Abstract
Background
Allogeneic hematopoietic stem-cell transplantation (ahsct) is associated with significant morbidity and mortality, but it can cure carefully selected patients with acute myeloid leukemia (aml) in second remission (cr2). In a cohort of patients with aml who underwent ahsct in cr2, we determined the pre-transplant factors that predicted for overall survival (os), relapse, and non-relapse mortality. We also sought to validate the prognostic risk groups derived by Michelis and colleagues in this independent population.
Methods
In a retrospective chart review, we obtained data for 55 consecutive patients who underwent ahsct for aml in cr2. Hazard ratios were used to describe the independent effects of pre-transplant variables on outcome, and Kaplan–Meier curves were used to assess outcomes in the three prognostic groups identified by Michelis and colleagues.
Results
At 1, 3, and 5 years post-transplant, os was 60%, 45.5%, and 37.5% respectively. Statistically significant differences in os, relapse mortality, and non-relapse mortality were not identified between the prognostic risk groups identified by Michelis and colleagues. Women were less likely than men to relapse, and a modified European Society for Blood and Marrow Transplantation (mebmt) score of 3 or less was associated with a lower non-relapse mortality.
Conclusions
The 37.5% 5-year os in this cohort suggests that, compared with other options, ahsct offers patients with aml in cr2 a better chance of cure. Our study supports the use of the mebmt score to predict non-relapse mortality in this population.
Keywords: Acute myelogenous leukemia, allogeneic hematopoietic stem-cell transplantation, hematology
INTRODUCTION
Acute myeloid leukemia (aml) is the most common form of acute leukemia in adults, median age at diagnosis being 65–70 years1–4. Without treatment, aml is typically fatal within weeks to months3.
The goal of aml therapy is to achieve and sustain remission, and treatment conventionally consists of two phases. In the first phase, the goal is to induce remission with conventional-dose chemotherapy; in the second phase, the goals are to prolong the remission and prevent relapse3,5,6. Options for the second phase of therapy include intensive consolidation chemotherapy, autologous hematopoietic stem-cell transplantation or allogeneic hematopoietic stem-cell transplantation (ahsct)3,6. The best consolidation for a specific patient remains controversial and depends on various patient-and disease-related factors, including cytogenetic abnormalities, patient age, comorbidities, and patient wishes7. A graft-versus-leukemia effect gives ahsct superior anti-leukemic activity, with a greater chance of maintaining remission than is achieved with consolidation chemotherapy8,9. However, its benefit is limited by greater treatment-related mortality, which can be as high as 20%–30%, and the morbidity and mortality associated with graft-versus-host disease (gvhd)6,8,10–12.
Patients with aml can generally be divided into three prognostic groups based on cytogenetics: favourable, intermediate, and adverse risk3,7,13,14. Although the cytogenetic risk group predicts the likelihood of prolonged remission after consolidation therapy, treatment-related mortality is relatively uniform across all cytogenetic risk groups7,15–17. Because patients without favourable-risk cytogenetics experience lesser survival with consolidation chemotherapy, ahsct is usually recommended for patients in the adverse prognostic group and sometimes for those in the intermediate prognostic group because the anti-leukemic effect of ahsct generally outweighs the risks and mortality associated with the treatment and is more likely to achieve a favourable result in those patient groups6–8,10,17–20.
Another prognostically important factor in patients with aml is age. Altered disease biology and adverse prognostic cytogenetics are more frequently associated with increased age, and older adults are often ineligible for the intensive myeloablative conditioning chemotherapy required for ahsct. Generally, they have more comorbidities that can preclude them from tolerating the intense therapies given to younger patients4,9,21,22. However, reduced-intensity conditioning (ric) transplant regimens have allowed for more elderly patients to become eligible for ahsct4,21,23. Because ric transplantation is associated with lesser initial transplant-related morbidity and mortality, it can also be more easily tolerated by people with comorbidities; such transplants particularly rely on the graft-versus-leukemia effect of ahsct to maintain remission4,12,21.
Even when a second complete remission (cr2) in aml is obtained, its duration is often shorter than that for the first complete remission (cr1), and overall survival (os) rates are quite low—in the range of 11%–19% at 5 years29,15–17,24. Because the chance of relapsing after consolidation chemotherapy is high—of patients who did not undergo transplantation in cr1 in three Medical Research Council trials, more than 30% relapsed16—and because ahsct after relapse is the only treatment that can potentially cure a patient with aml20,25–27, the classification of relapsed patients into risk groups to predict outcome after ahsct would be useful. Several patient characteristics such as age, cytogenetics at diagnosis, length of cr1, presence and extent of comorbidities, and whether the donor is related or unrelated to the recipient have been shown to influence survival in ahsct patients after relapse7,9,14,17,25,28.
