for the comparison between men with nonclassical FD and women with classical FD (Figure 5). Taking all patients together, higher lysoGb3 concentrations at first visit were associated with a higher event rate in the past (HR, 1.01; 95% CI, 1.01 to 1.02; P<0.001). In the analyses of subgroups, an association was found in men with nonclassical FD (HR, 1.05; 95% CI, 1.01 to 1.10; P<0.05) and women with nonclassical FD (HR, 1.13; 95% CI, 1.03 to 1.25), whereas no relation was found in men and women with classical FD. When data from men with nonclassical FD and all women were combined, a similar relation was found (HR, 1.05; 95% CI, 1.03 to 1.08; P<0.001). In this combined group of men with nonclassical FD and all women, a ten-point increase in lysoGb3 resulted in a more rapid decrease in eGFR (additional decline of −0.34 ml/min per 1.73 m2 per year; 95% CI, −0.55 to −0.12; P<0.01).
Figure 5.
Boxplot of lysoGb3 stratified for sex and phenotype. The horizontal line inside the box from the 25th to 75th percentile depicts the median, the whiskers extend to the most extreme data point that is no more than 1.5 times the interquartile range. Outliers are indicated by squares. ***P<0.001.
In addition, a ten-point increase in lysoGb3 was associated with a 20.7% higher LVM (95% CI, 14.6 to 27.1; P<0.001) on echocardiography. LysoGb3 was not associated with eGFR or LVM in men with classical FD phenotype. Of note, the lysoGb3 concentration was not available for the N215S patient with classical FD.
Pediatric Cohort
Data on 42 pediatric patients (age <18 years old) were available (boys with classical FD: n=16; boys with nonclassical FD: n=4; girls with classical FD: n=15; girls with nonclassical FD: n=7). Median age was 16.1 years old (range =5–18). No events had occurred before the first visit in these children. Median eGFR was 123.8 ml/min per 1.73 m2 (range =92–165), and median LVM on echocardiography was 28.1 g/m2.7 (range =11–48; all within age– and sex–specific reference intervals14). The eGFR and LVM did not differ between children with the classical or nonclassical phenotype. WMLs were found in four children with classical FD (three boys and one girl) at ages 11, 15, 17, and 15 years old, respectively. The 15-year-old girl has been described before.15
Discussion
In this study, we compared the natural courses of men and women with classical and nonclassical FD. Our results confirm that the disease course of patients with the classical phenotype clearly differs from that of patients with nonclassical FD. In particular, men with classical FD have a much higher risk of developing events than both men with nonclassical FD and women. In addition, renal function is worse and LVM is higher in this group of patients. Of note, natural history in classically affected women resembles that of nonclassical disease in men, whereas nonclassically affected women have the mildest disease course. Interestingly, there was a strong relationship between the presence of cardiac hypertrophy and LGE, indicating fibrosis, in men but not women. Women often developed fibrosis in the absence of cardiac hypertrophy, which is in line with earlier findings in FD.16 The significance of an increased RWT but normal LVM in a subset of patients needs additional, preferably longitudinal studies.
Although these outcomes are not unexpected, this study shows, for the first time, the slopes of deterioration for different variables in the different phenotypic groups. Taking these findings together, it should be stressed that it is very important to stratify results of therapeutic studies by sex and phenotype. Our findings may also be used to predict disease course in patients with either classical or nonclassical FD, and as such, they may be helpful in counseling patients and their family members. However, the considerable variation in disease course, even within genotypes, should be taken into account.17
Most studies on the natural course of FD are from the pre-ERT era.12,13 Although not explicitly stated, the subjects in these studies were most likely to have a classical phenotype, because awareness and screening studies led to a marked increase in the identification of patients with nonclassical FD.5 These natural history studies showed that complications occurred at a mean age of approximately 40 years old12 and that men with FD had a reduced lifespan, with a median survival of 50–55 years.12,13 In a more recent study, the median event–free survival was 41 years.18 In our cohort, men with classical FD had their first event at a median age of 50 years old. This difference can be partly ascribed to the fact that a wider definition of clinical events was used in the previous studies, including bradyarrhythmia and an increase in creatinine of >50%.18 Another explanation could be that the retrospective design of our study has resulted in an underestimation of the event rate. We collected data from before the first visit to estimate the number of clinical events. Consequently, we may have missed or misclassified clinical events if they were not properly documented.
