Abstract
Introduction
Oncologists have traditionally been responsible for providing routine follow-up care for cancer survivors; in recent years, however, primary care providers (pcps) are taking a greater role in care during the follow-up period. In the present study, we used a longitudinal multi-province retrospective cohort study to examine how primary care and specialist care intersect in the delivery of breast cancer follow-up care.
Methods
Various databases (registry, clinical, and administrative) were linked in each of four provinces: British Columbia, Manitoba, Ontario, and Nova Scotia. Population-based cohorts of breast cancer survivors were identified in each province. Physician visits were identified using billings or claims data and were classified as visits to primary care (total, breast cancer–specific, and other), oncology (medical oncology, radiation oncology, and surgery), and other specialties. The mean numbers of visits by physician type and specialty, or by combinations thereof, were examined. The mean numbers of visits for each follow-up year were also examined by physician type.
Results
The results showed that many women (>64%) in each province received care from both primary care and oncology providers during the follow-up period. The mean number of breast cancer–specific visits to primary care and visits to oncology declined with each follow-up year. Interprovincial variations were observed, with greater surgeon follow-up in Nova Scotia and greater primary care follow-up in British Columbia. Provincial differences could reflect variations in policies and recommendations, relevant initiatives, and resources or infrastructure to support pcp-led follow-up care.
Conclusions
Optimizing the role of pcps in breast cancer follow-up care might require strategies to change attitudes about pcp-led follow-up and to better support pcps in providing survivorship care.
Keywords: Breast cancer, survivors, follow-up care, primary care
INTRODUCTION
Although the cancer incidence in Canada continues to rise as a result of an increasing and aging population, advances in screening, diagnostic technologies, and treatment have contributed to decreased cancer mortality1. With more new cases of cancer being diagnosed and fewer people dying of the disease, the number of cancer survivors is growing rapidly. That increase in the survivor population is particularly evident in the case of breast cancer: 25,000 women were estimated to have been diagnosed with breast cancer in 2015, with a current 5-year relative survival rate of 88%1.
Women diagnosed with breast cancer who successfully complete surgery with or without adjuvant or neoadjuvant therapy (that is, treatment with curative intent) typically transition into what is called “routine follow-up care” or “survivorship care.” According to the U.S. Institute of Medicine2, follow-up care should involve these essential components:
■ Prevention and detection of new and recurrent cancer
■ Surveillance for cancer spread, recurrence, or second cancers
■ Intervention for the consequences of cancer and its treatment (for example, physical issues such as such as lymphedema, pain, and fatigue, and psychosocial issues such as anxiety and distress)
■ Coordination between specialists and primary care providers (pcps) to ensure that a survivor’s overall health needs are met
Although specialists and pcps both play a role in addressing the myriad health care needs of survivors (which include not only cancer-related follow-up care, but also care for non-cancer-related health issues such as chronic disease screening and management), their roles are changing. Traditionally, cancer-related follow-up care was considered the responsibility of oncology specialists. However, several studies have found that primary care physicians are both willing to take on a greater role in follow-up care3 and able to provide follow-up that is as effective as specialist follow-up care4–6. In addition, follow-up care led by pcps has resulted in higher satisfaction for patients7. In light of those findings, many jurisdictions across Canada have implemented formal initiatives to transition routine follow-up to pcps. Examples of such initiatives are the Juravinski Cancer Centre well follow-up clinics in Ontario, the Moving Forward After Cancer Treatment program in Manitoba, the Provincial Integrated Cancer Survivorship Program in Alberta, and the Family Practice Oncology Network in British Columbia8.
The aim of the present multi-province study was to examine the use of physician services during the survivorship phase of breast cancer care, with a specific emphasis on how primary care and specialist care intersect. These specific questions were investigated:
■ How are various specialties involved in follow-up care for Canadian women who have been diagnosed with breast cancer?
■ Does the involvement differ between provinces or by years of follow-up?
METHODS
The work reported here was conducted as part of a longitudinal multi-province retrospective cohort study of breast cancer care using linked registry, clinical, and administrative health data9. Data linkage and analyses were carried out separately in each of the participating provinces. Approvals were received from all relevant institutional research ethics boards (University of British Columbia–BC Cancer Agency Research Ethics Board, University of Manitoba Health Research Ethics Board, Health Sciences and Affiliated Hospitals Research Ethics Board at Queen’s University in Ontario, Nova Scotia Health Authority Research Ethics Board) and from all relevant data access and privacy committees (BC Cancer Agency, B.C. Data Stewardship Committee, Manitoba Health’s Health Information Privacy Committee, ices–Queen’s Privacy Office, N.S. Department of Health and Wellness Data Access Committee, Health Data Nova Scotia Data Access Committee).
