Abstract
Purpose
Anti-hormonal therapy (tamoxifen) is recommended for estrogen receptor (er)–positive breast cancer (bca); however, its effect on low-receptor cancers is unclear. We retrospectively evaluated the effect of adjuvant tamoxifen in patients with weakly er-positive bca.
Methods
We identified 2221 bca patients who had been er-tested by ligand-based assay (lba) during 1976–1995 and who had been treated and followed until 2008. Cox proportional hazards models adjusted for age, body mass index, tumour size, nodal status, surgery, and chemotherapy were used to assess the effect of er level on bca survival in patients who received tamoxifen.
Results
Overall, 17% (383) of patients were within 0–3 fmol/mg cytosol protein, and 12% (266) were within 4–9 fmol/mg cytosol protein. Patients with er levels of 0–3, 4–9, 10–19, 20–49, and 50 fmol/mg or more cytosol protein had 20-year bca survival rates of 56%, 56%, 63%, 71%, and 60% respectively. Of the 2221 patients studied, 661 (29.8%) received anti-hormonal therapy. Within the latter group, er levels of 0–3, 4–9, 10–19, 20–49, and 50 fmol/mg or more cytosol protein were associated with a hazard ratio for lower bca mortality: respectively, 1.00 (reference), 0.59 (p = 0.09), 0.19 (p < 0.0001), 0.26 (p < 0.0001), and 0.31 (p < 0.0001)—the risk reduction being significant only for er levels of 10 fmol/mg or more cytosol protein.
Conclusions
Tamoxifen use in bca patients with a weakly positive er status (4–9 fmol/mg cytosol protein), compared with those having higher er levels (≥10 fmol/mg cytosol protein), is not associated with a significantly lower bca-specific mortality. Our results do not support treatment with anti-hormonal therapy for bca patients with a weakly positive er status as identified by lba.
Keywords: Breast cancer, estrogen receptor positivity, tamoxifen, cytosol protein
INTRODUCTION
The presence of estrogen receptors (ers) in breast cancer has been known for more than 30 years. Since that discovery, a number of studies have shown that er status is the single most important predictive and prognostic biomarker in breast cancer1–9. It is recognized that er-positive tumours should be treated with adjuvant tamoxifen or aromatase inhibitors, because anti-hormonal therapy is associated with an important survival benefit. Women with er-positive breast cancer treated with 5 years of adjuvant tamoxifen have a 29% decreased risk for death from the disease and a 50% decreased risk for contralateral breast cancer10.
In breast cancer, er was originally assessed using biochemical ligand-binding assays (lbas). That method has repeatedly been validated in the literature and was considered the “gold standard” for a long time2,8,11–16. A threshold of 10 fmol/mg or more cytosol protein was generally accepted to consider a tumour er-positive10. Starting in the mid-1990s, lbas were progressively replaced by immunohistochemistry (ihc) assays, which use highly specific monoclonal antibodies directed against er to assess tumour hormonal status. Results are generally reported as the percentage of stained cells combined with the intensity of staining. Until recently, 10% or more stained cells was considered the threshold for er positivity. However, a number of studies have reported up to 20% inter-laboratory variability in ihc results when testing er status in the same breast cancers17–19. Those divergent results might reflect variations in pre-analytic variables, thresholds for positivity, and interpretation criteria. To reduce the number of misclassified patients, the American Society of Clinical Oncology and the College of American Pathologists in 2010 published guideline recommendations concerning ihc testing for er in breast cancer. After a review of the evidence, the panel recommended that er assays be considered positive if at least 1% of tumour nuclei are positive in the sample when tested in the presence of controls2,8,11–16,20. Since 2010, patients with weakly positive er tumours (1%–9% of stained cells) have therefore been offered anti-hormonal therapy, because they are considered er-positive. However, little is known about the real benefit of treating weakly positive er tumours with anti-hormonal therapies.
To assess the prognostic significance of er, the primary endpoint of the present study was breast cancer–specific survival and mortality according to er level (femtomoles per milligram cytosol protein). As a secondary endpoint, survival in patients having different er concentration levels expressed by breast cancers treated or not with tamoxifen were compared to determine whether adjuvant anti-hormonal treatment of er-poor breast cancer patients is really beneficial.
METHODS
Study Population
After ethics approval was obtained from the institutional research ethics committee (Comité d’éthique de la recherche du chu de Québec), a retrospective study of women who attended our tertiary breast cancer centre (Centre des maladies du sein Deschêne–Fabia) was performed. Because the study had no direct patient involvement, patient consent for the study was not required. Patients included in the study were women diagnosed and treated for an infiltrative breast cancer between February 1976 and May 1995 and followed until 2008 at our centre. We excluded women with noninvasive breast cancer, those who were metastatic at diagnosis, those who had a prior diagnosis of cancer at any site (except non-melanoma skin cancer and cervical intraepithelial neoplasia), and those whose er status was unknown.
Data Collection
To identify and extract patient characteristics, we used the registry of our institution (cms registry). The cms registry is one of the most important breast cancer registries in Canada; it has information on more than 10,000 women diagnosed with breast cancer and treated at our institution since 197621.
