Abstract
Background
We used administrative health data to explore the impact of primary care physician (pcp) visits on acute-care service utilization by women receiving adjuvant chemotherapy for early-stage breast cancer (ebc).
Methods
Our population-based retrospective cohort study examined pcp visits and acute-care use [defined as an emergency room (er) visit or hospitalization] by women diagnosed with ebc between 2007 and 2009 and treated with adjuvant chemotherapy. Multivariate regression analysis was used to identify the effect of pcp visits on the likelihood of experiencing an acute-care visit.
Results
Patients receiving chemotherapy visited a pcp significantly more frequently than they had before their diagnosis [relative risk (rr): 1.48; 95% confidence interval (ci): 1.44 to 1.53; p < 0.001] and significantly more frequently than control subjects without cancer (rr: 1.51; 95% ci: 1.46 to 1.57; p < 0.001). More than one third of pcp visits by chemotherapy patients were related to breast cancer or chemotherapy-related side effects. In adjusted multivariate analyses, the likelihood of experiencing an er visit or hospitalization increased in the days immediately after a pcp visit (rr: 1.92; 95% ci: 1.76 to 2.10; p < 0.001).
Conclusions
During chemotherapy treatment, patients visited their pcp more frequently than control subjects did, and they visited for reasons related to their breast cancer or to chemotherapy-related side effects. Visits to a pcp by patients receiving chemotherapy were associated with an increased frequency of er visits or hospitalizations in the days immediately after the pcp visit. Those results suggest an opportunity to institute measures for early detection and intervention in chemotherapy side effects.
Keywords: Breast cancer, chemotherapy, primary care physicians, emergency room, hospitalization
INTRODUCTION
Cancer creates a high burden of suffering and cost worldwide1. Excluding cancers of the skin, breast cancer is the most frequently diagnosed cancer in women, and it is second only to lung cancer as a cause of cancer death in women2. Adjuvant chemotherapy is commonly used for early-stage breast cancer (ebc) and is associated with an increase in overall survival3; however, research has shown that a high proportion of women receiving adjuvant chemotherapy for ebc visit the emergency room (er) or are hospitalized during treatment4,5.
Primary care plays a pivotal role in the health care system, often serving as the first point of access to care. A strong primary care system is consistently associated with better and more equitable health outcomes, higher patient satisfaction, and lower costs6. Cancer services are usually provided within a large and complex delivery system involving many different providers. Primary care physicians (pcps) have been found to have an important role to play in each phase of the cancer care continuum7. Furthermore, studies have shown that pcps are interested in being more involved and are keen to be team players for their patients with cancer8–10. Despite the growing burden of cancer and interest on the part of pcps to be involved, information about pcp involvement in cancer care during the active treatment phase is scarce.
The overall aim of the present research was to further an understanding of the role of pcps during active cancer treatment, including management of chemotherapy-related side effects. Three specific objectives were considered:
■ To describe patterns (such as frequency, type, and reason) of pcp visits by ebc patients receiving adjuvant chemotherapy, and to compare those patterns with the patterns demonstrated by control subjects
■ To describe patterns (such as variations in time of day, day of week, and er visit urgency) of acute-care use (defined as an er visit or hospitalization) by ebc patients receiving adjuvant chemotherapy
■ To explore the association between pcp visits and acute-care use by ebc patients receiving adjuvant chemotherapy
METHODS
Study Cohort
We used deterministically linked administrative health databases in Ontario to conduct a population-based retrospective cohort study. The cohort for analysis was identified from the Ontario Cancer Registry as part of a study by Enright et al.5. Patients diagnosed with ebc between January 2007 and December 2009 who received at least 1 cycle of adjuvant chemotherapy within 4 months of their breast cancer surgery date were included. Exclusions were age less than 18 years, male sex, no record of curative breast cancer surgery, neoadjuvant-intent chemotherapy, stage iv disease, and history of any cancer (excluding non-melanoma skin cancer) within the 5 years preceding the breast cancer diagnosis. The resulting cohort consisted of 8359 women, hereinafter identified as the chemotherapy patient cohort.