A number of studies have described prognostically significant categories meant to determine which patients will benefit most from ahsct in cr2; those studies include a large single-centre retrospective cohort study from the Princess Margaret Hospital in Toronto by Michelis et al. and a German study investigating the prognostic implications of a modified European Society for Blood and Marrow Transplantation (mebmt) risk score17,25,27,29,30.
For patients with aml in cr2, Michelis et al.29 assessed the influence of several pre-transplant variables on os, relapse rate, and non-relapse mortality. Those investigators used a multivariable analysis to identify three prognostically significant categories including patient age, duration of first remission, and score on the hematopoietic cell transplantation–specific comorbidity index (hct-ci)28. The tool is attractive because it is simple to use and combines several readily available prognostic factors into a prognostic model (Table i).
TABLE I.
The three prognostic risk groups defined by Michelis et al.29
| Risk group | Age (years) | Duration of first remission (months) | HCT-CI score |
|---|---|---|---|
| Favorable | <55 | ≥6 | ≤3 |
| Intermediate | <55 | ≥6 | >3 |
| Poor | ≥55 | ≥6 | Any |
| Poor | <55 | <6 | Any |
| Poor | ≥55 | <6 | Any |
HCT-CI = hematopoietic cell transplantation–specific comorbidity index.
We analyzed outcomes in a cohort of patients undergoing transplantation at a single centre to determine whether the prognostically significant categories derived from the Michelis et al.29 study apply to an independent group of patients with aml in cr2, and we assessed other patient characteristics to determine which pre-transplant factors predicted os, relapse rate, and non-relapse mortality after ahsct in our aml cohort in cr2.
METHODS
Patients
The transplantation database in Halifax, Nova Scotia, was reviewed to identify patients with de novo or secondary aml in cr2 who had undergone a first ahsct from a matched related or unrelated donor (10/10 or 6/6 match, or 9/10 or 5/6 mismatch) between 1992 and 2013. Patients had to have been between 17 and 65 years of age at transplantation and to have received a myeloablative or ric regimen. After excluding patients who had received a syngeneic transplant, a cord blood transplant, a haploidentical transplant, or in vitro T-cell depletion, 55 patients were identified. Although the Michelis et al.29 study included patients up to the age of 70 years, patients over the age of 65 years were not included in the study because the upper age limit for ahsct at our centre is 65. All patients had consented to the use of their disease and transplant-related information for research purposes. The study protocol was approved by the research ethics board at the Capital District Health Authority.
Data
In a retrospective chart review, the following patient and disease characteristics were obtained: patient age at transplantation, sex, date of initial aml diagnosis, date of achievement of first remission, date of relapse before blood and marrow transplantation, date of blood and marrow transplantation, cytogenetic risk group at diagnosis and at relapse, conditioning regimen, primary induction success, presence of pre-existing myelodysplastic syndrome, related or unrelated donor, age of donor, human leucocyte antigen match characteristics, cytomegalovirus serostatus of patient and donor, blood type of patient and donor, co-morbidities and laboratory values to calculate the hct-ci score28, and remaining parameters to calculate the mebmt risk score30. These post-transplant variables and outcomes were also recorded: gvhd prophylaxis, development of acute gvhd or chronic gvhd and grade, date of last follow-up or death, and cause of death (if applicable).
Data were obtained from the hospital electronic medical records system (Horizon Patient Folder: McKesson, San Francisco, CA, U.S.A.), hospital paper records, and the OneMatch program operated by Canadian Blood Services.
Definitions of Clinical Parameters
Second complete remission, incomplete second remission, and relapse were defined as they were in the Michelis et al. study29,31.
Conditioning Chemotherapy Used for Myeloablative and RIC Regimens
Patients received conditioning treatments for ahsct according to protocols used by the Division of Hematology, Department of Medicine, at Capital Health in Halifax, Nova Scotia:
■ Busulfan and cyclophosphamide conditioning consisted of oral busulfan 1 mg/kg every 6 hours for 16 doses, starting at 06h00 on day −7 and ending at 24h00 on day −4, and intravenous cyclophosphamide 60 mg/kg daily on days −3 and −2, with stem-cell infusion at least 48 hours after completion of chemotherapy.
■ Cyclophosphamide and fractionated total body irradiation (ftbi) conditioning consisted of intravenous cyclophosphamide 60 mg/kg daily on days −4 and −3; ftbi on days −2, −1, and 0 (6 fractions of 200 cGy each, midplane, for 1200 cGy total). Bone marrow or peripheral blood was infused after the last fraction of radiation.