Patient characteristics extracted from the cms registry included age at diagnosis (years) and body mass index (kilograms per meters squared). The er and progesterone (pr) receptors were measured by biochemistry (dextran-coated charcoal assay) until the beginning of the 1990s; thereafter they were measured by ihc. The cut-off point for positivity was established at 10 fmol/mg cytosol protein for the dextran-coated charcoal assay. Patients with very low er levels (1–3 fmol/mg cytosol protein) were considered er-negative based on earlier studies that had used 3 fmol/mg cytosol protein as the threshold for negativity8,10,22. In the present study, survival for patients with er levels of 1–3 fmol/mg cytosol protein was analyzed and shown to be similar to that for patients with er levels of 0 fmol/mg cytosol protein. Thus, patients with er levels of 0 fmol/mg and within 1–3 fmol/mg cytosol protein were combined to constitute the er-negative group. Patients with er levels within 4–9 fmol/mg cytosol protein were deemed the weakly positive er group. Patients with er levels exceeding 9 fmol/mg cytosol protein were considered er-positive and were analyzed in groups (10–19 fmol/mg, 20–49 fmol/mg, and 50 fmol/mg or more cytosol protein).
Extent of disease at diagnosis was assessed by tumour size and regional or distant involvement. Tumour size (millimetres) corresponds to the largest diameter of the primary tumour considering the invasive component of the tumour only. Axillary lymph node involvement (node-negative or node-positive) and the absolute number of involved nodes, when applicable, was based on axillary dissection.
Initial locoregional treatments and neoadjuvant or adjuvant systemic therapies were documented. The most invasive breast surgery was reported and classified as mastectomy (including simple mastectomy, modified radical mastectomy, or radical mastectomy) or breast-conserving surgery (including lumpectomy and partial or segmental mastectomy). Women classified as having had no breast surgery included those who had an incisional biopsy as the most invasive breast procedure. Axillary surgery included mostly axillary dissection, because sentinel lymph node biopsy was not done before 1996.
Adjuvant endocrine therapy included tamoxifen almost exclusively. Neoadjuvant and adjuvant chemotherapy regimens were classified as traditional polychemotherapy (cyclophosphamide, methotrexate, fluorouracil, and in rare instances, melphalan), anthracycline-containing regimens (doxorubicin or epirubicin), or anthracycline-containing regimens plus taxanes (docetaxel or paclitaxel).
Follow-Up
The cms conducts active follow-up of all breast cancer patients to assess vital status. Vital status was updated by linkage of the cms register with the database of beneficiaries of the Quebec universal health insurance system (Régie de l’assurance maladie du Québec) and with the Quebec mortality database held by the Institut de la statistique du Québec. Compared with the Quebec mortality database, the Régie de l’assurance maladie du Québec database allows for the identification of a date at which an individual was still known to be alive. The 2221 women included in the present study were followed for periods ranging from 4 days to 20 years (median: 14.7 years) to the end of October 2008, which was the termination date for the present analysis.
In addition, to detect if a bias was present in er-negative patients who did or did not receive tamoxifen as adjuvant therapy, we extracted medical files for all er-negative patients treated with tamoxifen, together with 10 cases of er-negative patients not treated with tamoxifen. Patients with negative or weakly positive er who received tamoxifen were generally patients within a trial studying the use of tamoxifen. We verified the patient characteristics together with their er and pr lba results.
Statistical Analysis
In order of priority, the date of the first histopathologic confirmation of malignancy was defined as the date of diagnosis, the date of the first positive cytology, or the date of the first clinical investigation (mainly mammography) showing malignancy.
We used the Kaplan–Meier method to estimate survival for breast cancer patients with tumours that were er-negative (0–3 fmol/mg cytosol protein), that had low er expression (4–9 fmol/mg cytosol protein), and that had higher er expression (10–19, 20–49, ≥50 fmol/mg cytosol protein). Analyses were performed separately for patients who did and did not receive anti-hormonal therapy (tamoxifen), as well as for both groups combined. Cox proportional hazards models were used to assess the effect of er level (0–3, 4–9, 10–19, 20–49, ≥50 fmol/mg cytosol protein) on breast cancer mortality in patients who did or did not receive anti-hormonal therapy. Breast cancer–specific mortality for the study patients is reported as hazard ratios with 95% confidence intervals. In addition, a multivariable Cox model was used to analyze all patients according to er level and the presence or absence of anti-hormonal treatment. Those patients were compared with a reference group [patients with er-negative breast cancer (0–3 fmol/mg cytosol protein) not treated with anti-hormonal therapy].
Follow-up for the patients included in the analyses started at breast cancer diagnosis and ended at death, end of follow-up, or at year 20 of follow-up, whichever came first. In the analyses, the event of interest was breast cancer–specific mortality. The Cox models were adjusted for age, body mass index, tumour size, nodal status, surgery, and chemotherapy. Further adjustment for pr status or end of follow-up at 15 years instead of 20 years (or both) did not materially change the results; those models are therefore not presented. All reported p values are 2-sided, with 95% confidence intervals. Statistical tests were performed using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.).
RESULTS
Table i presents patient characteristics according to anti-hormonal treatment status. Anti-hormonal therapy was given to 661 patients; 1560 patients did not receive any anti-hormonal therapy.
TABLE I.