Also, the phenomenon of immortal (or survivor) bias, which is inherent to the study design that we used, may have led to an underestimation of the event rate19: we calculated the event rate by using retrospective data from before the first visit. Consequently, deceased patients were not included, and these patients were more likely to have severe disease and thus, a history of clinical events.
The problem of immortal bias probably plays a less prominent role in women with FD and men with nonclassical FD, because a near–normal life expectancy may be assumed in these subgroups. Interestingly, men with nonclassical FD and women with classical FD showed substantial heterogeneity: some had only very few symptoms, whereas others had severe cardiac or renal disease at first visit, which is in line with previous findings.20–22 In these patients, it is important to rule out comorbidities that could explain severe disease manifestations. However, if additional investigations have shown that organ involvement is, at least partly, due to FD, treatment with ERT may be considered.23 Women with nonclassical FD were, in general, very mildly affected, with only a minority having clinical signs and symptoms.
Although it is clear that the disease course differs between patients with classical and nonclassical FD, the definition of these phenotypes is still subject of debate. We used residual enzyme activity (men only) and the presence of characteristic FD symptoms to classify patients. It was considered inappropriate to base the classification on the type of mutation, because it is known that phenotypes may differ between patients with the same mutation and even between patients within the same family. This is assumed to be caused by disease modifiers or in the case of women, skewed X inactivation.7–9 As an alternative, plasma lysoGb3 concentrations are a valuable tool in the classification of FD.4,24 In line with earlier findings,4 lysoGb3 concentrations can, indeed, be used to differentiate between phenotypes in men with FD. Concentrations of >45 nmol/L are strongly indicative for a classical FD phenotype in men with FD. Four men who were classified as patients with classical FD had a plasma lysoGb3 concentration of <45 nmol/L. Most likely, (part of) these four patients are misclassified due to misinterpretation of the retrospectively collected clinical information. Furthermore, one man with nonclassical FD had a plasma lysoGb3 concentration of 47 nmol/L, which is just above the cutoff value of 45 nmol/L. His brother, who was also classified as nonclassical, had a lysoGb3 concentration of 40 nmol/L, which suggests that their genotype (G325S) results in relatively high levels of plasma lysoGb3. More generally, similar lysoGb3 concentrations were found in men with nonclassical FD and women with classical FD, which is consistent with the observation that the disease course in women with classical FD resembles that of nonclassical disease in men. In addition, we showed that plasma lysoGb3 concentrations were associated with the disease severity in men and women with nonclassical FD. The absence of such an association in patients with classical FD may be due to a ceiling effect; above a certain threshold concentration, higher lysoGb3 levels are not predictive for more severe disease. These results support previous findings that the concentration of lysoGb3 is a reliable predictor of the disease course.25
For the pediatric patients in our cohort, no clinical events were reported. Also, we did not observe any abnormal value for the eGFR or LVM at first visit. However, four children with classical FD presented with one or more WMLs. The presence of early cerebrovascular disease in children with FD has been described in previous reports.26,27 Other early findings may include subtle electrocardiography abnormalities, mild left ventricular hypertrophy, and mild albuminuria.28–30
In conclusion, sex, phenotype, and plasma lysoGb3 concentrations are strongly associated with the rate of clinical events as well as cardiac, renal, and cerebral involvement. Men with classical FD have an increased risk of developing complications, more severe cardiac and renal disease, and higher lysoGb3 values compared with patients with nonclassical FD and women. Women with classical FD also have a higher risk of developing complications compared with women with nonclassical FD. Of interest, women may develop cardiac fibrosis in the absence of left ventricular hypertrophy according to reference values. These data are of high importance for supporting the development of guidelines for follow-up and treatment and studying effects of intervention per subgroup of patients.
