Abstract
Purpose
Elderly patients make up a large percentage of the individuals newly diagnosed with glioblastoma (gbm), but they face particular challenges in tolerating standard therapy, and compared with younger patients, they experience significantly shorter survival. We set out to compare clinical characteristics, treatment patterns, and outcomes in a non-elderly group (<65 years) and an elderly group (≥65 years) of patients diagnosed with gbm.
Methods
This retrospective population-based study used a province-wide cancer registry to identify patients with a new diagnosis of gbm within a 6-year period (2006–2012). Of the 138 patients identified, 56 (40.6%) were 65 years of age or older. Demographic characteristics, treatment patterns, and overall survival (os) in the elderly and non-elderly groups were compared. Predictors of os were determined using multivariate analysis.
Results
Elderly patients were more likely to present with a poor performance status (Eastern Cooperative Oncology Group ≥ 2), to undergo biopsy without resection, and to receive whole-brain or hypofractionated radiotherapy. Compared with non-elderly patients, the elderly patients were less likely to receive adjuvant temozolomide. Survival time was significantly shorter in the elderly than in the non-elderly patients (7.2 months vs. 11.2 months). In multivariate analysis, surgical resection, hypofractionated radiotherapy (compared with whole-brain or conventional radiotherapy), and chemotherapy were predictive of os in older patients. Among elderly patients receiving radiation, survival was improved with the use of combined therapy compared with the use of radiation only (11.3 months vs. 4.6 months).
Conclusions
Overall survival was shorter for elderly patients with gbm than for non-elderly patients; the elderly patients were also less likely to receive intensive surgical or adjuvant therapy. Our population-based analysis demonstrated improved os with surgical resection, hypofractionated radiotherapy, and temozolomide, and supports the results of recent clinical trials demonstrating a benefit for combination chemoradiotherapy in older patients.
Keywords: Glioblastoma, elderly patients, survival, chemoradiotherapy, toxicity, temozolomide, radiotherapy
INTRODUCTION
Glioblastoma (gbm) is the most common primary central nervous system malignancy diagnosed in adults. Despite advances in neurosurgical techniques and the use of adjuvant radiation with concomitant chemotherapy, disease recurrence remains essentially inevitable, and overall survival (os) is poor even in the fittest and most aggressively treated patients. The optimal management of elderly patients with gbm remains an area of significant interest because approximately half of all patients diagnosed with gbm are more than 65 years of age, and the rate of gbm in the elderly population is increasing1. Thus, the aging of the population will result in a growing number of older patients being diagnosed with gbm in years to come.
Recently, the results of the Elderly Glioblastoma Trial (Canadian Cancer Trials Group ce.6 study, European Organization for Research and Treatment of Cancer 26062-22061 study, and Trans Tasman Radiation Oncology Group 08.02 study)2, presented at the 2016 American Society of Clinical Oncology annual general meeting, provided further insight into the role of combined chemoradiotherapy in older patients with gbm. However, many questions remain unresolved, including the optimal fractionation schedule for radiotherapy, the role of temozolomide as monotherapy, and the most appropriate definition of “elderly” for clinical decision-making in this setting.
Standard adjuvant therapy for newly diagnosed gbm is based on the European Organization for Research and Treatment of Cancer–ncic trial, which showed a survival benefit for radiotherapy (60 Gy in 30 fractions) plus concurrent temozolomide, followed by adjuvant temozolomide3,4. That trial, in combination with earlier studies demonstrating a benefit for adjuvant radiotherapy in gbm5, established the foregoing treatment protocol as the current standard of care in newly diagnosed gbm.
Age is a well-established risk factor for poor outcome, with shorter os observed in elderly patients with gbm. That shorter os is likely multifactorial and reflects differing tumour biology in elderly patients, as well as differences in treatment patterns and tolerance of therapy6–8. Many elderly or frail patients are unable to tolerate combined chemoradiotherapy, and toxicities—including severe fatigue, myelosuppression, and infections—are common9. As a result, investigators have been led to explore alternative treatment strategies in older gbm patients, including hypofractionated radiotherapy10,11 and temozolomide monotherapy12. Those studies are beginning to address the overall lack of data with respect to the optimal adjuvant therapy in elderly patients.