■ Reduced-intensity conditioning ahsct entailed intravenous cyclophosphamide 300 mg/m2 daily, day −7 to day −3 inclusive, and intravenous fludarabine 30 mg/m2 daily, day −7 to day −3 inclusive. Stem-cell infusion occurred at least 72 hours after completion of chemotherapy.
One patient received intravenous etoposide (60 mg/kg) and ftbi because an anthracycline-induced cardiomyopathy precluded the use of cyclophosphamide.
GVHD Prophylaxis
Patients who underwent a myeloablative ahsct received cyclosporine starting on day −2 (6.25 mg/kg orally every 12 hours, or oral dose/2.5 intravenously over 4 hours every 12 hours) and intravenous methotrexate (15 mg/m2 on day 1 and 10 mg/m2 on days +3, +6, and +11) as gvhd prophylaxis. Patients who underwent a non-myeloablative ahsct received oral tacrolimus 3 mg twice daily starting on day −7, adjusted to maintain trough tacrolimus levels between 5 μmol/L and 15 μmol/L, with taper to start at day +50, and oral mycophenolate mofetil (CellCept: Genentech, South San Francisco, CA, U.S.A.) 1000 mg twice daily starting on day +1 and stopping on day +50 (started at least 24 hours after the last stem-cell infusion). One patient who received a myeloablative ahsct was switched from cyclosporine to tacrolimus on day +3 because of a reaction to cyclosporine. Acute gvhd was graded according to established criteria32, and chronic gvhd was graded as either limited or extensive33.
Statistical Analysis
Categorical variables are summarized as counts and percentages, and continuous variables are summarized as medians and ranges. The os, relapse rate, and non-relapse mortality were measured from the date of ahsct until the event date (death, relapse, or last follow-up). The hct-ci28 and mebmt30 scores were calculated before transplantation.
Patients were grouped into the three risk categories described by Michelis et al.29. Patients who were less than 55 years of age and whose duration of first remission was at least 6 months fell into the favourable-risk group if their hct-ci score was 3 or less; they fell into the intermediate-risk group of their hct-ci score was greater than 3. Patients who were more than 55 years of age or whose duration of first remission was less than 6 months (or both) fell into the poor-risk group regardless of hct-ci score.
Survival was calculated using the Kaplan–Meier method, and the log-rank test was used to compare variables of interest. Cox proportional hazards regression was used to assess the independent effect of possible predictors of os, relapse, and non-relapse mortality. Assumptions of proportionality were tested using the log-minus-log plot and were met. All analyses were conducted using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.). Two-sided p values were calculated, and p < 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
The 55 consecutive patients with aml who were transplanted in cr2 between June 1992 and May 2013 had a median age of 48 years (range: 17–64 years), and 30 of them (54.5%) were men. The median duration of cr1 was 12 months (range: 1–64 months). In 5 patients (9.1%), aml had developed from a pre-existing myelodysplastic syndrome; 4 patients (7.3%) experienced primary induction failure—that is, they required more than 1 induction chemotherapy regimen to achieve cr1. The cytogenetic risk profile at diagnosis was favourable in 12 patients (21.8%), intermediate in 33 (60%), poor in 3 (5.5%), and unavailable in 7 (12.7%). Scores on the hct-ci ranged from 0 to 8, with a median score of 3. Scores on the mebmt ranged from 2 to 5, with a median score of 3.
Transplant and Donor Characteristics
Myeloablative conditioning chemotherapy was given to 20 patients (36.4%) with ftbi and to 19 patients (34.5%) without ftbi; 16 patients (29.1%) received ric. In 28 patients (50.9%), the ahsct donation came from a sibling; in 26 (47.3%), it came from a matched unrelated individual; and in 1 (1.8%), it came from a related non-sibling donor (a double cousin). For 49 patients (89.1%), the ahsct graft came from a completely matched donor (6/6 or 10/10); for 6 patients (10.9%), the graft came from a 5/6 or 9/10 matched donor. For 34 of the recipient–donor pairs (61.8%), an ABO blood group match was attained; 12 pairs (21.8%) had a minor ABO mismatch, and 9 pairs (16.4%) had a major ABO mismatch. In 26 patients (47.3%), a risk for post-transplant cytomegalovirus infection was present, as defined by the serostatus of patient and donor. Median donor age was 40 years (range: 20–69 years). Of the 30 male recipients, 12 (40%) had a female donor.
Post-transplant Events
After ahsct, 69.1% of patients were diagnosed with acute or chronic gvhd. Grade ii–iv acute gvhd was diagnosed in 17 patients (30.9%) and grade i acute gvhd in 10 patients (18.2%); 28 of patients (50.9%) did not have acute gvhd. Extensive chronic gvhd was diagnosed in 12 patients (21.8%) and limited chronic gvhd in 15 patients (27.3%); 28 patients (50.9%) did not have chronic gvhd.