Concise Methods
Study Design
Data from three FD centers of excellence (Academic Medical Center, Amsterdam, The Netherlands; Royal Free London NHS Foundation Trust, London, United Kingdom; and the University Hospital Wuerzburg, Wuerzburg, Germany) were merged into one database. For this study, we used retrospectively gathered clinical data from before the first visit, which were retrieved from medical records and clinical letters, as well as data collected at first visit.
To study the natural course of FD, we included data before start of ERT. No event data for untreated patients subsequent to the first visit were included, because this would lead to bias by indication (i.e., only patients with less severe disease would be selected for untreated follow-up). Data at first visit were used to study the relation between phenotype, sex, age, and clinical and laboratory measurements.
Data from consecutive patients with a confirmed FD diagnosis by combination of phenotypic features, enzymatic assay (men), and DNA analysis (men and women; see below) were used. Subjects who were classified as no FD and patients for whom insufficient data were available for phenotypic classification or to assess the occurrence of events were excluded from the analysis. Subjects with one of the following genetic variants, with exceptions outlined below, were classified as no FD on the basis of previous reports, in which pathology studies confirmed that there is no characteristic storage in relevant organs: A143T,31,32 P60L,31 D313Y,33–35 and R118C.36 Three additional mutations were considered neutral variants on the basis of the high frequency in the general population,37,38 >50% residual enzyme activity, and/or lysoGb3 concentrations of <0.7 nmol/L: T385A, IVS0–10 C>T, and the complex haplotype IVS0–10 C>T/IVS4–16A>G/IVS6–22C>T.
Phenotype
Patients were classified as classical or nonclassical FD on the basis of their enzyme activity (men only) and the presence or absence of characteristic symptoms (Table 3).39 Men were considered to have a classical phenotype when they met the following criteria: (1) a GLA mutation, (2) enzyme activity ≤5% of the mean reference range, and (3) one or more characteristic FD symptoms (i.e., Fabry neuropathic pain, angiokeratoma, and/or cornea verticillata; definitions are in van der Tol et al.40). Men not fulfilling these criteria were categorized as nonclassical FD.
Table 3.
Criteria for phenotypic classification
| Men | Women |
|---|---|
| Classical FD | |
| A mutation in the GLA genea | A mutation in the GLA gene |
| One or more of the following characteristic FD symptoms: Fabry neuropathic pain, angiokeratoma, and/or cornea verticillata | One or more of the following characteristic FD symptoms: Fabry neuropathic pain, angiokeratoma, and/or cornea verticillata |
| Severely decreased or absent leukocyte aGAL activity (<5% of the normal mean) | |
| Nonclassical FD | |
| A mutation in the GLA gene and not fulfilling the criteria for classical FD | |
aGAL, α-galactosidase A.
The following genetic variants were considered no FD (neutral variants): A143T, P60L, D313Y, R118C, T385A, IVS0–10 C>T, and the complex haplotype IVS0–10 C>T/IVS4–16A>G/IVS6–22C>T. In patients in whom classification on the basis of these criteria was not feasible, the final judgement was made by the treating physician.
Women with a GLA mutation and one or more characteristic FD symptoms (i.e., Fabry neuropathic pain, angiokeratoma, and/or cornea verticillata40) were classified as having a classical phenotype. Women without these characteristic FD symptoms were classified as nonclassical FD.
Classification on the basis of phenotypic features and residual enzyme activity was challenging in two groups of patients. It was decided that, in these patients, a final judgement was made by the treating physician. These groups were as follows.