Study Population
Using the respective provincial cancer registries, a population-based cohort of women who had been diagnosed with a first-ever invasive carcinoma of the breast were identified in each of British Columbia, Manitoba, Ontario, and Nova Scotia. The years from which the cohorts were identified varied slightly by province depending on data availability (British Columbia: 2007–2010; Manitoba: 2007–2011; Ontario: 2007–2010; Nova Scotia: 2007–2012). For women having more than 1 cancer diagnosed in the same breast on the same day, the following hierarchy was used to select just 1 diagnosis: the highest stage, the highest histology priority, or the first malignancy number. Women were excluded if they did not have a unique health identifier, were non-residents of the province, were diagnosed with an in situ or stage 0 cancer, had a previous cancer diagnosis other than non-melanoma skin cancer, or had histology not specific to the breast, such as a non-solid tumour.
From the resulting cohort of women diagnosed with breast cancer, survivor cohorts were created. In each province, individuals were included in the survivorship phase analysis if
■ they had received curative surgery (that is, lumpectomy or mastectomy),
■ they had no metastases within 1 year of the diagnosis date,
■ they were alive 2 years after the diagnosis date (that is, they had at least 1 full year of routine survivor data, assuming a treatment phase of 1 year), and
■ they were registered in their provincial health insurance program from the date of diagnosis to the end date (that is, censoring or end of study).
Given that inclusion in the cohort was based on receipt of lumpectomy or mastectomy rather than on stage, a small number of individuals with stage iv breast cancers (who might have been clinical stage iii) were included in each province, although those women accounted for a maximum of 2.0% of each provincial cohort.
Individuals were censored from the study 6 months before their date of death or 3 months before diagnosis of a new primary cancer or evidence of recurrence. Recurrence was determined using documentation of recurrence within the provincial cancer registry or evidence from within physician billings or cancer registry data of receipt of chemotherapy or radiotherapy 2 years after the original diagnosis date. The end-of-study date was the date 6 months preceding the most recent death clearance data in each province.
Variables
In each province, multiple databases—for example, provincial cancer registries, physician billings or claims and scheduling databases, hospitalization databases, census data—were accessed to obtain cohort demographics and health care utilization information. Within each province, databases were linked deterministically via encrypted health card numbers providing longitudinal, individual-level data.
Age at diagnosis was derived using date of birth and date of diagnosis from provincial cancer registries. Provincial cancer registries provided TNM stage10,11. Comorbidity was computed using the Johns Hopkins Aggregated Diagnosis Groups (adg) system12. Rural residence was determined using the classifications developed by Statistics Canada13. Treatments were defined as follows:
■ Mastectomy Uni- or bilateral breast removal (that is, total or radical mastectomy) was determined using procedure codes from hospitalization and physician billings or claims data (Manitoba, Ontario, Nova Scotia) or from cancer registry data (British Columbia) within 2 weeks before to 9 months after diagnosis. In British Columbia, for referred cases, if a woman had a lumpectomy and mastectomy, the more definitive procedure (mastectomy) was reported.
■ Lumpectomy Excision of breast lesion, including partial or total excision of nipple or lactiferous duct, was determined using procedure codes from hospitalization and billings or claims data (Manitoba, Ontario, Nova Scotia) or cancer registry data (British Columbia) within 2 weeks before to 9 months after diagnosis.
■ Chemotherapy Delivery of any chemotherapy (neoadjuvant, adjuvant, or not otherwise specified) was determined using procedure codes for the administration of intravenous chemotherapy within physician billings or claims data (Manitoba, Ontario, Nova Scotia), provincial cancer pharmacy data [British Columbia (data held by the BC Cancer Agency)], or patterns of visits to medical oncology using physician billings data and data from the cancer centre scheduling database (Nova Scotia only).
■ Radiotherapy Delivery of nonpalliative radiotherapy within 9 months of the date of diagnosis, was determined from databases held by the provincial cancer program or agency (for example, cancer registries, scheduling databases).
Physician visits were identified from physician billings or claims data. Outpatient visits (that is, visits to home, office, or long-term care facility) were classified based on physician specialty into three physician types: primary care, oncology, or other. Primary care visits included visits to physicians whose main specialty was family, community, or general medicine. Using the diagnosis codes contained within physician billings data, primary care visits were subdivided into breast cancer–specific and other. Oncology visits included visits to physicians whose main specialty was medical oncology or radiation oncology and visits to a select group of surgeons in each province. In British Columbia, Manitoba, and Ontario, visits to all surgeons contained within the dataset who had completed at least 1 breast surgery were considered oncology visits. Consistent with previous work on follow-up care for colorectal cancer14, all visits in Nova Scotia to a general surgeon were considered oncology visits. Visits to all other specialties were considered “other.”
Statistical Analyses
Analyses were conducted separately in each province. Physician visits were examined during the survivorship phase, which was defined as the 4-year period beginning 1 year after the date of diagnosis and ending 5 years after the date of diagnosis or at censoring, whichever came first. To assess the volume of visits to each specialty, we calculated the mean number of visits per person over that person’s total follow-up time and, separately, the percentage of individuals with at least 1 visit to each specialty.
