Abstract
Inappropriate activation of the renin-angiotensin system (RAS) exacerbates renal and vascular injury. Accordingly, treatment with global RAS antagonists attenuates cardiovascular risk and slows the progression of proteinuric kidney disease. By reducing BP, RAS inhibitors limit secondary immune activation responding to hemodynamic injury in the target organ. However, RAS activation in hematopoietic cells has immunologic effects that diverge from those of RAS stimulation in the kidney and vasculature. In preclinical studies, activating type 1 angiotensin (AT1) receptors in T lymphocytes and myeloid cells blunts the polarization of these cells toward proinflammatory phenotypes, protecting the kidney from hypertensive injury and fibrosis. These endogenous functions of immune AT1 receptors temper the pathogenic actions of renal and vascular AT1 receptors during hypertension. By counteracting the effects of AT1 receptor stimulation in the target organ, exogenous administration of AT2 receptor agonists or angiotensin 1–7 analogs may similarly limit inflammatory injury to the heart and kidney. Moreover, although angiotensin II is the classic effector molecule of the RAS, several RAS enzymes affect immune homeostasis independently of canonic angiotensin II generation. Thus, as reviewed here, multiple components of the RAS signaling cascade influence inflammatory cell phenotype and function with unpredictable and context-specific effects on innate and adaptive immunity.
Keywords: immunology, hypertension, renin angiotensin system
The renin-angiotensin system (RAS) is a critical hormonal signaling cascade engaged in body fluid and BP homeostasis (Figure 1). Reductions in kidney perfusion stimulate the RAS with consequent renal sodium retention and intravascular volume expansion.1–3 Inappropriate activation of the RAS therefore leads to hypertension and progression of kidney and cardiovascular disease. Accordingly, medicines that block the actions of the RAS are among the most effective classes of agents used to reduce BP and ameliorate diabetic and nondiabetic kidney disease.4–7 Although the classically recognized functions of the RAS to promote renal sodium retention and vasoconstriction are mediated through the binding of angiotensin II (Ang II) to type 1 angiotensin (AT1) receptors,8,9 other peptides, enzymes, and receptors in this cascade have received increased scrutiny for their independent contributions to developmental biology, renal and vascular function, and immunity. The discovery that the production of RAS components in kidney parenchymal cells is regulated independently of RAS peptide levels in the circulation introduced a paradigm shift in our understanding of how the RAS contributes to the pathogenesis of hypertension.10 Tissue-specific regulation and functions of the RAS are similarly evident in other organs including those engaged in innate and adaptive immune responses. Indeed, cell lineages that constitute the immune system have the capacity to express RAS components,11,12 and the effects of the RAS peptides and enzymes on inflammatory responses are quite diverse. However, one recurring theme that emerges from the work of several laboratories including our own is that activating AT1 receptors directly on hematopoietic cells may provide a feedback, immunosuppressive signal to temper or limit the pathogenic actions of inappropriate RAS activation in the kidney, vasculature, and nervous system. Below, we highlight several of the immunologic effects of the RAS.
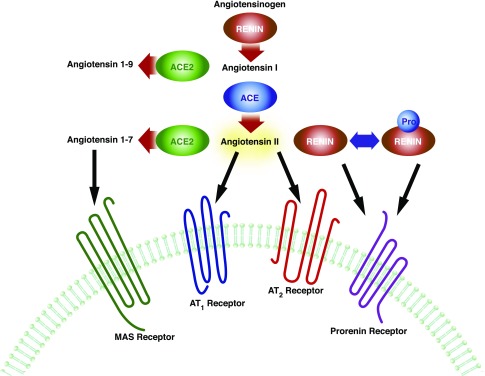
Figure 1.
The renin angiotensin system. The RAS is composed of multiple substrates and enzymes that can act in concert or separately to exert physiologic actions. The most well described function of the RAS is regulation of BP homeostasis. Angiotensinogen is converted to Ang I by renin in the circulation. Ang I is subsequently cleaved by ACE to produce Ang II, an effector molecule that increases vascular tone and promotes sodium reabsorption in the kidney by ligating the AT1 receptor. Apart from these canonic functions, several components of the RAS modulate immune responses. (Pro)renin and ACE regulate hematopoietic cell differentiation. AT1 receptor activation in immune cells versus the kidney exerts divergent effects on tissue inflammation. By degrading Ang II and/or by catalyzing the generation of Ang 1–7, ACE2 ameliorates inflammatory injury in the kidney and vasculature.
Immunologic Effects of Global RAS Activation
Preclinical and clinical studies using AT1 receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACEIs) have indicated that global RAS activation can drive inflammation in the kidney and vasculature through BP-independent mechanisms. For example, quinapril therapy reduces renal glomerular and tubular injury and inflammatory cell accumulation in rodent models of immune-complex GN,13–15 whereas ACEI or ARB treatment ameliorates murine lupus nephritis to a greater extent than amlodipine.16 In atherosclerosis, angiotensin converting enzyme (ACE) inhibition attenuates the vascular expression of the mononuclear cell chemokine CCL2 and the intralesional accumulation of inflammatory macrophages.17 Moreover, RAS-mediated induction of the profibrotic cytokine TGF-β has been recognized as a fundamental driver of scar formation in the kidney and, more recently, autoimmune inflammation in the brain.18–20 In human patients with CKD, RAS inhibition limits renal inflammation and oxidative stress independently of BP.21 These types of experiments developed the thesis that global RAS activation instigates tissue damage in part by stimulating cellular immune responses.
Although RAS-dependent hypertension largely accrues from activation of AT1 receptors in the kidney and its vasculature,22,23 upregulated immune responses in this setting can also contribute to tissue injury and even BP elevation. Accordingly, lymphocyte or cytokine blockade prolongs survival and blunts hypertensive renal damage in RAS activation models,24 whereas rodents lacking lymphocytes are protected from RAS-dependent hypertension and have preserved vasodilatory and natriuretic responses.25,26 Collectively, these studies would suggest that subclinical kidney injury or even salt retention triggered by renal AT1 receptor ligation invokes an inflammatory milieu that exacerbates BP elevation and tissue damage.27–30
Indeed, experiments using mice with genetic deletion of the dominant murine AT1 isoform, AT1A, have largely confirmed that the proinflammatory effects of RAS activation accrue from stimulating AT1 receptors in the target organ. For example, after bone marrow transfer between Agtr1a−/− mice lacking the AT1A receptor and wild-type controls, susceptibility to immune-mediated kidney injury and renal macrophage accumulation arose from AT1A receptor expression in the host rather than the bone marrow donor.31,32 In our own hands, AT1A receptor expression on bone marrow cells did not influence the progression of murine lupus nephritis. Rather, augmented AT1 receptor activation in the glomerular podocyte triggered robust renal inflammation in this model.33 Likewise, inflammation in the atherosclerotic lesion depended on AT1 receptor activation in the blood vessel rather than the bone marrow.34,35 Thus, the effects of the RAS to promote inflammation appeared to accrue from activation of AT1 receptors in the kidney and vasculature rather than in infiltrating hematopoietic cells. However, these studies belied a more complex set of interactions between the immune system and individual RAS components that became evident through gene deletion experiments discussed below.
