Abstract
Thyroid hormones (TH) are endocrine messengers essential for normal development and function of virtually every vertebrate. The hypothalamic-pituitary-thyroid axis is exquisitely modulated to maintain nearly constant TH (T4 and T3) concentrations in circulation. However peripheral tissues and the CNS control the intracellular availability of TH, suggesting that circulating concentrations of TH are not fully representative of what each cell type sees. Indeed, recent work in the field has identified that TH transporters, deiodinases and thyroid hormone receptor coregulators can strongly control tissue-specific sensitivity to a set amount of TH. Furthermore, the mechanism by which the thyroid hormone receptors regulate target gene expression can vary by gene, tissue and cellular context. This review will highlight novel insights into the machinery that controls the cellular response to TH, which include unique signaling cascades. These findings shed new light into the pathophysiology of human diseases caused by abnormal TH signaling.
Introduction
Thyroid hormones modulate gene expression in virtually every vertebrate tissue; their actions are finely tuned by a series of conserved pathways, which orchestrate the onset of crucial physiological processes for normal development, growth and energy metabolism. Since the cloning of the thyroid hormone receptors in 1986 three decades of intense research have enlightened our knowledge on the molecular basis of thyroid hormone action. In the present review we will address the new insights into thyroid hormone action that have been published within last four years.
The Hypothalamic-Pituitary-Thyroid axis
Thyroid hormones (TH) are essential for growth and development in virtually every vertebrate including humans. TH synthesis and secretion is finely modulated by the hypothalamic-pituitary-thyroid (HPT) axis. It is well appreciated that thyroid hormone production is governed by this central axis that begins in the paraventricular nucleus of the hypothalamus and proceeds through the pituitary before engaging the thyroid. Indeed, human mutations have been described at every level of the axis and all cause hypothyroidism (Medici, Visser, Visser, & Peeters, 2015). Importantly, circulating thyroid hormones (TH) negatively feedback to the central components of the axis to regulate levels. Today, pituitary-secreted thyroid-stimulating hormone (TSH) remains the most important and universal biomarker of TH action in humans.
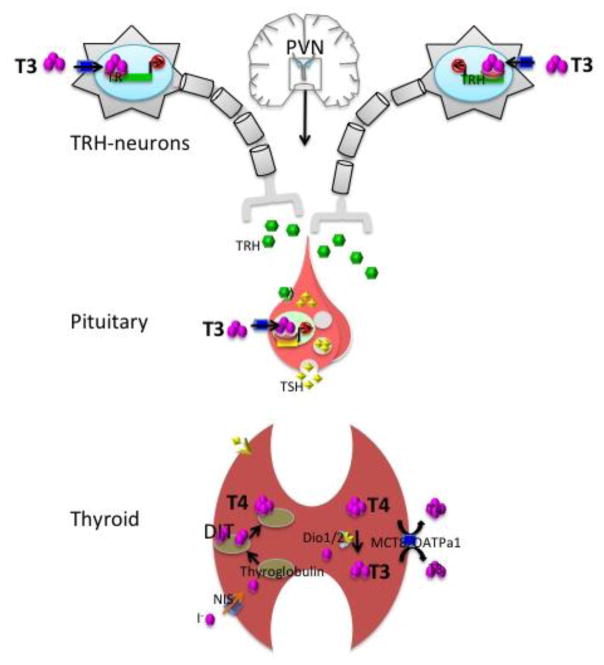
The hypothalamic section of the axis is represented by the thyrotropin-releasing hormone (TRH)-neurons in the paraventricular nucleus (PVN), which secrete TRH in response to a series of environmental and physiological stimuli. Interestingly, TRH neurons in the PVN co-secrete the neuropeptide cocaine- and amphetamine-related transcript (CART) but its role remains unclear (Hollenberg, 2008). Several other important neuronal populations exist in the PVN including corticotropin-releasing hormone (CRH), vasopressin and oxytocin neurons, which each have an important endocrine function. The TRH gene encodes a prepro-hormone that is ultimately processed to a tripeptide with the sequence of glu-his-pro (Nillni & Sevarino, 1999). Released into the median eminence, TRH induces the secretion of thyroid-stimulating hormone (TSH) from the anterior pituitary, which in turn accentuates the synthesis and secretion of thyroid hormones from the thyroid gland into the circulation (Figure 1). TRH released into the median eminence acts on the TRH-receptor located in the membrane of thyrotrophs, the TSH-producing cell in the pituitary. Thyrotrophs lose their ability to secrete TSH when disaggregated in cell culture, suggesting a functional role for the architecture of the anterior hypophysis of the pituitary and the possible participation of other signaling factors to modulate thyrotroph sensitivity to TRH in vivo (Bargi-Souza et al., 2015). The HPT axis plays the major role in maintaining the homeostasis of circulating TH levels as circulating T4 and T3 feed back to both the hypothalamus and the pituitary to regulate TRH and TSH production (see Negative Regulation by Thyroid Hormone).
Figure 1.
The hypothalamus-pituitary-thyroid (HPT) axis maintains thyroid hormone homeostasis. The PVN neurons secret TRH in response to low circulating T3/T4 ratio. In turn, TRH signaling in the pituitary stimulates the secretion of TSH, which triggers the release of T4 and T3 from the thyroid gland into the bloodstream. T3 negative feedback upon expression of TRH and TSH genes in the PVN and the pituitary respectively keeps the T3/T4 ratio virtually constant in circulation.
Remarkably, the set point of the HPT axis differs between individuals (Fitzgerald & Bean, 2016). This is likely due to genetic factors. Genome-wide association studies (GWAS) analyzing different populations of euthyroid humans have identified polymorphisms associated with differences in circulating levels of TSH and T4 within the normal range of concentration. The loci associated with TSH levels are PDE8B, PDE10A, CAPZB, MAF/LOC440389, VEGFA, NR3C2, IGFBP5, NFIA, SOX9, PRDM11, FGF7, INSR, ABO, MIR1179, NRG1, MBIP, ITPK1, SASH1, GLIS3, whereas free T4 (FT4) was associated with DIO1, LHX3, FOXE1, AADAT, NETO1/FBXO15, LPCAT2/CAPNS2, (Malinowski et al., 2014; Porcu et al., 2013; Taylor et al., 2015; Zhan et al., 2014). The role of these polymorphisms in the modulation of TSH and FT4 are just starting to be elucidated, however many of these genes appear to have their actions in the thyroid. For example, polymorphisms in PDE8B, a phosphodiesterase expressed in the thyroid, are associated with high circulating levels of TSH together with slightly decreased FT4 levels (G. Roef et al., 2013). This is consistent with PDE8B regulating TSH action in regulating the synthesis of thyroid hormone (Figure 2). In thyroid tumor patients only, polymorphisms in another thyroid specific gene CAPZB were found to be associated with lower TSH levels (Feng et al., 2015). Polymorphisms in NRG1 and FOXE1 were associated with a risk of follicular adenoma (Rogounovitch et al., 2015). Polymorphisms in FOXE1 are also associated with congenital hypothyroidism (Carre et al., 2014). Some polymorphisms have little to no effect in patient care such as the type 1 deiodinase (DIO1), which does not change the dose of T4 required for TSH suppression in thyroidectimized patients (Santoro et al., 2014). Recently, hypothyroidism has been asociated with both neurological disease and a longer life span. Levels of TSH have been asociated with longevity, schizophrenia or bipolar disorder in two independent studies, suggesting that octogenarians and/or patients with schizoprenia have higher concentrations of circulating TSH (Jansen et al., 2015; Wysokinski & Kloszewska, 2014). Despite the fact that TSH has long been interpreted as a major marker of thyroid status it only reflects the effects of thyroid hormone signaling in the hypothalamus and pituitary, thus identifying novel markers of thyroid hormone action in the periphery becomes paramount.
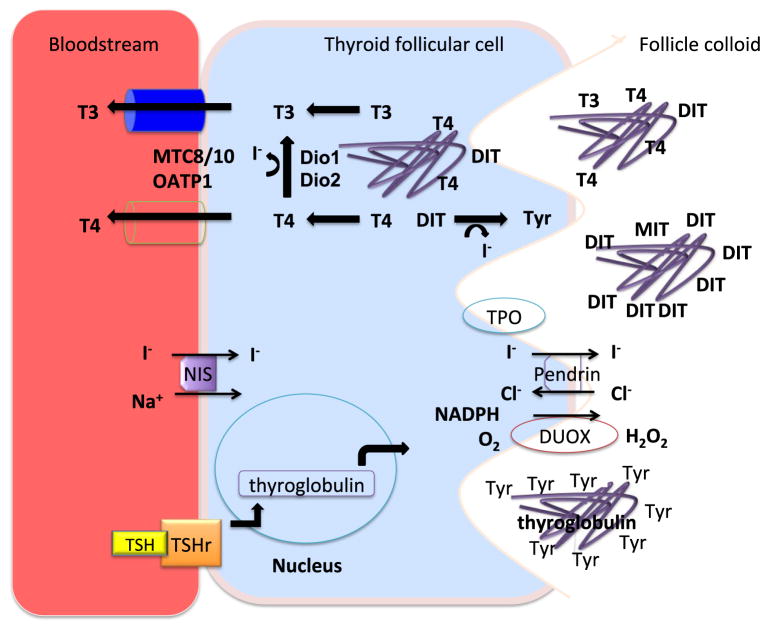
Figure 2. The thyroid gland secretes T3 and T4 into the circulation.
The thyroid follicular cells concentrate iodine within the colloid by using NIS/Pendrin transporters, which captures circulating I− into the cytoplasm, followed by secretion to the colloid respectively. In the colloid the I− is organified and coupled to thyroglobulin-tyrosine residues by TPO catalyzed oxidation, which requires of H2O2 generated by DUOX as cofactor; thus generating DIT and MIT, which form T4 and T3. TSH stimulation promotes endocytosis of the T4/T3-containing thyroglobulin, which releases the hormones after proteolysis in the cytosol. TSH receptor is coupled to G-protein and increases cAMP, which in turn is hydrolyzed by PDE8B. The MCT8 appears necessary for the transport of T4 from the thyroid gland while it and other transporters may play a role in T3 transport form the gland.
Thyroid Hormone Transporters
Thyroid hormones (T4 and T3) are primarily protein bound moieties in the circulation and are in equilibrium with free T4 and T3, which are biologically active (Figure 3). The ratio of protein bound T4 to FT4 is higher than T3, which partly explains its much longer half-life and role as a pro-hormone. In recent years, it has become clear that circulating T4 and T3 do not passively cross cell membranes such as those present in liver, thyroid follicular cells or astrocytes and neurons in the brain. Several T4 and T3 transport proteins have been identified including: 1. The monocarboxylate transporters MCT8 and MCT10; 2. The organic anion transporter OATP1 and 3: L-type amino acid transporter LAT. MCT8 has preference for T3, whereas T4 and rT3 are preferentially transported by OATP1. LAT transports both T4 and T3 but with relative lower affinity (Jayarama-Naidu et al., 2015; Schwabedissen et al., 2011).