To assess how the various physician types and specialties, particularly primary care and oncology, intersected during follow-up care, we calculated the percentage of unique individuals with visits to specific physician types or specialties, or combinations thereof (that is, each individual appeared in only one category). The mean number of visits (mean per person per follow-up year) was calculated for each physician type (primary care, oncology, and other) and for each province and year of follow-up. To account for variations in cohort demographics across provinces, we conducted post hoc stratified analyses of primary care visits by stage and comorbidity (number of adgs). No statistical tests were performed, because the results describe a census of patients in those years. Instead, our interpretation of the results focused on demographically and clinically important differences.
RESULTS
The mean follow-up time was 3.5 years in British Columbia and Manitoba, 4.7 years in Ontario, and 2.9 years in Nova Scotia. The shorter follow-up time in Nova Scotia is largely the result of an earlier end-of-study date there than in other provinces (that is, death clearance data were up-to-date as of 31 March 2014, resulting in an end-of-study date of 1 October 2013), which excluded all individuals diagnosed in 2012 from the analyses.
Cohort Characteristics
Table i presents the characteristics of the survivorship cohort. The average age of each cohort at diagnosis was approximately 60 years, and the age distribution was similar across provinces. The percentage of the cohort diagnosed with stages i and ii breast cancer was greater in Manitoba (87.6%) and Nova Scotia (86.9%) than in British Columbia (77.9%) and Ontario (78.6%). British Columbia and Ontario both had a greater percentage of patients with disease of unknown stage. Comorbidity was lowest in British Columbia, as evidenced by the greatest percentage of individuals with 0–3 adgs and the lowest percentage with 10 or more adgs. A greater percentage of women in Nova Scotia received mastectomies, and a lower percentage of women in British Columbia received lumpectomies (some might have been captured in the “unknown surgery” category). A lower percentage of women appear to have received chemotherapy in British Columbia; however, that observation might reflect the fact that chemotherapy data were not available for 14.5% of the B.C. cohort.
TABLE I.
Characteristics of the survivorship cohort
| Characteristic | Cohort years by province | |||
|---|---|---|---|---|
|
| ||||
| British Columbia 2007–2010 | Manitoba 2007–2011 | Ontario 2007–2010 | Nova Scotia 2007–2012 | |
| Cohort size (n)a | 9,338 | 2,688 | 23,700 | 2,735 |
| Mean age at diagnosis (years) | 60.6±13.2 | 61±13.17 | 60.0±13.0 | 60.8±12.9 |
| Age group (%) | ||||
| <40 Years | 4.3 | 4.2 | 5.7 | 3.9 |
| 40–49 Years | 18.2 | 16.1 | 17.2 | 17.9 |
| 50–59 Years | 25.5 | 26.6 | 26.7 | 23.8 |
| 60–69 Years | 26.2 | 27.0 | 26.0 | 28.9 |
| 70–74 Years | 9.2 | 9.5 | 9.4 | 10.1 |
| ≥75 Years | 16.6 | 16.6 | 15.0 | 15.4 |
| Rural residenceb (%) | 14.1 | 28.1 | 12.1 | 34.5 |
| Stage at diagnosis (%) | ||||
| I | 44.7 | 47.2 | 42.3 | 50.0 |
| II | 33.2 | 40.4 | 36.3 | 36.9 |
| III | 11.5 | 11.8 | 11.2 | 11.5 |
| IV | 2.0 | 0.3 | 0.4 | 0.8 |
| Unknown | 8.7 | 0.4 | 9.8 | 0.8 |
| Comorbidity (%) | ||||
| 0–3 ADGs | 31.2 | 22.3 | 24.9 | 20.0 |
| 4–5 ADGs | 24.5 | 24.4 | 23.1 | 20.9 |
| 6–7 ADGs | 20.2 | 20.8 | 21.9 | 21.1 |
| 8–9 ADGs | 13.2 | 16.2 | 15.4 | 17.6 |
| ≥10 ADGs | 10.9 | 16.4 | 14.8 | 20.4 |
| Treatment received (%)c | ||||
| Lumpectomy | 51.6 | 72.5 | 72.9 | 72.0 |
| Mastectomy | 37.1 | 33.9 | 35.0 | 49.7 |
| Chemotherapy (any) | 35.8 | 46.6 | 45.9 | 42.1d |
| Radiotherapy (adjuvant) | 64.1 | 57.7 | 64.6 | 57.0 |
Total number of individuals with at least 1 year of complete follow-up data.
All categories except urban.
Complete treatment data were not available for all cohort members in British Columbia. Where data were incomplete, a “flag” was assigned by the BC Cancer Agency to indicate receipt of mastectomy or lumpectomy, permitting inclusion of the individual in the cohort.
In Nova Scotia, chemotherapy was not complete in physician billings data. Where chemotherapy was not present in the billings data, it was considered to have been administered if the individual had 2 visits to medical oncology within 6 months of first medical oncology consultation as determined in physician billings or in the cancer centre scheduling database.