Two control populations were identified: the chemotherapy patients as own-control subjects during an identical calendar period 2 years before their breast cancer diagnosis, and control subjects without cancer drawn from a random sample of the general population residing in Ontario during the same study time frame and matched 1:1 to the chemotherapy patient cohort based on age, comorbidity, and geographic location.
Data Sources and Measures
Population-level administrative data were obtained from a variety of databases held at the Institute for Clinical Evaluative Sciences. Visits to the er and hospital admissions were identified using, respectively, the National Ambulatory Care Reporting System and the Discharge Abstract Database maintained by the Canadian Institute for Health Information. We used the Ontario Health Insurance Plan (ohip) database to identify claims for physician services and the Physician Database at the Institute for Clinical Evaluative Sciences to identify physician specialty. Basic demographic information was obtained from the Registered Persons Database. Socioeconomic status was an ecological variable estimated for each patient by linking their postal code to Statistics Canada census data to obtain an income quintile11. Comorbidity burden was assessed using the Charlson comorbidity index12.
Visits to a pcp were determined by identifying, for each patient, all ohip physician claims between their first day of chemotherapy and the 30 days after the last day of chemotherapy. Physician types were then determined by linking the ohip physician numbers to the Physician Database. The ohip visits with a main physician specialty code equal to “general practitioner/family practitioner” or “family practitioner/emergency medicine” were selected. Exclusions included ohip visits with an ohip location code equal to “inpatient,” “emergency room,” or “unknown.” Acute care visits occurring between the first day of chemotherapy and the 30 days after the last day of chemotherapy were divided into three categories: er visit only, er visit resulting in hospitalization, and direct hospitalization.
Statistical Analysis
Descriptive analyses and test statistics were calculated to compare pcp visit patterns between the chemotherapy patients and each of the two control cohorts. A negative binomial model was used to compare rates of pcp visits per study period for the chemotherapy patient cohort and the control cohorts, controlling for potentially confounding variables (age, rural or urban setting, income quintile, and Charlson comorbidity index). The resulting estimates accounted for the matching between the chemotherapy patients and the control subjects, and for multiple records for each patient13.
Descriptive statistics were also produced to determine variations in acute-care use by time of day and day of week, as well as by the urgency of er visits. Urgency was determined by the triage-level variable for the er visit (available in National Ambulatory Care Reporting System); values of that variable correspond to the levels outlined in the Canadian Emergency Department Triage and Acuity Scale. We evaluated less-urgent visits to the er (levels 4 and 5) in relation to more-urgent visits (levels 1–3)14,15.
Using a negative binomial model, the rates of acute-care visits by the chemotherapy patient cohort were compared by pcp-exposed time (defined as within the 2 days after a pcp visit, because a pcp visit was identified to potentially have a lesser effect on the outcome after those 2 days) and unexposed time, controlling for potentially confounding variables (age, rural or urban setting, income quintile, Charlson comorbidity index, and chemotherapy regimen type). Generalized estimating equation methods were used to account for the repeated measures for an individual16.
All statistical analyses were conducted at Institute for Clinical Evaluative Sciences using the SAS software application for unix (version 9.3: SAS Institute, Cary, NC, U.S.A.). All tests were 2-sided, and p values less than 0.05 were considered statistically significant. The research study was approved by Sunnybrook Health Sciences Centre Research Ethics Board and by the University of Toronto Research Ethics Board.
RESULTS
Cohort Characteristics
Table i presents the characteristics of the chemotherapy patient cohort (n = 8359) and the control cohort without cancer (n = 8359). The average age of the chemotherapy patients at diagnosis was 53.7 ± 10.5 years. Patients were followed for the duration of their chemotherapy plus 30 days (mean: 131 ± 41 days).
TABLE I.