(1) Patients with the N215S mutation: this group is especially prevalent in the United Kingdom. According to the literature and physician experience, patients exhibit a nonclassical (mostly cardiac) phenotype, but exceptions may occur. In this group of 90 patients, 12 had a characteristic symptom but without confirmatory deficiency of GLA activity in leukocytes in men (n=5). Furthermore, one of the N215S patients presented with renal disease at young age (with no other cause). Renal disease was observed in his family (not included in our cohort). According to the judgement of the treating physician, this patient was classified as having classical FD, whereas the other N215S patients were all classified as having nonclassical FD. Similarly, three patients with characteristic symptoms and the P389A (one man and one woman) or R112H (one woman) mutation were discussed with the treating physician.
These patients all had late-onset presentation, only minimal cornea verticillata (no other characteristic FD symptoms), and a family history of nonclassical FD. Consequently, they were classified as nonclassical FD.
(2) Men with slightly >5% enzyme activity in the presence of one or more characteristic symptoms (n=13): residual enzyme activity ranged from 6% to 10% in leukocytes (n=10) and from 6% to 20% in plasma (n=3). All had at least one characteristic FD symptom, and the majority had a relative with classical FD and consequently, were considered having classical FD. In four men, the enzyme activity and/or the data on characteristic FD symptoms were missing. These patients were classified as classical FD according to the opinion of the treating physician, which was mainly on the basis of their family history.
We also included three patients (one man and two women; all from the same family) with the A143T mutation. They were classified as having classical FD on the basis of the combination of characteristic deposits on renal biopsy or postmortem biopsy, the presence of one or more characteristic FD symptoms, low enzyme activity (3.9%, 21%, and 38% respectively), and high–plasma lysoGb3 concentrations (man: 35–50 nmol/L while receiving ERT; woman 1: 16 nmol/L while receiving ERT; and woman 2: 8 nmol/L while not receiving ERT). In these patients, a combination of the A143T mutation and an unknown mutation and/or other (genetic) disease modifiers may have caused the classical FD presentation.
Outcomes
We assessed the clinical event rates from birth to first visit. It is important to note that this is not a mortality study, because patients were included at first visit. Furthermore, clinical and laboratory measurements at first visit were analyzed.
Clinical Events
Renal events were defined as CKD stage G5 (eGFR<15 ml/min per 1.73 m2), renal transplantation, or RRT. Cardiac events included atrial fibrillation, admission for any rhythm disturbance, admission for congestive heart failure, implantation of an implantable cardiac defibrillator or pacemaker, myocardial infarction, coronary artery bypass graft surgery, or a percutaneous transluminal angioplasty. Cerebral events were defined as stroke or transient ischemic attack diagnosed by a neurologist.
Clinical and Laboratory Measurements
Renal function was evaluated by the eGFR and the amount of protein excretion in urine. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation in adults41 and the Schwartz formula in children up to 17 years of age.42 The eGFR of patients who had received a renal transplant or were undergoing RRT was set at 10 ml/min per 1.73 m2. Albuminuria and proteinuria excretion was categorized following CKD guidelines: A1, normal to mildly increased (albumin excretion rate [AER] <30 mg/24 h, protein excretion rate [PER] <150 mg/24 h, albumin-to-creatinine ratio [ACR] <3 mg/mmol, and/or protein-to-creatinine ratio <15 mg/mmol); A2, moderately increased (AER=30–300, PER=150–500, ACR=3–30, and/or protein-to-creatinine ratio =15–50); and A3, severely increased (AER>300, PER>500, ACR>30, and/or protein-to-creatinine ratio >50).41
Cardiac involvement was assessed by echocardiography and cardiac MRI. LVM on echocardiography was calculated using the Devereux formula and corrected for height (meters2.7).43 Left ventricular hypertrophy was defined as LVM≥49 and ≥45 g/m2.7 in men and women, respectively.43 The upper reference limit of the RWT was defined as >0.42.43 The LVM (not including papillary muscles) measured by cardiac MRI was corrected for body surface area using the Dubois formula. The upper reference limits,44 adjusted for not including papillary muscles (9% on average),45,46 for men and women were estimated at 78 and 74 g/m2, respectively. In addition, the presence of LGE on cardiac MRI was assessed as a marker of fibrosis.
The presence of WMLs/ischemic lesions was investigated by cerebral MRI.