Renin/Prorenin
In converting angiotensinogen to angiotensin I (Ang I), renin catalyzes the rate-limiting step in the generation of the RAS effector molecule, Ang II (Figure 1). Accordingly, renin is the proximate driver of AT1 receptor-dependent inflammation in the vascular wall.34,36 However, in 2002, Nguyen and colleagues cloned the (pro)renin receptor (PRR) through which renin and its precursor (pro)renin activate the extracellular signal-regulated kinase 1/2 signaling cascade independently of canonic Ang II generation.37 Whereas the PRR is part of a Wnt/β-catenin signaling complex that is critical for planar development,38 the PRR is also broadly expressed in cardiovascular control organs where its ligation can directly promote inflammation. For example, PRR stimulation enhances proinflammatory cytokine levels in the vasculature and microglia.39,40 Within the immune system, the PRR pathway is active in human monocytes and is required in T lymphocytes for their acquisition of peripheral T cell markers including CD4 and CD8 during thymic education.41,42 Moreover, the PRR is expressed on both macrophages and T cells infiltrating the glomerulus during human crescentic GN, and renin-mediated induction of IL-6 and cyclooxygenase-2 in human mononuclear cells requires extracellular signal-regulated kinase 1/2 phosphorylation but not angiotensin receptor ligation.43 These data support a new paradigm in which RAS components other than the effector molecule Ang II modulate inflammation in the target organ. However, the net effects of renin’s classic proteolytic actions combined with its nonproteolytic signaling via the PRR in inflammatory disease await further clarification.
ACE
As the dominant enzyme that converts Ang I to the RAS effector molecule Ang II, ACE promotes inflammation in the heart, kidney, and vasculature that is attributable to Ang II (Figure 1). Accordingly, ACE inhibition not only ameliorates cardiac damage after myocardial infarction and slows the progression of proteinuric kidney disease, but also reduces circulating and urinary levels of inflammatory markers.5,44–48 Early studies investigating the immune functions of ACE focused on the role of ACE in granulomatous disease. Secreted by histiocytes in granulomata, circulating ACE became a marker to support the diagnosis of sarcoidosis.49 In turn, ACE inhibition could shrink the granulomata induced by Schistosoma mansoni infection, suggesting that ACE similarly contributes to the infectious inflammatory response.50 Nevertheless, ACE, like (pro)renin, has other pleiotropic effects on immunity that have emerged more recently. First, separate from its functions as a proteolytic enzyme, ACE acts as a transcription factor to direct the emergence of endothelial, myeloid, erythroid, and lymphoid cell lineages from hemangioblast colonies.51 This biology may contribute to the development of anemia in some ACEI-treated patients although alterations in erythropoietin levels also play a role.52 Second, ACE edits the carboxyl terminus of peptide antigens presented to CD8+ T cells in the context of class I major histocompatibility molecules.53 This function of ACE would have unpredictable effects on adaptive immune responses, depending on specific alterations in antigen sequences mediated through ACE’s carboxypeptidase activity (Figure 2). Thus, whereas ACE-mediated generation of Ang II in the target organ triggers damage to invoke a secondary inflammatory response, the direct actions of ACE within immune cells and their progenitors are more nuanced and context-specific.
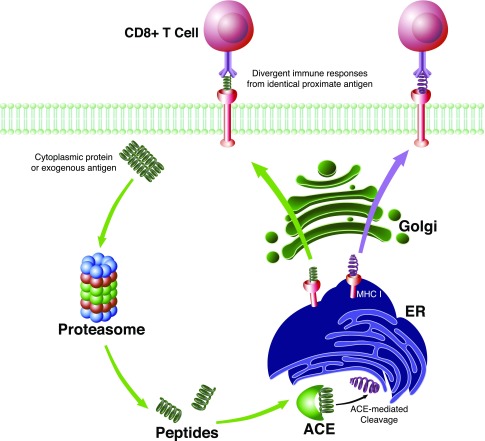
Figure 2.
ACE-mediated cleavage of MHC I peptides in antigen presenting cells modulates adaptive immunity. Cytoplasmic proteins or exogenous antigens (in the case of dendritic cell cross presentation) are fragmented by proteasomes. Peptide products are then shuttled to the endoplasmic reticulum (ER) where ACE can alter the peptide sequence via C-terminal cleavage. After MHC I loading, the peptide is transported to the cell surface. CD8+ T cells scan the MHC I complex and, upon recognition, initiate an adaptive immune response. Alterations made by ACE to the peptide sequence can thereby alter the specificity of the immune response.
ACE2/Angiotensin 1–7
The immunologic effects of the alternate ACE enzyme, ACE2, could accrue from its catabolism of Ang II or the consequent generation of Ang 1–7. By reducing Ang II levels in the kidney and vasculature, ACE2 might be predicted to blunt inflammatory responses elicited by Ang II–mediated hemodynamic injury. Indeed, in the murine apoE-deficient model of atherosclerosis, ACE2 attenuates the formation of aortic plaques in vivo and also limits macrophage expression of several proinflammmatory cytokines in vitro, including TNF-α and IL-6, after an LPS challenge, suggesting that ACE2 can directly blunt the proinflammatory polarization of myeloid cells.54 These anti-inflammatory effects of ACE2 depend at least in part on local reductions in Ang II levels as concomitant ACE inhibition ameliorates some of the tissue damage and immune activation attributable to ACE2 deficiency. Consistent with the capacity of Ang II to drive tissue fibrosis, ACE2 similarly protects against kidney inflammation and fibrosis in the obstructive uropathy model in part by limiting induction of renal TGF-β expression.55
Although the notion that ACE2 blunts inflammatory responses by raising levels of Ang 1–7 is still debated, treatment with Ang 1–7 has successfully reduced inflammatory damage in the heart and kidney in several preclinical studies. For example, Ang 1–7 infusion limits inflammatory cardiac injury in a diabetic hypertensive rat model56 and attenuates glomerular injury and renal expression of inflammatory markers in a rat MPGN model.57 Whether the benefits of Ang 1–7 accrue from activation of the putative Ang 1–7, or Mas, receptor is less clear as Ang 1–7 formulations can mitigate tissue inflammation even in Mas-deficient rodents.58 Moreover, the favorable actions of Ang 1–7 are not universally seen in all forms of renal damage as experimental kidney fibrosis and AKI worsen with Ang 1–7 treatment.59 Thus, the immunologic effects of Ang 1–7 may depend not only on its dose and formulation but also on disease context, and careful translational studies will be required to determine if Ang 1–7 represents a novel anti-inflammatory therapeutic to ameliorate specific renal and vascular diseases.