Characteristics of the study cohorts
| Characteristic | EBC patients receiving CTx (n=8359) | Control subjects without cancer (n=8359) |
|---|---|---|
| Age at diagnosis (years) | ||
| Mean | 53.7±10.5 | 53.7±10.5 |
| Range | 18–99 | 19–99 |
| Income quintile [n (%)] | ||
| Q1 (lowest) | 1294 (15.5) | 1413 (16.9) |
| Q2 | 1543 (18.5) | 1613 (19.3) |
| Q3 | 1720 (20.6) | 1804 (21.6) |
| Q4 | 1877 (22.5) | 1749 (20.9) |
| Q5 | 1925 (23.0) | 1780 (21.3) |
| Geographic location [n (%)] | ||
| Urban | 7353 (88.0) | 7312 (87.5) |
| Rural | 1006 (12.0) | 1047 (12.5) |
| Score on the CCI [n (%)] | ||
| 0 | 7685 (91.9) | 7686 (91.9) |
| 1 | 548 (6.6) | 454 (5.4) |
| 2+ | 126 (1.5) | 219 (2.6) |
| Stage [n (%)] | ||
| I | 1844 (22.1) | |
| II | 4477 (53.6) | |
| III | 1587 (19.0) | |
| Unavailable | 451 (5.4) | |
| Regime type [n (%)] | ||
| Anthracycline only | 1673 (20.0) | |
| Docetaxel | 5504 (65.8) | |
| Paclitaxel | 1165 (13.9) |
EBC = early-stage breast cancer; CTx = chemotherapy; CCI = Charlson comorbidity index.
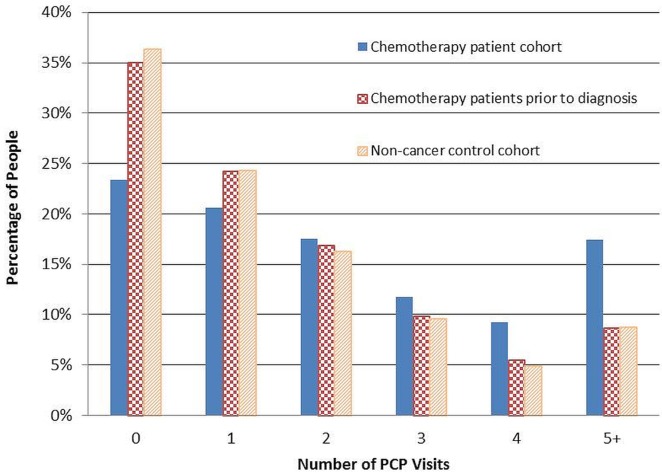
PCP Utilization Patterns
Figure 1 shows the percentage of pcp visits by cohort. The mean number of pcp visits was significantly higher for the chemotherapy patients, both compared with themselves before diagnosis (2.54 vs. 1.73, p < 0.001) and compared with the control cohort without cancer (mean: 2.54 vs. 1.71; p < 0.001). In addition, chemotherapy patients were significantly more likely to have made at least 1 pcp visit during their chemotherapy compared with the period before diagnosis (76.6% vs. 65.0%, p < 0.001) and compared with the control cohort (76.6% vs. 63.7, p < 0.001). The rate of pcp visits for the chemotherapy patient cohort during chemotherapy was 1.48 times their own rate before diagnosis (95% confidence interval: 1.44 to 1.53; p < 0.001) and 1.51 times the rate for the control cohort (95% confidence interval: 1.46 to 1.57; p < 0.001).
FIGURE 1.
Percentage of primary care physician (PCP) visits by cohort.
The ohip diagnosis code 174, which has a corresponding description of “malignant neoplasms, female breast,” was the top diagnosis code reported for the chemotherapy patient cohort and accounted for 33.7% of pcp visits. The ohip diagnosis code 977, which has a corresponding description of “adverse effects of drugs and medications including allergy, overdose, and reactions,” was the 11th most frequent code for chemotherapy patients, compared with 98th most frequent for non-cancer controls. That observation suggests that a portion of the claims associated with the chemotherapy patient cohort were likely related to chemotherapy-related side effects.