As with any clinical trial, application of the results is limited by the fact that clinical trial patients are, by virtue of enrolment criteria, frequently more fit than are patients of a similar age encountered in the community setting. The effect is likely even more pronounced in elderly patients, who frequently have comorbid conditions and competing causes of mortality. Thus, the goal of the present study was to evaluate the characteristics, treatment patterns, and outcomes of elderly patients in a population-based setting and to compare them with those of their younger counterparts.
METHODS
Patient Population
The study included patients who had a new diagnosis of gbm according to the World Health Organization classification for brain tumours13 and who were referred to our tertiary care cancer institution during a 6-year period.
The Dr. H. Bliss Murphy Cancer Centre, located in St. John’s, serves as the primary referral site for newly diagnosed patients with cancer in the province of Newfoundland and Labrador. It is affiliated with the Health Sciences Centre, which provides tertiary care services including neurosurgery, medical oncology, and radiation oncology for the province’s estimated population of 514,536 people14. This site is the only one to deliver neurosurgery and radiotherapy in the province. Systemic therapy can be delivered at satellite clinics under the direction of medical oncologists based in St. John’s.
Patients were identified using the Newfoundland and Labrador Cancer Registry, a provincial database of all patients with malignant tumours pathologically diagnosed within the province. Patients were included if they were referred to the Dr. H. Bliss Murphy Cancer Centre and received at least 1 complete evaluation by a medical or radiation oncologist during the period 1 January 2006 to 1 January 2012, inclusive. Patients were excluded if the diagnosis was made on imaging alone without pathology correlation, or if initial adjuvant therapy or the greater proportion of treatment was administered out of province.
In a retrospective chart review, clinical and treatment information was abstracted from hospital and cancer centre electronic and paper records. Those data were supplemented with publically available information (obituaries, for example) where applicable. Full institutional ethics approval was obtained before the study commenced.
Definition of Variables
The “elderly” cohort in this study was defined as patients 65 years of age or older at the time of pathology diagnosis. The Eastern Cooperative Oncology Group (ecog) performance status (ps) was defined as the ps recorded by the attending clinician at the initial clinical encounter. Surgical intervention was classified as documented in operative reports, with any extent of debulking classified as “surgical resection.” Stereotactic biopsy alone, without attempt at debulking, was classified as “biopsy.”
In patients who received radiotherapy, conventional radiation therapy was defined as 50 Gy or more delivered in 20 or more fractions. Hypofractionated (40 Gy in 15 fractions) and whole-brain radiotherapy were classified separately for the purposes of the analysis.
Overall survival was defined as the interval between initial surgery or pathology diagnosis and date of death (where applicable). In patients still alive at the end of the study period, data were censored at 1 January 2012.
Patient status with respect to O6-methylguanine dna methyltransferase (mgmt) promoter methylation and IDH1/2 mutation were not available, because those tests were not routinely performed during the study period.
Statistical Methods
Summary statistics are provided for patient demographics and for disease and treatment factors. Estimates of os were calculated using the Kaplan–Meier method. Survival differences between groups were examined using the log-rank test. Multivariable analyses were performed for all patients and for the elderly and non-elderly patients separately using the Cox proportional hazards model. Variables included in the analysis were age, sex, ecog ps, extent of surgery, type of radiotherapy administered, and administration of adjuvant chemotherapy. All analyses were conducted using the IBM SPSS Statistics software application (version 23: IBM, Armonk, NY, U.S.A.). All tests were considered statistically significant at p < 0.05.