AT1 Receptors
In the adult organism, the AT1 receptor is more highly expressed in cells of the immune system than the type 2 angiotensin receptor (AT2) receptor.11,60,61 Given the capacity of global RAS activation to drive renal and vascular inflammation during hypertension, several groups have examined the role of AT1 receptors on immune cells in regulating hypertensive target organ damage through the generation of murine Agtr1a−/− bone marrow chimeras. The Tsukuba mouse harbors the human renin and angiotensinogen genes and therefore serves as a model of hypertension induced by chronic RAS stimulation. Tsukuba mouse chimeras lacking the AT1A receptor on bone marrow–derived cells have a preserved hypertensive response but more severe atherosclerosis in the aorta compared with wild-type transplant controls.62 Similarly, in a chronic Ang II infusion model of hypertension, we found that AT1A receptor–deficient bone marrow chimeras have exaggerated BP elevation, albuminuria, and accumulation of T cells and macrophages in the kidney.60 Thus, in contrast to the pathogenic actions of renal and vascular AT1 receptors in hypertension, AT1 receptors on immune cells appear to play a protective role (Figure 3).
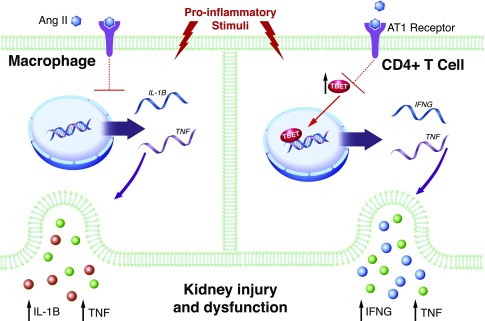
Figure 3.
Functions of the AT1 receptor on immune cells. Proinflammatory stimuli drive the differentiation of M1 macrophages (left) and Th1 T lymphocytes (right) that release pathogenic cytokines such as IL-1β, TNF, and IFN-γ (IFNG). In preclinical studies, activation of the AT1 receptor on macrophages and T cells limits their differentiation toward proinflammatory lineages, thus inhibiting the production and release of these cytokines that drive hypertensive kidney injury and fibrosis.
To further investigate these favorable actions of immune cell AT1 receptors in hypertension, we generated mice lacking the AT1A receptor selectively on T lymphocytes (T cell KO) or myeloid cells (Macro KO) and then subjected these mice and their wild-type littermates to our Ang II–dependent hypertension model.63,64 The chronic hypertensive responses were similar in the T cell KO and control cohorts. However, the T cell KO animals had augmented albuminuria and exaggerated perivascular accumulation of CD4+ T lymphocytes in the hypertensive kidney, just as seen in the AT1A receptor–deficient bone marrow chimeras. Moreover, AT1 receptor deficiency on T cells during hypertension upregulated renal mRNA expression of the kidney injury marker Ngal (Lcn2) and exacerbated podocyte dropout as detected by reduced glomerular staining for WT1.64 Thus, activating AT1 receptors on T lymphocytes attenuates hypertensive injury to the kidney glomerulus through a BP-independent mechanism. To explain these findings, CD4+ T cells lacking the AT1A receptor isolated from the hypertensive kidney or spleen expressed higher levels of the proinflammatory cytokines IFN-γ and TNF-α as well as the Th1 transcription factor T-bet (Tbx21) that drives expression of these cytokines in T cells.64 Thus, AT1 receptor stimulation on T cells suppresses T-bet–dependent differentiation of the CD4+ T helper cell toward the proinflammatory Th1 cell lineage. Subsequent studies revealed that T-bet deficiency limits albuminuria, podocyte loss, and renal Ngal expression, confirming a role for the Th1 immune response to instigate glomerular damage during hypertension.64,65
In our chronic Ang II infusion model, the Macro KO animals lacking myeloid AT1A receptors also had a preserved hypertensive response.63 On the basis of these data, AT1 receptors on T cells and macrophages do not play a key role in BP homeostasis. Nevertheless, the Macro KO cohort had more severe renal tubular injury and interstitial fibrosis than controls after 4 weeks of hypertension. This capacity of the macrophage AT1 receptor to mitigate kidney fibrosis was similarly evident in the normotensive ureteral obstruction model,63 consistent with Ichikawa’s experiments using AT1A receptor–deficient bone marrow chimeras.66 Analogous to the effects of the AT1 receptor on T cell polarization, we found that activating the AT1 receptor on macrophages suppresses their proinflammatory M1 polarization with consequent reductions in TNF and IL-1β expression. In a murine obesity model, Ma and Fogo similarly detected enhanced expression of several M1 markers on AT1A receptor–deficient macrophages.67 M1 cytokines have been implicated in renal fibrogenesis, and in kidney crosstransplant studies we were able to confirm that the macrophage AT1 receptor ameliorates kidney fibrosis by limiting IL-1 generation and thereby abrogating IL-1 receptor stimulation in renal parenchymal cells.63 Thus, on the basis of bone marrow chimera and conditional gene targeting studies, AT1 receptors on myeloid and lymphoid populations play an immunomodulatory role that tempers the pathogenic actions of AT1 receptors in the kidney and vasculature during hypertension. However, considerable work will be required to elucidate cellular signaling pathways governing these effects and develop translational approaches that exploit divergent, cell-specific actions of AT1 receptors in patients with hypertension and/or kidney fibrosis.