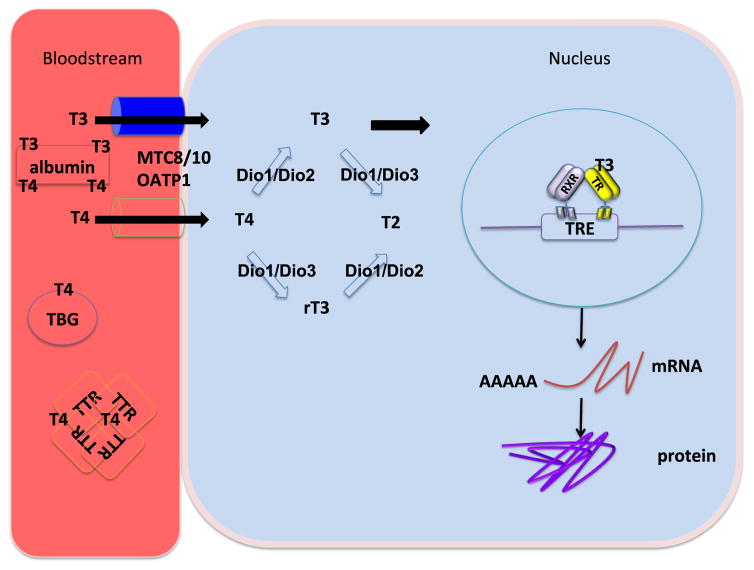
Figure 3. T3 modulates gene expression in virtually every vertebrate.
T4/T3 circulates attached to serum proteins including thyroxine binding globulin (TBG) transthyretin (TTR), and albumin. A small fraction of circulating T4 is free to be transported into the cytoplasm, where it is activated to T3 by outer-ring deiodination catalyzed by Dio1 or Dio2. The resulting T3 is thought to be diffused into the nucleus to bind the thyroid hormone receptors (TR). Upon binding to the thyroid hormone receptors, T3 modulates the rate of mRNA synthesis and ultimately the protein levels of thousands of genes in virtually every cell.
The most clinically relevant thyroid hormone transporter is the MCT8 (Friesema, Visser, & Visser, 2010). Mutations in this transporter have been found in patients with the Allan-Herndon Dudley Syndrome (AHDS). AHDS is an X-linked disorder, which is heralded by potentially severe neurologic complications including mental retardation and movement disorders. Patients with AHDS also exhibit abnormal thyroid function tests that include a normal TSH with a low free T4 and high levels of circulating T3. It is very likely that the neuronal disorder is secondary to the inability for T3 to gain access to neurons during development. This was shown in a recent study of human brains from a fetus and child with AHDS. Both brains had anatomic and structural changes consistent with severe abnormalities of development concurrent with greatly diminished cerebral T3 levels (Lopez-Espindola et al., 2014). Also, the relationship of AHDS to a deficiency of T3 transport has been solidified by studies showing that human fetal and neonatal brain from patients with AHDS look remarkably similar to brain from humans with severe hypothyroidism (Gagliardi et al., 2015; J. H. Kim et al., 2015). The primary structure of MCT8 forms twelve trans-membrane subunits that are predicted to form a canal that facilitates the bidirectional transport of T3 in favor of its gradient of concentration. Interestingly, dimerization of MCT8 also appears to be necessary for its function (Fischer et al., 2014). The inner part of the canal contains residues of several amino acids, which appear to interact directly with the ligand. For example histidine 192 is required for T3-transport specificity (Braun, Lelios, Krause, & Schweizer, 2013) (Groeneweg, Lima de Souza, Visser, Peeters, & Visser, 2013), whereas mutation of Arg445 and/or Asp498 induced complete ablation of TH transport in cell lines. Both of these mutations have been found in patients with Allan-Herndon Dudley Syndrome (Groeneweg et al., 2014). Transporters may also play a role in determining circulating levels of thyroid hormones. Indeed, MCT8 is expressed on the basolateral membrane of thyrocytes and appears to be necessary for T4 efflux from the gland (de Souza et al., 2015) (Trajkovic-Arsic et al., 2010). Furthermore, two SNPs in MCT8 have been associated with differences in circulating T3: the first SNP causes a missense mutation in serine107 to proline; the second is intronic and the effect on transporter expression is not yet understood (G. L. Roef et al., 2013).
Above all, TH transporters are key to TH action and function in humans. Their patterns of expression in stages of development and tissue-type modulate circulating and peripheral thyroid hormone availability. As discussed, mutations in the MCT8 gene highlight the necessity of transporters. In mice, mutation of the MCT8 recapitulates the thyroid function phenotype found in humans; however, the underlying cause of increased T3 and low T4 remains an open question. Remarkably MCT8 deficient mice are neurologically normal, most likely due to the co-expression of the OATP1 transporter during development. Mice that lack both MCT8 and OATP1 develop neurological disorders similar to the human disease (Mayerl et al., 2014). Human neurons express only MCT8 and do not have the redundancy of OATP1.
Studies in which the type 1 deiodinase (see Deiodinases section below) was ablated specifically in liver of MCT8KO mice did not prevent the increase in circulating T3 and low T4 (Wirth, Rijntjes, Meyer, Kohrle, & Schweizer, 2015), whereas the global ablation of Dio1 in MCT8KO mice normalized T3 and T4 in circulation. Thus, Dio1 activity in tissues other than liver, accounts for the pathologic thyroid status in MCT8KO mice (Liao et al., 2011).
As mentioned above, the relationship of AHDS to T3 transport deficiency has been highlighted by several studies in humans. Thus, the available mouse models of AHDS provide important pre-clinical utility in testing novel approaches and treatments. Indeed, therapeutic strategies to overcome the poor transport of TH in MCT8 deficiencies include the use of thyroid hormone analogs such as TRIAC, TETRAC and DITPA as the cellular uptake of these T3 analogs is MCT8-independent (de Vrieze et al., 2014) (Ferrara et al., 2014) (Mayerl et al., 2014). In addition to mouse, zebrafish have also been shown to be a suitable model to analyze the neurologic phenotype of MCT8 ablation (de Vrieze et al., 2014), (Vatine et al., 2012).
New insights have also determined that TH transport can be regulated. Indeed, the expression of MCT8 and OATP1 in the mouse brain was transiently diminished in response to an acute inflammatory challenge by lipopolysaccharide LPS (Wittmann et al., 2015). Moreover, studies in the developing brain of the chicken show that transporter mRNA expression mirrors the increasing intracellular T3/T4 ratio (Van Herck et al., 2015). Particularly, thyroid status modulates the expression of MCT8, MCT10 and OATP1 in the brain of teleosts. High levels of T3 down-regulated the expression of these transporters whereas methimazole induced hypothyroidism and increased transporter expression. Take together, this suggests that transporter genes are negatively regulated by thyroid hormone (Muzzio, Noyes, Stapleton, & Lema, 2014). At the protein level, long-term iodine deficiency increased the localization of MCT8 to the membrane of thyrocytes, and this effect was prevented by treatment with T3 (Hu et al., 2014). In contrast, short term iodine overload down-regulated MCT8 mRNA but not LAT2 in the thyroid gland (de Souza et al., 2015).
The Deiodinases
Once thyroid hormones are synthesized they are subjected to further metabolism by a family of deiodinases. Deiodination is the process by which T4 is converted to its bioactive form T3, which can take place within the thyroid. However, TH levels are predominantly regulated by deiodinases in target tissues or cell types (St Germain, Galton, & Hernandez, 2009). Three iodothyronine deiodinases catalyze the stereospecific removal of iodine from T4 and T3 (Figure 3). The type 2 deiodinase (D2) activates T4 by outer-ring deiodination (ORD) to produce T3. The type 3 deiodinase (D3) deactivates T4 and T3 by inner-ring deiodination (IRD) to produce rT3 or 3′,3-T2 respectively. The type 1 deiodinase (D1) catalyzes both ORD and IRD (Gereben, McAninch, Ribeiro, & Bianco, 2015). Recently, elucidation of the crystal structure of a portion of D3 (its globular domain) allowed us to understand that the deiodinases may recognize iodothyronines through a similar histidine-arginine clamp that is employed by both transporters such as the MCT8 and by the thyroid hormone receptors (Schweizer, Schlicker, Braun, Kohrle, & Steegborn, 2014).
As outlined the cell specific expression of deiodinases has the ability to determine the amount of T3 available. D3 activity is higher during late developmental and early postnatal stages, however it decreases to almost undetectable levels in most tissues after birth. In the cerebellum, mice that lack D3 mice show impaired foliation characterized by thinner external and internal germinal layers (EGL IGL) (Peeters et al., 2013). D2 and D3 play a role in the developing cochlea: D3 acts prior to the onset of hearing by preventing the premature response to TH and after birth D2 amplifies T3 levels to triggers the onset of auditory function (Ng et al., 2004; Ng et al., 2009). Silencing D3 during development has detrimental effects as observed in zebrafish, which results in delayed hatching, significantly smaller size, and decreased inflation of the swim bladder (Heijlen et al., 2014). Striking studies in different species show that reactivation of D3 expression occurs in early phases of tissue regeneration e.g. liver, muscle and fin. Notably, muscle satellite cell-specific ablation of D3 prevented muscle regeneration after cardiotoxin analogue III (CTX) injection due to apoptosis of satellite-cells (Dentice et al., 2014). Moreover, D3 activity has been shown to increase in a mouse model of myocardial infarction, which may be an adaptive response to decreased metabolism or a marker of tissue regeneration (Janssen et al., 2013). In both development and tissue regeneration, D3 activity is high and tissue concentrations of T3 are low suggesting that cellular differentiation occurs and/or prefers a hypothyroid environment.
The majority of human hypothyroidism is treated with L-thyroxine (T4) and thus requires deiodination to T3 for physiological activity. There has been significant interest in whether changes in deiodinase function could impair L-thyroxine therapy. A recently identified polymorphism in D2 segregates with decreased clinical effect of T4 therapy in humans (Panicker et al., 2009). This Ala92 change in the enzyme has been predicted to impair function of the enzyme. For example, patients with mild cognitive impairment carrying the Ala92-D2 SNP showed decreased levels of circulating T3 (Luo, Zhou, Zou, Keyim, & Dong, 2015). However, four SNPs identified in patients with primary hypothyroidism on T4-treatment showed no effect on circulating TSH and fT3 (Al-azzam, Alkhateeb, Al-Azzeh, Alzoubi, & Khabour, 2013). To further address how the Ala92-D2 SNP affects D2 function, McAninch et al analyzed the expression of gene targets in human brain from individuals known to express this polymorphism. Surprisingly, the Ala92-D2 polymorphism modified the profile of gene expression in human brain without modifying the expression of TH-target genes suggesting that D2 could influence the expression of other genes through TH independent pathways (McAninch et al., 2015).