Enzyme activity was expressed as percentage of the mean of the lower and upper reference values. Plasma lysoGb3 levels were measured with an (adjusted) method on the basis of tandem mass spectrometry with glycine- (all samples from the Royal Free Hospital and the University Hospital Wuerzburg as well as all samples after August of 2015 from the Academic Medical Center) or isotope-labeled lysoGb3 (samples from before August of 2015 from the Academic Medical Center) as an internal standard.47 Results from both internal standards correlated very well (Supplemental Figures E1 and E2).
Statistical Analyses
R (version 3.1.5) was used for statistical analysis. The retrospectively collected data on events were used to assess the event-free survival by using survival curves and Cox proportional hazard models. Models were fitted for first renal, first cardiac, and first cerebral events separately as well as any first event. The proportional hazard assumption was visually tested by using Schoenfeld residuals and including a time-dependent variable as covariate. In addition, cross-sectional analyses were performed on data collected at or close to the first visit. A linear regression model was used to analyze the relation between age, LVM, and RWT. Data on LVM were first log transformed. Not meeting the assumption of normality, a robust linear regression model (package robustbase) using MM-type estimation48 was used for the analysis of the relation between age and eGFR. Logistic regression was used to analyze the presence of CKD stage A3, LGE, and WMLs. Sex and phenotype were included as covariates in all models. The Kruskal–Wallis test with Dunn test for post hoc comparison was used to compare lysoGb3 concentrations at first visit between subgroups. Logistic and linear regression models were used to assess the relation between lysoGb3 and disease severity. P values <0.05 were considered statistically significant. Where appropriate, 95% CIs are given.
Disclosures
M.A. and O.T.W. have no competing interests to declare. C.W. has received honoraria for lecturing from Sanofi Genzyme (Cambridge, MA, United States) and a grant to the institution from Sanofi Genzyme and Shire (Dublin, Ireland). D.H. has received honoraria for speaking and participating on advisory boards and support for research from Shire, Sanofi Genzyme, Amicus (Cranbury, NJ, United States), and Protalix (Carmiel, Israel). Also, D.H. has a consultancy arrangement through UCL Consultants (London, United Kingdom) to support, in part, laboratory research. A.M. has received honoraria for consultancy and educational activities as well as research grant support from Shire, Sanofi Genzyme, Protalix/Pfizer (New York City, NY, United States), and Amicus. D.O. received travel assistance from Sanofi Genzyme and Shire. P.M.E. has received speaker fees from Shire and consultancy and speaker fees from Sanofi Genzyme, Pfizer, and Gilead Sciences (Foster City, CA). G.E.L., M.B., and C.E.H. have received travel support, honoraria for consultancies, and educational grants from Sanofi Genzyme, Shire, Protalix, Actelion (Allschwil, Switzerland), and Amicus. All financial arrangements are made with the AMC Medical Research BV in accordance with the Research Code of the Academic Medical Center. F.A.W. has received honoraria for presentations and board meetings, travel expenses to meetings, and honoraria for consultancy work from Sanofi Genzyme and Shire. F.A.W. has also received unrestricted educational grants and research grants from Sanofi Genzyme.
Supplementary Material
Acknowledgments
We thank the Fabry Support and Information Group of The Netherlands for their cooperation and critical review. We acknowledge Prof. Dr. F. Weidemann (Katharinen Hospital Unna, Unna, Germany and University Hospital Wuerzburg, Wuerzburg, Germany), Dr. Christiane Drechsler, and Dr. P. Nordbeck (University Hospital Wuerzburg, Wuerzburg, Germany) for their support. In addition, we thank Tracey Clarke, Kim de Gier, Shirley Klein–van Loon, Mark Mckie, Els Ormel, Matthew Reed, and Irina Turkin for their help with the data and sample collection.
This study was supported by a grant 836011009 from the Ministry of Health.
Researchers worked independently from the funders. The funding source was not involved in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit an article for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016090964/-/DCSupplemental.