Outcomes
At 1, 3, and 5 years post-transplantation, os was 60%, 45.5%, and 37.5% respectively. Of the 36 patients who died after ahsct, 19 (52.8%) died in relapse. For those 19 patients, time to relapse ranged from 1.5 months to 22.5 months (median: 5.6 months). Death from non-relapse causes, including complications of gvhd, occurred in 17 of the 36 patients (47.2%).
Pre-transplant Characteristics and Outcomes
None of the pre-transplant variables examined were significantly associated with increased os (Table ii). The only variable significantly associated with a lower relapse rate was female sex (p = 0.04); no other pre-transplant variable was significantly associated with the relapse rate (Table iii). The only variable found to be significantly associated with non-relapse mortality was a mebmt score of 3 or less (p = 0.049, Table iv).
TABLE II.
Univariate analysis of the effect of pre-transplantation variables on overall survival
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Sex | |||
| Men | 1 | ||
| Women | 0.6 | 0.3 to 1.19 | 0.14 |
| Duration of first remission | |||
| <6 Months | 1 | ||
| ≥6 Months | 0.88 | 0.38 to 2.04 | 0.77 |
| Patient age group | |||
| ≤55 Years | 1 | ||
| >55 Years | 0.71 | 0.32 to 1.55 | 0.39 |
| Cytogenetic risk category | |||
| Favourable | 1.05 | 0.48 to 2.29 | 0.91 |
| Intermediate or poor | 1 | ||
| Post myelodysplastic syndrome | |||
| No | 1 | ||
| Yes | 0.67 | 0.2 to 2.24 | 0.52 |
| Primary induction failure | |||
| No | 1 | ||
| Yes | 0.45 | 0.1 to 1.92 | 0.28 |
| Regimen | |||
| Myeloablative conditioning and total body irradiation | 1.1 | 0.47 to 2.58 | 0.83 |
| Myeloablative conditioning | 0.91 | 0.4 to 2.06 | 0.83 |
| Reduced-intensity conditioning | 1 | ||
| Donor type | |||
| Matched unrelated donor | 1 | ||
| Related (sibling or non-sibling) | 0.84 | 0.43 to 1.65 | 0.61 |
| Human leukocyte antigen | |||
| Match | 1.31 | 0.46 to 3.77 | 0.62 |
| Mismatch | 1 | ||
| Blood type match | |||
| Match | 1 | ||
| Minor mismatch | 1 | 0.46 to 2.2 | 1 |
| Major mismatch | 0.8 | 0.3 to 2.12 | 0.65 |
| Cytomegalovirus risk | |||
| No | 1 | ||
| Yes | 0.57 | 0.29 to 1.14 | 0.11 |
| HCT-CI score | |||
| ≤3 | 1 | ||
| >3 | 1.4 | 0.73 to 2.7 | 0.31 |
| Female donor, male recipient | |||
| No | 1 | ||
| Yes | 1.36 | 0.63 to 2.92 | 0.43 |
| Age of donor | |||
| ≤40 Years | 1 | ||
| >40 Years | 0.6 | 0.29 to 1.21 | 0.15 |
| mEBMT score | |||
| ≤3 | 1 | ||
| >3 | 1.74 | 0.89 to 3.4 | 0.11 |
HR = hazard ratio; CI = confidence interval; HCT-CI = hematopoietic cell transplantation–specific comorbidity index; mEBMT = modified European Society for Blood and Marrow Transplantation.
TABLE III.