Patient characteristics by anti-hormonal treatment status
| Characteristic | Anti-hormonal treatment | ||
|---|---|---|---|
|
| |||
| Yes | No | Overall | |
| Patients (n) | 661 | 1560 | 2221 |
| Mean age (years) | 59.4±11.1 | 53.5±13.1 | 55.3±12.8 |
| Mean BMI (kg/m2) | 24.7±4.4 | 23.7±4.0 | 24.0±4.2 |
| Missing [n (%)] | 11 (1.6) | 47 (3.0) | 58 (2.6) |
| Tumour size [n (%)] | |||
| ≤20 mm | 301 (45.5) | 819 (52.5) | 1120 (50.4) |
| 21–50 mm | 225 (43.1) | 585 (37.5) | 870 (39.2) |
| >50 mm | 52 (7.8) | 104 (6.7) | 156 (7.0) |
| Missing | 23 (3.5) | 52 (3.3) | 75 (3.4) |
| Axillary nodes [n (%)] | |||
| 0 | 248 (37.5) | 870 (55.8) | 1118 (50.3) |
| 1–3 | 225 (34.0) | 347 (22.2) | 572 (25.8) |
| >3 | 128 (19.4) | 219 (14.0) | 347 (15.6) |
| Unknown | 60 (9.1) | 124 (8.0) | 184 (8.3) |
| Estrogen receptor [n (%)] | |||
| 0–3 fmol/mg | 40 (6.1) | 343 (22.0) | 383 (17.2) |
| 4–9 fmol/mg | 46 (7.0) | 220 (14.1) | 266 (12.0) |
| 10–19 fmol/mg | 100 (15.1) | 196 (12.6) | 296 (13.3) |
| 20–49 fmol/mg | 171 (25.9) | 267 (17.1) | 438 (19.7) |
| ≥50 fmol/mg | 304 (46.0) | 534 (34.2) | 838 (37.7) |
| Progesterone receptor [n (%)] | |||
| 0–9 fmol/mg | 205 (31.0) | 627 (40.2) | 832 (37.5) |
| ≥10 fmol/mg | 421 (63.7) | 804 (51.5) | 1225 (55.2) |
| Unknown | 35 (5.3) | 129 (8.27) | 164 (7.4) |
| Surgery type [n (%)] | |||
| Total mastectomy | 282 (42.7) | 788 (50.5) | 1070 (48.2) |
| Lumpectomy and RT | 294 (44.5) | 615 (39.4) | 909 (41.0) |
| Other | 85 (12.9) | 157 (10.1) | 242 (10.9) |
| Chemotherapy [n (%)] | |||
| Yes | 100 (15.1) | 450 (28.9) | 550 (24.8) |
| No | 561 (84.9) | 1110 (71.2) | 1671 (75.2) |
| Death | |||
| Breast cancer | 216 (32.7) | 556 (35.6) | 772 (34.8) |
| Overall | 336 (50.8) | 830 (53.2) | 1166 (52.5) |
BMI = body mass index; RT = radiotherapy.
Patients treated with anti-hormonal therapy were older than patients who did not receive such therapy (mean age: 59.4 years vs. 53.5 years). Overall, tumour size in the patients was, in order of frequency, 20 mm or less, 21–50 mm, and more than 50 mm (50.4%, 39.2%, and 7.0% respectively), and a high proportion of patients had positive axillary lymph node involvement (41.4%). Among patients treated with anti-hormonal therapy, 6.1% were er-negative (0–3 fmol/mg cytosol protein), and 7.0% were weakly er-positive (4–9 fmol/ mg cytosol protein). As expected, patients not treated with anti-hormonal therapy had a higher prevalence of negative and weakly positive er results (frequency: 22.0% and 14.1% respectively). Compared with patients not treated with anti-hormonal therapy, those who were treated had higher er levels. The proportion of patients with er test results in the ranges 10–19 fmol/mg, 20–49 fmol/mg, and 50 fmol/mg or more cytosol protein were, for those treated with tamoxifen, 15.1%, 25.9%, and 46% respectively, and for patients not receiving tamoxifen, 12.6%, 17.1%, and 34.2% respectively. The proportions of patients with pr values of 0–9 fmol/mg and 10 fmol/mg or more cytosol protein were 31.0% and 63.7% respectively for those receiving anti-hormonal treatment and 40.2% and 51.5% respectively for those not so treated. In both groups, most patients did not receive chemotherapy in addition to anti-hormonal therapy (84.9% and 71.2%). After a median follow-up of 14.7 years, breast cancer–related deaths numbered 216 among the 661 patients in the anti-hormonal therapy group (32.7%) and 556 among the 1560 patients in the group not receiving anti-hormonal therapy (35.6%).
When comparing patients with weakly positive er results (4–9 fmol/mg cytosol protein) and patients with er results showing 10 fmol/mg or more cytosol protein, patients with weakly positive er had more weakly positive or negative pr results (53.8% vs. 26.4%), were more likely to receive systemic chemotherapy (39.5% vs. 19.5%), and experienced higher breast cancer–specific mortality (41.7% vs. 31.7%). When er-negative patients were compared with weakly positive er patients, no major differences were found (Table ii).
TABLE II.