AT2 Receptors
Actions of the AT2 receptor counteract the actions of the AT1 receptor in several organ systems.68 For example, in contrast to AT1 receptor–mediated vasoconstriction, AT2 receptor activation in the vasculature prompts vasodilation with potential beneficial effects in the renal microcirculation during pathogenic conditions.69 In our hands and others, the AT2 receptor is not highly expressed on cells of the immune system,61,63,64 but has been detected on human T and NK cells.12 In a rat model of myocardial infarction, intracardiac transfer of CD4+ or CD8+ T cell populations expressing the AT2 receptor reduced infarct size and improved cardiac function.70,71 The protective effects of AT2 receptor activation in the target organ can also have downstream beneficial effects on the tissue’s inflammatory milieu. The development of a selective AT2 receptor agonist, compound 21 (C21),72 has highlighted the potential broad anti-inflammatory effects of AT2 receptor stimulation. For example, in a rat myocardial infarction model, AT2 receptor stimulation with C21 reduced scar size, preserved ejection fraction, and prevented upregulation of the proinflammatory cytokines IL-1β and IL-6 in the border zone of the infarcts, all of which was reversed by concomitant treatment with the AT2 receptor blocker PD 123319.73 AT2 receptor activation similarly reduced renal inflammation measured by TNF and IL-6 expression and oxidative stress in the Zucker rat model of renal injury.74 Moreover, treatment of cultured proximal tubule epithelial cells with C21 reduced TNF and IL-6 production in response to LPS activation.75 The immunosuppressive effects of AT2 receptor stimulation accrue from enhanced epoxyeicosatrienoic acid formation and direct inhibition of the NF-κB inflammatory signaling cascade.76 These preclinical studies indicate that the immunomodulatory actions of the AT2 receptor occur both within infiltrating immune cells and within the target organ’s parenchymal cells.
Immunologic Effects of RAS Manipulation in Humans
The discrepant and partially independent effects of the various RAS peptides and enzymes on immune responses should allow discrete, optimized interventions to limit RAS-dependent inflammation in humans. AT2 receptor and Ang 1–7 agonists, if carefully engineered and tested in specific cardiovascular and renal disease contexts, may complement current ACEI and ARB treatment, possibly by mitigating the induction of inflammatory responses that can result from blocking AT1 receptors on immune cells. Inasmuch as noncanonic renin/prorenin signaling provokes inflammatory responses independently of Ang II, direct renin inhibition may similarly complement standard RAS blockade by suppressing immunity. In this regard, human studies confirm the favorable effects of direct renin inhibition on parameters of immune activation. For example, in 27 patients with type 1 diabetes mellitus and no nephropathy, treatment with aliskerin for 30 days reduced urinary excretion of IFN-α2 and IL-2.77 Similarly, in 30 patients with hypertension and CKD, aliskerin treatment for 8 weeks reduced serum C reactive protein (CRP) levels.78 Nevertheless, the addition of direct renin inhibitors to ARB therapy has not yielded consistent improvements in clinical outcomes,79 diminishing enthusiasm for this strategy unless alternative, more efficacious modulators of noncanonic PRR signaling can be developed.
On the basis of preclinical data from others and our group, the incomplete efficacy of global AT1 receptor blockade in arresting human kidney disease progression80 could accrue in part from inhibition of AT1 receptors on immune cells that provokes inflammation and thereby tempers the beneficial effects of RAS blockade directly in the target organ. Although the components of the RAS are expressed in human inflammatory cells,12 examining the in vivo effects of activating the RAS specifically within these cells poses a considerable challenge. In cultures of human T cells activated in vitro, Ang II did not alter production of the proinflammatory cytokines IFN-γ or IL-17 and had no effect on dendritic cell–mediated T cell proliferation.81 However, in vitro stimulation of human PBMCs with Ang II suppressed their generation of TGF-β,82 despite the well documented effects of Ang II to drive TGF-β expression in kidney cells.18 Moreover, in vivo captopril treatment of human kidney transplant recipients enhanced TGF-β release from their PBMCs,82 consistent with our murine studies in which abrogating AT1 receptor signals in myeloid cells induced profibrotic gene expression programs, augmenting renal scar formation.63
In the absence of tools to modulate the RAS selectively within human immune cell lineages, studies in which patients are treated with ACEIs or ARBs reveal the net effect of concomitantly disrupting AT1 receptor signals in both the target organ and the immune system. In most cases, ACEI or ARB monotherapy in patients with hypertension has had favorable effects on circulating markers of inflammation. For example, in a randomized crossover trial in 45 patients with mild-to-moderate hypertension, candesartan reduced plasma levels of CCL2 and TNF after 2 months.83 Similarly, in 20 young, newly diagnosed patients with hypertension, 3 months of therapy with losartan normalized BP and reduced the proliferative responses of T lymphocytes drawn from the circulation.84 Finally, in a randomized, crossover trial of 47 hypertensive patients with hypercholesterolemia, the addition of losartan to simvastatin for 2 months reduced CCL2 levels more than simvastatin alone.85 However, in these types of studies, global RAS blockade in all tissues could obscure opposing tissue-specific immunologic effects, and indirect reductions in systemic inflammation during global RAS blockade could accrue from the hemodynamic protection of target organs.
Consistent with these hemodynamic benefits, ACEI or ARB treatment for diseases localized to the blood vessel wall has largely attenuated circulating markers of inflammation. For example, in an uncontrolled study of 77 patients with known coronary artery disease, 6 months of ramipril reduced circulating CRP levels.86 Similarly, in 27 postangioplasty patients, irbesartan reduced IL-6 and CRP concentrations after 3 months compared with baseline.45 However, not all studies indicate that RAS inhibition reduces vascular inflammation through effects on BP. In an observational study of 507 patients with stroke, ramipril treatment was associated with lower CRP levels at the time of the stroke compared with other antihypertensive regimens.87 Regardless of the mechanism, disrupting pathologic vascular remodeling with RAS blockade has, on balance, improved parameters of systemic inflammation.
By contrast, in patients with hypertension and renal inflammation, global RAS blockade has had mixed effects on immune responses. In 29 patients with hypertension and chronic GN, irbesartan therapy for 26 weeks yielded variable effects on immune parameters with reductions in circulating CRP levels but no significant effects on TNF or IL-6 levels.88Moreover, in a 16-week randomized study of 109 hypertensive diabetic patients, high-dose valsartan did not reduce serum IL-6 or TNF levels.89 Patients undergoing hemodialysis exhibit evidence of systemic inflammation, and in a randomized study of 15 hemodialysis patients,90 ramipril raised circulating IL-1β concentrations, consistent with our murine studies,63 but lowered IL-10 and IL-6 concentrations. Both ramipril and valsartan increased systemic oxidant stress measured by F(2)-isoprostane levels without modulating BP during dialysis. Thus, it is possible that in the context of renal inflammation, preserving a putative protective action of immune AT1 receptors while blocking AT1 receptors in cardiovascular control centers may become more relevant. Conversely, as inflammatory cells have lower levels of AT1 receptor expression than the kidney,64 vigorous levels of RAS activation may be required to invoke the protective actions of AT1 receptors on immune cells that partially abate the pathogenic actions of AT1 receptors in the kidney and vasculature.