References
- 1.Kint JA: Fabry’s disease: Alpha-galactosidase deficiency. Science 167: 1268–1269, 1970 [DOI] [PubMed] [Google Scholar]
- 2.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L: Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med 276: 1163–1167, 1967 [DOI] [PubMed] [Google Scholar]
- 3.Desnick RJ, Ioannou YA, Eng CM: A-galactosidase a deficiency: Fabry disease. In: The Online Metabolic and Molecular Bases of Inherited Diseases, edited by Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson K, Mitchell G, New York, McGraw-Hill, 2014; Available at http://ommbid.mhmedical.com/content.aspx?bookid=971&Sectionid=62644837. Accessed November 24, 2016 [Google Scholar]
- 4.Smid BE, van der Tol L, Biegstraaten M, Linthorst GE, Hollak CE, Poorthuis BJ: Plasma globotriaosylsphingosine in relation to phenotypes of Fabry disease. J Med Genet 52: 262–268, 2015 [DOI] [PubMed] [Google Scholar]
- 5.van der Tol L, Smid BE, Poorthuis BJ, Biegstraaten M, Deprez RH, Linthorst GE, Hollak CE: A systematic review on screening for Fabry disease: Prevalence of individuals with genetic variants of unknown significance. J Med Genet 51: 1–9, 2014 [DOI] [PubMed] [Google Scholar]
- 6.MacDermot KD, Holmes A, Miners AH: Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet 38: 769–775, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echevarria L, Benistan K, Toussaint A, Dubourg O, Hagege AA, Eladari D, Jabbour F, Beldjord C, de Mazancourt P, Germain DP: X chromosome inactivation in female patients with Fabry disease. Clin Genet 89: 44–54, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Elstein D, Schachamorov E, Beeri R, Altarescu G: X-inactivation in Fabry disease. Gene 505: 266–268, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Mauer M, Glynn E, Svarstad E, Tøndel C, Gubler MC, West M, Sokolovskiy A, Whitley C, Najafian B: Mosaicism of podocyte involvement is related to podocyte injury in females with Fabry disease. PLoS One 9: e112188, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rombach SM, Smid BE, Bouwman MG, Linthorst GE, Dijkgraaf MG, Hollak CE: Long term enzyme replacement therapy for Fabry disease: Effectiveness on kidney, heart and brain. Orphanet J Rare Dis 8: 47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weidemann F, Niemann M, Störk S, Breunig F, Beer M, Sommer C, Herrmann S, Ertl G, Wanner C: Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: Evidence for disease progression towards serious complications. J Intern Med 274: 331–341, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDermot KD, Holmes A, Miners AH: Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet 38: 750–760, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin Iii HA, Kopp JB: Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81: 122–138, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR: Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Bouwman MG, Rombach SM, Linthorst GE, Poorthuis BJ, Deprez RH, Aerts JM, Wijburg FA: Early cerebral manifestations in a young female with Fabry disease with skewed X-inactivation. Clin Genet 80: 500–502, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Niemann M, Herrmann S, Hu K, Breunig F, Strotmann J, Beer M, Machann W, Voelker W, Ertl G, Wanner C, Weidemann F: Differences in Fabry cardiomyopathy between female and male patients: Consequences for diagnostic assessment. JACC Cardiovasc Imaging 4: 592–601, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Pan X, Ouyang Y, Wang Z, Ren H, Shen P, Wang W, Xu Y, Ni L, Yu X, Chen X, Zhang W, Yang L, Li X, Xu J, Chen N: Genotype: A crucial but not unique factor affecting the clinical phenotypes in Fabry disease. PLoS One 11: e0161330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, Sorensen SA, Wilcox WR, Desnick RJ: Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant 24: 2102–2111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lévesque LE, Hanley JA, Kezouh A, Suissa S: Problem of immortal time bias in cohort studies: Example using statins for preventing progression of diabetes. BMJ 340: b5087, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, Tanaka H: An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med 333: 288–293, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Takenaka T, Teraguchi H, Yoshida A, Taguchi S, Ninomiya K, Umekita Y, Yoshida H, Horinouchi M, Tabata K, Yonezawa S, Yoshimitsu M, Higuchi K, Nakao S, Anan R, Minagoe S, Tei C: Terminal stage cardiac findings in patients with cardiac Fabry disease: An electrocardiographic, echocardiographic, and autopsy study. J Cardiol 51: 50–59, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, Kanzaki T, Enriquez AL, Eng CM, Tanaka H, Tei C, Desnick RJ: Fabry disease: Detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int 64: 801–807, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Biegstraaten M, Arngrímsson R, Barbey F, Boks L, Cecchi F, Deegan PB, Feldt-Rasmussen U, Geberhiwot T, Germain DP, Hendriksz C, Hughes DA, Kantola I, Karabul N, Lavery C, Linthorst GE, Mehta A, van de Mheen E, Oliveira JP, Parini R, Ramaswami U, Rudnicki M, Serra A, Sommer C, Sunder-Plassmann G, Svarstad E, Sweeb A, Terryn W, Tylki-Szymanska A, Tøndel C, Vujkovac B, Weidemann F, Wijburg FA, Woolfson P, Hollak CE: Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: The European Fabry Working Group consensus document. Orphanet J Rare Dis 10: 36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira S, Auray-Blais C, Boutin M, Lavoie P, Nunes JP, Martins E, Garman S, Oliveira JP: Variations in the GLA gene correlate with globotriaosylceramide and globotriaosylsphingosine analog levels in urine and plasma. Clin Chim Acta 447: 96–104, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rombach SM, Dekker N, Bouwman MG, Linthorst GE, Zwinderman AH, Wijburg FA, Kuiper S, Vd Bergh Weerman MA, Groener JE, Poorthuis BJ, Hollak CE, Aerts JM: Plasma globotriaosylsphingosine: Diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta 1802: 741–748, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wijburg FA, Bénichou B, Bichet DG, Clarke LA, Dostalova G, Fainboim A, Fellgiebel A, Forcelini C, An Haack K, Hopkin RJ, Mauer M, Najafian B, Scott CR, Shankar SP, Thurberg BL, Tøndel C, Tylki-Szymańska A, Ramaswami U: Characterization of early disease status in treatment-naive male paediatric patients with Fabry disease enrolled in a randomized clinical trial. PLoS One 10: e0124987, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrera-Salazar MA, O’Rourke E, Charria-Ortiz G, Barranger JA: Radiological evidence of early cerebral microvascular disease in young children with Fabry disease. J Pediatr 147: 102–105, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Ries M, Gupta S, Moore DF, Sachdev V, Quirk JM, Murray GJ, Rosing DR, Robinson C, Schaefer E, Gal A, Dambrosia JM, Garman SC, Brady RO, Schiffmann R: Pediatric Fabry disease. Pediatrics 115: e344–e355, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Havranek S, Linhart A, Urbanova Z, Ramaswami U: Early cardiac changes in children with anderson-fabry disease. JIMD Rep 11: 53–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgwardt L, Feldt-Rasmussen U, Rasmussen AK, Ballegaard M, Meldgaard Lund A: Fabry disease in children: Agalsidase-beta enzyme replacement therapy. Clin Genet 83: 432–438, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Smid BE, Hollak CE, Poorthuis BJ, van den Bergh Weerman MA, Florquin S, Kok WE, Lekanne Deprez RH, Timmermans J, Linthorst GE: Diagnostic dilemmas in Fabry disease: A case series study on GLA mutations of unknown clinical significance. Clin Genet 88: 161–166, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Terryn W, Vanholder R, Hemelsoet D, Leroy BP, Van Biesen W, De Schoenmakere G, Wuyts B, Claes K, De Backer J, De Paepe G, Fogo A, Praet M, Poppe B: Questioning the pathogenic role of the GLA p.Ala143Thr “Mutation” in Fabry disease: Implications for screening studies and ERT. JIMD Rep 8: 