Patient characteristics by estrogen receptor status
| Characteristic | Estrogen receptor status (fmol/mg) | ||
|---|---|---|---|
|
| |||
| 0–3 | 4–9 | ≥10 | |
| Patients (n) | 383 | 266 | 1572 |
| Mean age (years) | 51.7±13.0 | 52.1±12.1 | 56.7±12.7 |
| Mean BMI (kg/m2) | 23.8±4.1 | 23.8±4.5 | 24.1±4.2 |
| Missing [n (%)] | 8 (2.1) | 5 (1.9) | 45 (2.9) |
| Tumour size [n (%)] | |||
| ≤20 mm | 168 (43.9) | 116 (43.6) | 836 (53.2) |
| 21–50 mm | 154 (40.2) | 117 (44.0) | 599 (38.1) |
| >50 mm | 38 (9.9) | 21 (7.9) | 97 (6.2) |
| Missing | 23 (6.0) | 12 (4.5) | 40 (2.5) |
| Axillary nodes [n (%)] | |||
| 0 | 186 (48.6) | 132 (49.6) | 800 (50.9) |
| 1–3 | 93 (24.3) | 65 (24.4) | 414 (26.3) |
| >3 | 76 (19.8) | 47 (17.7) | 224 (14.3) |
| Unknown | 28 (7.3) | 22 (8.3) | 134 (8.5) |
| Progesterone receptor [n (%)] | |||
| 0–9 fmol/mg | 274 (71.5) | 143 (53.8) | 415 (26.4) |
| ≥10 fmol/mg | 76 (19.8) | 121 (45.5) | 1028 (65.4) |
| Unknown | 33 (8.6) | 2 (0.8) | 129 (8.2) |
| Surgery [n (%)] | |||
| Total mastectomy | 190 (49.6) | 123 (46.2) | 757 (48.2) |
| Lumpectomy and RT | 158 (41.3) | 112 (42.1) | 639 (40.6) |
| Other | 35 (9.1) | 31 (11.7) | 176 (11.2) |
| Chemotherapy [n (%)] | |||
| Yes | 139 (36.3) | 105 (39.5) | 306 (19.5) |
| No | 244 (63.7) | 161 (60.5) | 1266 (80.5) |
| Death [n (%)] | |||
| Breast cancer | 162 (42.3) | 111 (41.7) | 499 (31.7) |
| Overall | 214 (55.9) | 140 (52.6) | 812 (51.7) |
BMI = body mass index; RT = radiotherapy.
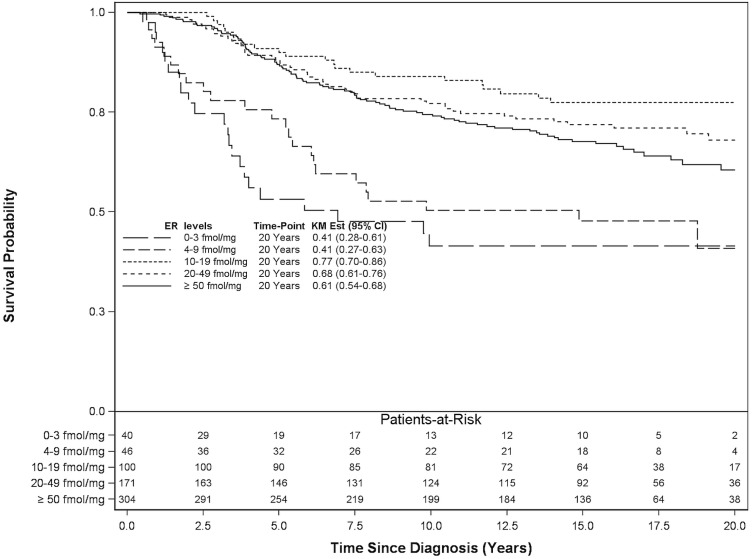
Among the 661 patients treated with anti-hormonal therapy, the 20-year breast cancer–specific survival results demonstrated a gap between the survival curves for the er groups corresponding to less than 10 fmol/mg and 10 fmol/mg or more cytosol protein (Figure 1). The 20-year breast cancer–specific mortality in patients treated with anti-hormonal therapy showed a significant risk reduction for er levels of 10 fmol/mg or more cytosol protein (Table iii). In the adjusted analysis, er levels of 0–3 fmol/mg, 4–9 fmol/mg, 10–19 fmol/mg, 20–49 fmol/mg, and 50 fmol/ mg or more cytosol protein were associated with a hazard ratio for lowered breast cancer mortality: 1.00 (reference), 0.59 (p = 0.09), 0.19 (p < 0.0001), 0.26 (p < 0.0001), and 0.31 (p < 0.0001) respectively.
FIGURE 1.
20-Year breast cancer–specific survival for 661 patients treated with anti-hormonal therapy. ER = estrogen receptor; KM Est = Kaplan–Meier estimate; CI = confidence interval.
TABLE III.
Breast cancer–specific mortality up to 20 years by estrogen receptor (ER) status in 661 patients treated with anti-hormonal therapy
| ER status (fmol/mg) | Pts (n) | Deaths (n) | Unadjusted analysis | Adjusted analysisa | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |||
| 0–3 | 40 | 22 | 1.00 | Reference | 1.00 | Reference | ||
| 4–9 | 46 | 24 | 0.75 | 0.42 to 1.34 | 0.33 | 0.59 | 0.33 to 1.08 | 0.09 |
| 10–19 | 100 | 22 | 0.22 | 0.12 to 0.41 | <0.0001 | 0.19 | 0.10 to 0.36 | <0.0001 |
| 20–49 | 171 | 49 | 0.31 | 0.19 to 0.52 | <0.0001 | 0.26 | 0.16 to 0.45 | <0.0001 |
| ≥50 | 304 | 99 | 0.38 | 0.24 to 0.60 | <0.0001 | 0.31 | 0.19 to 0.51 | <0.0001 |
Adjusted for age, body mass index, tumour size, axillary lymph node involvement, surgery, radiation therapy, and chemotherapy.