Gene polymorphism studies from patients with autoimmune disease are consistent with protective actions of the RAS in immune cells. In several series of human patients with SLE, lower serum ACE levels as a proxy for diminished systemic RAS activation have been associated with more severe renal disease.91,92 Similarly, lower serum ACE levels in patients with pulmonary sarcoidosis associated with a more vigorous immunogenic response to influenza vaccination.93 However, not all human cohorts confirm this inverse association,94,95 and such observational gene association studies must be interpreted with caution.
An intriguing example of apparent immune amplification during RAS blockade has been reported in patients with an idiosyncratic ARB-associated inflammatory bowel disease, a disorder classically driven by hyperactive Th1 immune responses. In these rare cases, treatment with the ARB olmesartan has been linked to a severe immune-mediated enteropathy.96–98 Consistent with a heightened Th1 response in this syndrome, intraepithelial CD3+ T cell accumulations are noted in the bowel wall, and disease responds to anti-TNF therapy. These clinical features are consistent with our finding that mice lacking the AT1 receptor on T lymphocytes have an exaggerated Th1 response with an augmented capacity for TNF generation.64 The enteropathy occurs more commonly in patients with other evidence of autoimmunity, suggesting a predisposition to ARB-induced immune activation.
Randomized controlled trials of RAS inhibition provide some indications that preserving a low level of RAS signaling may be protective. In the ONTARGET and NEPHRON-D studies, complete RAS blockade with concomitant ACEI and ARB treatment led to higher levels of kidney dysfunction than ACEI or ARB monotherapy, despite no difference in systolic BPs between ARB treatment and dual blockade.99,100 In a smaller double-blinded randomized study of 56 patients with macroalbuminuric diabetic nephropathy, combination therapy increased the adjusted risk for worsening proteinuria and increased urinary excretion of CCL2 compared with ACEI or ARB monotherapy.101 These trials would indicate that a threshold level of RAS inhibition rather than maximal, dual blockade is optimal for the treatment of hypertension and kidney disease.
If permitting RAS activation in immune cells is beneficial, then blocking RAS signals selectively within the end organ, such as the heart or kidney, could ameliorate disease in these organs more effectively than global, systemic RAS blockade. Some human biopsy studies support the notion that RAS blockade reduces inflammation by acting directly within the target organ. For example, analysis of heart biopsy specimens from 58 patients with ischemic heart disease randomized to ramipril, valsartan, or placebo for 6 days before coronary bypass surgery showed that treatment with either the ACEI or the ARB was associated with lower TNF and IL-6 levels in the cardiac tissue.102 RAS inhibitors with inherent specificity for target organs could offer one strategy to combat local inflammation while preserving any beneficial RAS signals in circulating hematopoietic cells. For example, in a small study of patients who had suffered myocardial infarctions, treatment with “high–tissue penetrating” quinapril reduced circulating CRP levels more than “low–tissue penetrating” enalapril.103 Although authors attributed the differences in efficacy to enhanced vascular wall penetration with quinapril, differences in potency would offer an alternative explanation.
In the absence of tissue-specific ARBs, exploring targeted, low-grade immunosuppression in those patients with target organ damage and evidence of ACEI- or ARB-provoked inflammation represents a feasible alternative, particularly in this era of precision medicine. Preclinical studies indicate that RAS inhibition concomitant with chemokine blockade can limit proteinuria and podocyte loss in CKD.104 In small human studies, lymphocyte suppression with mycophenolate mofetil or even TNF blockade can reduce BP in patients with rheumatologic disease.105,106 Nevertheless, the risks of prolonged immunosuppression could outweigh cardiovascular benefits unless reserved for persistently hypertensive patients who sustain renal injury and a fingerprint of immune activation despite ARB or ACEI therapy. For example, patients appropriate for pilot intervention studies could be identified on the basis of recorded BPs, documented albuminuria, and serum or PBMC levels of TNF or IL-1β in large captive patient cohorts such as the Veterans Affairs health system. Further adoption of electronic medical records linked to biobanks of stored blood samples should facilitate the assembly of such cohorts. On the other hand, just as intrarenal RAS augmentation occurs independently of the circulating RAS,107 induction of cytokine signaling pathways that promote hypertension and renal damage may be circumscribed within the kidney65 and escape detection in samples of peripheral blood. Accordingly, additional preclinical studies are needed to (1) identify more selective targets within inflammatory signaling cascades whose blockade during RAS inhibition can reduce BP and/or protect end organs with limited impairment of systemic immunity, and (2) elucidate the cellular sources of immune mediators that promote hypertension and/or renal injury during ARB or ACEI treatment. Ultimately, advances in bioengineering, implemented with thoughtful ethical guidance, may yield tissue-specific therapies in humans to abrogate pathogenic AT1 receptor–mediated actions in the kidney and/or vasculature without invoking off-target immune activation.108,109
Conclusion
Complementing its central role in BP homeostasis, the RAS has diverse and complex effects on innate and adaptive immunity. Through coordinated regulation of Ang II levels, the RAS proteolytic cascade affects hemodynamic injury in the heart, kidney, and vasculature leading to profound, global upregulation of inflammatory responses. However, through actions unrelated to Ang II, the (pro)renin receptor and ACE affect the development and differentiation of individual immune cell lineages, the consequences of which will require further elucidation. Moreover, by altering the peptide sequences of antigens presented to CD8+ T cells, ACE may have profound effects on antiviral immunity and even tumor surveillance. Although activating AT1 receptors in the kidney and vasculature instigates damage that secondarily engages the immune system, bone marrow chimera and conditional gene targeting studies in mice indicate that stimulating AT1 receptors directly on inflammatory cells paradoxically tempers this immune activation in an apparent feedback paradigm. This phenomenon cannot be easily ascribed to AT1 receptor–mediated immune cell “exhaustion” as we do not detect markers of exhaustion on T lymphocytes subjected to chronic RAS activation.64 Moreover, we see divergent, pathogenic, and protective actions of renal and T cell AT1 receptors, respectively, in a single murine model of AKI well before T cell exhaustion should emerge.110 Confirmation that AT1 receptor activation on immune cells suppresses the inflammation and injury induced by activation of AT1 receptors in the kidney and vasculature would open new avenues for novel immunomodulatory therapies to be used in conjunction with global ARBs. Indeed, TNF and IL-1, both regulated by the immune AT1 receptor in our experiments, have been linked to hypertension and/or vascular dysfunction in preclinical and clinical studies.25,65,106,111,112 Other potential anti-inflammatory RAS therapies that warrant further translational investigation include Ang 1–7 analogs and specific AT2 receptor agonists.113 Finally, because of space constraints, this review has not addressed important immunologic effects of the RAS effector aldosterone.114 Because the RAS signaling cascade interacts at multiple levels with the immune system, developing incisive and cell lineage–specific “cocktails” for RAS-dependent immune modulation should lead to improved protection of the kidney and vasculature through BP-dependent and -independent mechanisms.