New insights into tissue-specific D2 activity have underlined the necessity for new biomarkers of TH action. D2 activity is modulated through T4-induced degradation via reversible ubiquitination, which is ER-localization specific and requires a D2 conserved lysine in the globular domain (Egri & Gereben, 2014). D2 ubiquitination is modulated by WD repeat and SOCS box containing 1 (WSB-1) in a tissue-specific manner. Within the brain, the hypothalamus D2 activity is not sensitive to T4, whereas the hippocampus and cortex showed increased D2 activity in astrocyte-specific WSB-1-KO mice (Werneck-de-Castro et al., 2015). Since most T3 in the brain is produced from T4, local T3 availability could be dependent upon neuronal sensitivity to D2 degradation. In the skeletal system, the expression of D2 is modified by SNPs and associated with osteoarthritis (OA). D2 up-regulation and D3 down-regulation causes locally increased levels of T3 in chondrocytes. This promotes the expression of the metalloproteinase ADAMTS5, which degrades the cartilage in joints (Nagase et al., 2013). The SNP rs225014 upstream of D2 has been associated with risk of osteoarthritis and carriers of this allele show defective epigenetic silencing of D2 in chondrocytes (Bomer et al., 2015). In contrast, D2 ablation in mice induced increased mineral content in bones with a normal cartilage phenotype (Waung, Bassett, & Williams, 2015). Further work will be required to delineate the role of deiodinases and T3 production in osteoarthritis.
D1 is the main deiodinase expressed in the thyroid gland and is highly upregulated during iodine deficiency (Lavado-Autric, Calvo, de Mena, de Escobar, & Obregon, 2013). Dio1 catalyzes both ORD and IRD with equimolar efficiency. Experimental evidence suggests that Dio1 functions as an iodine scavenger in the kidney whereas in the thyroid gland and liver it generates significant amounts of circulating T3 particularly in hyperthyroid patients. Insights from transgenic models show that Dio1 is a TRβ1-target (Forrest et al., 1996). Thus, the global ablation of TRβ1 downregulates Dio1. Dio1 is the most highly regulated T3-target, however the role of Dio1 on circulating T3 in euthyroidism remains controversial. Nevertheless SNPs in DIO1 found in several independent GWAS studies indicate that Dio1 plays a primary role in determining the set point of circulating T3 in euthyroid patients. Also, testosterone may play a role in Dio1 activity as observed in orchidectomized rats as the replacement with testosterone after surgery was shown to maintain normal Dio1 activity in liver (Sosic-Jurjevic et al., 2015).
Thyroid Hormone Receptors
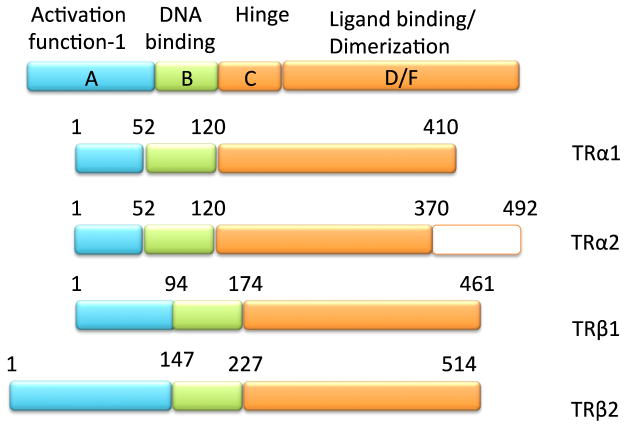
T3 action is completely dependent upon its cognate receptors, the thyroid hormone receptor isoforms (TRs) (Figure 4). Members of the nuclear receptor superfamily, the TRs are ligand-activated transcription factors that interact with T3 via a C-terminal ligand-binding domain (LBD) (Figure 5). Conformational change in the LBD dictates action of the TR based upon its recruitment of coregulator proteins, which mediate epigenetic change (see below). There are three T3-binding isoforms: TRβ1 and TRβ2 are alternatively spliced products of a single gene while TRα1 is encoded for by a separate gene but shares a very high degree of structural homology with the TRβ isoforms. TRα2 has a separate C-terminal extension and does not bind T3. The functional role of each TR isoform appears to be most dictated by its tissue-specific expression as shown by multiple mouse models where individual receptor isoforms have been knocked-out (Brent, 2012).
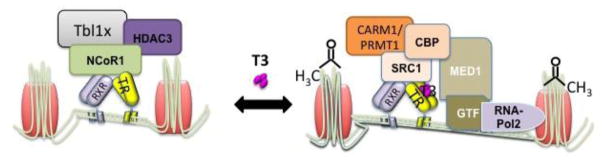
Figure 4. T3 binds to the thyroid hormone receptors to modulate gene expression.
In the absence of ligand the TR recruits NCoR1, which forms a multiprotein corepressor complex including Tbl1 and HDAC3. Binding of T3 induces the dismissal of the corepressor complex and allows the recruitment of coactivators (SRC1/CBP, CARM1/PRMT1), which include histone acetyl and methyl transferase activity thus facilitating the activation of the general transcription machinery and mRNA synthesis.
Figure 5. Schematic representation of the thyroid hormone receptor isoforms.
TRs are encoded by the THRA and THRB genes, which produce multiple isoforms, Depicted are the major isoforms that modulated the actions of T3, with exception of TRalpha2 which does not bind T3 but acts as negative dominant by competing for the TRE with other TRs. TR isoforms share high sequence homology within their functional domains.
Resistance to thyroid hormone (RTH) syndrome has gleaned significant insight into the role of TR isoforms in humans. RTH is due to mutations in the TR isoforms that cause distinct syndromes based on the tissue distribution of the receptor. For example mutations in the TRβ isoforms lead to the syndrome of resistance to thyroid hormone beta (RTHβ), which is characterized by inappropriately elevated levels of TSH in the face of high levels of T4 and T3 due to defective TH feedback at the level of the pituitary and hypothalamus (Forrest et al., 1996). Additionally, there is significant RTH in the liver while tissues such as the heart are in fact hyperthyroid. This variable phenotype is due to the presence of the TRβ isoforms in the pituitary, hypothalamus and liver while TRα is primarily expressed in other tissues including the heart. Thus, TRα expressing tissues see the high TH levels and become functionally hyperthyroid.
In contrast, RTH in patients due to TRα mutations has a phenotype that includes short stature with constipation, bradycardia and mild intellectual impairment due to the primary presence of TRα in the GI tract, heart, bone and many neuronal subtypes in the brain (Schoenmakers et al., 2013). Importantly, RTH secondary to TRα mutations were not easily identified because only mild abnormalities were found in thyroid function tests unlike RTHβ. Still more cases of RTH due to TRα are likely to be identified given the potential for some mutations to cause high-normal T3 levels with low-normal T4 levels and low reverse T3 levels. Similar to RTH due to TRβ, there is a phenotypic spectrum in humans with TRα mutations depending upon the severity of the mutation in context of impairing T3-binding. Finally, as will be discussed later, the deleterious function of mutant TRs in both RTHα and RTH β is likely due to excessive recruitment of nuclear corepressors and ultimately deacetylation and repression of TR target genes (C. Moran & Chatterjee, 2016; Carla. Moran & Chatterjee, 2015; Schoenmakers et al., 2013; Vlaeminck-Guillem, Espiard, Flamant, & Wemeau, 2015).
To better understand how the TR isoforms mediate their transcriptional properties investigators now use novel approaches to characterize their role on a genome wide level (Table 1). Studies in cell culture using Hela or HepG2 cells stably expressing TRα1 or TRβ1 have shown that both TRs can modulate the same genes in the same fashion, however TR isoform specific signaling patterns were observed on some target genes (Lin et al., 2013). Similarly, specific sets of genes had a receptor-selective response to T3 in the neural cell line C17.2 stably expressing TRβ1 or TRα1 (Chatonnet, Guyot, Benoit, & Flamant, 2013). In this study, each isoform had a separate cistrome indicating that some of the differences in gene expression could be secondary to differential binding properties of the individual receptors. Further analysis of the isoform-specific effects of the individual TRs will require genetic swapping experiments in vivo.
Table 1.
TRB1 chip-seq studies
| Reference | Gronved, L. et al 2015 | Ramadoss, P. et al 2014 | Ayers, S. et al 2014 | Chatonnet, F. et al 2013 |
|---|---|---|---|---|
| Model | mouse liver | mouse liver | cell culture | cell culture |
| Detection method | Monoclonal AB C1 | biotin-streptavidin | biotin-streptavidin | biotin-streptavidin |
| Findings | ||||
| DR4 is the most common TRE in positive targets | ☑ | ☑ | ☑ | ☑ |
| DR0 is associated with negative targets | ☑ | |||
| T3 induced recruitment of TR to TRE | ☑ | ☑ | ☑ | |
| T3 independent interaction between TR and TRE | ☑ | ☑ | ☑ | |
| Majority of T3 binding sites found in intragenic regions | ☑ | ☑ | ☑ | ☑ |
| Little TRB binding sites near negative target genes | ☑ | ☑ | ||
| RXRalpha heterodimer partner | Not done | ☑ | Not done | Not done |
| TR isoform specific binding sites | Not done | Not done | Not done | ☑ |
| Motif enrichment analysis for TF other than TR | ☑ | ☑ | ||
| T3 regulated DNAase hypersensitive sites | ☑ | ☑ | Not done | Not done |
To determine how the TRs function in vivo investigators have now characterized the binding sites of the TRβ isoform in the mouse liver using a variety of techniques. Mice expressing a biotin-tagged TRβ1 in the liver showed enrichment of binding sites mostly in intergenic regions but also demonstrated binding sites in proximal regions or introns of target genes (Ramadoss et al., 2014). Likewise, in HepG2 cells that stably express TRβ, the TR bound to clusters of binding sites in intergenic, promoter and upstream regions of responsive genes (Ayers et al., 2014). Of note, these studies used motif enrichment analysis and determined that a DR+4 motif was the most common target for the TR (Ramadoss et al., 2014) (Chatonnet et al., 2013; Grontved et al., 2015). Finally, genome wide Chip-seq and DNase hypersensitivity analysis showed extensive chromatin remodeling in response to T3 stimulation, which indicates ligand-induced recruitment of TRβ1 to binding sites. Ultimately, this suggested that not all TRs are bound to DNA in the absence of ligand as proposed by the current consensus model (Ramadoss et al., 2014). This notion is supported by data showing that subcellular localization of the TR is facilitated by a conserved nuclear localization sequence that is harbored in the helix 3 and 6 of the LBD (Mavinakere, Powers, Subramanian, Roggero, & Allison, 2012). Recently it has been shown that the importin α1, β1 and 7 translocate TRα1 from the cytosol into the nucleus (Roggero et al., 2016).
These genome wide binding studies have also established that RXRα ChIP peaks overlap with TRβ1 ChIP peaks in both the hypothyroid and hyperthyroid state. This further reinforces the notion that TRβ1 functions as heterodimer with RXRα(Ramadoss et al., 2014) (Grontved et al., 2015). Notably, these studies also suggest that TR homodimers or monomers could be bound to TREs in vivo, which strengthens previous in vitro studies that showed gene repression is preferentially mediated by TR homodimers (Machado et al., 2009; Makowski, Brzostek, Cohen, & Hollenberg, 2003). In contrast to positive targets, negative targets of T3 were not as commonly associated with TRβ1 binding peaks, suggesting that many T3-negative targets do not bind TRβ1 directly (Grontved et al., 2015). However in some instances the ligand-bound TRβ1 was recruited to certain negative T3 targets. This implies that there could be a role for direct TR binding in some instances of negative regulation (J. Y. Kim, Son, Kim, & Lee, 2010) (Ramadoss et al., 2014). While genome-wide studies are lacking in vivo data for TRα1, Dudazy-Gralla et al took advantage of a mouse model that expresses a TRα1-GFP fusion protein from the endogenous locus. In vivo ChIP assays using this model confirmed TR binding sites in known T3-regulated genes in the brain such as Hairless and identify a novel T3-regulated gene (RNF166) via its ability to recruit TRα1 to its 3′untranslated region (Dudazy Gralla et al., 2013).