ADGs = Johns Hopkins Aggregated Diagnosis Groups
Physician Visits by Physician Type or Specialty
As shown in Table ii, the most frequently visited physician type during the follow-up period (that is, the period beginning 1 year after diagnosis and ending 5 years after diagnosis) was primary care. In all provinces, the mean number of visits was higher for pcps than for other specialities, and nearly all individuals in each province had at least 1 visit to a pcp during follow-up. The mean number of breast cancer–specific visits to primary care was low (approximately 1 or less), with only between half and two thirds of women visiting a pcp for a breast cancer–specific issue. In contrast, upward of 65% of women made at least 1 visit to oncology, with medical oncology being the most usual and frequent specialty visited. Most women in each province also had visits to other specialities during the follow-up period.
TABLE II.
Physician visits during the follow-up period
| Visit designation | British Columbia (n=9,338) | Manitoba (n=2,688) | Ontario (n=23,700) | Nova Scotia (n=2,735) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mean visitsa (n) | Pts with 1 visit or more (%) | Mean visitsa (n) | Pts with 1 visit or more (%) | Mean visitsa (n) | Pts with 1 visit or more (%) | Mean visitsa (n) | Pts with 1 visit or more (%) | |
| Primary care | ||||||||
| All | 5.2±4.0 | 97.9 | 5.9±4.4 | 97.7 | 5.3±4.8 | 97.6 | 4.4±3.6 | 95.9 |
| Breast cancer–specificb | 0.6±0.8 | 64.0 | 0.5±0.8 | 55.2 | 0.9±1.7 | 67.8 | 1.3±1.5 | 65.9 |
| Other | 4.6±3.9 | 97.1 | 5.4±4.2 | 97.4 | 4.5±4.3 | 96.2 | 3.7±3.2 | 93.0 |
| Oncology | ||||||||
| All | 1.2±1.8 | 65.4 | 2.0±2.5 | 83.5 | 2.7±2.1 | 95.3 | 1.6±1.6 | 82.3 |
| Medical | 0.8±1.4 | 42.2 | 1.5±2.3 | 66.3 | 2.6±4.1 | 82.5 | 1.7±2.1 | 23.5 |
| Radiation | 0.1±0.5 | 15.0 | 0.2±0.4 | 32.4 | 1.4±2.0 | 39.0 | 0.7±0.5 | 18.0 |
| Surgeryc | 0.3±0.7 | 35.9 | 0.3±0.6 | 36.8 | 1.8±2.8 | 66.9 | 1.1±0.7 | 71.1 |
| Other | 1.7±2.3 | 83.3 | 3.9±5.4 | 94.4 | 2.9±3.6 | 65.6 | 2.5±3.0 | 81.2 |
Mean number of visits to each physician type per patient per year. This was calculated as the total number of visits to each type or speciality during the follow-up duration (that is, beginning of follow-up to study end date for each individual) divided by the total follow-up time for that individual.
Breast cancer–specific visits were those with an associated diagnosis code corresponding to the following categories of diagnosis codes within the billings or claims data: breast neoplasms, benign neoplasms and carcinoma in situ, and infectious and inflammatory conditions of the breast. In Nova Scotia and Ontario, anxiety and lymph system–related conditions were used, as were breast-related procedure codes in Ontario.
Visits to a surgeon included all visits to a physician with a main speciality of general surgery as shown in the billing or claims data in Nova Scotia. In other provinces, visits to a surgeon included only visits to a surgeon who performed at least 1 breast cancer–related procedure during the study period.
Pts = patients.
Most women received care from a combination of physician types during the follow-up period (Table iii). Between 64.6% and 93.1% of women made visits to both primary care and oncology. The most common combination was “primary care and multiple” in all provinces except Nova Scotia, where the most common combination was “primary care and surgery.” British Columbia had the highest percentage of women with visits to primary care only (33.3%). In other words, those women received no care from oncology during the follow-up period. In all provinces, follow-up from primary care only was more common than follow-up care from oncology only.
TABLE III.
Unique patients with visits to each physician type or specialty or combination thereof during the follow-up period
| Physician type or specialty | Patients with visits during follow-up (%) | |||
|---|---|---|---|---|
|
| ||||
| British Columbia (n=9,338) | Manitoba (n=2,688) | Ontario (n=23,700) | Nova Scotia (n=2,735) | |
| Primary care onlya | 33.3 | 15.8 | 4.7 | 16.2 |
| Oncology only | ||||
| Medical | 0.4 | 0.8 | 0.3 | 0.4 |
| Radiation | 0.1 | 0.1 | 0.1 | 0.3 |
| Surgery | 0.1 | 0.2 | 0.2 | 1.2 |
| Multiple | 0.2 | 0.5 | 1.2 | 0.7 |
| TOTAL | 0.7 | 1.6 | 1.7 | 2.6 |
| Primary care and oncologyb | ||||
| Primary care and medical | 20.0 | 26.3 | 16.8 | 5.4 |
| Primary care and radiation | 5.4 | 6.8 | 2.5 | 3.8 |
| Primary care and surgeryc | 15.1 | 7.1 | 6.8 | 44.2 |
| Primary care and multiple | 24.2 | 41.7 | 67.0 | 26.3 |
| TOTAL | 64.6 | 81.9 | 93.1 | 79.7 |
| Other onlyd | 0.2 | 0.3 | 0.1 | 0.3 |
| No physician | 1.2 | 0.4 | 0.3 | 1.2 |
With or without visits to “other”; no visits to “oncology.”