Pts = patients; HR = hazard ratio; CI = confidence interval.
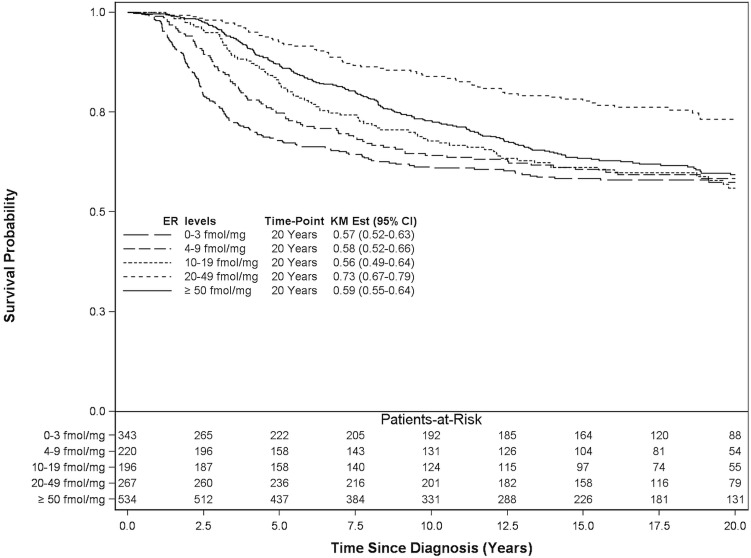
Among the 1560 patients not receiving anti-hormonal therapy, the 20-year breast cancer–specific survival was similar to that for patients taking anti-hormonal therapy, with the exception of patients with er levels in the range 20–49 fmol/mg cytosol protein, who seemed to experience improved survival (73%, Figure 2). The 20-year breast cancer–specific mortality in patients not receiving anti-hormonal therapy demonstrated a significant risk reduction only for er levels of 20 fmol/mg or more cytosol protein (Table iv). In the adjusted analysis, er levels of 0–3 fmol/mg, 4–9 fmol/mg, 10–19 fmol/mg, 20–49 fmol/mg, and 50 fmol/mg or more cytosol protein were associated with a hazard ratio for lowered breast cancer mortality: 1.00 (reference), 0.91 (p = 0.52), 0.86 (p = 0.30), 0.47 (p < 0.0001), and 0.78 (p = 0.03) respectively.
FIGURE 2.
20-Year breast cancer–specific survival for 1560 patients treated without anti-hormonal therapy. ER = estrogen receptor; KM Est = Kaplan–Meier estimate; CI = confidence interval.
TABLE IV.
Breast cancer–specific mortality up to 20 years by estrogen receptor (ER) status in 1560 patients treated without anti-hormonal therapy
| ER status (fmol/mg) | Pts (n) | Deaths (n) | Unadjusted analysis | Adjusted analysisa | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |||
| 0–3 | 343 | 140 | 1.00 | Reference | 1.00 | Reference | ||
| 4–9 | 220 | 87 | 0.89 | 0.68 to 1.16 | 0.39 | 0.91 | 0.70 to 1.20 | 0.52 |
| 10–19 | 196 | 80 | 0.86 | 0.65 to 1.12 | 0.25 | 0.86 | 0.65 to 1.14 | 0.30 |
| 20–49 | 267 | 62 | 0.44 | 0.33 to 0.60 | <0.0001 | 0.47 | 0.35 to 0.63 | <0.0001 |
| ≥50 | 534 | 187 | 0.74 | 0.60 to 0.92 | 0.01 | 0.78 | 0.62 to 0.98 | 0.03 |
Adjusted for age, body mass index, tumour size, axillary lymph node involvement, surgery, radiation therapy, and chemotherapy.
Pts = patients; HR = hazard ratio; CI = confidence interval.
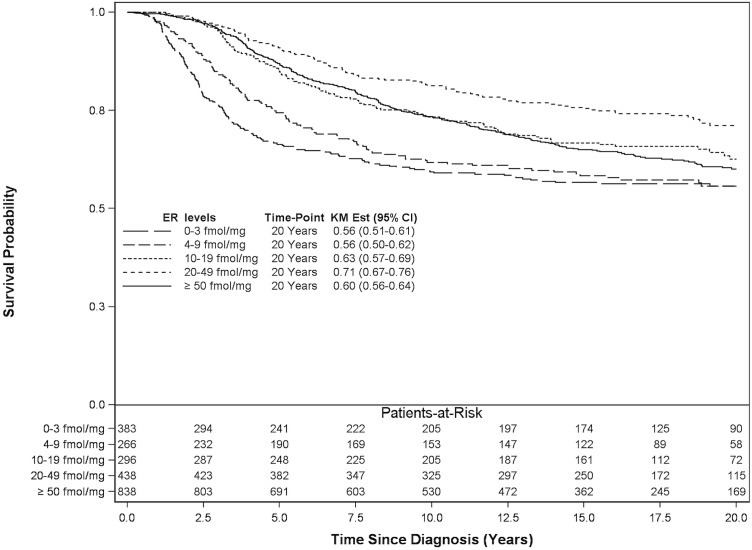
Figure 3 shows that, for all 2221 patients (regardless of therapy), the 20-year breast cancer survival for patients with er levels in the ranges 0–3 fmol/mg, 4–9 fmol/mg, 10–19 fmol/mg, 20–49 fmol/mg, and 50 fmol/mg or more cytosol protein was 56%, 56%, 63%, 71%, and 60% respectively. In the adjusted analysis of all patients, the 20-year breast cancer–specific mortality revealed a significant protective effect of anti-hormonal therapy, particularly in patients treated for tumours with er levels of 10 fmol/ mg or more cytosol protein compared with those having er-negative tumours (0–3 fmol/mg cytosol protein) not treated with anti-hormonal therapy (p < 0.0001, Table v).