RESULTS
Of the 148 patients diagnosed with gbm in the province between 1 January 2006 and 1 January 2012, 10 were excluded because of a lack of tissue diagnosis; thus, 138 patients were identified and met the inclusion criteria. As of 1 January 2012, 122 os events (88.4%) had occurred, 13 patients were known to be alive, and 3 patients had been lost to follow-up. Median duration of follow-up was 9.9 months.
Table i summarizes demographic and treatment characteristics for the elderly and non-elderly patients. At the time of diagnosis, 56 patients (40.6%) were 65 years of age or older. Mean age at diagnosis in the elderly cohort was 72 years (range: 65–85 years); in the non-elderly cohort, it was 54 years (range: 20–64 years). The elderly and non-elderly cohorts both showed a slight male predominance, and the sex distribution was similar in both groups. At diagnosis, 54.3% of all patients had an ecog ps less than 2; however, the percentage of patients with a ps less than 2 was greater in the non-elderly patients (67.1%) than in the elderly patients (35.7%).
TABLE I.
Demographic and clinical characteristics by age category
| Characteristic | Age group | p Value | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Overall | <65 Years | ≥65 Years | |||||
|
|
|
|
|||||
| (n) | (%) | (n) | (%) | (n) | (%) | ||
| Patients | 138 | 82 | 56 | — | |||
| Male sex | 84 | 60.9 | 49 | 59.8 | 35 | 62.5 | 0.746 |
| ECOG performance status < 2 | 75 | 54.3 | 55 | 67.1 | 20 | 35.7 | <0.001 |
| Surgery | |||||||
| Biopsy | 23 | 16.7 | 9 | 11.0 | 14 | 25.0 | 0.03 |
| Resection | 115 | 83.3 | 73 | 89.0 | 42 | 75.0 | |
| Adjuvant therapy | |||||||
| Best supportive care | 10 | 7.2 | 4 | 4.9 | 6 | 10.7 | 0.001 |
| Radiotherapy alone | 39 | 28.3 | 11 | 13.4 | 28 | 50.0 | |
| Chemoradiotherapy | 81 | 58.7 | 63 | 76.8 | 18 | 32.1 | |
| Temozolomide monotherapy | 8 | 5.8 | 4 | 4.9 | 4 | 7.1 | |
| Radiotherapy fractionation | |||||||
| No radiotherapy | 18 | 13.0 | 8 | 9.8 | 10 | 17.9 | |
| Whole-brain radiotherapy | 13 | 9.4 | 3 | 3.7 | 10 | 17.9 | |
| Hypofractionated (40 Gy, 15 fr) | 41 | 29.7 | 12 | 14.6 | 29 | 51.8 | |
| Conventional (59.4–60 Gy, 30–33 fr) | 66 | 47.8 | 59 | 72.0 | 7 | 12.5 | 0.001 |
ECOG = Eastern Cooperative Oncology Group; fr = fractions.
Of the 138 patients diagnosed with gbm during the study period, 115 (83.3%) underwent gross total or subtotal resection; the remaining 23 (16.7%) underwent stereotactic biopsy without resection. As Table i shows, elderly patients were more likely to undergo biopsy only (p = 0.03). Elderly patients were also more likely to receive best supportive care rather than adjuvant therapy after surgery (p = 0.001). Concomitant chemoradiotherapy with or without adjuvant temozolomide was given to 81 patients (58.7%). Combination therapy was more commonly given to non-elderly patients (76.8%) than to elderly patients (32.1%, p < 0.0001). Planned radiation fractionation schedules also differed between the elderly and non-elderly patients (p = 0.001). Significantly more non-elderly patients were prescribed conventional radiotherapy (72.0% vs. 12.5%); elderly patients were more likely to receive hypofractionated or whole-brain radiotherapy.