Univariate analysis of the effect of pre-transplantation variables on relapse rate
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Sex | |||
| Men | 1 | ||
| Women | 0.34 | 0.12 to 0.93 | 0.04 |
| Duration of first remission | |||
| <6 Months | 1 | ||
| ≥6 Months | 0.74 | 0.25 to 2.22 | 0.59 |
| Patient age group | |||
| ≤55 Years | 1 | ||
| >55 Years | 1.13 | 0.43 to 2.94 | 0.81 |
| Cytogenetic risk category | |||
| Favourable | 0.98 | 0.35 to 2.71 | 0.96 |
| Intermediate or poor | 1 | ||
| Post myelodysplastic syndrome | |||
| No | 1 | ||
| Yes | 1.2 | 0.28 to 5.19 | 0.81 |
| Primary induction failure | |||
| No | 1 | ||
| Yes | 1.71 | 0.5 to 5.83 | 0.39 |
| Regimen | |||
| Myeloablative conditioning and total body irradiation | 0.4 | 0.12 to 1.34 | 0.14 |
| Myeloablative conditioning | 0.65 | 0.24 to 1.74 | 0.39 |
| Reduced-intensity conditioning | 1 | ||
| Donor type | |||
| Matched unrelated donor | 1 | ||
| Related (sibling or non-sibling) | 1.57 | 0.62 to 3.93 | 0.34 |
| Human leukocyte antigen | |||
| Match | 2.99 | 0.4 to 22.32 | 0.29 |
| Mismatch | 1 | ||
| Blood type match | |||
| Match | 1 | ||
| Minor mismatch | 0.8 | 0.26 to 2.42 | 0.69 |
| Major mismatch | 0.5 | 0.11 to 2.18 | 0.35 |
| Cytomegalovirus risk | |||
| No | 1 | ||
| Yes | 0.77 | 0.32 to 1.87 | 0.56 |
| HCT-CI score | |||
| ≤3 | 1 | ||
| >3 | 0.98 | 0.4 to 2.4 | 0.97 |
| Female donor, male recipient | |||
| No | 1 | ||
| Yes | 1.89 | 0.72 to 4.97 | 0.2 |
| Age of donor | |||
| ≤40 Years | 1 | ||
| >40 Years | 0.67 | 0.27 to 1.68 | 0.39 |
| mEBMT score | |||
| ≤3 | 1 | ||
| >3 | 0.99 | 0.37 to 2.61 | 0.98 |
HR = hazard ratio; CI = confidence interval; HCT-CI = hematopoietic cell transplantation–specific comorbidity index; mEBMT = modified European Society for Blood and Marrow Transplantation.
TABLE IV.
Univariate analysis of the effect of pre-transplantation variables on non-relapse mortality
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Sex | |||
| Male | 1 | ||
| Female | 0.96 | 0.37 to 2.51 | 0.94 |
| Duration of first remission | |||
| <6 Months | 1 | ||
| ≥6 Months | 0.73 | 0.23 to 2.3 | 0.59 |
| Patient age | |||
| ≤55 Years | 1 | ||
| >55 Years | 0.5 | 0.14 to 1.77 | 0.29 |
| Cytogenetic risk category | |||
| Favourable | 1.03 | 0.31 to 3.39 | 0.97 |
| Intermediate or poor | 1 | ||
| Post myelodysplastic syndrome | |||
| No | 1 | ||
| Yes | 0.89 | 0.19 to 4.13 | 0.88 |
| Primary induction failure | |||
| No | 1 | ||
| Yes | NA | NA | 0.99 |
| Regimen | |||
| Myeloablative conditioning and total body irradiation | 2.78 | 0.71 to 10.93 | 0.14 |
| Myeloablative conditioning | 1.4 | 0.35 to 5.64 | 0.63 |
| Reduced-intensity conditioning | 1 | ||
| Donor type | |||
| Matched unrelated donor | 1 | ||
| Related (sibling or non-sibling) | 0.53 | 0.19 to 1.45 | 0.21 |
| Human leukocyte antigen | |||
| Match | 0.86 | 0.24 to 3.1 | 0.81 |
| Mismatch | 1 | ||
| Blood type match | |||
| Match | 1 | ||
| Minor mismatch | 1.33 | 0.44 to 4.08 | 0.62 |
| Major mismatch | 1.05 | 0.28 to 3.99 | 0.94 |
| Cytomegalovirus risk | |||
| No | 1 | ||
| Yes | 0.44 | 0.15 to 1.26 | 0.13 |
| HCT-CI score | |||
| ≤3 | 1 | ||
| >3 | 1.93 | 0.73 to 5.08 | 0.18 |
| Female donor, male recipient | |||
| No | 1 | ||
| Yes | 0.81 | 0.23 to 2.9 | 0.75 |
| Age of donor | |||
| ≤40 Years | 1 | ||
| >40 Years | 0.53 | 0.18 to 1.59 | 0.26 |
| mEBMT score | |||
| ≤3 | 1 | ||
| >3 | 2.68 | 1.01 to 7.16 | 0.049 |
HR = hazard ratio; CI = confidence interval; NA = not applicable; HCT-CI = hematopoietic cell transplantation–specific comorbidity index; mEBMT = modified European Society for Blood and Marrow Transplantation.
Prognostic Groups
After patients were divided into the three risk groups defined by Michelis et al.29, the favourable-risk group included 25 patients (cr1 ≥ 6 months, age < 55 years, hct-ci score ≤ 3), the intermediate-risk group included 20 patients (cr1 ≥ 6 months, age < 55, hct-ci score > 3), and the poor-risk group included 10 patients (cr1 < 6 months or age ≥ 55, or both).