FIGURE 3.
20-Year breast cancer–specific survival for all 2221 study patients. ER = estrogen receptor; KM Est = Kaplan–Meier estimate; CI = confidence interval.
TABLE V.
Breast cancer–specific mortality up to 20 years by estrogen receptor (ER) status and anti-hormonal (A-H) treatment status in 2221 patients
| ER status (fmol/mg) | Unadjusted analysis | Adjusted analysisa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| No A-H treatment | A-H treatment | No A-H treatment | A-H treatment | |||||||||
|
|
|
|
|
|||||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| 0–3 | 1.00 | Reference | 1.79 | 1.14 to 2.81 | 0.01 | 1.00 | Reference | 1.55 | 0.97 to 2.46 | 0.07 | ||
| 4–9 | 0.89 | 0.68 to 1.16 | 0.39 | 1.37 | 0.89 to 2.11 | 0.16 | 0.92 | 0.71 to 1.21 | 0.56 | 1.03 | 0.66 to 1.60 | 0.90 |
| 10–19 | 0.85 | 0.65 to 1.12 | 0.25 | 0.41 | 0.26 to 0.65 | 0.0001 | 0.86 | 0.66 to 1.14 | 0.30 | 0.34 | 0.22 to 0.54 | <0.0001 |
| 20–49 | 0.44 | 0.33 to 0.60 | <0.0001 | 0.57 | 0.41 to 0.79 | 0.0008 | 0.47 | 0.35 to 0.63 | <0.0001 | 0.46 | 0.33 to 0.65 | <0.0001 |
| ≥50 | 0.74 | 0.60 to 0.92 | 0.008 | 0.69 | 0.53 to 0.89 | 0.004 | 0.77 | 0.61 to 0.96 | 0.02 | 0.55 | 0.42 to 0.73 | <0.0001 |
Adjusted for age, body mass index, tumour size, axillary lymph node involvement, surgery, radiation therapy, and chemotherapy HR = hazard ratio; CI = confidence interval.
DISCUSSION
In the present study, we demonstrated a relationship between er expression and survival, confirming that er is a strong prognostic factor in patients with breast cancer. Multivariable analyses of breast cancer survival according to er expression and anti-hormonal treatment (tamoxifen) showed an er threshold of 10 fmol/mg cytosol protein, which was associated with a statistically significant benefit for anti-hormonal therapy. Even though a survival trend seemed to be present, no benefit from anti-hormonal adjuvant therapy was observed for breast cancers with poor er expression (4–9 fmol/mg cytosol protein) compared with those having negative er expression (0–3 fmol/mg cytosol protein). In addition, no significant benefit from anti-hormonal therapy was observed for breast cancers having negative or weak er expression (0–3 fmol/mg and 4–9 fmol/mg cytosol protein) compared with breast cancers having negative er expression (0–3 fmol/mg cytosol protein) that were not treated with anti-hormonal therapy.
The benefit of anti-hormonal therapy in breast cancers expressing hormone receptors has been recognized for more than 30 years. During those years, assays to determine the presence or absence of hormone receptors on cancer cells have progressively been refined, and thresholds have been lowered. Recently, the American Society of Clinical Oncology and the College of American Pathologists jointly recommended that any tumour expressing 1% or more er should be considered positive17–20. However, determining a relevant threshold is difficult, and uncertainty about a threshold for er expression at which the clinical benefits of anti-hormonal therapy are outweighed by the misclassification of a tumour (hormone-sensitive vs. hormone-resistant) will always be present. For instance, patients with an er-positive breast cancer misclassified as er-negative will be denied the benefit of anti-hormonal therapy, and patients with an er-negative breast cancer misclassified as er-positive with be exposed to the risks of anti-hormonal therapy and be denied the benefits of other treatments.
A number of studies have shown a direct linear relationship between er concentration and survival3,8,14,16,18,23–26. However, very few studies have supported the use of anti-hormonal treatment in breast cancer patients whose tumours are weakly positive for er (3–9 fmol/mg cytosol protein by lba or 1%–9% by ihc assay). The six studies used to establish the guidelines published by the American Society of Clinical Oncology and the College of American Pathologists had different scoring systems, different threshold values, limited follow-up, and a low number of patients with er-poor tumours8,16,18,23,24,27. Recently, the Early Breast Cancer Trialists’ Collaborative Group published a meta-analysis studying the relevance of breast cancer hormone receptors and the efficacy of adjuvant tamoxifen10. They included more than twenty randomized controlled trials with 21,457 patients that examined the effects of 5 years of adjuvant tamoxifen in nonmetastatic breast cancer patients with variable er positivity thresholds tested by biochemical assay. The authors showed that patients with er levels below 10 fmol/mg cytosol protein did not benefit from anti-hormonal therapy.