Acute-Care Use Patterns
We identified 6697 acute-care visits in the chemotherapy patient cohort. Of those visits, 68.7% were er-only visits, 24.5% were er visits that resulted in a hospitalization, and 6.8% were direct hospitalizations. Most acute care visits (71%) occurred outside of regular weekday office hours (08h00–16h00). The triage-level variable showed that 20.0% of er visits were considered less urgent. Compared with chemotherapy patients residing in urban areas, those residing in rural areas made a higher proportion of er visits that were considered less urgent (43.5% vs. 14.2%, p < 0.001).
Primary Care and Acute Care Association
Using a negative binomial model, we compared the rates of all acute-care visits by the chemotherapy patient cohort according to pcp-exposed and -unexposed time (Table ii). Holding the other variables constant, the intensity of acute-care visits after a pcp visit was 1.92 times the intensity of visits during the period not following a pcp visit (95% confidence interval: 1.76 to 2.10; p < 0.001). The adjusted rate of acute-care visits per 1000 days was 10.6 during the pcp-exposed period and 7.6 during the pcp-unexposed period. Significant variations based on comorbidity score, chemotherapy regimen, and rural compared with urban setting were observed.
TABLE II.
Negative binomial regression analysis of acute-care use
| Variable | RR | 95% CI | p Value |
|---|---|---|---|
| Exposure to PCP | |||
| Yes | 1.92 | 1.76 to 2.10 | <0.001 |
| No | Reference | ||
| Age group | |||
| ≤39 Years | Reference | ||
| 40–49 Years | 0.81 | 0.72 to 0.92 | 0.0006 |
| 50–59 Years | 0.79 | 0.71 to 0.89 | <0.001 |
| 60–69 Years | 0.88 | 0.78 to 0.99 | 0.0311 |
| ≥70 Years | 1.06 | 0.90 to 1.24 | 0.4917 |
| Income quintile | |||
| Q1 (lowest) | 1.17 | 1.06 to 1.29 | 0.0022 |
| Q2 | 1.1 | 0.99 to 1.22 | 0.0519 |
| Q3 | 1.18 | 1.08 to 1.30 | 0.0005 |
| Q4 | 1.1 | 1.00 to 1.20 | 0.0446 |
| Q5 | Reference | ||
| Geographic location | |||
| Urban | Reference | ||
| Rural | 1.42 | 1.29 to 1.56 | <0.001 |
| Score on the CCI | |||
| 0 | Reference | ||
| 1 | 1.31 | 1.17 to 1.46 | <0.001 |
| 2+ | 1.44 | 1.16 to 1.78 | 0.0009 |
| Regimen type | |||
| Anthracycline only | Reference | ||
| Docetaxel | 1.64 | 1.49 to 1.80 | <0.001 |
| Paclitaxel | 0.9 | 0.78 to 1.03 | 0.1140 |
RR = relative risk; CI = confidence interval; PCP = primary care physician; CCI = Charlson comorbidity index.
DISCUSSION
Our study shows that patients continue to visit their pcp during chemotherapy treatment and that they consult the pcp for reasons related to their breast cancer. Furthermore, pcp visits by chemotherapy patients were significantly more frequent than they were in the two control groups studied (the same women 2 years before their breast cancer diagnosis, and control subjects without cancer). Those results align with research showing that patients continue to see their pcp throughout the cancer care continuum7,17. Using ohip diagnosis codes, we were not able to quantify precisely the percentage of visits that were likely related to breast cancer or chemotherapy treatment, but we estimated it to be in the 33.7%–39.2% range. With a growing awareness of the important role that pcps play during the cancer care continuum, emphasis by a patient’s oncologist that the patient should continue to see their pcp throughout their cancer treatment might result in increased pcp visits.