101–108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froissart R, Guffon N, Vanier MT, Desnick RJ, Maire I: Fabry disease: D313Y is an alpha-galactosidase A sequence variant that causes pseudodeficient activity in plasma. Mol Genet Metab 80: 307–314, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Yasuda M, Shabbeer J, Benson SD, Maire I, Burnett RM, Desnick RJ: Fabry disease: Characterization of alpha-galactosidase A double mutations and the D313Y plasma enzyme pseudodeficiency allele. Hum Mutat 22: 486–492, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Niemann M, Rolfs A, Giese A, Mascher H, Breunig F, Ertl G, Wanner C, Weidemann F: Lyso-Gb3 indicates that the alpha-galactosidase A mutation D313Y is not clinically relevant for Fabry disease. JIMD Rep 7: 99–102, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira S, Ortiz A, Germain DP, Viana-Baptista M, Caldeira-Gomes A, Camprecios M, Fenollar-Cortes M, Gallegos-Villalobos A, Garcia D, Garcia-Robles JA, Egido J, Gutierrez-Rivas E, Herrero JA, Mas S, Oancea R, Peres P, Salazar-Martin LM, Solera-Garcia J, Alves H, Garman SC, Oliveira JP: The alpha-galactosidase A p.Arg118Cys variant does not cause a Fabry disease phenotype: Data from individual patients and family studies. Mol Genet Metab 114: 248–258, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bono C, Nuzzo D, Albeggiani G, Zizzo C, Francofonte D, Iemolo F, Sanzaro E, Duro G: Genetic screening of Fabry patients with EcoTILLING and HRM technology. BMC Res Notes 4: 323, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA; 1000 Genomes Project Consortium : An integrated map of genetic variation from 1,092 human genomes. Nature 491: 56–65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smid BE, van der Tol L, Cecchi F, Elliott PM, Hughes DA, Linthorst GE, Timmermans J, Weidemann F, West ML, Biegstraaten M, Lekanne Deprez RH, Florquin S, Postema PG, Tomberli B, van der Wal AC, van den Bergh Weerman MA, Hollak CE: Uncertain diagnosis of Fabry disease: Consensus recommendation on diagnosis in adults with left ventricular hypertrophy and genetic variants of unknown significance. Int J Cardiol 177: 400–408, 2014 [DOI] [PubMed] [Google Scholar]
- 40.van der Tol L, Cassiman D, Houge G, Janssen MC, Lachmann RH, Linthorst GE, Ramaswami U, Sommer C, Tøndel C, West ML, Weidemann F, Wijburg FA, Svarstad E, Hollak CE, Biegstraaten M: Uncertain diagnosis of fabry disease in patients with neuropathic pain, angiokeratoma or cornea verticillata: Consensus on the approach to diagnosis and follow-up. JIMD Rep 17: 83–90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA: Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 17: 29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel-Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch-Herold M, Pearson G, Sinha S, Lima JA, Bluemke DA: Left ventricular papillary muscle mass: Relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr 30: 426–432, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Gommans DH, Bakker J, Cramer GE, Verheugt FW, Brouwer MA, Kofflard MJ: Impact of the papillary muscles on cardiac magnetic resonance image analysis of important left ventricular parameters in hypertrophic cardiomyopathy. Neth Heart J 24: 326–331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold H, Mirzaian M, Dekker N, Joao Ferraz M, Lugtenburg J, Codée JD, van der Marel GA, Overkleeft HS, Linthorst GE, Groener JE, Aerts JM, Poorthuis BJ: Quantification of globotriaosylsphingosine in plasma and urine of fabry patients by stable isotope ultraperformance liquid chromatography-tandem mass spectrometry. Clin Chem 59: 547–556, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Koller M, Stahel WA: Sharpening Wald-type inference in robust regression for small samples. Comput Stat Data Anal 55: 2504–2515, 2011 [Google Scholar]
- 49.Maceira AM, Prasad SK, Khan M, Pennell DJ: Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 8: 417.–, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.