The ability to technically perform ChIP-seq in vivo has certainly emphasized the importance of the genomic actions of the TR isoforms. Still, it is clear that the TRs can function in a non-genomic fashion. Indeed many actions of T3 may occur too quickly to be mediated by enhanced or repressed target gene expression. Martin et al have identified two tyrosine motifs in the second zinc finger of TRβ1 that are not present in TRα1. In the absence of T3 the TRβ1 isoform can bind the regulatory subunit of PI3K. The addition of ligand dissociates this interaction and causes an increase in PI3K signaling. Remarkably, mutating one of these tyrosines in the TRβ1 isoform in vivo using a knock-in strategy did not impair classical nuclear TRβ1 signaling in context of thyroid function or regulation of genomic targets. When hippocampal T3-signaling was examined in this mutation, there were significant impairments in synaptic strength and plasticity demonstrating a developmental defect in PI3K signaling likely caused by the absence of TRβ1 (Martin et al., 2014). A role for TR β1 signaling via PI3K has also been demonstrated in the thyroid where a mutant TRβ1 can activate growth via PI3K (Furuya, Lu, Guigon, & Cheng, 2009).
Post-translational modifications have been shown to play a major role in modulation of transcription factor activity including TRs. Both TR isoforms can undergo sumoylation via separate pathways but on specific lysine residues. Importantly, sumoylation appears to be critical for the release of corepressors and the recruitment of coactivators and is required for a normal response to hormone stimulation. In this context, TR- sumoylation is needed for normal differentiation and growth of a human preadipocyte cell line, 3T3L1 cells. Targeted mutation of several lysine residues known to be sumoylated in human TRB1 impairs growth, differentiation and lipid storage of these cells (Y. Y. Liu, Kogai, Schultz, Mody, & Brent, 2012). Moreover, this phenotype was attenuated by blocking the TR’s interaction with the nuclear receptor corepressor, which indicates that TR-sumoylation is needed for a normal interaction with coregulators (see Coregulators section below) (Y.-Y. Liu et al., 2015)
While these signaling and genomic strategies are beginning to illuminate novel functions of thyroid hormone receptors in vivo, mouse models are elucidating their isoform-specific effects. Using a unique conditionally – targeted thrb mouse model, Selma-Ruby et al have demonstrated a role for TRβ directly in thyroid follicular cells. Thyrocyte-specific deletion of TRβ in mice leads to increased circulating levels of T4 and rT3, low TSH concentration and normal free T3 levels (Selmi-Ruby et al., 2014). Clearly, mice that lack all TRs develop follicular cells. Thus, it is likely that the TRs play little role in follicular cell development. Instead TRs may provide another layer of feedback to the HPT axis. In addition to the thyroid follicular cell, evidence exists for TRβ expression in the adrenal gland. Huang et al used a mouse model that marked TRβ1 expression with β–galactosidase and saw expression in a region of the adrenal cortex that expresses 20-α-hydroxysteroid dehydrogenase. Interestingly, this region in mouse regresses early in life in males but not in females. This differentiation process appears to be T3-dependent via TR β1. Whether there is role for T3-signaling in adrenal function in humans remains unknown (Huang, Kraft, Moy, Ng, & Forrest, 2015).
As discussed previously, TH has profound impact on the developing brain. While neurons express both TR isoforms, resolving their specific roles in individual neurons has been difficult. To overcome this Fauquier et al have developed a mouse model where a dominant negative TRα1 allele can be expressed in a cell-specific manner. Using the cerebellum, the investigators induced the mutant TR in a variety of cell-types. Remarkably, its expression in Purkinje cells and Bergman cells was able to affect all cerebellum development suggesting that TH-signaling can control cell function indirectly by regulating the environment or other signaling pathways (Fauquier et al., 2014). Using a different TRα1 mutant mouse model, Mittag et al demonstrated another example of the neuronal effects of TRα1. A select group of TRα1 expressing neurons in the anterior hypothalamus exerts specific control over blood pressure and heart rate. This indicates that TRα1 effects in the cardiovascular system may be also controlled indirectly via the CNS (Mittag et al., 2013). Finally, this same mouse model has demonstrated a unique role for TRα1 in mediating endothelial vasoconstriction in the tail vein of mice. This work has profound implications for energy expenditure studies using animal models. In this particular study, enhanced heat loss from the tail was compensated for by an increase in energy expenditure. This underlines the importance of accounting for tail heat loss in animal studies. If not done, it could be incorrectly assumed that the model has a primary increase in energy expenditure (Warner et al., 2013).
Coregulators
The classic view of TH action on positively regulated TR targets is that gene expression is mediated at the genomic level by the TRs either in the presence or absence of T3. In the absence of T3 or when present in low abundance, the TRs recruit a multiprotein complex that includes the nuclear corepressors, NCoR1 and SMRT, which in turn recruit HDAC3 and other proteins to mediate transcriptional repression via histone deacetylation (Sun et al., 2013; You et al., 2013). The presence of T3 leads to dismissal of the corepressor complex and recruitment of group of coregulators that include the SRC family of coactivators as well as p300/CBP and other transcriptional activators that lead to histone acetylation and transcriptional activation (Astapova, 2016) (Figure 4). However, recent findings have challenged this model and the exact mechanisms of transcriptional repression in hypothyroidism and activation in hyperthyroidism remain unclear.
To understand the role of corepressors and coactivators in TH action, Vella et al developed a model disrupting both NCoR1 and SRC-1 action. An SRC-1 knockout mouse was crossed to a mouse expressing a mutant NCoR1 allele, NCORΔID, that cannot interact with the TR (Figure 6) (Astapova et al., 2008). Previous work had already established that NCoR1 and SRC-1 played specific roles in TH action in the liver (Vella et al., 2014). In original studies characterizing expression of NCORΔID, NCoR1 played a key role in mediating sensitivity to the hormone such that in its absence there was increased sensitivity to T3 with enhanced activation of classic T3-target genes (Astapova et al., 2008). In contrast, deletion of SRC-1 led to a decreased response in context of gene activation to a set level of T3(Takeuchi et al., 2002). Taken together these data demonstrate that the balance between corepressors and coactivators determines the target set point of gene expression (Vella et al., 2014). Interestingly, expression of NCoRΔID and deletion of SRC-1 together led to the re-establishment of normal T3-sensitivity as this allowed for the recruitment of SRC-2 to target genes. Overall this model suggests that cellular levels of both TR-specific corepressors and coactivators determine T3-sensitivity. This is supported further by the discovery of splice variants of corepressors that have variable interaction with the TRs (Snyder, Goodson, Schroeder, & Privalsky, 2015). Furthermore, corepressor specificity does exist as SMRT plays little role in modulating TH action both in the liver and systemically in context of the thyroid axis (Shimizu et al., 2015). Thus, T3 action in a target cell can be functionally determined by the amount of NCoR1 present in conjunction with coactivators present. Further support for the notion of corepressor specificity comes from experiments where the hypomorphic NCoRΔID allele has been crossed into mice that express mutant TRβ or TRα alleles (PV mutation). The TRβ and TRα PV mutations are akin to those found in human syndromes of RTH where the mutant TR constitutively bound to NCoR1(Yen, 2003). In both cases the presence of the NCoRΔID allele improves the RTH phenotype. In the TRβPV mice, NCoRΔID reverses and normalizes the HPT axis abnormalities. In TRαPV mice, presence of the NCoRΔID allele allows previously infertile mothers to produce live pups. While these models suggest that the constitutive recruitment of NCoR1 by mutant TRs is responsible for the phenotype of RTH, much work remains to further characterize the mechanism of action of both the TRs and NCoR1 in the tissues studied (Fozzatti, Park, Zhao, Willingham, & Cheng, 2013; Laura. Fozzatti et al., 2013).
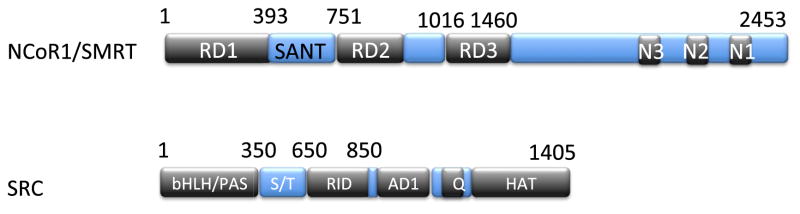
Figure 6. Corepressors and coactivators harbor nuclear receptor interacting domains.
NCoR1 and SMRT harbor three nuclear receptor interacting domains; N3 and N2 interact preferentially with unliganded TR, whereas SRC harbors an RID that interacts with the TR upon adoption of liganded conformation.
Despite the established roles of NCoR1/SMRT and the SRC family in context of TH action in the liver and the HPT axis, there are numerous other potential coregulators that may play a role in TH action. Each of the T3-binding TR isoforms has a distinct amino-terminus or A/B domain, which have been shown to potentially recruit a host of distinct proteins and suggest that isoform specificity could be dictated by coregulator recruitment (Yi et al., 2015). Moreover, mass spectrometry studies have demonstrated that TRs display isoform specific interaction with a wide set of nuclear proteins that is potentially dependent on the AF1 domain, which has the highest variability amongst the domains of the TR isoforms (Hahm & Privalsky, 2013; Hahm, Schroeder, & Privalsky, 2014). Additional coregulators that may play a role in T3 action include the histone deacetylase Sirt1 and the mediator subunit Med1 (Fondell, 2013; Suh et al., 2013). It is expected that multiple T3-responsive tissues will allow for their own code of regulators, which then mediate T3 action. For example the set of co-regulators that may act in the heart is not known as experiments that express NCoRΔID or delete SRC-1 globally have little effect on the response of T3-target genes in the heart (Vella et al., 2014).
Even though the recruitment of coregulators is paramount for TH action, the mechanism by which they mediate their effects is key to understanding the molecular components of TH action. Current thought involves TR isoforms binding NCoR1/SMRT, which then recruits the active histone deacetylase HDAC3. However, in mouse models where the NCoR1 or SMRT domain required for the deacetylase function of HDAC3 is mutated the activation of gene targets in the liver is much less than that seen in HDAC3 KO mice or in mice that lack both NCoR1 and SMRT function (Sun et al., 2013) (Shimizu et al., 2015). This implies that NCoR1/HDAC3 function may require more than deacetylation to silence gene expression in hypothyroidism. Indeed, deletion of HDAC3 or NCoR1/SMRT in murine liver cells in culture or in vivo leads to the strong activation of gene targets important in lipogenesis. Many of these genes are direct TH targets. In addition, HDAC3 deletion leads to activation of histone acetylation on these targets. However, the re-expression of a modified HDAC3 that can no longer deacetylate restores repression of these genes independently of changes in histone acetylation (Sun et al., 2013). This suggests that HDAC3 represses gene expression independently of histone acetylation, though this needs to be established directly on thyroid hormone targets. Taken together these data suggest that while NCoR1 and HDAC3 are necessary for the repressive effects of the TR, the exact molecular mechanism remains unclear. This is further complicated by the fact that the TR has the ability to activate certain targets such as TRH in the hypothalamus or FBXO21 in the liver in the hypothyroid state (Joseph-Bravo, Jaimes-Hoy, & Charli, 2015) (Astapova et al., 2008). The role of coregulators in so-called ligand-independent activation is not understood at all.