Disclosures
None.
Acknowledgments
This work was supported by National Institutes of Health grants DK087893, HL128355, and P30DK096493; Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Grant BX000893; and the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Goldblatt H, Lynch J, Hanzal RF, Summerville WW: Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347–379, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyton AC: Blood pressure control--special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Hall JE, Brands MW, Henegar JR: Angiotensin II and long-term arterial pressure regulation: The overriding dominance of the kidney. J Am Soc Nephrol 10[Suppl 12]: S258–S265, 1999 [PubMed] [Google Scholar]
- 4.Taal MW, Brenner BM: Renoprotective benefits of RAS inhibition: From ACEI to angiotensin II antagonists. Kidney Int 57: 1803–1817, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P; The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group : Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group : Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 359: 995–1003, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM: Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA 92: 3521–3525, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliverio MI, Best CF, Smithies O, Coffman TM: Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension 35: 550–554, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA: Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM: Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest 104: 1693–1701, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R: Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol 18: 1093–1102, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J: Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int 62[Suppl 82]: S12–S22, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Ortega M, Bustos C, Hernández-Presa MA, Lorenzo O, Plaza JJ, Egido J: Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J Immunol 161: 430–439, 1998 [PubMed] [Google Scholar]
- 15.Guo S, Kowalewska J, Wietecha TA, Iyoda M, Wang L, Yi K, Spencer M, Banas M, Alexandrescu S, Hudkins KL, Alpers CE: Renin-angiotensin system blockade is renoprotective in immune complex-mediated glomerulonephritis. J Am Soc Nephrol 19: 1168–1176, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez De Lema G, De Wit C, Cohen CD, Nieto E, Molina A, Banas B, Luckow B, Vicente AB, Mampaso F, Schlöndorff D: Angiotensin inhibition reduces glomerular damage and renal chemokine expression in MRL/lpr mice. J Pharmacol Exp Ther 307: 275–281, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hernández-Presa M, Bustos C, Ortego M, Tuñon J, Renedo G, Ruiz-Ortega M, Egido J: Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 95: 1532–1541, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Border WA, Noble NA: Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension 31: 181–188, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Wolf G, Mueller E, Stahl RA, Ziyadeh FN: Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest 92: 1366–1372, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L: Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest 120: 2782–2794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R: Proinflammatory effects of oxidative stress in chronic kidney disease: Role of additional angiotensin II blockade. Am J Physiol Renal Physiol 284: F863–F869, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Sparks MA, Stegbauer J, Chen D, Gomez JA, Griffiths RC, Azad HA, Herrera M, Gurley SB, Coffman TM: Vascular type 1A angiotensin II receptors control BP by regulating renal blood flow and urinary sodium excretion. J Am Soc Nephrol 26: 2953–2962, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC: Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG: Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P: Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 298: R1089–R1097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco M, Tapia E, Santamaría J, Zafra I, García-Torres R, Gordon KL, Pons H, Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J: Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol 12: 2263–2271, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA: Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T: Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Ozawa Y, Kobori H, Suzaki Y, Navar LG: Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–F339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hisada Y, Sugaya T, Tanaka S, Suzuki Y, Ra C, Kimura K, Fukamizu A: An essential role of angiotensin II receptor type 1a in recipient kidney, not in transplanted peripheral blood leukocytes, in progressive immune-mediated renal injury. Lab Invest 81: 1243–1251, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K: Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest 103: 627–635, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM: Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest 119: 943–953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H, Rateri DL, Feldman DL, Charnigo RJ Jr, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A: Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest 118: 984–993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga J, Egashira K, Matoba T, Kubo M, Ihara Y, Iwai M, Horiuchi M, Sunagawa K: Essential role of angiotensin II type 1a receptors in the host vascular wall, but not the bone marrow, in the pathogenesis of angiotensin II-induced atherosclerosis. Hypertens Res 31: 1791–1800, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN: Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ Res 101: 268–276, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C: Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, Liu M, Raizada MK, Sumners C: Direct pro-inflammatory effects of prorenin on microglia. PLoS One 9: e92937, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S: (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes 58: 1625–1633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Jan Danser AH, Bader M, Nguyen G, Luft FC, Muller DN: Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension 51: 682–688, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Geisberger S, Maschke U, Gebhardt M, Kleinewietfeld M, Manzel A, Linker RA, Chidgey A, Dechend R, Nguyen G, Daumke O, Muller DN, Wright MD, Binger KJ: New role for the (pro)renin receptor in T-cell development. Blood 126: 504–507, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Narumi K, Hirose T, Sato E, Mori T, Kisu K, Ishikawa M, Totsune K, Ishii T, Ichihara A, Nguyen G, Sato H, Ito S: A functional (pro)renin receptor is expressed in human lymphocytes and monocytes. Am J Physiol Renal Physiol 308: F487–F499, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Morton Hawkins C; The SAVE Investigators : Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med 327: 669–677, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Schieffer B, Bünte C, Witte J, Hoeper K, Böger RH, Schwedhelm E, Drexler H: Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol 44: 362–368, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Brull DJ, Sanders J, Rumley A, Lowe GD, Humphries SE, Montgomery HE: Impact of angiotensin converting enzyme inhibition on post-coronary artery bypass interleukin 6 release. Heart 87: 252–255, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Yamada S, Ueda Y, Koide H: Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. Am J Med Sci 343: 46–51, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Mandelia A, Bajpai M, Agarwala S, Gupta AK, Kumar R, Ali A: The role of urinary TGF-β1, TNF-α, IL-6 and microalbuminuria for monitoring therapy in posterior urethral valves. Pediatr Nephrol 28: 1991–2001, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Lieberman J: Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med 59: 365–372, 1975 [DOI] [PubMed] [Google Scholar]