Emergency rooms offer accessible care 24 hours per day and serve a diverse range of patients with a wide variety of care needs. Some patients have life-threatening or urgent conditions for which the er is the most appropriate location. Other patients have less-urgent conditions that could potentially be treated within other areas of the health care system18. The link between er visits and access to primary care is mediated by several factors that are difficult to measure, including the availability and appropriateness of local resources such as walk-in clinics; patient preference for the place of care; distance to facilities; and hours of operation19. In many cases, the er is the appropriate care setting for cancer patients experiencing adverse side effects such as febrile neutropenia; however, the er might not always be the most appropriate care setting for cancer patients needing support and management of some side effects4. The results of the present study show that approximately 20% of er visits are identified as less urgent. Patients could have chosen the er for a range of reasons, such as symptoms that might have required er care or lack of access to primary care or oncology care outside of regular weekday office hours. After-hours access to primary care has been identified as a challenge for some Canadian jurisdictions, including Ontario19. Our results highlight an opportunity to focus on reducing less-urgent er visits during chemotherapy treatment.
Visits to a pcp during chemotherapy treatment were associated with an increased frequency of acute-care use immediately after the pcp visit. However, given the methods of the present study, no causal relationship can be established. Possible explanations include a patient experiencing adverse effects that require er care, a patient potentially experiencing challenges in accessing primary or oncology care, and a pcp potentially not having the tools available to manage their patient’s treatment-related side effects. Visits to a pcp might also be viewed as a marker for sicker patients. Our research was not able to distinguish between patients experiencing treatment-related side effects amenable to primary care and patients experiencing adverse effects that required er care. Enright et al.5 showed significant variations in acute-care visits by chemotherapy patients based on comorbidity, type of chemotherapy regimen, geographic region, and rural residence. Our findings represent an opportunity to focus interventions on regions with high rates of er visits, as well as on patients receiving regimens associated with greater er use. A comparison of acute-care use after a pcp visit by ebc patients who did not receive chemotherapy during the same time period might provide valuable insights, but was not feasible because of the baseline characteristics of the cohort.
Knowing that patients continue to visit their pcp during chemotherapy treatment emphasizes the need to support pcps in that role. The results of our research demonstrate the high burden of care created by cancer and highlight the continuous involvement of pcps in the care of ebc patients. Interventions aimed at preventing and managing certain treatment-related side effects in the primary care setting might lower the number of less-urgent er visits and improve the patient experience.
Administrative database studies are limited by a lack of detailed clinical information in the datasets. We were unable to identify potentially important factors such as use of supportive care medications. Given the limitations of the ohip data, we were unable to determine the precise clinical reasons for pcp visits. Furthermore, ohip includes both fee-for-service billings and shadow billings submitted by pcps participating in capitation models20. If shadow billings are incomplete, the pcp visits reported here could be underestimated. Lastly, because of limitations in the ohip data, we were unable to differentiate between a family medicine clinic visit and a walk-in clinic visit11.
The design and methodology chosen for the study allowed us to determine the likelihood of an acute-care visit immediately following a pcp visit. However, we were unable to identify chemotherapy-related events that did not lead to care in an er because the patient was managed in primary care or another care setting. Lastly, because our study focused on ebc patients, the question of whether our findings can be generalized to patients with metastatic disease or to patients with other types of cancer requires further research.
CONCLUSIONS
To our knowledge, this population-based cohort study is the first to examine the association between pcp visits and acute care use by ebc patients. Our study provides insight into the role of pcps during the active treatment phase of breast cancer and preliminary insights into the association between pcp visits and acute-care use. Our findings present an opportunity to implement interventions for the early detection and management of chemotherapy-related side effects, such as initiatives at the primary care level.