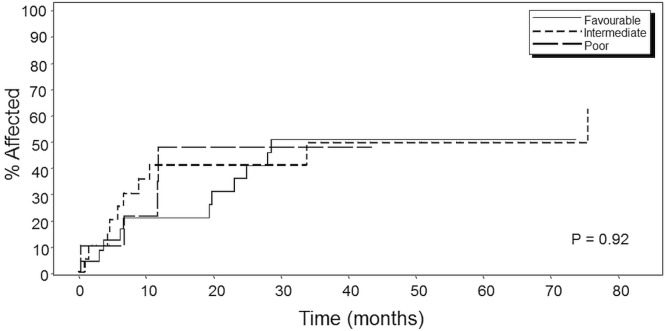
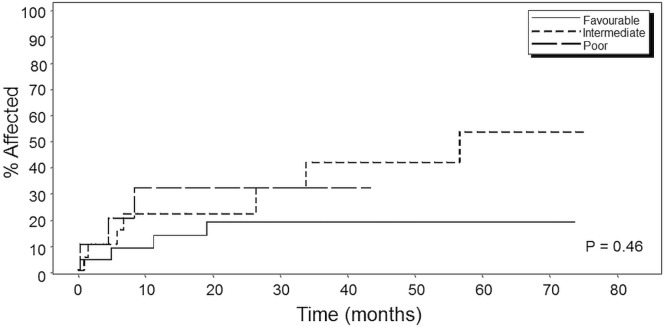
In a departure from the Michelis et al. study, in which a significant (p = 0.0001) difference in os based on log-rank statistics was seen between the groups, our analysis did not reveal any statistically significant differences in os (p = 0.85, Figure 1), although at 5 years, 5 of 25 patients in the favourable-risk group and 4 of 20 patients in the intermediate-risk group were still alive (20% in both groups). In the poor-risk group, only 1 of the 10 patients was still alive (10%). Similar analyses revealed no significant differences either in relapse mortality (p = 0.92, Figure 2) or in non-relapse mortality (p = 0.46, Figure 3) between the three prognostic groups.
FIGURE 1.
Kaplan–Meier curve for overall survival in patients by Michelis et al.29 risk category. Favourable = first remission (CR1) of 6 months or more, age less than 55 years, and hematopoietic cell transplantation–specific comorbidity index (HCT-CI) score of 3 or less (n = 25); intermediate = CR1 of 6 months or more, age less than 55 years, and HCT-CI score greater than 3 (n = 20); poor = CR1 of less than 6 months or age 55 years or more (n = 10), or both.
FIGURE 2.
Kaplan–Meier curve for relapse mortality in patients by Michelis et al.29 risk category. Favourable = first remission (CR1) of 6 months or more, age less than 55 years, and hematopoietic cell transplantation–specific comorbidity index (HCT-CI) score of 3 or less (n = 25); intermediate = CR1 of 6 months or more, age less than 55 years, and HCT-CI score greater than 3 (n = 20); poor = CR1 of less than 6 months or age 55 years or more (n = 10), or both.
FIGURE 3.
Kaplan–Meier curve for non-relapse mortality in patients by Michelis et al.29 risk category. Favourable = first remission (CR1) of 6 months or more, age less than 55 years, and hematopoietic cell transplantation–specific comorbidity index (HCT-CI) score of 3 or less (n = 25); intermediate = CR1 of 6 months or more, age less than 55 years, and HCT-CI score greater than 3 (n = 20); poor = CR1 of less than 6 months or age 55 years or more (n = 10), or both.
DISCUSSION
For patients with relapsed aml, ahsct is the only current treatment that offers the possibility of cure17,20,25–27. Unfortunately, ahsct is associated with high treatment-related morbidity and mortality6,8,10–12, and investigational treatments or palliation are sometimes more appropriate options for certain patients. The 5-year post-transplantation os of 37.5% in this cohort is comparable to rates published in other reports16,25 and further suggests that that, compared with other options, ahsct offers carefully selected patients with aml in cr2 a better chance of long-term survival.
Our study confirms the usefulness of the mebmt score in predicting non-relapse mortality in this population (p = 0.049). Because the mebmt score is a pre-transplantation prognostic indicator, it would also be expected to predict the relapse rate and os30, but it did not significantly predict either outcome in our cohort (p = 0.98 and p = 0.11 respectively). An explanation for the fact that the likelihood of relapse was significantly lower for women than for men (p = 0.04) was not found in the literature, although it has previously been reported that men receiving ahsct donations from women tend to experience a greater incidence of gvhd, lesser survival, and a lower relapse rate because of graft-versus-leukemia effect than do men receiving their donation from a man, or women receiving a donation from a donor or either sex34–36. However, our analyses did not reveal a significant effect on outcomes of a match consisting of a female donor and male recipient, and even if such an effect had been seen, it would not explain the lower relapse rate seen in the women in our cohort. This particular association might have been spurious.