- 50.Weinstock JV, Ehrinpreis MN, Boros DL, Gee JB: Effect of SQ 14225, an inhibitor of angiotensin I-converting enzyme, on the granulomatous response to Schistosoma mansoni eggs in mice. J Clin Invest 67: 931–936, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, Pryzhkova M, Péault B: Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood 112: 3601–3614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gossmann J, Thürmann P, Bachmann T, Weller S, Kachel HG, Schoeppe W, Scheuermann EH: Mechanism of angiotensin converting enzyme inhibitor-related anemia in renal transplant recipients. Kidney Int 50: 973–978, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, Bernstein KE: The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat Immunol 12: 1078–1085, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, Tikellis C: Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res 107: 888–897, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Huang XR, Chen HY, Penninger JM, Lan HY: Loss of angiotensin-converting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab Invest 92: 650–661, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Al-Maghrebi M, Benter IF, Diz DI: Endogenous angiotensin-(1-7) reduces cardiac ischemia-induced dysfunction in diabetic hypertensive rats. Pharmacol Res 59: 263–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Noble NA, Border WA, Huang Y: Infusion of angiotensin-(1-7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am J Physiol Renal Physiol 298: F579–F588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, Bretas TL, Bader M, de Sousa LP, da Silva TA, dos Santos RA, Simões e Silva AC, Teixeira MM: Anti-inflammatory effects of the activation of the angiotensin-(1-7) receptor, MAS, in experimental models of arthritis. J Immunol 185: 5569–5576, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Esteban V, Heringer-Walther S, Sterner-Kock A, de Bruin R, van den Engel S, Wang Y, Mezzano S, Egido J, Schultheiss HP, Ruiz-Ortega M, Walther T: Angiotensin-(1-7) and the g protein-coupled receptor MAS are key players in renal inflammation. PLoS One 4: e5406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P: A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55: 99–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG: Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296: R208–R216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato H, Ishida J, Nagano K, Honjo K, Sugaya T, Takeda N, Sugiyama F, Yagami K, Fujita T, Nangaku M, Fukamizu A: Deterioration of atherosclerosis in mice lacking angiotensin II type 1A receptor in bone marrow-derived cells. Lab Invest 88: 731–739, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Zhang JD, Patel MB, Griffiths R, Dolber PC, Ruiz P, Sparks MA, Stegbauer J, Jin H, Gomez JA, Buckley AF, Lefler WS, Chen D, Crowley SD: Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest 124: 2198–2203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD: A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res 110: 1604–1617, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD: Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64: 1275–1281, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishida M, Fujinaka H, Matsusaka T, Price J, Kon V, Fogo AB, Davidson JM, Linton MF, Fazio S, Homma T, Yoshida H, Ichikawa I: Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest 110: 1859–1868, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma LJ, Corsa BA, Zhou J, Yang H, Li H, Tang YW, Babaev VR, Major AS, Linton MF, Fazio S, Hunley TE, Kon V, Fogo AB: Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am J Physiol Renal Physiol 300: F1203–F1213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siragy HM: The angiotensin II type 2 receptor and the kidney. J Renin Angiotensin Aldosterone Syst 11: 33–36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arendshorst WJ, Brännström K, Ruan X: Actions of angiotensin II on the renal microvasculature. J Am Soc Nephrol 10[Suppl 11]: S149–S161, 1999 [PubMed] [Google Scholar]
- 70.Curato C, Slavic S, Dong J, Skorska A, Altarche-Xifró W, Miteva K, Kaschina E, Thiel A, Imboden H, Wang J, Steckelings U, Steinhoff G, Unger T, Li J: Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. J Immunol 185: 6286–6293, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Skorska A, von Haehling S, Ludwig M, Lux CA, Gaebel R, Kleiner G, Klopsch C, Dong J, Curato C, Altarche-Xifró W, Slavic S, Unger T, Steinhoff G, Li J, David R: The CD4(+) AT2R(+) T cell subpopulation improves post-infarction remodelling and restores cardiac function. J Cell Mol Med 19: 1975–1985, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlén A, Pettersson A, Nyberg F, Fändriks L, Gallo-Payet N, Hallberg A, Alterman M: Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem 47: 5995–6008, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschöpe C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlöf B, Kintscher U, Unger T, Steckelings UM: Angiotensin II type 2 receptor stimulation: A novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 118: 2523–2532, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Sabuhi R, Ali Q, Asghar M, Al-Zamily NR, Hussain T: Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: Opposing effects in lean and obese Zucker rats. Am J Physiol Renal Physiol 300: F700–F706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dhande I, Ali Q, Hussain T: Proximal tubule angiotensin AT2 receptors mediate an anti-inflammatory response via interleukin-10: Role in renoprotection in obese rats. Hypertension 61: 1218–1226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, Thöne-Reineke C, Reichenbach A, Schacherl J, Dahlöf B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck WH, Unger T, Steckelings UM: Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 55: 924–931, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Cherney DZ, Reich HN, Scholey JW, Daneman D, Mahmud FH, Har RL, Sochett EB: The effect of aliskiren on urinary cytokine/chemokine responses to clamped hyperglycaemia in type 1 diabetes. Diabetologia 56: 2308–2317, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Morishita Y, Hanawa S, Chinda J, Iimura O, Tsunematsu S, Kusano E: Effects of aliskiren on blood pressure and the predictive biomarkers for cardiovascular disease in hemodialysis-dependent chronic kidney disease patients with hypertension. Hypertens Res 34: 308–313, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA; ALTITUDE Investigators : Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ: Activation of human T cells in hypertension: Studies of humanized mice and hypertensive humans. Hypertension 68: 123–132, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Paolo S, Schena A, Stallone G, Grandaliano G, Soccio M, Cerullo G, Gesualdo L, Paolo Schena F: Captopril enhances transforming growth factor (TGF)-beta1 expression in peripheral blood mononuclear cells: A mechanism independent from angiotensin converting enzyme inhibition? A study in cyclosporine-treated kidney-transplanted patients. Transplantation 74: 