With or without visits to “other.”
Visits to a surgeon included all visits to a physician with a main speciality of general surgery as shown in the billing or claims data in Nova Scotia. In other provinces, visits to a surgeon included only visits to a surgeon who performed at least 1 breast cancer–related procedure during the study period.
For example, optometry, podiatry, hematology.
Variations by Province and Year
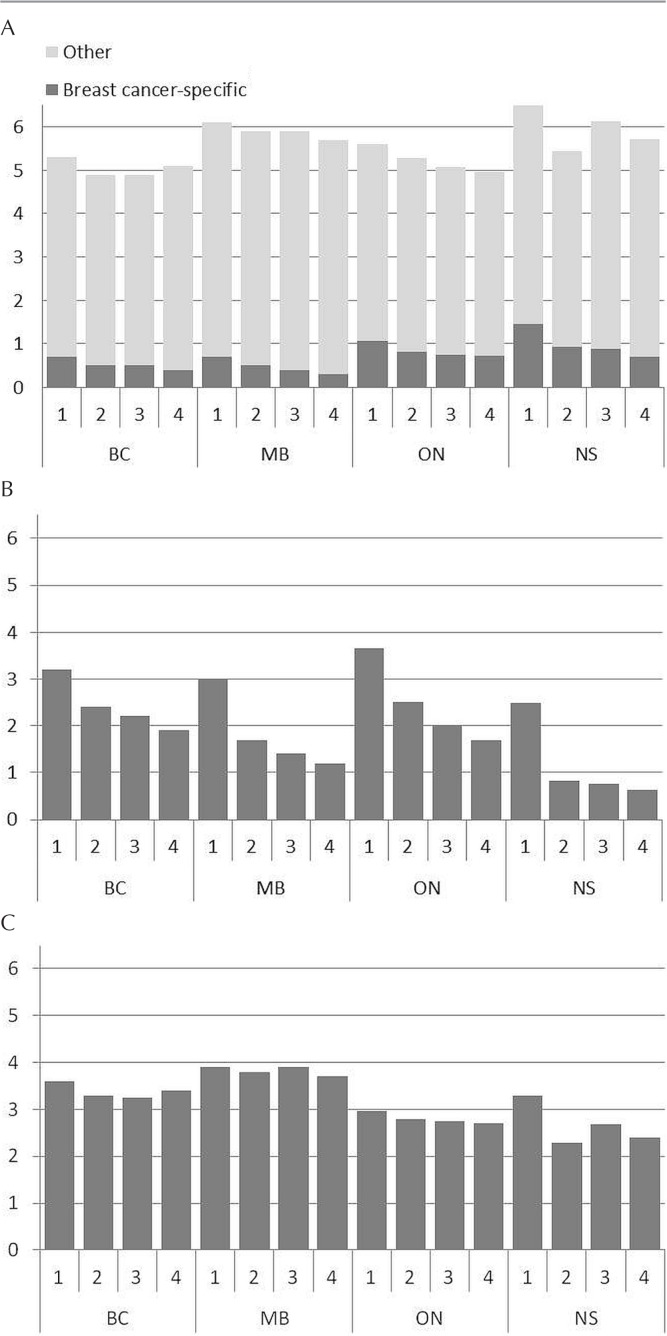
In all provinces, the mean number of breast cancer–specific primary care visits per person declined from year 1 to year 4 [Figure 1(A)]. The greatest mean number of breast cancer–specific primary care visits for each year of follow-up occurred in Nova Scotia, except for year 4, when Nova Scotia and Ontario each had a mean of 0.7 visits. The mean number of visits to oncology also declined from year 1 to year 4 of follow-up in each province [Figure 1(B)]. The decline in oncology visits occurred gradually in all provinces except Nova Scotia, where a sharp decline after year 1 was evident (to 0.82 from 2.49 visits), with only a marginal decline thereafter (to 0.64 in year 4 from 0.82 in year 2). For other specialities, the mean number of visits per person was greatest in Manitoba [Figure 1(C)]. Although number of visits to primary care and oncology declined over the course of the follow-up period in all provinces, such a decline did not occur for other specialties, except in Ontario.
FIGURE 1.
Mean visits per person to each physician type by year of follow-up and province. The analyses include only individuals with complete data for each follow-up year. (A) Primary care. Breast cancer–specific = visits having a diagnosis code corresponding to the following categories of diagnosis codes within billings or claims data: breast neoplasms, benign neoplasms and carcinoma in situ, and infectious and inflammatory conditions of the breast. In Nova Scotia and Ontario, anxiety and lymph system–related conditions were used, as were breast-related procedure codes in Ontario. (B) Oncology. In Nova Scotia, visits to a surgeon included all visits to a physician with a main speciality of general surgery as shown in the billing or claims data. In other provinces, visits to a surgeon included only visits to a surgeon who performed at least 1 breast cancer–related procedure during the study period. (C) Other.