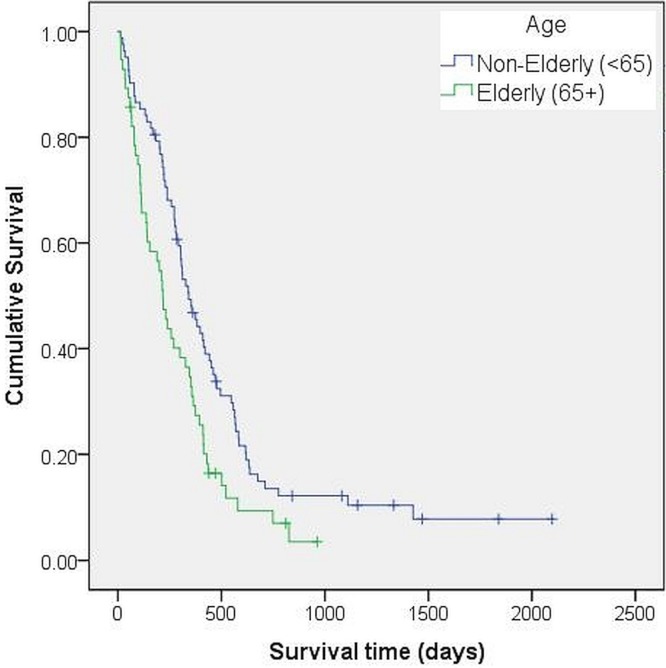
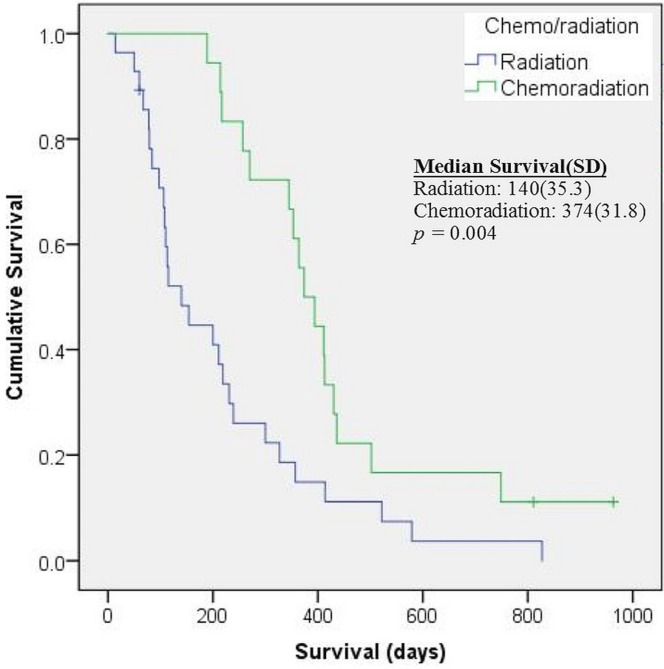
Compared with the elderly patients, non-elderly patients experienced significantly longer os. As Figure 1 shows, median survival was 217 days [95% confidence interval (ci): 165.3 days to 268.7 days] in elderly patients compared with 342 days (95% ci: 270.4 days to 413.6 days) in non-elderly patients (p = 0.006). When the analysis was limited to patients 65 years of age or older who received radiation with or without temozolomide (n = 46, Figure 2), median survival was 374 days (95% ci: 311.6 days to 436.4 days) in those who received combined treatment and 140 days (95% ci: 70.8 days to 209.2 days) in those who received radiation only. Because of the small number of elderly patients receiving radiotherapy, no comparison could be made between the patients who received conventional radiotherapy (60 Gy in 30 fractions) and those who received hypofractionated radiotherapy.
FIGURE 1.
Survival by age group. The Kaplan–Meier survival curve shows significantly longer median survival in non-elderly compared with elderly patients with glioblastoma.
FIGURE 2.
Survival in 46 elderly patients treated with radiation with or without chemotherapy. When the survival analysis was limited to elderly patients (65 years of age and older) who received radiotherapy, median survival was significantly longer for patients who received combined chemoradiotherapy than for those who underwent radiotherapy alone. SD = standard deviation.