Unique Paradigms of TH Action – Negative Regulation by TH
As discussed extensively, TH is the major regulator of the set point of the HPT axis and it exerts its biologic effects through feedback inhibition at the level of the hypothalamus and pituitary. In addition, TH down regulates more genes in the liver in the presence of T3 that it activates (Ramadoss et al., 2014; Sasaki et al., 1999). While positive regulation by T3 is well explained by the models described above negative regulation remains a paradox. The expression of TRH in the paraventricular nucleus of the hypothalamus and the TSH subunit genes in the thyrotroph of the pituitary are regulated at the level of transcription mainly through TRβ2 though more recent data suggests that TRβ1 also plays a role (Ng et al., 2015). In addition, it has recently been observed that the ablation of TRα2, a non-T3 binding receptor in hypothalamus of mice, resulted in the overexpression of TRα1. These mice had a phenotype of low of free T3 and T4 levels and normal TSH suggesting a role of this previously neglected TR isoform in the regulation of HPT axis. Normally, TRα2 acts as a dominant inhibitor of T3-binding TR isoforms by competing for DNA binding sites. In vivo experiments of using siRNA to ablate or overexpress TRα2 show abrogated T3 action on TH-reporter genes in the hypothalamus consistent with the hypothesis that TRα2 can block WT receptor function (Guissouma et al., 2014). Further involvement of TRα isoforms in the regulation of the HPT axis can be garnered from RTH patients with mutations in TRα. Some patients have a subnormal free T4/T3 ratio with a normal or low TSH, though this may depend on the mutation present (C. Moran & Chatterjee, 2016; Carla. Moran et al., 2014). Taken together, these findings suggest that both THRB and THRA are required for normal set point of the HPT axis and negative regulation by T3.
While the TRs are required for negative regulation, the mechanism by which it is mediated is not clear. Genome wide ChIP studies in the liver (see Table 1) demonstrate that negatively regulated targets are much less likely to have TR-binding sites in the region surrounding them suggesting that negative regulation could be indirect. In contrast, direct evidence of TRβ2 binding to the TSHβ promoter has been obtained in a pituitary cell line (Chiamolera et al., 2012). Thus, it is likely that both direct and indirect mechanisms will explain how the TRs are able to mediate negative regulation. The role of the coregulators also remains enigmatic in negative regulation. Paradoxically, deletion of SRC-1 causes a RTH syndrome similar to that seen with a mutated TRβ isoform with inappropriate TSH secretion in the setting of elevated TH levels (Weiss et al., 1999). This phenotype suggests that SRC-1 plays a direct role in T3-mediated repression. Indeed, co-expression of NCoRΔID in SRC-1 −/− mice normalizes the HPT axis and re-establishes normal negative regulation of the TSHβ subunit gene (Vella et al., 2014). Thus, in the pituitary the coregulators appear to play paradoxical roles in negative regulation of the TSHβ gene by T3 (Costa-e-Sousa & Hollenberg, 2012). Whether the co-regulators have similar effects on TRH expression in the PVN remains unclear. Indeed many uncertainties remain in context of T3-mediated regulation of TRH. For example it is not clear how T3 accesses the PVN as local T3 is primarily derived in the region of the tanycytes at the base of the hypothalamus(Hanon et al., 2008; Watanabe et al., 2007). Thus, it remains possible that T3 may act indirectly on the TRH neurons in the PVH (Hollenberg, 2008). Remarkably T3 has no effect on TRH gene expression in neurons in the lateral hypothalamus, which is in much closer proximity to the tanycytes where T3 is produced (Sugrue, Vella, Morales, Lopez, & Hollenberg, 2010).
While SRC-1 and NCoR1 clearly play a role in the regulation of TSHβ gene expression and the set point of the HPT axis, the deletion of SRC-1 or NCoR1 has no effect on negative T3 targets such as Gsta1 or Fbxo21 in the liver (Vella et al., 2014). Thus, tissue specific and coregulator specific mechanisms must be in place to allow for negative regulation by T3 in a target cell. Further studies on epigenetic marks and their role in negative regulation are required in order to better understand the molecular mechanisms in play in negative regulation.
Summary and Conclusions
Over the last number of years work on thyroid hormone action has firmly established that the intracellular actions of the hormone are quite discernible from circulating levels. While the HPT axis moves to keep circulating TH levels within a tight range based on a genetic set point with environmental cues, the intracellular availability of T3 is based on a myriad of other determinants. We now realize that a multitude of transporters mediate TH access to distinct cell types and that these transporters can be regulated themselves. Next, the deiodinases play a distinct role in regulating cell-specific levels of T3 that can reach the nucleus. They, too, are regulated in pathophysiologic states at both the level of transcription and translation. Finally, the intranuclear milieu of TRs and co-regulators is highly varied depending upon the cell-type studied. This variability undoubtedly leads to changes in response to a set level of T3. Remarkably, the model of an unliganded versus a liganded TR in context of co-regulator function is no longer valid. Clearly the TRs have the ability to bind to DNA in the presence of T3 or leave in its absence. Yet how this is regulated remains unclear. Despite the advances made, a unifying model of negative regulation by TH and the TRs remains elusive. It is likely that numerous models will be required to explain this phenomenon. Although questions remain, it is also clear that our ability to better understand tissue-specific thyroid hormone action at the molecular level will lead to better biomarkers in humans so that therapy can be better tailored in different pathophysiologic states.
Acknowledgments
A. M. is supported by CONACyT Postdoctoral Fellowship 250095. ANH is supported by NIH grants DK056123, DK098525, DK105029. We thank Inna Astapova, PhD and Kristen Vella, PhD for their critical review of the manuscript.
Abbreviations
- TH
Thyroid hormones
- T4
thyroxine
- T3
Triiodothyronine
- CNS
central nervous system
- HPT
hypothalamic-pituitary-thyroid axis
- TRH
thyrotropin-releasing hormone
- PVN
paraventricular nucleus
- CART
Cocaine- and amphetamine-regulated transcript
- CRH
corticotrophin releasing hormone
- TSH
thyroid stimulating hormone
- GWAS
(genome-wide association studies)
- PDE8B
Phosphodiesterase 8B
- PDE10A
Phosphodiesterase 10A
- CAPZB
Capping Actin Protein Of Muscle Z-Line Beta Subunit
- MAF/LOC440389
V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homolog
- VEGFA
Vascular Endothelial Growth Factor A
- NR3C2
Nuclear Receptor Subfamily 3 Group C Member 2
- IGFBP5
Insulin Like Growth Factor Binding Protein 5
- NFIA
Nuclear Factor I A
- SOX9
SRY-Box 9
- PRDM11
PR Domain 11
- FGF7
Fibroblast Growth Factor 7
- INSR
Insulin Receptor
- ABO
ABO Blood Group
- MIR1179
MicroRNA 1179
- NRG1
Neuregulin 1
- MBIP
MAP3K12 Binding Inhibitory Protein 1
- ITPK1
Inositol-Tetrakisphosphate 1-Kinase
- SASH1
SAM And SH3 Domain Containing 1
- GLIS3
GLIS Family Zinc Finger 3
- DIO1
Deiodinase, Iodothyronine Type I
- LHX3
LIM Homeobox 3
- FOXE1
Forkhead Box E1
- AADAT
Aminoadipate Aminotransferase
- NETO1/FBXO15
Neuropilin And Tolloid Like 1/F-Box Protein 15
- LPCAT2/CAPNS2
Lysophosphatidylcholine Acyltransferase 2/Calpain Small Subunit 2
- FT4
free thyroxin
- MCT8
monocarboxylate transporter 8
- MCT10
monocarboxylate transporter 10
- OATP1
organic anion transporter
- LAT
L-type amino acid transporter
- AHS
Allan-hendon dudley syndrome
- SNP
single nucleotide polymorphism
- TRIAC
3,5,3′-Triiodothyroacetic acid
- TETRAC
Tetraiodothyroacetic acid
- DITPA
3,5-Diiodothyropropionic Acid
- LPS
lipopolysaccharide
- LAT2
L-type amino acid transporter 2
- D2
type 2 deiodinase
- D3
type 3 deiodinase
- IRD
inner-ring deiodination
- ORD
outer ring deiodination
- D1
type 1 deiodinase
- EGL
external germinal layers
- IGL
internal germinal layers
- CTX
cardiotoxin analogue III
- WSB-1
WD Repeat And SOCS Box Containing 1
- OA
osteoarthritis
- ADAMTS5
ADAM Metallopeptidase With Thrombospondin Type 1 Motif 5
- TR
thyroid hormone receptor
- LBD
ligand binding domain
- TRB1
thyroid hormone receptor beta 1
- TRB2
thyroid hormone receptor beta 2
- TRa1
thyroid hormone receptor alpha 1
- RTHB
resistance to thyroid hormone beta
- RTH
resistance to thyroid hormones
- GI
gastrointestinal
- RXRα
retinoic X receptor alpha
- GFP
green fluorescent protein
- RNF166
Ring Finger Protein 166
- PI3K
Fosfoinositol 3-quinasa
- NCoR1
Nuclear receptor corepressor 1
- SMRT
silencing mediator of retinoic acid and thyroid hormone receptor
- HDAC3
Histone Deacetylase 3
- SRC-1
steroid receptor coactivator 1
- CBP
CREB-binding protein
- NCORΔID
Nuclear receptor corepressor 1 that lacks nuclear receptor interaction domains
- SRC-2
steroid receptor coactivator 2
- FBXO21
F-Box Protein 21
- Gsta1
Glutathione S-Transferase Alpha 1
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-azzam S, Alkhateeb A, Al-Azzeh O, Alzoubi K, Khabour O. The Role of Type II Deiodinase Polymorphisms in Clinical Management of Hypothyroid Patients Treated with Levothyroxine. Experimental and Clinical Endocrinology\& Diabetes. 2013;121(05):300–305. doi: 10.1055/s-0032-1331695. [DOI] [PubMed] [Google Scholar]
- Astapova I. Role of co-regulators in metabolic and transcriptional actions of thyroid hormone. Journal of Molecular Endocrinology. 2016;56(3):R73–R97. doi: 10.1530/jme-15-0246. [DOI] [PubMed] [Google Scholar]
- Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19544–19549. doi: 10.1073/pnas.0804604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers S, Switnicki MP, Angajala A, Lammel J, Arumanayagam AS, Webb P. Genome-Wide Binding Patterns of Thyroid Hormone Receptor Beta. Plos One. 2014;9(2) doi: 10.1371/journal.pone.0081186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargi-Souza P, Kucka M, Bjelobaba I, Tomic M, Janjic MM, Nunes MT, Stojilkovic SS. Loss of Basal and TRH-Stimulated Tshb Expression in Dispersed Pituitary Cells. Endocrinology. 2015;156(1):242–254. doi: 10.1210/en.2014-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomer Nils, den Hollander Wouter, Ramos Yolande FM, Bos Steffan D, van der Breggen Ruud, Lakenberg Nico, … Meulenbelt Ingrid. Underlying molecular mechanisms of DIO2susceptibility in symptomatic osteoarthritis. Annals of the Rheumatic Diseases. 2015;74(8):1571–1579. doi: 10.1136/annrheumdis-2013-204739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Doreen, Lelios Iva, Krause Gerd, Schweizer Ulrich. Histidines in Potential Substrate Recognition Sites Affect Thyroid Hormone Transport by Monocarboxylate Transporter 8 (MCT8) Endocrinology. 2013;154(7):2553–2561. doi: 10.1210/en.2012-2197. [DOI] [PubMed] [Google Scholar]
- Brent GA. Mechanisms of thyroid hormone action. Journal of Clinical Investigation. 2012;122(9):3035–3043. doi: 10.1172/jci60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre A, Hamza RT, Kariyawasam D, Guillot L, Teissier R, Tron E, … Polak M. A Novel FOXE1 Mutation (R73S) in Bamforth-Lazarus Syndrome Causing Increased Thyroidal Gene Expression. Thyroid. 2014;24(4):649–654. doi: 10.1089/thy.2013.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatonnet F, Guyot R, Benoit G, Flamant F. Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(8):E766–E775. doi: 10.1073/pnas.1210626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamolera MI, Sidhaye AR, Matsumoto S, He QY, Hashimoto K, Ortiga-Carvalho TM, Wondisford FE. Fundamentally Distinct Roles of Thyroid Hormone Receptor Isoforms in a Thyrotroph Cell Line Are due to Differential DNA Binding. Molecular Endocrinology. 2012;26(6):926–939. doi: 10.1210/me.2011-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-e-Sousa Ricardo H, Hollenberg Anthony N. Minireview: The Neural Regulation of the Hypothalamic-Pituitary-Thyroid Axis. Endocrinology. 2012;153(9):4128–4135. doi: 10.1210/en.2012-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza E, Dias G, Cardoso R, Lima L, Fortunato R, Visser T, … Carvalho D. MCT8 is Downregulated by Short Time Iodine Overload in the Thyroid Gland of Rats. Hormone and Metabolic Research. 2015;47(12):910–915. doi: 10.1055/s-0035-1550008. [DOI] [PubMed] [Google Scholar]
- de Vrieze Erik, van de Wiel Sandra MW, Zethof Jan, Flik Gert, Klaren Peter HM, Arjona Francisco J. Knockdown of Monocarboxylate Transporter 8 ( mct8) Disturbs Brain Development and Locomotion in Zebrafish. Endocrinology. 2014;155(6):2320–2330. doi: 10.1210/en.2013-1962. [DOI] [PubMed] [Google Scholar]
- Dentice Monica, Ambrosio Raffaele, Damiano Valentina, Sibilio Annarita, Luongo Cristina, Guardiola Ombretta, … Salvatore Domenico. Intracellular Inactivation of Thyroid Hormone Is a Survival Mechanism for Muscle Stem Cell Proliferation and Lineage Progression. Cell Metabolism. 2014;20(6):1038–1048. doi: 10.1016/j.cmet.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudazy Gralla Susi, Nordstrm Kristina, Hofmann Peter Josef, Meseh Dina Abdul, Schomburg Lutz, Vennstrm Bjrn, Mittag Jens. Identification of thyroid hormone response elements in vivousing mice expressing a tagged thyroid hormone receptor α1. Bioscience Reports. 2013;33(2):94. doi: 10.1002/prot.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egri P, Gereben B. Minimal requirements for ubiquitination-mediated regulation of thyroid hormone activation. Journal of Molecular Endocrinology. 2014;53(2):217–226. doi: 10.1530/JME-14-0156. [DOI] [PubMed] [Google Scholar]
- Fauquier T, Chatonnet F, Picou F, Richard S, Fossat N, Aguilera N, … Flamant F. Purkinje cells and Bergmann glia are primary targets of the TR alpha 1 thyroid hormone receptor during mouse cerebellum postnatal development. Development. 2014;141(1):166–175. doi: 10.1242/dev.103226. [DOI] [PubMed] [Google Scholar]
- Feng Shouhao, Lin Shengli, Zou Jidong, Wang Yulong, Ji Qinghai, Lv Zhenghua. Association between rs12045440 Polymorphism in the CAPZB Intron and Serum TSH Concentrations in Chinese Thyroid Tumor Patients. International Journal of Endocrinology. 2015;2015(9):1–7. doi: 10.1093/hmg/dds136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara Alfonso Massimiliano, Liao Xiao-Hui, Gil-Ibanez Pilar, Bernal Juan, Weiss Roy E, Dumitrescu Alexandra M, Refetoff Samuel. Placenta Passage of the Thyroid Hormone Analog DITPA to Male Wild-Type and Mct8-Deficient Mice. Endocrinology. 2014;155(10):4088–4093. doi: 10.1210/en.2014-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Kleinau G, Muller A, Kuhnen P, Zwanziger D, Kinne A, … Biebermann H. Modulation of monocarboxylate transporter 8 oligomerization by specific pathogenic mutations. Journal of Molecular Endocrinology. 2014;54(1):39–50. doi: 10.1530/JME-14-0272. [DOI] [PubMed] [Google Scholar]
- Fitzgerald SP, Bean NG. The Relationship between Population T4/TSH Set Point Data and T4/TSH Physiology. Journal of Thyroid Research. 2016 doi: 10.1155/2016/6351473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell JD. The Mediator complex in thyroid hormone receptor action. Biochimica Et Biophysica Acta-General Subjects. 2013;1830(7):3867–3875. doi: 10.1016/j.bbagen.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: Evidence for tissue-specific modulation of receptor function. Embo Journal. 1996;15(12):3006–3015. [PMC free article] [PubMed] [Google Scholar]
- Fozzatti Laura, Park Jeong Won, Zhao Li, Willingham Mark C, Cheng Sheue-yann. Oncogenic Actions of the Nuclear Receptor Corepressor (NCOR1) in a Mouse Model of Thyroid Cancer. Plos One. 2013;8(6) doi: 10.1371/journal.pone.0067954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzatti Laura, Kim Dong Wook, Park Jeong Won, Willingham Mark C, Hollenberg Anthony N, Cheng Sheue-Yann. Nuclear receptor corepressor (NCOR1) regulates in vivo actions of a mutated thyroid hormone receptor alpha. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(19):7850–7855. doi: 10.1073/pnas.1222334110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema ECH, Visser WE, Visser TJ. Genetics and phenomics of thyroid hormone transport by MCT8. Molecular and Cellular Endocrinology. 2010;322(1–2):107–113. doi: 10.1016/j.mce.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Furuya F, Lu CX, Guigon CJ, Cheng SY. Nongenomic activation of phosphatidylinositol 3-kinase signaling by thyroid hormone receptors. Steroids. 2009;74(7):628–634. doi: 10.1016/j.steroids.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi L, Nataren N, Feng JH, Schreiber AW, Hahn CN, Conwell LS, … Scott HS. Allan-Herndon-Dudley Syndrome with Unusual Profound Sensorineural Hearing Loss. American Journal of Medical Genetics Part A. 2015;167(8):1872–1876. doi: 10.1002/ajmg.a.37075. [DOI] [PubMed] [Google Scholar]
- Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nature Reviews Endocrinology. 2015;11(11):642–652. doi: 10.1038/nrendo.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg Stefan, Friesema Edith CH, Kersseboom Simone, Klootwijk Wim, Visser W Edward, Peeters Robin P, Visser Theo J. The Role of Arg445 and Asp498 in the Human Thyroid Hormone Transporter MCT8. Endocrinology. 2014;155(2):618–626. doi: 10.1210/en.2013-1521. [DOI] [PubMed] [Google Scholar]
- Groeneweg Stefan, Lima de Souza Elaine C, Visser W Edward, Peeters Robin P, Visser Theo J. Importance of His192 in the Human Thyroid Hormone Transporter MCT8 for Substrate Recognition. Endocrinology. 2013;154(7):2525–2532. doi: 10.1210/en.2012-2225. [DOI] [PubMed] [Google Scholar]
- Grontved L, Waterfall JJ, Kim DW, Baek S, Sung MH, Zhao L, … Cheng SY. Transcriptional activation by the thyroid hormone receptor through ligand-dependent receptor recruitment and chromatin remodelling. Nature Communications. 2015:6. doi: 10.1038/ncomms8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guissouma Hajer, Ghaddab-Zroud Rym, Seugnet Isabelle, Decherf Stphanie, Demeneix Barbara, Clerget-Froidevaux Marie-Stphanie. TR Alpha 2 Exerts Dominant Negative Effects on Hypothalamic Trh Transcription In Vivo. PLoS ONE. 2014;9(4):e95064. doi: 10.1371/journal.pone.0095064.g004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JB, Privalsky ML. Research Resource: Identification of Novel Coregulators Specific for Thyroid Hormone Receptor-beta 2. Molecular Endocrinology. 2013;27(5):840–859. doi: 10.1210/me.2012-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JB, Schroeder AC, Privalsky ML. The two major isoforms of thyroid hormone receptor, TR alpha 1 and TR beta 1, preferentially partner with distinct panels of auxiliary proteins. Molecular and Cellular Endocrinology. 2014;383(1–2):80–95. doi: 10.1016/j.mce.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Current Biology. 2008;18(15):1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- Heijlen Marjolein, Houbrechts Anne M, Bagci Enise, Van Herck Stijn LJ, Kersseboom Simone, Esguerra Camila V, … Darras Veerle M. Knockdown of Type 3 Iodothyronine Deiodinase Severely Perturbs Both Embryonic and Early Larval Development in Zebrafish. Endocrinology. 2014;155(4):1547–1559. doi: 10.1210/en.2013-1660. [DOI] [PubMed] [Google Scholar]
- Hollenberg Anthony N. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid. 2008;18(2):131–139. doi: 10.1089/thy.2007.0251. [DOI] [PubMed] [Google Scholar]
- Hu Zhimei, Zhuo Xiaohua, Shi Yanan, Liu Xin, Yuan Jihong, Li Lanying, Sun Yina. Iodine deficiency up-regulates monocarboxylate transporter 8 expression of mouse thyroid gland. Chinese medical journal. 2014;127(23):4071–4076. doi: 10.3760/cma.j.issn.0366-6999.20141314. [DOI] [PubMed] [Google Scholar]
- Huang CCJ, Kraft C, Moy N, Ng L, Forrest D. A Novel Population of Inner Cortical Cells in the Adrenal Gland That Displays Sexually Dimorphic Expression of Thyroid Hormone Receptor-beta 1. Endocrinology. 2015;156(6):2338–2348. doi: 10.1210/en.2015-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen SW, Akintola AA, Roelfsema F, van der Spoel E, Cobbaert CM, Ballieux BE, … van Heemst D. Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Scientific Reports. 2015;5:11. doi: 10.1038/srep11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen Rob, Zuidwijk Marian, Muller Alice, Mulders Joyce, Oudejans Cees BM, Simonides Warner S. Cardiac Expression of Deiodinase type 3 (Dio3) Following Myocardial Infarction Is Associated With the Induction of a Pluripotency microRNA Signature from the Dlk1-Dio3 Genomic Region. Endocrinology. 2013;154(6):1973–1978. doi: 10.1210/en.2012-2017. [DOI] [PubMed] [Google Scholar]