ACKNOWLEDGMENTS
This study was conducted with the support of the Ontario Institute for Cancer Research and Cancer Care Ontario through funding provided by the Government of Ontario. This study was presented as a poster at the Applied Research in Cancer Control conference, Toronto, ON, 12 May 2014; The Cancer and Primary Care Research International Network, Winnipeg, MB, 10–13 June 2014; and the 6th Annual Meeting of the Ontario Institute for Cancer Research/Cancer Care Ontario Health Services Research Program, Toronto, ON, 19 June 2014.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Quality Council of Ontario (cqco) Unplanned Hospital Visits During Chemotherapy [Web page] Toronto, ON: CQCO; 2016. [Available at: http://www.csqi.on.ca/by_patient_journey/treatment/unplanned_hospital_visits_during_chemotherapy/; cited 17 December 2016] [Google Scholar]
- 5.Enright KA, Grunfeld E, Yun L, et al. Population-based assessment of emergency room visits and hospitalizations among women receiving adjuvant chemotherapy for early breast cancer. J Oncol Pract. 2015;11:126–32. doi: 10.1200/JOP.2014.001073. [DOI] [PubMed] [Google Scholar]
- 6.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Giudice L, Bondy S, Chen Z, et al. Physician care of cancer patients. In: Jaakkimainen L, Upshur R, Klein-Geltink JE, editors. Primary Care in Ontario: ICES Atlas. Toronto, ON: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 8.Aubin M, Vezina L, Verreault R, et al. Patient, primary care physician and specialist expectations of primary care physician involvement in cancer care. J Gen Intern Med. 2012;27:8–15. doi: 10.1007/s11606-011-1777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sussman J, Evans W, Whelan T, Bainbridge D, Schiff S, Hasler A. Integration between primary care providers and the cancer system: gaps and opportunities [abstract 6584] J Clin Oncol. 2009;27:15s. [Google Scholar]
- 10.Grunfeld E. Primary care physicians and oncologists are players on the same team. J Clin Oncol. 2008;26:2246–7. doi: 10.1200/JCO.2007.15.7081. [DOI] [PubMed] [Google Scholar]
- 11.Schultz S, Tepper J, Guttmann A, Jaakkimainen L. Characteristics of primary care practice. In: Jaakkimainen L, Upshur R, Klein-Geltink JE, et al., editors. Primary Care in Ontario: ICES Atlas. Toronto, ON: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comor-bidity index for use with icd-9-cm administrative databases. J Clin Epidemiol. 1992;45:613–19. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.Alexander MT, Kufera JA. Butting heads on matched cohort analysis using SAS software. Presented at: NESUG 2007 (NorthEast SAS Users Group); Baltimore, MD, U.S.A.. 9–14 November 2007; [Available online at: http://lexjansen.com/nesug/nesug07/sa/sa01.pdf; cited 11 December 2016] [Google Scholar]
- 14.Glazier RH, Klein-Geltink J, Kopp A, Sibley LM. Capitation and enhanced fee-for-service models for primary care reform: a population-based evaluation. CMAJ. 2009;180:E72–81. doi: 10.1503/cmaj.081316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field S, Lantz A. Emergency department use by ctas levels iv and v patients. CJEM. 2006;8:317–22. doi: 10.1017/S1481803500013968. [DOI] [PubMed] [Google Scholar]
- 16.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 17.Grunfeld E, Hodgson DC, Del Giudice ME, Moineddin R. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Pract. 2010;6:174–81. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beveridge R, Clarke B, Janes L, Savage N, Thompson J, Dodd G. Canadian emergency department triage and acuity scale: implementation guidelines. CJEM. 1999;1(suppl 3):S2–28. [Available online at: http://www.caep.ca/resources/ctas/implementation-guidelines; cited 17 December 2016] [Google Scholar]
- 19.Glazier RH, Kopp A, Schultz SE, Kiran T, Henry DA. All the right intentions but few of the desired results: lessons on access to primary care from Ontario’s patient enrolment models. Healthc Q. 2012;15:17–21. doi: 10.12927/hcq.2013.23041. [DOI] [PubMed] [Google Scholar]
- 20.Jaakkimainen L, Upshur R, Klein-Geltink JE, et al., editors. Primary Care in Ontario: ICES Atlas. Toronto, ON: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]