Table ii shows hazard ratios and significance levels for the multivariate analysis. In the overall analysis, surgical resection (compared with biopsy) and receipt of adjuvant chemotherapy were the strongest predictors of os. Receiving either hypofractionated or conventional radiotherapy was also predictive of survival; receiving whole-brain radiation was not associated with improved survival.
TABLE II.
Multivariate analysis for overall survival
| Variable | Age group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall (n=132) | <65 Years (n=82) | ≥ 65 Years (n=56) | |||||||
|
|
|
|
|||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Sex | |||||||||
| Women | 1.00 | 1.00 | 1.00 | ||||||
| Men | 0.90 | 0.61 to 1.32 | 0.59 | 1.19 | 0.72 to 1.96 | 0.502 | 0.87 | 0.44 to 1.70 | 0.676 |
| Age | 1.02 | 1.00 to 1.04 | 0.117 | 1.03 | 1.00 to 1.06 | 0.059 | 1.06 | 1.00 to 1.13 | 0.052 |
| ECOG performance status ≥ 2 | 1.13 | 0.68 to 1.89 | 0.64 | 1.74 | 0.88 to 3.44 | 0.109 | 0.77 | 0.37 to 1.64 | 0.501 |
| Surgery | |||||||||
| Biopsy | 1.00 | 1.00 | 1.00 | ||||||
| Resection | 0.42 | 0.24 to 0.73 | 0.002 | 1.09 | 0.47 to 2.52 | 0.85 | 0.26 | 0.11 to 0.64 | 0.004 |
| Chemotherapy | |||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 0.20 | 0.11 to 0.38 | <0.001 | 0.06 | 0.02 to 0.21 | <0.001 | 0.20 | 0.09 to 0.47 | <0.001 |
| Radiotherapy fractionation | |||||||||
| No radiotherapy | 1.00 | 1.00 | 1.00 | ||||||
| Whole-brain radiotherapy | 0.62 | 0.27 to 1.41 | 0.251 | 1.48 | 0.35 to 6.25 | 0.596 | 0.53 | 0.17 to 1.67 | 0.28 |
| Hypofractionated (40 Gy, 15 fr) | 0.32 | 0.17 to 0.60 | <0.001 | 0.31 | 0.11 to 0.89 | 0.03 | 0.40 | 0.16 to 0.98 | 0.044 |
| Conventional (59.4–60 Gy, 30–33 fr) | 0.38 | 0.18 to 0.78 | 0.008 | 0.22 | 0.08 to 0.57 | 0.002 | 0.54 | 0.16 to 1.85 | 0.325 |
HR = hazard ratio; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; fr = fractions.
In non-elderly patients, chemotherapy, but not the extent of surgery, was associated with improved survival. Administration of hypofractionated or conventional radiotherapy was associated with survival; administration of whole-brain radiotherapy was not.
When the analysis was limited to patients 65 years of age and older, extent of surgery and receipt of chemotherapy were associated with improved survival, as was hypofractionated radiation therapy, but conventional or whole-brain radiotherapy were not. After controlling for the treatment administered (any combination of surgery, radiation, and chemotherapy), age, sex, and ecog ps at presentation were not predictive in any of the cohorts (overall, non-elderly, elderly).
DISCUSSION
Although advances in the multimodality treatment of gbm have led to modest improvements in os, prognosis remains poor, particularly in older patients. Older age at diagnosis is associated with shorter survival and increased toxicity from treatment. The goal of the present study was to add to the body of literature about the management of such patients in a non-trial, community-based setting. In the present population-based study, we reviewed clinical characteristics, treatment patterns, and survival for patients diagnosed with gbm in the province of Newfoundland and Labrador during 2006–2012. This setting offers a relatively unique opportunity to capture a truly population-based sample, with minimal referral bias. The greatest proportion of the province’s population resides on the island of Newfoundland, and the entire province is served by a single tertiary care centre for most specialty services such as neurosurgery and radiation oncology. Although a small proportion of patients might have chosen to travel outside the province for surgery, the complexities of doing so after a diagnosis of gbm make such travel relatively unfeasible. In addition, during the period in question, there was a tendency toward early (pre-discharge) referral to oncology in patients with a new diagnosis of gbm, independent of ps, increasing the number of patients captured and reducing the selection bias generally seen in patients at tertiary referral centres.