Primary Care Visits Stratified by Stage and Comorbidity
The mean number of visits per person per year to primary care did not vary substantially with stage (Table iv). The mean number of visits per person to primary care did consistently increase with comorbidity (that is, greater number of adgs) for all provinces.
TABLE IV.
Visits per person per year to primary care by stage, comorbidity, and province
| Variable | Mean visits during follow-upa (n) | |||
|---|---|---|---|---|
|
| ||||
| British Columbia (n=9,338) | Manitoba (n=2,688) | Ontario (n=23,700) | Nova Scotia (n=2,735) | |
| Stage | ||||
| I | 5.2±3.8 | 5.8±4.1 | 5.2±4.5 | 4.2±3.5 |
| II | 5.1±4.0 | 6.0±4.7 | 5.4±4.7 | 4.4±3.9 |
| III | 5.1±4.1 | 5.6±4.1 | 5.2±5.3 | 4.0±3.3 |
| Unknown | 5.2±4.3 | 6.7±3.9 | 6.0±5.2 | 5.5±3.8 |
| Comorbidity | ||||
| 0–3 ADGs | 3.4±2.7 | 3.7±2.5 | 3.2±3.2 | 2.6±2.4 |
| 4–7 ADGs | 5.0±3.3 | 5.4±3.4 | 4.8±3.9 | 3.7±3.0 |
| ≥8 ADGs | 7.7±5.0 | 8.0±5.4 | 7.8±5.8 | 5.7±4.2 |
Calculated based on the number of complete years for which an individual had complete follow-up data (that is, does not include partial years) and the total number of visits during those years.
ADGs = Johns Hopkins Aggregated Diagnosis Groups
DISCUSSION
The present study focused on visits to pcps and oncology specialists in the delivery of routine follow-up care. Our findings confirm that pcps are active and presumably play an important role in the provision of health care to breast cancer survivors during the follow-up period; however, the pcp role seems to be less specific to breast cancer follow-up than to providing care for other health issues. Our observations also revealed that, for all four provinces studied, most breast cancer survivors receive care from multiple physician specialties during the follow-up period, and the number of visits to both pcps and oncologists decline with each successive year of follow-up.
Primary care providers are increasingly accepted as providers of follow-up breast cancer care, and evidence from randomized controlled trials has demonstrated that pcp-led follow-up care is equivalent to oncologist-led care for this population4–6. Nonetheless, our findings suggest that oncologists continue to deliver most of the cancer-related follow-up care for breast cancer survivors, as evidenced by the low mean number of breast cancer-specific pcp visits (see Table i and Figure 1). However, not all visits to oncologists during this time are necessarily for routine follow-up care. Certainly, many people might visit oncologists because of complex late effects of treatment or might visit medical oncologists in particular for surveillance related to hormonal therapy. Importantly, although upward of 65% of the study cohort in each province had at least 1 oncology visit, very few individuals received care solely from oncologists during the follow-up period (specifically, fewer than 3% in each province). In Manitoba, Ontario, and Nova Scotia, nearly all women (approximately 80% or more) made visits to both pcps and oncologists.
The fact that many women receive follow-up from both oncology and primary care is likely related to many factors. Some women might require ongoing contact with oncologists for treatment-related issues, but others might simply prefer to continue to receive care from their oncologist beyond the treatment phase. That hypothesis is supported by numerous studies that have reported concerns on the part of cancer survivors about the ability of pcps to provide follow-up care15–17. Oncologists might also be reluctant to completely transition follow-up care to pcps. In fact, prior research has shown that oncologists lack confidence in the ability of pcps to detect recurrence and to care for late effects of cancer treatment18 and that most do not believe that pcps should be responsible for follow-up care19. Multiple-provider follow-up care might also be a result of confusion on the part of providers and survivors alike about who is responsible for follow-up care. Breast cancer follow-up guidelines have not made specific recommendations about who should provide follow-up care20,21, and policies addressing this issue are not consistently in place—factors that might contribute to a lack of role clarity for physicians. Cancer survivors have also expressed confusion about which provider is responsible for cancer follow-up care22–24. Moreover, any effects of policy shifts encouraging pcp-led follow-up care might not yet be reflected in population-based patterns of care.
A smaller percentage of women in British Columbia (compared with women in Manitoba, Ontario, and Nova Scotia) made visits to both primary care and oncology during the follow-up period, but a larger percentage made visits to primary care only. Although that province has no formal policy for the transition of follow-up care to primary care, the practice is recommended. The situation is similar to that in other provinces, in which, to varying degrees, formal and informal efforts have been made to transition follow-up to primary care (for example, institutional policies, disease site recommendations, training for general practitioners). What is unique in British Columbia is that a comprehensive General Practitioners in Oncology Training Program was established in 200425. Notably, the program provides general practitioners with 8 weeks of training (a 2-week classroom-based module followed by a 6-week clinical component), which is focused on improving the ability of general practitioners to provide cancer-specific care to individuals, including survivorship care. Through the provision of resources and infrastructure, that program might be facilitating the implementation of local recommendations and contributing to the higher rates of pcp-led follow-up care observed in British Columbia. When visits were examined by follow-up year, women in British Columbia, compared with those in other provinces, typically made fewer pcp visits in each year. That observation might be related to the fact that the B.C. cohort was healthier than those in the other provinces, which was confirmed by the stratified analysis of pcp visits, showing that the provincial differences are diminished after stratification by comorbidity.