In 2011, Khoshnoud et al.36 randomized postmenopausal breast cancer patients to receive either adjuvant tamoxifen or placebo. The er status of their patients was re-analyzed with ihc so that cytosol-based assays could be compared with ihc for the determination of er status and the prediction of response to adjuvant tamoxifen. The authors observed high concordance between the cytosol assays and the ihc assays (88%). At a median follow-up of 17 years, no clinical benefit of tamoxifen was observed in er-negative patients (<0.05 fmol/μg dna or <10% on ihc), and lesser recurrence-free survival was observed in er-positive patients treated with tamoxifen (hazard ratio: 0.53; p < 0.001). However, very few patients with er-poor tumours were included in that study (7 patients with 1%–9% er positivity on ihc), and the er scale used was different from those used in most previous studies (femtomoles per microgram of dna rather than femtomoles per milligram of cytosol protein)26.
In 2009, Merglen et al.28 found that, for patients with less than 10% er on ihc assay, the risk of death from their disease was significantly increased among those treated with tamoxifen compared with those treated without it. That observation raises the hypothesis that tumours with low levels of er or negative er seem to be not only more aggressive in nature but also resistant to anti-hormonal treatments.
Anti-hormonal treatments are associated with a number of side effects. Tamoxifen and aromatase inhibitors have been shown to be associated with increased risks of thromboembolic events, uterine cancer, bone fractures, and many other effects shown to decrease quality of life (fatigue, hot sweats, depression, and so on)10,29. Given that new evidence now shows a survival benefit for patients receiving tamoxifen for 10 years rather than 5 years, correctly classifying breast cancers as er-positive or -negative is of crucial importance30. Based on our findings and on the absence of evidence supporting extended therapy for patients with er-poor tumours, the duration of tamoxifen therapy should not be extended to 10 years in this subgroup of patients.
Considering all studies about this subject, the present study encompasses the largest number of patients followed for the longest period of time at a single centre. Moreover, the present study has the largest number of patients with weakly positive er concentrations in the published literature. Only 31 of the 2221 study patients (1.4%) were lost to follow-up, causes of death were known for almost all (97.2%) patients, and very few variables had missing values. Unlike previous studies, the present study had a sample size large enough to permit analyses of subgroups of patients with many different er concentration levels.
However, some limitations must be taken into account when interpreting our results. This was a retrospective study, and in addition, er concentrations were analyzed by biochemical assay—a technique that is no longer used, but that has been shown to be equivalent to ihc. However, the use of biochemical results was necessary because follow-up for breast cancer patients whose er concentrations were analyzed by ihc is currently insufficient. In addition, misclassification of er status could have occurred, and unreported deaths in the registry could potentially have underestimated a real treatment effect. It was also not possible to determine how many patients did not complete 5 years of tamoxifen therapy because of complications, side effects, or patient preference. Such drop-outs could also have biased the results, resulting in an underestimated treatment effect. Moreover, comorbidities, tumour histology, tumour grade, and menopausal status were not included in the adjusted analyses because those variables were missing for several patients. We performed additional sensitivity analyses to determine whether excluding those variables affected our results. By comparing models that included and excluded those variables, we found that treatment effects were attenuated, meaning that our results might have been overestimated.
CONCLUSIONS
Results of the present study suggest that, compared with breast cancer patients whose tumours express er at 10 fmol/mg cytosol protein on lba, those with weaker er expression (<10 fmol/mg cytosol protein) do not significantly benefit from adjuvant anti-hormonal therapy (tamoxifen). Caution should therefore be applied when treating breast cancer patients with low er expression measured by lba; treatment allocation should be decided on a case-by-case basis. Because we analyzed patients treated with tamoxifen, it is still uncertain whether our findings could apply to patients taking aromatase inhibitors. In addition, it is also uncertain whether our findings apply to weakly er-positive breast cancers tested by ihc, given that the study used er concentrations analyzed by lba. A similar study that includes patients treated with aromatase inhibitors and patients whose er expression was analyzed by ihc should be done to confirm whether our findings are valid in current patient populations.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Alexieva-Figusch J, Van Putten WL, Blankenstein MA, Blonk-Van Der Wijst J, Klijn JG. The prognostic value and relationships of patient characteristics, estrogen and progestin receptors, and site of relapse in primary breast cancer. Cancer. 1988;61:758–68. doi: 10.1002/1097-0142(19880215)61:4<758::AID-CNCR2820610421>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Kinsel LB, Szabo E, Greene GL, Konrath J, Leight GS, McCarty KS., Jr Immunocytochemical analysis of estrogen receptors as a predictor of prognosis in breast cancer patients: comparison with quantitative biochemical methods. Cancer Res. 1989;49:1052–6. [PubMed] [Google Scholar]