Although a cr1 duration of 6 or more months, a hct-ci score of 3 or less, and an age of less than 55 years were not associated with improved os in our cohort, we observed a trend toward improved os with both a cr1 of more than 6 months and a hct-ci score of 3 or less. Unexpectedly, patient age less than 55 years at the time of transplantation was associated with a trend toward decreased os. A donor age of 40 years or more was also associated with a trend toward improved os. The unexpectedly improved survival in older patients and donors likely reflects the careful selection of patients in this age group for transplantation and the use of ric in those patients. The Michelis et al. study included patients 65–70 years of age, but patients older than 65 years have not been considered for ahsct at our centre. Matched related donors tended to be older than matched unrelated donors, with median ages of 44 years (range: 20–69 years) and 35.5 years (range: 20–55 years) respectively. Aside from the extra time it might take to arrange an unrelated ahsct, transplants involving related donors are, compared with those involving unrelated donors, associated with lower toxicity despite improvements in matching34.
Our analysis is limited by sample size and the fact that our study included patients undergoing transplantation over a period of 21 years (because only a small number of patients who received ahsct at our centre were in cr2). It would have been interesting to analyze patients based on the era in which they were transplanted; however, given the already small sample size, such an analysis was not feasible. Despite the long duration of our study in a rapidly evolving field, we observed no statistically significant association between year of transplantation and os, relapse rate, or non-relapse mortality in our cohort.
The favourable-, intermediate-, and poor-risk prognostic groups derived by Michelis et al.29 were not independently validated in our patient population, likely because of the relatively small number of patients in our study. Moreover, some patients were transplanted more recently, and their follow-up is therefore relatively short [median: 20 months (range: 0–263 months)]. The prognostic scoring derived by Michelis et al. uses patient age, duration of first remission, and hct-ci score to predict patient outcome after ahsct for aml in cr2 because those variables all independently significantly affected patient os in the original publication. In our cohort, those three variables were not independently predictive of os, and so it was not unexpected that the multivariate analysis also did not yield significant results. A large national study including our data was recently completed to validate the prognostic categories described by Michelis et al.29. That study found, on a national level, that duration of cr1 and hct-ci score, but not age, predicts survival for aml patients transplanted in cr2, and the prognostic groups were then altered to reflect those findings37. The larger study that included our population might be more representative than the original Michelis et al. cohort and might be useful to consider when deciding whether a patient with aml in cr2 should receive ahsct. As more patients in cr2 are transplanted at our centre, it might be beneficial to use a larger sample size in an attempt to validate the new prognostic groups in our distinct population. The fact that we were unable to reproduce the original Michelis et al. prognostic groups29 in our cohort—in addition to the fact that Michelis et al. had to modify their prognostic groups when looking at a national population37—illustrates the observation that a prognostic score significant in one population might not apply in all populations.
Nevertheless, having a variety of tools available, such as the hct-ci score28, the mebmt score30, and the Michelis et al. groups37, can aid in decision-making when a clinician is faced with uncertainty about whether to pursue ahsct in a patient with aml in cr2.
CONCLUSIONS
Our study supports the mebmt score as a useful tool to predict non-relapse mortality in patients with aml in cr2. The 37.5% 5-year os in our cohort suggests that ahsct is a reasonable option and therefore worthwhile pursuing in carefully selected patients. Further work should be done to determine useful prognostic categories that will help to guide clinical decision-making about whether to pursue this treatment in patients with aml in cr2.
ACKNOWLEDGMENTS
Summer studentship funding was provided by the Dalhousie Faculty of Medicine Summer Research Studentship. This work was also supported by the Richard Payzant Ward Travel Fund.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 2.Redaelli A, Lee JM, Stephens JM, Pashos CL. Epidemiology and clinical burden of acute myeloid leukemia. Expert Rev Anticancer Ther. 2003;3:695–710. doi: 10.1586/14737140.3.5.695. [DOI] [PubMed] [Google Scholar]
- 3.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 4.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–67. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 6.Rowe JM. Optimal induction and post-remission therapy for aml in first remission. Hematology Am Soc Hematol Educ Program. 2009:396–405. doi: 10.1182/asheducation-2009.1.396. [DOI] [PubMed] [Google Scholar]
- 7.Chalandon Y, Barnett MJ, Horsman DE, et al. Influence of cytogenetic abnormalities on outcome after allogeneic bone marrow transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2002;8:435–43. doi: 10.1053/bbmt.2002.v8.pm12234169. [DOI] [PubMed] [Google Scholar]
- 8.Stelljes M, Krug U, Beelen DW, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol. 2014;32:288–96. doi: 10.1200/JCO.2013.50.5768. [DOI] [PubMed] [Google Scholar]