- Jayarama-Naidu Roopa, Johannes Jrg, Meyer Franziska, Wirth Eva Katrin, Schomburg Lutz, Kohrle Josef, Renko Kostja. A Nonradioactive Uptake Assay for Rapid Analysis of Thyroid Hormone Transporter Function. Endocrinology. 2015;156(7):2739–2745. doi: 10.1210/en.2015-1016. [DOI] [PubMed] [Google Scholar]
- Joseph-Bravo P, Jaimes-Hoy L, Charli JL. Regulation of TRH neurons and energy homeostasis-related signals under stress. Journal of Endocrinology. 2015;224(3):R139–R159. doi: 10.1530/joe-14-0593. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim YM, Yum MS, Choi JH, Lee BH, Kim GH, Yoo HW. Clinical and Endocrine Features of Two Allan-Herndon-Dudley Syndrome Patients with Monocarboxylate Transporter 8 Mutations. Hormone Research in Paediatrics. 2015;83(4):288–292. doi: 10.1159/000371466. [DOI] [PubMed] [Google Scholar]
- Kim JY, Son YL, Kim JS, Lee YC. Molecular Determinants Required for Selective Interactions between the Thyroid Hormone Receptor Homodimer and the Nuclear Receptor Corepressor N-CoR. Journal of Molecular Biology. 2010;396(3):747–760. doi: 10.1016/j.jmb.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Lavado-Autric Rosalia, Calvo Rosa Maria, de Mena Raquel Martinez, de Escobar Gabriella Morreale, Obregon Maria Jesus. Deiodinase activities in thyroids and tissues of iodine-deficient female rats. Endocrinology. 2013;154(1):529–536. doi: 10.1210/en.2012-1727. [DOI] [PubMed] [Google Scholar]
- Liao XH, Di Cosmo C, Dumitrescu AM, Hernandez A, Van Sande J, St Germain DL, … Refetoff S. Distinct Roles of Deiodinases on the Phenotype of Mct8 Defect: A Comparison of Eight Different Mouse Genotypes. Endocrinology. 2011;152(3):1180–1191. doi: 10.1210/en.2010-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Jean Z, Sieglaff Douglas H, Yuan Chaoshen, Su Jing, Arumanayagam AnithaChristy S, Firouzbakht Sharareh, … Webb Paul. Gene Specific Actions of Thyroid Hormone Receptor Subtypes. Plos One. 2013;8(1) doi: 10.1371/journal.pone.0052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Kogai T, Schultz JJ, Mody K, Brent GA. Thyroid Hormone Receptor Isoform-specific Modification by Small Ubiquitin-like Modifier (SUMO) Modulates Thyroid Hormone-dependent Gene Regulation. Journal of Biological Chemistry. 2012;287(43):36499–36508. doi: 10.1074/jbc.M112.344317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yan-Yun, Ayers Stephen, Milanesi Anna, Teng Xiaochun, Rabi Sina, Akiba Ysutada, Brent Gregory A. Thyroid Hormone Receptor Sumoylation Is Required for Preadipocyte Differentiation and Proliferation. Journal of Biological Chemistry. 2015;290(12):7402–7415. doi: 10.1074/jbc.M114.600312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espindola D, Morales-Bastos C, Grijota-Martinez C, Liao XH, Lev D, Sugo E, … Guadano-Ferraz A. Mutations of the Thyroid Hormone Transporter MCT8 Cause Prenatal Brain Damage and Persistent Hypomyelination. Journal of Clinical Endocrinology & Metabolism. 2014;99(12):E2799–E2804. doi: 10.1210/jc.2014-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Zhou XH, Zou T, Keyim K, Dong LM. Type II deiodinase polymorphisms and serum thyroid hormone levels in patients with mild cognitive impairment. Genetics and Molecular Research. 2015;14(2):5407–5416. doi: 10.4238/2015.May.22.10. [DOI] [PubMed] [Google Scholar]
- Machado DS, Sabet A, Santiago LA, Sidhaye AR, Chiamolera MI, Ortiga-Carvalho TM, Wondisford FE. A thyroid hormone receptor mutation that dissociates thyroid hormone regulation of gene expression in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(23):9441–9446. doi: 10.1073/pnas.0903227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski A, Brzostek S, Cohen RN, Hollenberg AN. Determination of nuclear receptor corepressor interactions with the thyroid hormone receptor. Molecular Endocrinology. 2003;17(2):273–286. doi: 10.1210/me.2002-0310. [DOI] [PubMed] [Google Scholar]
- Malinowski Jennifer R, Denny Joshua C, Bielinski Suzette J, Basford Melissa A, Bradford Yuki, Peissig Peggy L, … Crawford Dana C. Genetic Variants Associated with Serum Thyroid Stimulating Hormone (TSH) Levels in European Americans and African Americans from the eMERGE Network. PLoS ONE. 2014;9(12):e111301. doi: 10.1371/journal.pone.0111301.s013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NP, de Velasco EMF, Mizuno F, Scappini EL, Gloss B, Erxleben C, … Armstrong DL. A Rapid Cytoplasmic Mechanism for PI3 Kinase Regulation by the Nuclear Thyroid Hormone Receptor, TR beta, and Genetic Evidence for Its Role in the Maturation of Mouse Hippocampal Synapses In Vivo. Endocrinology. 2014;155(9):3713–3724. doi: 10.1210/en.2013-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavinakere Manohara S, Powers Jeremy M, Subramanian Kelly S, Roggero Vincent R, Allison Lizabeth A. Multiple novel signals mediate thyroid hormone receptor nuclear import and export. The Journal of biological chemistry. 2012;287(37):31280–31297. doi: 10.1074/jbc.M112.397745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl Steffen, Mller Julia, Bauer Reinhard, Richert Sarah, Kassmann Celia M, Darras Veerle M, … Heuer Heike. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. The Journal of clinical investigation. 2014;124(5):1987–1999. doi: 10.1172/JCI70324DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAninch Elizabeth A, Jo Sungro, Preite Nailliw Z, Farkas Erzsbet, Mohcsik Petra, Fekete Csaba, … Bianco Antonio C. Prevalent Polymorphism in Thyroid Hormone-Activating Enzyme Leaves a Genetic Fingerprint That Underlies Associated Clinical Syndromes. The Journal of Clinical Endocrinology\& Metabolism. 2015;100(3):920–933. doi: 10.1210/jc.2014-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici M, Visser WE, Visser TJ, Peeters RP. Genetic Determination of the Hypothalamic-Pituitary-Thyroid Axis: Where Do We Stand? Endocrine Reviews. 2015;36(2):214–244. doi: 10.1210/er.2014-1081. [DOI] [PubMed] [Google Scholar]
- Mittag J, Lyons DJ, Sallstrom J, Vujovic M, Dudazy-Gralla S, Warner A, … Vennstrom B. Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. Journal of Clinical Investigation. 2013;123(1):509–516. doi: 10.1172/jci65252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Chatterjee K. Resistance to Thyroid Hormone alpha-Emerging Definition of a Disorder of Thyroid Hormone Action. Journal of Clinical Endocrinology & Metabolism. 2016;101(7):2636–2639. doi: 10.1210/jc.2016-2317. [DOI] [PubMed] [Google Scholar]
- Moran Carla, Agostini Maura, Visser W Edward, Schoenmakers Erik, Schoenmakers Nadia, Offiah Amaka C, … Krishna K Chatterjee. Resistance to thyroid hormone caused by a mutation in thyroid hormone receptor (TR)α1 and TRα2 : clinical, biochemical, and genetic analyses of three related patients. THE LANCET Diabetes\& Endocrinology. 2014;2(8):619–626. doi: 10.1016/S2213-8587(14)70111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Carla, Chatterjee Krishna. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Practice\& Research Clinical Endocrinology\& Metabolism. 2015;29(4):647–657. doi: 10.1016/j.beem.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzio Amanda M, Noyes Pamela D, Stapleton Heather M, Lema Sean C. Tissue distribution and thyroid hormone effects on mRNA abundance for membrane transporters Mct8, Mct10, and organic anion-transporting polypeptides (Oatps) in a teleost fish. Comparative Biochemistry and Physiology, Part A. 2014;167(C):77–89. doi: 10.1016/j.cbpa.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Nagasawa Y, Tachida Y, Sakakibara S, Okutsu J, Suematsu N, … Shimada K. Deiodinase 2 upregulation demonstrated in osteoarthritis patients cartilage causes cartilage destruction in tissue-specific transgenic rats. Osteoarthritis and Cartilage. 2013;21(3):514–523. doi: 10.1016/j.joca.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Ng L, Cordas E, Wu XF, Vella KR, Hollenberg AN, Forrest D. Age-Related Hearing Loss and Degeneration of Cochlear Hair Cells in Mice Lacking Thyroid Hormone Receptor beta 1. Endocrinology. 2015;156(10):3853–3865. doi: 10.1210/en.2015-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, … Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hernandez A, He WX, Ren TY, Srinivas M, Ma M, … Forrest D. A Protective Role for Type 3 Deiodinase, a Thyroid Hormone-Inactivating Enzyme, in Cochlear Development and Auditory Function. Endocrinology. 2009;150(4):1952–1960. doi: 10.1210/en.2008-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni EA, Sevarino KA. The biology of pro-thyrotropin-releasing hormone-derived peptides. Endocrine Reviews. 1999;20(5):599–648. doi: 10.1210/er.20.5.599. [DOI] [PubMed] [Google Scholar]
- Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common Variation in the DIO2 Gene Predicts Baseline Psychological Well-Being and Response to Combination Thyroxine Plus Triiodothyronine Therapy in Hypothyroid Patients. Journal of Clinical Endocrinology & Metabolism. 2009;94(5):1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- Peeters Robin P, Hernandez Arturo, Ng Lily, Ma Michelle, Sharlin David S, Pandey Mritunjay, … Forrest Douglas. Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor α1. Endocrinology. 2013;154(1):550–561. doi: 10.1210/en.2012-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, … Naitza S. A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function. Plos Genetics. 2013;9(2):20. doi: 10.1371/journal.pgen.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss P, Abraham BJ, Tsai L, Zhou YM, Costa-e-Sousa RH, Ye FL, … Hollenberg AN. Novel Mechanism of Positive versus Negative Regulation by Thyroid Hormone Receptor beta 1 (TR beta 1) Identified by Genome-wide Profiling of Binding Sites in Mouse Liver. Journal of Biological Chemistry. 2014;289(3):1313–1328. doi: 10.1074/jbc.M113.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roef GL, Rietzschel ER, De Meyer T, Bekaert S, De Buyzere ML, Van Daele C, … Taes YE. Associations between single nucleotide polymorphisms in thyroid hormone transporter genes (MCT8, MCT10 and OATP1C1) and circulating thyroid hormones. Clinica Chimica Acta. 2013;425(C):227–232. doi: 10.1016/j.cca.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Roef G, Taes Y, Toye K, Goemaere S, Fiers T, Verstraete A, Kaufman JM. Heredity and lifestyle in the determination of between-subject variation in thyroid hormone levels in euthyroid men. European Journal of Endocrinology. 2013;169(6):835–844. doi: 10.1530/eje-13-0265. [DOI] [PubMed] [Google Scholar]