- 3.Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50:7057–61. [PubMed] [Google Scholar]
- 4.McGuire WL. Breast cancer prognostic factors: evaluation guidelines. J Natl Cancer Inst. 1991;83:154–5. doi: 10.1093/jnci/83.3.154. [DOI] [PubMed] [Google Scholar]
- 5.Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol. 1996;14:2843–77. doi: 10.1200/JCO.1996.14.10.2843. [DOI] [PubMed] [Google Scholar]
- 6.Molino A, Micciolo R, Turazza M, et al. Prognostic significance of estrogen receptors in 405 primary breast cancers: a comparison of immunohistochemical and biochemical methods. Breast Cancer Res Treat. 1997;45:241–9. doi: 10.1023/A:1005769925670. [DOI] [PubMed] [Google Scholar]
- 7.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 8.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists consensus statement 1999. Arch Pathol Lab Med. 2000;124:966–78. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 10.Davies C, Godwin J, Gray R, et al. on behalf of the Early Breast Cancer Trialists’ Collaborative Group Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire WL, De La Garza M, Chamness GC. Evaluation of estrogen receptor assays in human breast cancer tissue. Cancer Res. 1977;37:637–9. [PubMed] [Google Scholar]
- 12.Lippman ME, Allegra JC, Thompson EB, et al. The relation between estrogen receptors and response rate to cytotoxic chemotherapy in metastatic breast cancer. N Engl J Med. 1978;298:1223–8. doi: 10.1056/NEJM197806012982203. [DOI] [PubMed] [Google Scholar]
- 13.Stewart J, King R, Hayward J, Rubens R. Estrogen and progesterone receptors: correlation of response rates, site and timing of receptor analysis. Breast Cancer Res Treat. 1982;2:243–50. doi: 10.1007/BF01806937. [DOI] [PubMed] [Google Scholar]
- 14.Rutqvist LE, Cedermark B, Glas U, et al. The Stockholm trial on adjuvant tamoxifen in early breast cancer. Correlation between estrogen receptor level and treatment effect. Breast Cancer Res Treat. 1987;10:255–66. doi: 10.1007/BF01805762. [DOI] [PubMed] [Google Scholar]
- 15.Blankenstein MA. Comparison of ligand binding assay and enzyme immunoassay of oestrogen receptor in human breast cancer cytosols. Experience of the eortc Receptor Group. Breast Cancer Res Treat. 1990;17:91–8. doi: 10.1007/BF01806289. [DOI] [PubMed] [Google Scholar]
- 16.Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996;74:1445–51. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes A, Jasani B, Barnes DM, Bobrow LG, Miller KD. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000;53:125–30. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regan MM, Viale G, Mastropasqua MG, et al. on behalf of the International Breast Cancer Study Group Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98:1571–81. doi: 10.1093/jnci/djj415. [DOI] [PubMed] [Google Scholar]
- 19.Badve SS, Baehner FL, Gray RP, et al. Estrogen- and progesterone-receptor status in ecog 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26:2473–81. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 20.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–7. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berube S, Provencher L, Robert J, et al. Quantitative exploration of possible reasons for the recent improvement in breast cancer survival. Breast Cancer Res Treat. 2007;106:419–31. doi: 10.1007/s10549-007-9503-1. [DOI] [PubMed] [Google Scholar]
- 22.Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2:1102–9. doi: 10.1200/JCO.1984.2.10.1102. [DOI] [PubMed] [Google Scholar]
- 23.Elledge RM, Green S, Pugh R, et al. Estrogen receptor (er) and progesterone receptor (pgr), by ligand-binding assay compared with er, pgr and pS2, by immunohistochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89:111–17. doi: 10.1002/(SICI)1097-0215(20000320)89:2<111::AID-IJC2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Thomson CS, Twelves CJ, Mallon EA, Leake RE, on behalf of the Scottish Cancer Trials Breast Group and the Scottish Cancer Therapy Network Adjuvant ovarian ablation vs cmf chemotherapy in premenopausal breast cancer patients: trial update and impact of immunohistochemical assessment of er status. Breast. 2002;11:419–29. doi: 10.1054/brst.2002.0451. [DOI] [PubMed] [Google Scholar]
- 25.Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: big 1–98. J Clin Oncol. 2007;25:3846–52. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 26.Khoshnoud MR, Lofdahl B, Fohlin H, et al. Immunohistochemistry compared to cytosol assays for determination of estrogen receptor and prediction of the long-term effect of adjuvant tamoxifen. Breast Cancer Res Treat. 2011;126:421–30. doi: 10.1007/s10549-010-1202-7. [DOI] [PubMed] [Google Scholar]
- 27.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–21. [PubMed] [Google Scholar]
- 28.Merglen A, Verkooijen HM, Fioretta G, et al. Hormonal therapy for oestrogen receptor–negative breast cancer is associated with higher disease-specific mortality. Ann Oncol. 2009;20:857–61. doi: 10.1093/annonc/mdn688. [DOI] [PubMed] [Google Scholar]
- 29.Visvanathan K, Chlebowski RT, Hurley P, et al. on behalf of the American Society of Clinical Oncology American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–58. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies C, Pan H, Godwin J, et al. on behalf of the Adjuvant Tamoxifen: Longer Against Shorter (atlas) Collaborative Group Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor–positive breast cancer: atlas, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [Erratum in: Lancet 2013;381:804] [DOI] [PMC free article] [PubMed] [Google Scholar]