1710–1715, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Koh KK, Ahn JY, Han SH, Kim DS, Jin DK, Kim HS, Shin MS, Ahn TH, Choi IS, Shin EK: Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol 42: 905–910, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Sonmez A, Kisa U, Uckaya G, Eyileten T, Comert B, Koc B, Kocabalkan F, Ozata M: Effects of losartan treatment on T-cell activities and plasma leptin concentrations in primary hypertension. J Renin Angiotensin Aldosterone Syst 2: 112–116, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, Kang MH, Ahn TH, Choi IS, Shin EK: Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation 110: 3687–3692, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Lopez Santi RG, Valeff EC, Duymovich CR, Mazziotta D, Mijailovsky NE, Filippa GC, Maltez R, Hernandez VA, Monroy AG, Borzi JG, Acheme RA, Etchegoyen MC; PROCORDIS investigators : Effects of an angiotensin-converting enzyme inhibitor (ramipril) on inflammatory markers in secondary prevention patients: RAICES Study. Coron Artery Dis 16: 423–429, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Di Napoli M, Papa F: Angiotensin-converting enzyme inhibitor use is associated with reduced plasma concentration of C-reactive protein in patients with first-ever ischemic stroke. Stroke 34: 2922–2929, 2003 [DOI] [PubMed] [Google Scholar]
- 88.Tsuruoka S, Kai H, Usui J, Morito N, Saito C, Yoh K, Yamagata K: Effects of irbesartan on inflammatory cytokine concentrations in patients with chronic glomerulonephritis. Intern Med 52: 303–308, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Kintscher U, Marx N, Martus P, Stoppelhaar M, Schimkus J, Schneider A, Walcher D, Kümmel A, Winkler R, Kappert K, Dörffel Y, Scholze J, Unger T: Effect of high-dose valsartan on inflammatory and lipid parameters in patients with Type 2 diabetes and hypertension. Diabetes Res Clin Pract 89: 209–215, 2010 [DOI] [PubMed] [Google Scholar]
- 90.Gamboa JL, Pretorius M, Todd-Tzanetos DR, Luther JM, Yu C, Ikizler TA, Brown NJ: Comparative effects of angiotensin-converting enzyme inhibition and angiotensin-receptor blockade on inflammation during hemodialysis. J Am Soc Nephrol 23: 334–342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato H, Akai Y, Iwano M, Kurumatani N, Kurioka H, Kubo A, Yamaguchi T, Fujimoto T, Dohi K: Association of an insertion polymorphism of angiotensin-converting enzyme gene with the activity of systemic lupus erythematosus. Lupus 7: 530–534, 1998 [DOI] [PubMed] [Google Scholar]
- 92.Akai Y, Sato H, Iwano M, Kurumatani N, Kurioka H, Kubo A, Yamaguchi T, Shiiki H, Fujimoto T, Dohi K: Association of an insertion polymorphism of angiotensin-converting enzyme gene with the activity of lupus nephritis. Clin Nephrol 51: 141–146, 1999 [PubMed] [Google Scholar]
- 93.Tavana S, Argani H, Gholamin S, Razavi SM, Keshtkar-Jahromi M, Talebian AS, Moghaddam KG, Sepehri Z, Azad TM, Keshtkar-Jahromi M: Influenza vaccination in patients with pulmonary sarcoidosis: Efficacy and safety. Influenza Other Respi Viruses 6: 136–141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prkacin I, Novak B, Sertić J, Mrzljak A: Angiotensin-converting enzyme gene polymorphism in patients with systemic lupus. Acta Med Croatica 55: 73–76, 2001 [PubMed] [Google Scholar]
- 95.Kaufman KM, Kelly J, Gray-McGuire C, Asundi N, Yu H, Reid J, Baird T, Hutchings D, Bruner G, Scofield RH, Moser K, Harley JB: Linkage analysis of angiotensin-converting enzyme (ACE) insertion/deletion polymorphism and systemic lupus erythematosus. Mol Cell Endocrinol 177: 81–85, 2001 [DOI] [PubMed] [Google Scholar]
- 96.Rubio-Tapia A, Herman ML, Ludvigsson JF, Kelly DG, Mangan TF, Wu TT, Murray JA: Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc 87: 732–738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ianiro G, Bibbò S, Montalto M, Ricci R, Gasbarrini A, Cammarota G: Systematic review: Sprue-like enteropathy associated with olmesartan. Aliment Pharmacol Ther 40: 16–23, 2014 [DOI] [PubMed] [Google Scholar]
- 98.Marthey L, Cadiot G, Seksik P, Pouderoux P, Lacroute J, Skinazi F, Mesnard B, Chayvialle JA, Savoye G, Druez A, Parlier D, Abitbol V, Gompel M, Eoche M, Poncin E, Bobichon R, Colardelle P, Wils P, Salloum H, Peschard S, Zerbib F, Méresse B, Cerf-Bensussan N, Malamut G, Carbonnel F: Olmesartan-associated enteropathy: Results of a national survey. Aliment Pharmacol Ther 40: 1103–1109, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C; ONTARGET Investigators : Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators : Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 101.Titan SM, M Vieira J Jr, Dominguez WV, Barros RT, Zatz R: ACEI and ARB combination therapy in patients with macroalbuminuric diabetic nephropathy and low socioeconomic level: A double-blind randomized clinical trial. Clin Nephrol 76: 273–283, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Neri Serneri GG, Boddi M, Modesti PA, Coppo M, Cecioni I, Toscano T, Papa ML, Bandinelli M, Lisi GF, Chiavarelli M: Cardiac angiotensin II participates in coronary microvessel inflammation of unstable angina and strengthens the immunomediated component. Circ Res 94: 1630–1637, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Tsikouris JP, Suarez JA, Simoni JS, Ziska M, Meyerrose GE: Exploring the effects of ACE inhibitor tissue penetration on vascular inflammation following acute myocardial infarction. Coron Artery Dis 15: 211–217, 2004 [PubMed] [Google Scholar]
- 104.Ayoub MA, Zhang Y, Kelly RS, See HB, Johnstone EK, McCall EA, Williams JH, Kelly DJ, Pfleger KD: Functional interaction between angiotensin II receptor type 1 and chemokine (C-C motif) receptor 2 with implications for chronic kidney disease. PLoS One 10: e0119803, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B: Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17[Suppl 3]: S218–S225, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T: Infliximab, a TNF-α inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens 28: 165–169, 2014 [DOI] [PubMed] [Google Scholar]
- 107.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG: AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension 43: 1126–1132, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woodard LE, Wilson MH: piggyBac-ing models and new therapeutic strategies. Trends Biotechnol 33: 525–533, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyagi A, Lu A, Humphreys BD: Gene editing: Powerful new tools for nephrology research and therapy. J Am Soc Nephrol 27: 2940–2947, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang J, Rudemiller NP, Patel MB, Wei Q, Karlovich NS, Jeffs AD, Wu M, Sparks MA, Privratsky JR, Herrera M, Gurley SB, Nedospasov SA, Crowley SD: Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J Am Soc Nephrol 27: 2257–2264, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD: Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 23: 360–368, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, Hung AM: IL-1 inhibition and vascular function in CKD [published online ahead of print September 19, 2016]. J Am Soc Nephrol doi: 10.1681/ASN.2016040453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petty WJ, Miller AA, McCoy TP, Gallagher PE, Tallant EA, Torti FM: Phase I and pharmacokinetic study of angiotensin-(1-7), an endogenous antiangiogenic hormone. Clin Cancer Res 15: 7398–7404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz G, Lumeng CN, Mortensen RM: Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 120: 3350–3364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]