For the purposes of the present work, “elderly” was defined as 65 years of age or older. That cut-off was chosen because it was used in earlier population-based studies of older gbm patients15,16, but it remains controversial, given that physiologic age and fitness vary significantly at the individual patient level. In addition, although the original Stupp et al. trial4 excluded patients more than 70 years of age, a subgroup analysis3 showed a lesser benefit from combined therapy in patients 65–70 years of age.
Older age has consistently been found to be a negative prognostic factor for os in patients with gbm7,15. An updated 5-year analysis of the Stupp et al. trial4 found a median survival of 14.6 months for all patients treated with combined chemoradiotherapy; patients more than 60 years of age survived for a median of 10.9 months with aggressive therapy3. However, the trial excluded patients who were more than 70 years of age and those with a poor ps, and thus it represented a highly selected population of older patients. The observed difference in os might therefore suggest a degree of more aggressive tumour biology in older patients, because those patients were otherwise matched for ps, comorbidities, and treatment. In population-based analyses, median survival in this age group has ranged between 4–9 months15–18. In a retrospective analysis of American patients more than 65 years of age with a new diagnosis of gbm between 1997 and 2009, median survival ranged between 2 months (for patients who received no postoperative therapy) and 11 months (for those who received standard combined chemoradiation). In addition, increasing age beyond 65 years was associated with shorter survival, with patients 65–75 years of age surviving a median of 8 months after diagnosis, compared with 4 months for patients 75 years of age and older17.
In the present study, elderly patients constituted 41% of the cohort diagnosed with gbm. Compared with the patients less than 65 years of age, the elderly patients had a poorer ps at diagnosis and were more likely to receive less aggressive interventions in the form of surgery, radiation, and chemotherapy. In particular, elderly patients were more likely to undergo stereotactic biopsy rather than partial or gross total resection, and to receive hypofractionated radiation rather than conventional radiotherapy or combined chemoradiotherapy.
As previously demonstrated, median os was shorter in elderly patients than in non-elderly patients with gbm: 7.2 months in the elderly group compared with 11.4 months in the non-elderly group. Those results are similar to findings reported in other retrospective analyses of elderly gbm patients15–18. Among elderly patients who received radiotherapy, os was found to be prolonged in those who had received combined-modality treatment: median survival was 11.3 months in patients who received chemoradiation compared with 4.6 months in those treated with radiation only. Notably, that difference is more pronounced than the difference reported in the ce.6 trial, which randomized patients 65 years and older to radiation (40 Gy in 15 fractions) with or without combined and adjuvant temozolomide. In that study, median survival was 9.3 months in the combination arm and 7.6 months in the radiation-only arm2. The relatively poor survival seen in our patients who received radiation only is likely a reflection of the nonrandomized nature of the study, with patients having a poorer ps and those having competing comorbidities being less likely to receive combination therapy. Nonetheless, our results parallel the ce.6 trial findings in that a significant benefit was seen with the addition of temozolomide to radiotherapy in elderly patients in a non-trial, population-based setting.
On multivariate analysis, we found that the strongest predictor for survival in elderly and non-elderly patients alike was receipt of chemotherapy—either as part of concomitant chemoradiotherapy or as temozolomide monotherapy. Administration of conventional radiotherapy (50–60 Gy in total) was strongly predictive for survival in non-elderly patients, but not in elderly patients. Hypofractionated radiation therapy was associated with improved survival in elderly patients. Those results support recent clinical trials in older gbm patients, in which hypofractionated radiotherapy, compared with conventional fractionation schedules of 60 Gy in 30 fractions, was associated with improved survival and decreased toxicity10,19.