- Roggero VR, Zhang JB, Parente LE, Doshi Y, Dziedzic RC, McGregor EL, … Allison LA. Nuclear import of the thyroid hormone receptor\& alpha;1 is mediated by importin 7, importin\& beta;1, and adaptor importin\& alpha;1. Molecular and Cellular Endocrinology. 2016;419(C):185–197. doi: 10.1016/j.mce.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogounovitch TI, Bychkov A, Takahashi M, Mitsutake N, Nakashima M, Nikitski AV, … Saenko VA. The Common Genetic Variant rs944289 on Chromosome 14q13.3 Associates with Risk of Both Malignant and Benign Thyroid Tumors in the Japanese Population. Thyroid. 2015;25(3):333–340. doi: 10.1089/thy.2014.0431. [DOI] [PubMed] [Google Scholar]
- Santoro AB, Vargens DD, Barros MD, Bulzico DA, Kowalski LP, Meirelles RMR, … Suarez-Kurtz G. Effect of UGT1A1, UGT1A3, DIO1 and DIO2 polymorphisms on L-thyroxine doses required for TSH suppression in patients with differentiated thyroid cancer. British Journal of Clinical Pharmacology. 2014;78(5):1067–1075. doi: 10.1111/bcp.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Lesoon-Wood LA, Dey A, Kuwata T, Weintraub BD, Humphrey G, … Ozato K. Ligand-induced recruitment of a histone deacetylase in the negative-feedback regulation of the thyrotropin beta gene. Embo Journal. 1999;18(19):5389–5398. doi: 10.1093/emboj/18.19.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers N, Moran C, Peeters RP, Visser T, Gurnell M, Chatterjee K. Resistance to thyroid hormone mediated by defective thyroid hormone receptor alpha. Biochimica Et Biophysica Acta-General Subjects. 2013;1830(7):4004–4008. doi: 10.1016/j.bbagen.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Schwabedissen Hemz, Ware JA, Finkelstein D, Chaudhry AS, Mansell S, Leon-Ponte M, … Kim RB. Hepatic Organic Anion Transporting Polypeptide Transporter and Thyroid Hormone Receptor Interplay Determines Cholesterol and Glucose Homeostasis. Hepatology. 2011;54(2):644–654. doi: 10.1002/hep.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer U, Schlicker C, Braun D, Kohrle J, Steegborn C. Crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism. Proceedings of the National Academy of Sciences. 2014;111(29):10526–10531. doi: 10.1073/pnas.1323873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi-Ruby Samia, Bouazza Lamia, Obregon Maria Jesus, Conscience Aude, Flamant Frederic, Samarut Jacques, … Rousset Bernard. The Targeted Inactivation of TRβ Gene in Thyroid Follicular Cells Suggests a New Mechanism of Regulation of Thyroid Hormone Production. Endocrinology. 2014;155(2):635–646. doi: 10.1210/en.2013-1435. [DOI] [PubMed] [Google Scholar]
- Shimizu Hiroaki, Astapova Inna, Ye Felix, Bilban Martin, Cohen Ronald N, Hollenberg Anthony N. NCoR1 and SMRT Play Unique Roles in Thyroid Hormone Action In Vivo. Molecular and Cellular Biology. 2015;35(3):555–565. doi: 10.1128/mcb.01208-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder Chelsea A, Goodson Michael L, Schroeder Amy C, Privalsky Martin L. Regulation of corepressor alternative mRNA splicing by hormonal and metabolic signaling. Molecular and Cellular Endocrinology. 2015;413(C):228–235. doi: 10.1016/j.mce.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosic-Jurjevic B, Filipovic B, Renko K, Miler M, Trifunovic S, Ajdzanovic V, … Milosevic V. Testosterone and estradiol treatments differently affect pituitary-thyroid axis and liver deiodinase 1 activity in orchidectomized middle-aged rats. Experimental Gerontology. 2015;72:85–98. doi: 10.1016/j.exger.2015.09.010. [DOI] [PubMed] [Google Scholar]
- St Germain DL, Galton VA, Hernandez A. Minireview: Defining the Roles of the Iodothyronine Deiodinases: Current Concepts and Challenges. Endocrinology. 2009;150(3):1097–1107. doi: 10.1210/en.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue Michelle L, Vella Kristen R, Morales Crystal, Lopez Marisol E, Hollenberg Anthony N. The Thyrotropin-Releasing Hormone Gene Is Regulated by Thyroid Hormone at the Level of Transcription in Vivo. Endocrinology. 2010;151(2):793–801. doi: 10.1210/en.2009-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Ji Ho, Sieglaff Douglas H, Zhang Aijun, Xia Xuefeng, Cvoro Aleksandra, Winnier Glenn E, Webb Paul. SIRT1 is a Direct Coactivator of Thyroid Hormone Receptor β1 with Gene-Specific Actions. PLoS ONE. 2013;8(7):e70097. doi: 10.1371/journal.pone.0070097.s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Zheng, Feng Dan, Fang Bin, Mullican Shannon E, You Seo-Hee, Lim Hee-Woong, … Lazar Mitchell A. Deacetylase-Independent Function of HDAC3 in Transcription and Metabolism Requires Nuclear Receptor Corepressor. Molecular Cell. 2013;52(6):769–782. doi: 10.1016/j.molcel.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Murata Y, Sadow P, Hayashi Y, Seo H, Xu JM, … Refetoff S. Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of T-3-regulated transcription of genes in vivo. Endocrinology. 2002;143(4):1346–1352. doi: 10.1210/en.143.4.1346. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Porcu E, Chew S, Campbell PJ, Traglia M, Brown SJ, … Wilson SG. Whole-genome sequence-based analysis of thyroid function. Nature Communications. 2015;6 doi: 10.1038/ncomms6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic-Arsic M, Muller J, Darras VM, Groba C, Lee S, Weih D, … Heuer H. Impact of Monocarboxylate Transporter-8 Deficiency on the Hypothalamus-Pituitary-Thyroid Axis in Mice. Endocrinology. 2010;151(10):5053–5062. doi: 10.1210/en.2010-0593. [DOI] [PubMed] [Google Scholar]
- Van Herck Stijn LJ, Delbaere Joke, Bourgeois Nele MA, McAllan Bronwyn M, Richardson Samantha J, Darras Veerle M. Expression of thyroid hormone transporters and deiodinases at the brain barriers in the embryonic chicken: Insights into the regulation of thyroid hormone availability during neurodevelopment. General and Comparative Endocrinology. 2015;214(C):30–39. doi: 10.1016/j.ygcen.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Vatine Gad David, Zada David, Lerer-Goldshtein Tali, Tovin Adi, Malkinson Guy, Yaniv Karina, Appelbaum Lior. Zebrafish as a model for monocarboxyl transporter 8-deficiency. The Journal of biological chemistry. 2012;288(1):169–180. doi: 10.1074/jbc.M112.413831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella Kristen R, Ramadoss Preeti, Costa-e-Sousa Ricardo H, Astapova Inna, Ye Felix D, Holtz Kaila A, … Hollenberg Anthony N. Thyroid Hormone Signaling In Vivo Requires a Balance between Coactivators and Corepressors. Molecular and Cellular Biology. 2014;34(9):1564–1575. doi: 10.1128/mcb.00129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeminck-Guillem V, Espiard S, Flamant F, Wemeau JL. TR alpha receptor mutations extend the spectrum of syndromes of reduced sensitivity to thyroid hormone. Presse Medicale. 2015;44(11):1103–1112. doi: 10.1016/j.lpm.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Warner A, Rahman A, Solsjo P, Gottschling K, Davis B, Vennstrom B, … Mittag J. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor alpha 1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(40):16241–16246. doi: 10.1073/pnas.1310300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, … Yoshimura T. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2007;292(1):R568–R572. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- Waung Julian A, Bassett JH Duncan, Williams Graham R. Adult Mice Lacking the Type 2 Iodothyronine Deiodinase Have Increased Subchondral Bone but Normal Articular Cartilage. Thyroid : official journal of the American Thyroid Association. 2015;25(3):269–277. doi: 10.1089/thy.2014.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RE, Xu JM, Ning G, Pohlenz J, O’Malley BW, Refetoff S. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. Embo Journal. 1999;18(7):1900–1904. doi: 10.1093/emboj/18.7.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneck-de-Castro Joo Pedro, Fonseca Tatiana L, Ueta Cintia B, McAninch Elizabeth A, Abdalla Sherine, Wittmann Gbor, … Bianco Antonio C. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. The Journal of clinical investigation. 2015;125(2):769–781. doi: 10.1172/JCI77588DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth EK, Rijntjes E, Meyer F, Kohrle J, Schweizer U. High T3, Low T4 Serum Levels in Mct8 Deficiency Are Not Caused by Increased Hepatic Conversion through Type I Deiodinase. European thyroid journal. 2015;4(Suppl 1):87–91. doi: 10.1159/000381021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann Gbor, Szabon Judit, Mohcsik Petra, Nouriel Shira S, Gereben Balzs, Fekete Csaba, Lechan Ronald M. Parallel Regulation of Thyroid Hormone Transporters OATP1c1 and MCT8 During and After Endotoxemia at the Blood-Brain Barrier of Male Rodents. Endocrinology. 2015;156(4):1552–1564. doi: 10.1210/en.2014-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysokinski A, Kloszewska I. Level of Thyroid-Stimulating Hormone (TSH) in Patients with Acute Schizophrenia, Unipolar Depression or Bipolar Disorder. Neurochemical Research. 2014;39(7):1245–1253. doi: 10.1007/s11064-014-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM. Molecular basis of resistance to thyroid hormone. Trends in Endocrinology and Metabolism. 2003;14(7):327–333. doi: 10.1016/s1043-2760(03)00114-0. [DOI] [PubMed] [Google Scholar]
- Yi P, Wang Z, Feng Q, Pintilie GD, Foulds CE, Lanz RB, … O’Malley BW. Structure of a Biologically Active Estrogen Receptor-Coactivator Complex on DNA. Molecular Cell. 2015;57(6):1047–1058. doi: 10.1016/j.molcel.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You SH, Lim HW, Sun Z, Broache M, Won KJ, Lazar MA. Nuclear receptor co-repressors are required for the histone-deacetylase activity of HDAC3 in vivo. Nature Structural\& Molecular Biology. 2013;20(2):182–187. doi: 10.1038/nsmb.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M, Chen G, Pan CM, Gu ZH, Zhao SX, Liu W, … Song HD. Genome-wide association study identifies a novel susceptibility gene for serum TSH levels in Chinese populations. Human Molecular Genetics. 2014;23(20):5505–5517. doi: 10.1093/hmg/ddu250. [DOI] [PubMed] [Google Scholar]