In all provinces, the mean numbers of visits to primary care and oncology both declined from year 1 to year 4 of follow-up. That observation is consistent with results in a prior study of breast cancer follow-up care in Ontario26 and could reflect practice that is adherent to guideline recommendations for follow-up care. For example, the 2006 guidelines from the American Society of Clinical Oncology20 recommended that that all women undergo a careful history and medical examination every 3–6 months for the first 3 years after diagnosis, but only every 6–12 months for the next 2 years. A decline in visits over time might therefore correspond to the delivery of guideline-adherent follow-up care.
Importantly, the present study represents the first inter-provincial comparison of patterns of use of physician services by breast cancer survivors in Canada. The strengths of the study include its use of population-based provincial cohorts and extensive work on the part of the participating provinces to optimize comparability. In addition to providing data that could be used to improve breast cancer care delivery in Canada, our work demonstrates the feasibility of parallel analyses across the country to assess equitable access to high-quality care.
Nonetheless, our work is not without limitations. Although the study involved collaboration across provinces, we were unable to achieve perfectly duplicated data processing and analyses from one jurisdiction to another because the work was done in parallel. For instance, there was provincial variation in terms of the codes used to identify breast cancer–specific primary care visits (that is, Nova Scotia used a broader range of diagnosis codes and Ontario included breast-related procedure codes). That shortcoming remained despite more than 2 years of collaboration to achieve duplication, and it is an example of why country-wide access to health care data is imperative.
A second issue related to the identification of breast-specific visits to primary care is that billings data might not capture all visits to a pcp in which breast cancer–related issues are addressed. In each province, a limited number of diagnoses (specifically, 3 in Nova Scotia and 1 elsewhere) can be recorded in the billings data for each visit with a primary care physician. As a result, if an individual discusses issues related to a prior breast cancer diagnosis during a visit to the pcp for another health issue, the billing claim that is submitted might reflect only the health issue that was the main purpose of the visit, resulting in an underestimate of breast cancer–specific pcp visits.
Finally, a small number of women with stage iv breast cancer were included in the cohorts because treatment- related inclusion criteria (that is, receipt of lumpectomy or mastectomy) rather than stage-related criteria were used during cohort identification. Patients with stage iv disease likely constituted a small set of surgically-treated, clinical stage iii patients whose subsequent pathologic stage increased to stage iv. Importantly, the inclusion of this small subset of stage iv patients did not substantially affect the study results. For example, assuming that, in British Columbia, women made an average of 3 visits per person to primary care in year 1 of follow-up, and stage iv patients made an average of 10 visits to primary care, exclusion of the stage iv patients from the cohort would result in a mean of 2.86 visits per person (that is, a decrease of only 0.14 visits). In other provinces, in which even fewer women with stage iv breast cancer were included, the effect would have been even less.
CONCLUSIONS
Patterns of physician utilization during the follow-up period vary. Specifically, our study suggests that surgeons in Nova Scotia might be involved to a greater extent in providing follow-up care than are surgeons in other provinces, and that primary care–led follow-up is more common in British Columbia than elsewhere. Those differences might be related to differences in policies and initiatives across provinces, but might also be the result of variations in resources and infrastructure to support pcp-led follow-up care. Across all provinces, follow-up care for breast cancer survivors is characterized by the involvement of both primary care and oncology despite many ongoing initiatives across the country to improve the transition of routine follow-up care from oncologists to pcps. It could be that the impact of those initiatives will be discernable in patterns of follow-up care in future years; however, the literature suggests that patient need is not the driving factor for the ongoing involvement of oncologists in follow-up care, thereby underscoring the need for continued efforts to improve the transitioning of follow-up care, including efforts to increase confidence in the ability of PCPs to deliver effective follow-up care and to better support pcps in taking on a greater role in survivorship care.
ACKNOWLEDGMENTS
The authors thank Dongdong Li, Emma Shu, Marlo Whitehead, and Yan Wang for conducting statistical analyses. This study was funded by the Canadian Institutes of Health Research (grant no. 128272). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funder.
This study was supported by the Institute for Clinical Evaluative Sciences (ices), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (mohltc). No endorsement by ices or the Ontario mohltc is intended or should be inferred.
Parts of this material are based on data and information provided by Cancer Care Ontario (cco). The opinions, results, views, and conclusions reported here are those of the authors and do not necessarily reflect those of cco. No endorsement by cco is intended or should be inferred.
Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (cihi). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not necessarily those of cihi.