In the present study, we found that extent of resection (biopsy vs. partial or gross total resection) was predictive for survival in elderly patients, but not in non-elderly patients. That finding contrasts with multiple earlier studies that demonstrated a strong predictive value for surgical resection in patients with gbm15,20,21. In our study, the number of non-elderly patients who did not undergo resection was quite small compared with the resected group (9 patients and 73 patients respectively). Thus, the lack of a significant effect here could be attributed to the small sample size and heterogeneity within the non-resected group.
We found that neither age nor ps was independently predictive of survival after controlling for extent of surgery, chemotherapy, and radiation. That observation likely reflects the fact that older and frailer patients were less likely to receive aggressive therapy, and thus their shorter os can be attributed both to their overall fitness for therapy and to the treatment received. Similar results were recently reported in a retrospective analysis by Tsang et al.15. However, the significant hazard ratios associated with administration of chemotherapy or radiotherapy, or both, support a role for active treatment in appropriately selected older patients with gbm.
Interpretation of the present findings is limited by the retrospective nature of the data. Given that patterns of treatment were significantly different for elderly patients than for their non-elderly counterparts, it is impossible to determine with any precision the relative contributions of age, ps, and treatment to the variation in survival observed between the elderly and non-elderly patients. In addition, neither mgmt methylation status nor IDH1/2 mutation status testing was available during the study period. Both markers have been shown to have significant prognostic and predictive value in patients with gbm, and mgmt methylation status in particular has been shown to predict response to temozolomide22–25. In ce.6, elderly patients with mgmt-methylated tumours showed the greatest survival benefit with combined therapy2. In elderly patients who are less likely to tolerate combined chemoradiotherapy, mgmt methylation status might be especially important. The use of temozolomide monotherapy in mgmt-methylated patients is an area of active study in elderly patients with gbm25.
In treating patients with gbm, the goal of therapy is to prolong survival if possible, while maintaining quality of life. In elderly patients, that principle is particularly vital because those patients are more susceptible to treatment-induced toxicities, and they benefit less overall from adjuvant therapies. To evaluate the real-world efficacy of existing and novel treatments, studies should ideally take into account quality-of-life measures such as functional independence. The field would benefit from a tool assessing treatment-related morbidity and its effects on quality of life. Treatment-induced toxicities and morbidity should be carefully balanced against any potential gains in terms of survival duration.
CONCLUSIONS
Further research is needed to determine the optimal management of older patients with gbm. In older patients with an adequate ps, recent data support the use of radiation combined with temozolomide compared with radiation only2, although the ideal intensity and duration of radiation given with chemotherapy in this population remains an ongoing question. Increased availability and utilization of molecular markers such as mgmt methylation status are now helping to select the patients most likely to benefit from temozolomide —particularly from among patients with a poor ps10,12. Further molecular and clinical markers are needed to help determine those likely to respond to treatment and those predisposed to significant toxicity from therapy. Finally, as long as gbm remains an incurable disease, priority should be given to discovering methods and tailoring treatments that maximize quality of life, particularly in older patients whose projected survival remains limited.
ACKNOWLEDGMENTS
Many thanks to Farah McCrate msc of the Dr. H. Bliss Murphy Cancer Centre for statistical advice.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: EM has received a travel grant from Bristol–Myers Squibb outside the submitted work. KL has received fees as an advisory board member for Roche outside the submitted work. MS has received research funding and honoraria from Roche outside the submitted work.
