Abstract
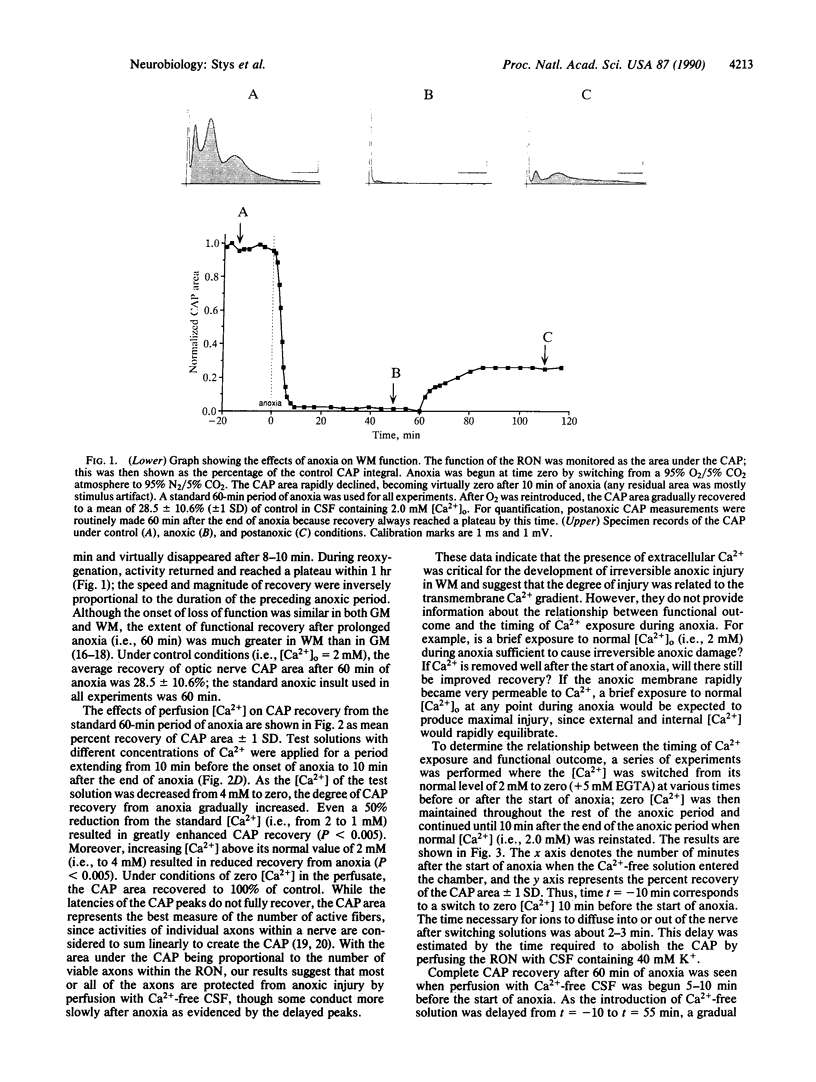
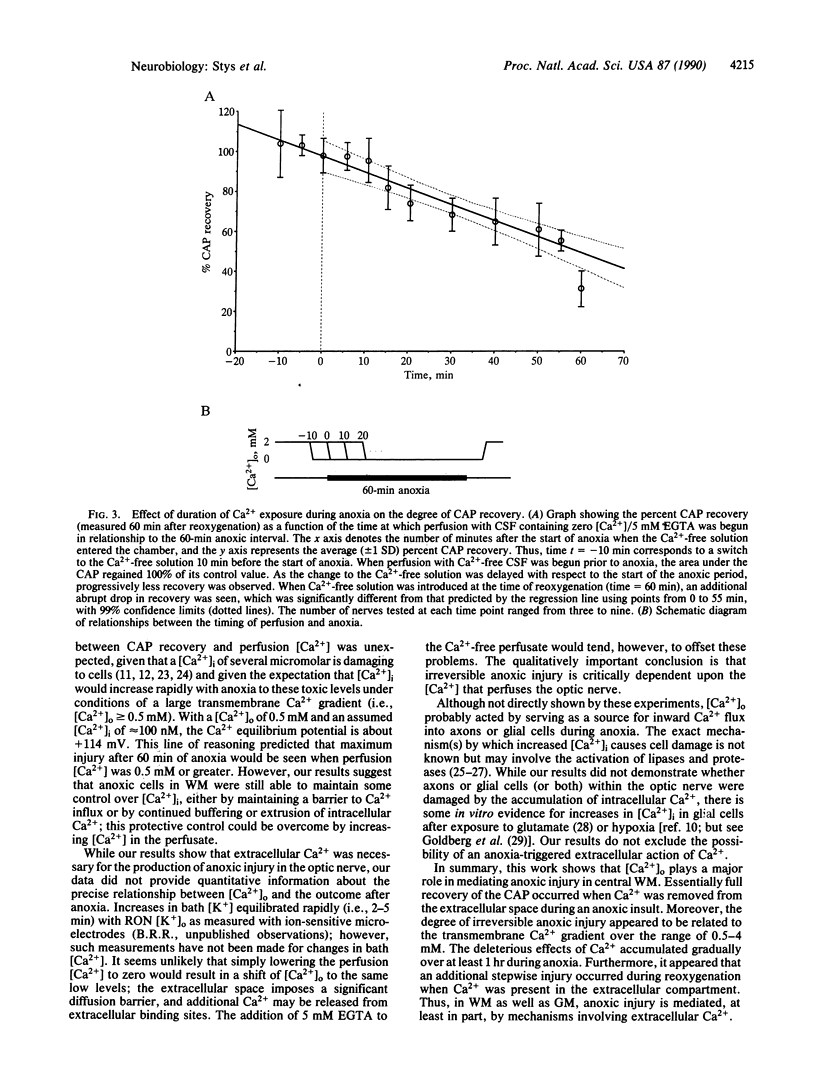
White matter (WM) of the mammalian brain is susceptible to anoxic injury, but little is known about the pathophysiology of this process. We studied the mechanisms of anoxic injury in WM using the isolated rat optic nerve, a typical central nervous system WM tract. Optic nerve function, measured as the area under the compound action potential, rapidly failed when exposed to anoxia and recovered to 28.5% of control after a standard 60-min period of anoxia. Irreversible anoxic injury was critically dependent on the molar concentration of extracellular calcium [( Ca2+]o); maintaining the tissue in Ca2(+)-free solution throughout the anoxic period resulted in 100% compound action potential recovery. Increasing perfusion [Ca2+] during anoxia from zero to 4 mM resulted in progressively less recovery. As the introduction of the Ca2(+)-free solution was progressively delayed with respect to the onset of anoxia, a graded decrease in recovery of function was observed, suggesting that in WM the deleterious effects of Ca2+ accumulate slowly during anoxia. At the time of reoxygenation, an additional stepwise increase in injury was observed that was also Ca2(+)-dependent. Mammalian WM, which is relatively resistant to anoxic injury compared with gray matter, is damaged by anoxia in a manner that appears to depend on the gradual accumulation of Ca2+ in a cytoplasmic compartment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste H., Jørgensen M. B., Diemer N. H., Hansen A. J. Calcium accumulation by glutamate receptor activation is involved in hippocampal cell damage after ischemia. Acta Neurol Scand. 1988 Dec;78(6):529–536. doi: 10.1111/j.1600-0404.1988.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985 Aug 5;58(3):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Ransom B. R., Kunis D. M., Gutnick M. J. Activity-dependent K+ accumulation in the developing rat optic nerve. Science. 1982 Jun 18;216(4552):1341–1343. doi: 10.1126/science.7079771. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cummins K. L., Perkel D. H., Dorfman L. J. Nerve fiber conduction-velocity distributions. I. Estimation based on the single-fiber and compound action potentials. Electroencephalogr Clin Neurophysiol. 1979 Jun;46(6):634–646. doi: 10.1016/0013-4694(79)90101-9. [DOI] [PubMed] [Google Scholar]
- Demopoulos H. B., Flamm E. S., Pietronigro D. D., Seligman M. L. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980;492:91–119. [PubMed] [Google Scholar]