We gratefully acknowledge CancerCare Manitoba for their ongoing support and Manitoba Health for the provision of data. The results and conclusions presented are those of the authors. No official endorsement by Manitoba Health is intended or should be inferred.
Nova Scotia data were provided by Health Data Nova Scotia and the N.S. Department of Health and Wellness; however, the observations and opinions expressed are those of the authors and do not represent those of either Health Data Nova Scotia or the N.S. Department of Health and Wellness.
Data for this study from the B.C. Ministry of Health (moh) and the Canadian Institute for Health Information (cihi) were provided by Population Data BC, a multi-university resource facilitating research on human health, well-being, and development. Data from these databases were provided: the moh Medical Services Plan (msp) Payment Information File, the cihi Discharge Abstract Database (Hospital Separations) file, and the moh Consolidation File (msp Registration and Premium Billing). All inferences, opinions, and conclusions drawn in this study are those of the authors and do not reflect the opinions or policies of the B.C. data stewards.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Steering Committee . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 2.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 3.Del Giudice ME, Grunfeld E, Harvey BJ, Piliotis E, Verma S. Primary care physicians’ views of routine follow-up care of cancer survivors. J Clin Oncol. 2009;27:3338–45. doi: 10.1200/JCO.2008.20.4883. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld E, Mant D, Yudkin P, et al. Routine follow-up of breast cancer in primary care: a randomised trial. BMJ. 1996;313:665–9. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol. 2006;24:848–55. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld E, Julian JA, Pond G, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011;29:4755–62. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- 7.Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Canadian Team to Improve Community-Based Cancer Care Along the Continuum, Knowledge Translation Team . Improving Coordination Between Primary Care Providers and Oncology Specialists: Cases from Canada. Toronto, ON: University of Toronto; 2015. [Available online at: http://canimpact.utoronto.ca/wp-content/uploads/2014/08/CanIMPACT-Casebook.pdf; cited 25 August 2016] [Google Scholar]
- 9.Grunfeld E, Petrovic B. Consultative workshop proceedings of the Canadian Team to Improve Community-Based Cancer Care Along the Continuum. Curr Oncol. 2017;24:135–40. [Google Scholar]
- 10.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002. [DOI] [Google Scholar]
- 11.Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumours. 6th ed. Hoboken, NJ: John Wiley and Sons; 2002. [Google Scholar]
- 12.Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (adgs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:932–9. doi: 10.1097/MLR.0b013e318215d5e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNiven C, Puderer H, Janes D. Census Metropolitan Area and Census Agglomeration Influenced Zones (MIZ): A Description of the Methodology. Ottawa, ON: Statistics Canada, Geography Division; 2000. [Available online at: http://publications.gc.ca/Collection/Statcan/92F0138M/92F0138MIE2000002.pdf; cited 22 August 2016] [Google Scholar]
- 14.Urquhart R, Folkes A, Porter G, et al. Population-based longitudinal study of follow-up care for patients with colorectal cancer in Nova Scotia. J Oncol Pract. 2012;8:246–52. doi: 10.1200/JOP.2011.000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan NF, Evans J, Rose PW. A qualitative study of unmet needs and interactions with primary care among cancer survivors. Br J Cancer. 2011;105(suppl 1):S46–51. doi: 10.1038/bjc.2011.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan ME, Butow P, Marven M, Spillane AJ, Boyle FM. Survivorship care after breast cancer treatment—experiences and preferences of Australian women. Breast. 2011;20:271–7. doi: 10.1016/j.breast.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Urquhart R, Folkes A, Babineau J, Grunfeld E. Views of breast and colorectal cancer survivors on their routine follow-up care. Curr Oncol. 2012;19:294–301. doi: 10.3747/co.19.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403–10. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung WY, Aziz N, Noone AM, et al. Physician preferences and attitudes regarding different models of cancer survivorship care: a comparison of primary care providers and oncologists. J Cancer Surviv. 2013;7:343–54. doi: 10.1007/s11764-013-0281-y. [DOI] [PubMed] [Google Scholar]
- 20.Khatcheressian JL, Wolff AC, Smith TJ, et al. on behalf of the American Society of Clinical Oncology American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–7. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 21.Grunfeld E, Dhesy-Thind S, Levine MN, on behalf of the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update) CMAJ. 2005;172:1319–20. doi: 10.1503/cmaj.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27:2489–95. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 23.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;2010:25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miedema B, MacDonald I, Tatemichi S. Cancer follow-up care. Patients’ perspectives. Can Fam Physician. 2003;49:890–5. [PMC free article] [PubMed] [Google Scholar]
- 25.BC Cancer Agency (bcca) General Practitioners in Oncology Training Program [Web page] Vancouver, BC: BCCA; 2016. [Available at: http://www.bccancer.bc.ca/health-professionals/networks/family-practice-oncology-network/general-practitioners-in-oncology-training-program; cited 28 August 2016] [Google Scholar]
- 26.Grunfeld E, Hodgson DC, Del Giudice E, Moineddin R. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Practice. 2010;6:174–81. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]