REFERENCES
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. cbtrus statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [Erratum in: Neuro Oncol 2013;15:646–7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry JR, Laperriere N, O’Callaghan CJ, et al. A phase iii randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (cctg ce.6, eortc 26062-22061 trog 08.02, NCT00482677) [abstract LBA2] J Clin Oncol. 2016;34 [Available online at: http://meetinglibrary.asco.org/content/163254-176; cited 6 February 2017] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. on behalf of the European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups and the ncic Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase iii study: 5-year analysis of the eortc–ncic trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. on behalf of the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the ncic Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Keime-Guibert F, Chinot O, Taillandier L, et al. on behalf of the Association of French-Speaking Neuro-Oncologists Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–35. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 6.Zarnett OJ, Sahgal A, Gosio J, et al. Treatment of elderly patients with glioblastoma: a systematic evidence-based analysis. JAMA Neurol. 2015;72:589–96. doi: 10.1001/jamaneurol.2014.3739. [DOI] [PubMed] [Google Scholar]
- 7.Kita D, Ciernik IF, Vaccarella S, et al. Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33:17–22. doi: 10.1159/000210017. [DOI] [PubMed] [Google Scholar]
- 8.Lowry JK, Snyder JJ, Lowry PW. Brain tumors in the elderly: recent trends in a Minnesota cohort study. Arch Neurol. 1998;55:922–8. doi: 10.1001/archneur.55.7.922. [DOI] [PubMed] [Google Scholar]
- 9.Sijben AE, McIntyre JB, Roldán GB, et al. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol. 2008;89:97–103. doi: 10.1007/s11060-008-9593-6. [DOI] [PubMed] [Google Scholar]
- 10.Malmström A, Grønberg BH, Marosi C, et al. on behalf of the Nordic Clinical Brain Tumour Study Group Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–26. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 11.Roa W, Kepka L, Kumar N, et al. International Atomic Energy Agency randomized phase iii study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33:4145–50. doi: 10.1200/JCO.2015.62.6606. [DOI] [PubMed] [Google Scholar]
- 12.Wick W, Platten M, Meisner C, et al. on behalf of the noa-08 Study Group of Neurooncology Working Group (noa) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the noa-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–15. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 13.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 who classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statistics Canada . Focus on Geography Series, 2011 Census [Web page] Ottawa, ON: Statistics Canada; 2012. [Available at: http://www12.statcan.gc.ca/census-recensement/2011/as-sa/fogs-spg/Facts-pr-eng.cfm?Lang=Eng&GK=PR&GC=10; cited 21 December 2015] [Google Scholar]
- 15.Tsang DS, Khan L, Perry JR, et al. Survival outcomes in elderly patients with glioblastoma. Clin Oncol (R Coll Radiol) 2015;27:176–83. doi: 10.1016/j.clon.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Arvold ND, Wang Y, Zigler C, Schrag D, Dominici F. Hospitalization burden and survival among older glioblastoma patients. Neuro Oncol. 2014;16:1530–40. doi: 10.1093/neuonc/nou060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton E, Ugiliweneza B, Woo S, Skirboll S, Boaky M. A Surveillance, Epidemiology and End Results–Medicare data analysis of elderly patients with glioblastoma multiforme: treatment patterns, outcomes and cost. Mol Clin Oncol. 2015;3:971–8. doi: 10.3892/mco.2015.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF. Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg. 2013;118:786–98. doi: 10.3171/2012.10.JNS112268. [DOI] [PubMed] [Google Scholar]
- 19.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–8. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 20.Chaichana KL, Martinez-Gutierrez JC, De la Garza-Ramos R, et al. Factors associated with survival for patients with glioblastoma with poor pre-operative functional status. J Clin Neurosci. 2013;20:818–23. doi: 10.1016/j.jocn.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu R, Komisarow JM, Agarwal VJ, et al. Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg. 2016;124:998–1007. doi: 10.3171/2015.4.JNS142200. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–7. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 24.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 25.Yin AA, Cai S, Dong Y, et al. A meta-analysis of temozolomide versus radiotherapy in elderly glioblastoma patients. J Neurooncol. 2014;116:315–24. doi: 10.1007/s11060-013-1294-0. [DOI] [PubMed] [Google Scholar]