- FISHER C. M., COLE M. HOMOLATERAL ATAXIA AND CRURAL PARESIS: A VASCULAR SYNDROME. J Neurol Neurosurg Psychiatry. 1965 Feb;28:48–55. doi: 10.1136/jnnp.28.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. M., Caplan L. R. Basilar artery branch occlusion: a cause of pontine infarction. Neurology. 1971 Sep;21(9):900–905. doi: 10.1212/wnl.21.9.900. [DOI] [PubMed] [Google Scholar]
- Fisher C. M. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979 Feb;36(2):65–73. doi: 10.1001/archneur.1979.00500380035003. [DOI] [PubMed] [Google Scholar]
- Flamm E. S., Demopoulos H. B., Seligman M. L., Poser R. G., Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978 Sep-Oct;9(5):445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- Foster R. E., Connors B. W., Waxman S. G. Rat optic nerve: electrophysiological, pharmacological and anatomical studies during development. Brain Res. 1982 Mar;255(3):371–386. doi: 10.1016/0165-3806(82)90005-0. [DOI] [PubMed] [Google Scholar]
- Goldberg M. P., Weiss J. H., Pham P. C., Choi D. W. N-methyl-D-aspartate receptors mediate hypoxic neuronal injury in cortical culture. J Pharmacol Exp Ther. 1987 Nov;243(2):784–791. [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mohr J. P., Kase C. S., Meckler R. J., Fisher C. M. Sensorimotor stroke due to thalamocapsular ischemia. Arch Neurol. 1977 Dec;34(12):739–741. doi: 10.1001/archneur.1977.00500240027004. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Davis G., Orrenius S. The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett. 1986 Dec 1;209(1):139–144. doi: 10.1016/0014-5793(86)81099-7. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Thor H., Orrenius S. Cytosolic-free Ca2+ and cell killing in hepatoma 1c1c7 cells exposed to chemical anoxia. FASEB J. 1989 Jan;3(1):59–64. doi: 10.1096/fasebj.3.1.2910738. [DOI] [PubMed] [Google Scholar]
- Ogura A., Miyamoto M., Kudo Y. Neuronal death in vitro: parallelism between survivability of hippocampal neurones and sustained elevation of cytosolic Ca2+ after exposure to glutamate receptor agonist. Exp Brain Res. 1988;73(3):447–458. doi: 10.1007/BF00406601. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Calcium and ischemic brain damage. Eur Neurol. 1986;25 (Suppl 1):45–56. doi: 10.1159/000116060. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Auer R. N., Siesjö B. K. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol. 1984;64(4):319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]