- 9.Smith ML, Hills RK, Grimwade D. Independent prognostic variables in acute myeloid leukaemia. Blood Rev. 2011;25:39–51. doi: 10.1016/j.blre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AK, Wheatley K, Goldstone AH, et al. on behalf of the Medical Research Council Adult and Paediatric Working Parties The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK mrc aml 10 trial. Br J Haematol. 2002;118:385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet aml Working Party consensus statement on allogeneic hsct for patients with aml in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9:579–90. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- 12.Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–44. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 13.Byrd JC, Mrozek K, Dodge RK, et al. on behalf of the Cancer and Leukemia Group B Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (calgb 8461) Blood. 2002;100:4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 14.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in aml: analysis of 1,612 patients entered into the mrc aml 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33. [PubMed] [Google Scholar]
- 15.Stone RM. Acute myeloid leukemia in first remission: to choose transplantation or not? J Clin Oncol. 2013;31:1262–6. doi: 10.1200/JCO.2012.43.4258. [DOI] [PubMed] [Google Scholar]
- 16.Burnett AK, Goldstone A, Hills RK, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013;31:1293–301. doi: 10.1200/JCO.2011.40.5977. [DOI] [PubMed] [Google Scholar]
- 17.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–78. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–61. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanada M, Matsuo K, Emi N, Naoe T. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer. 2005;103:1652–8. doi: 10.1002/cncr.20945. [DOI] [PubMed] [Google Scholar]
- 20.Armistead PM, de Lima M, Pierce S, et al. Quantifying the survival benefit for allogeneic hematopoietic stem cell transplantation in relapsed acute myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15:1431–8. doi: 10.1016/j.bbmt.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krauter J, Wagner K, Stadler M, et al. Prognostic factors in allo-sct of elderly patients with aml. Bone Marrow Transplant. 2011;46:545–51. doi: 10.1038/bmt.2010.145. [DOI] [PubMed] [Google Scholar]
- 22.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett AJ, Savani BN. Stem cell transplantation with reduced-intensity conditioning regimens: a review of ten years experience with new transplant concepts and new therapeutic agents. Leukemia. 2006;20:1661–72. doi: 10.1038/sj.leu.2404334. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Tallman MS, Oken MM, et al. Duration of second complete remission compared with first complete remission in patients with acute myeloid leukemia. Eastern Cooperative Oncology Group. Leukemia. 2000;14:1345–8. doi: 10.1038/sj.leu.2401853. [DOI] [PubMed] [Google Scholar]
- 25.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Société Française de Greffe de Moelle (sfgm) Bone Marrow Transplant. 2000;26:1157–63. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- 26.Gale RP, Horowitz MM, Rees JK, et al. Chemotherapy versus transplants for acute myelogenous leukemia in second remission. Leukemia. 1996;10:13–19. [PubMed] [Google Scholar]
- 27.Wong R, Shahjahan M, Wang X, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–14. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (hct)–specific comorbidity index: a new tool for risk assessment before allogeneic hct. Blood. 2005;106:2912–19. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelis FV, Atenafu EG, Gupta V, et al. Duration of first remission, hematopoietic cell transplantation–specific comorbidity index and patient age predict survival of patients with aml transplanted in second cr. Bone Marrow Transplant. 2013;48:1450–5. doi: 10.1038/bmt.2013.71. [DOI] [PubMed] [Google Scholar]
- 30.Hemmati PG, Terwey TH, le Coutre P, et al. A modified ebmt risk score predicts the outcome of patients with acute myeloid leukemia receiving allogeneic stem cell transplants. Eur J Haematol. 2011;86:305–16. doi: 10.1111/j.1600-0609.2011.01580.x. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell MR, Abboud CN, Altman J, et al. nccn Clinical Practice Guidelines: acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984–1021. doi: 10.6004/jnccn.2012.0103. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis. 2007;2:35. doi: 10.1186/1750-1172-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 34.Gahrton G. Risk assessment in haematopoietic stem cell transplantation: impact of donor–recipient sex combination in allogeneic transplantation. Best Pract Res Clin Haematol. 2007;20:219–29. doi: 10.1016/j.beha.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Gratwohl A, Hermans J, Niederwieser D, van Biezen A, van Houwelingen HC, Apperley J, on behalf of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation EBMT Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J. 2001;2:363–70. doi: 10.1038/sj.thj.6200117. [DOI] [PubMed] [Google Scholar]
- 36.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of hla-matched, related hematopoietic stem cell transplants. Blood. 2004;103:347–52. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 37.Michelis FV, Atenafu EG, Couban S, et al. Duration of first remission and hematopoietic cell transplantation-specific comorbidity index but not age predict survival of patients with aml transplanted in cr2: a retrospective multicenter study. Bone Marrow Transplant. 2016;51:1019–21. doi: 10.1038/bmt.2016.60. [DOI] [PubMed] [Google Scholar]