Abstract
Background
Interleukin-15 is a pleiotropic cytokine that is critical for the development and survival of multiple hematopoietic lineages. Mice lacking IL-15 have selective defects in populations of several pro-allergic immune cells including natural killer (NK) cells, NKT cells, and memory CD8+T cells. We therefore hypothesized that IL-15−/− mice will have reduced inflammatory responses during the development of allergic airway disease (AAD).
Objective
To determine whether IL-15−/− mice have attenuated allergic responses in a mouse model of AAD.
Methods
C57BL/6 wild-type (WT) and IL-15−/− mice were sensitized and challenged with ovalbumin (OVA) and the development of AAD was ascertained by examining changes in airway inflammatory responses, Th2 responses, and lung histopathology.
Results
Here we report that IL-15−/− mice developed enhanced allergic responses in an OVA-induced model of AAD. In the absence of IL-15, OVA-challenged mice exhibited enhanced bronchial eosinophilic inflammation, elevated IL-13 production, and severe lung histopathology in comparison with WT mice. In addition, increased numbers of CD4+T and B cells in the spleens and broncholaveolar lavage (BAL) were also observed. Examination of OVA-challenged IL-15Rα−/− animals revealed a similar phenotype resulting in enhanced airway eosinophilia compared to WT mice. Adoptive transfer of splenic CD8+T cells from OVA-sensitized WT mice suppressed the enhancement of eosinophilia in IL-15−/− animals to levels observed in WT mice, but had no further effects.
Conclusion and Clinical Relevance
These data demonstrate that mice with an endogenous IL-15 deficiency are susceptible to the development of severe, enhanced Th2-mediated AAD, which can be regulated by CD8+T cells. Furthermore, the development of disease as well as allergen-specific Th2 responses occurs despite deficiencies in several IL-15-dependent cell types including NK, NKT, and γδ T cells, suggesting that these cells or their subsets are dispensable for the induction of AAD in IL-15-deficient mice.
INTRODUCTION
Allergic airway disease (AAD) is a chronic inflammatory disease of the lung, characterized by bronchial airway inflammation, reversible airway obstruction, bronchial hyperreactivity, mucus plugging, and airway remodeling. Although CD4+T cells of the Th2 phenotype and their production of the cytokines IL-4, IL-5 and IL-13 are considered pivotal in the development of AAD, it is now well established that both innate and adaptive components of the immune response contribute to the overall manifestation of the disease in mice and humans [1–3]. Accordingly, innate effector cells such as innate lymphoid cells, NK cells, NKT cells, and γδ T cells have all been implicated in the development of AAD in murine experimental systems [4–9].
One cytokine essential to both innate and adaptive immune responses is IL-15. IL-15 is a member of the common γ chain (γC) cytokine family and has specific effects on the regulation of hematopoietic lineages [10–12]. It plays a critical role in the development, maturation, and homeostasis of NK and NKT cells [13–22] and also promotes the activation of dendritic cells (DCs) [23]. In addition, the cytokine helps regulate the homeostasis and survival of peripheral pools of memory CD8+T cells [24–29]. Mice lacking IL-15 (IL-15−/− mice) or its specific private receptor IL-15Rα (IL-15Rα−/− mice) have selective defects in the generation of NK and NKT cells, memory CD8+T cells, subsets of γδT cells, and intestinal intraepithelial lymphocytes [30, 31]. We have previously demonstrated a proinflammatory role for NK cells in asthma [6], and since NKT cells, γδ T cells, and CD8+T cells have all been shown to induce allergic disease, we hypothesized that potential deficiencies of these cell types or their subsets in IL-15−/− mice may attenuate the manifestations of AAD in these animals.
The present study investigated the development of AAD in IL-15−/− and IL-15Rα−/− mice using a well-characterized OVA-sensitization and challenge model [9, 32]. Contrary to expectations, our results demonstrate that in the absence of IL-15, IL-15−/− and IL-15Rα−/− mice demonstrated enhanced AAD consisting of airway eosinophilia and lung histopathology, suggesting that endogenous IL-15 is not required for the development of AAD. Furthermore, the development of allergic inflammation in IL-15−/− mice was accompanied by a robust Th2-mediated response including increases in the numbers of CD4+T cells and B cells, elevated levels of Th2 cytokines, and the presence of OVA-specific IgE antibodies, suggesting that the induction of allergen-specific Th2 responses can occur in these animals despite known deficiencies in pro-allergic innate cell types such as NK and NKT cells.
MATERIALS AND METHODS
Animals
Animals used for this study include adult male and female IL-15−/− and IL-15Rα−/− mice (kindly provided by Dr. Leo Lefrancois, University of Connecticut Health Center, CT) and C57BL/6J (B6) mice (purchased from The Jackson Laboratory, Bar Harbor, ME). All mice were bred and maintained in accordance with respective Institutional Animal Care and Use Committee- approved protocols at the University of Connecticut Health Center (animal protocol numbers 97-044 and 2000-005-03) or at Western New England University (animal protocol number 2014-S1).
Animal model of AAD
We have previously described an OVA-induced model of murine AAD [32]. Briefly, mice were sensitized with three weekly i.p. injections of 25 μg OVA, grade V (Sigma, St. Louis, MO) and 2 mg of alum (Aluminium hydroxide) suspended in saline. One week after the last injection, animals were placed in a nose only exposure chamber and challenged with a 1% OVA aerosol generated by a BANG nebulizer (CH Technologies, Westwood, NJ) for 1 hr. for 3 or 7 days as indicated in the text. The following experimental groups were used: OVA-OVA mice (sensitized and aerosol challenged with OVA); SAL-OVA (sensitized with saline and aerosol challenged with OVA), OVA i.p. (sensitized but not aerosol challenged with OVA) and naive mice (neither sensitized nor aerosol challenged with OVA). Animals were sacrificed 24 hours after the last OVA-aerosol challenge, and cells in broncho-alveolar lavage (BAL), spleen and lung were analyzed.
Isolation of Cells
Animals were weighed, anesthetized and euthanized by cardiac exsanguination and/or perfusion when necessary. Organs were obtained and processed as follows: spleens were mashed between frosted glass slides and washed with phosphate buffered saline (PBS); BAL was obtained by lavaging the lungs with five 1 ml aliquots of PBS; whole lungs were minced after BAL isolation and lung cells were obtained after treatment with 1.3 mM EDTA in HBSS at 37oC for 30 minutes with occasional shaking, followed by digestion with 100 U/ml collagenase (Life Technologies, Rockville, MD) in RPMI containing 1 mM MgCl2, 1 mM CaCl2, and 5% FCS, at 37°C for 1 hour. After suspensions were made, erythrocytes were lysed by hypotonic shock and leukocytes were resuspended in staining medium (SM) containing Hank’s balanced salt solution (HBSS) supplemented with HEPES buffer pH 7.2, at a final concentration of 10mM and FCS at a final concentration of 2%. Total leukocytes were enumerated with a hemocytometer and viability was assessed on the basis of trypan blue exclusion.
Cell Surface Phenotypic Analysis
Leukocytes from spleen, lung, and BAL were analyzed by flow cytometry for cell surface markers. Cells were stained with monoclonal antibodies (mAbs) reactive against different hematopoietic cell surface molecules, and conjugated with either fluorescein isothiocyanate, phycoerythrin or allophycocyanin: NK1.1 (clone PK136), CD3 (clone 145-2C11), DX5, CD4 (clone RM4–5), CD8 (clone 53–6.7), B220/CD45R (clone RA3-6B2), and CD11b (clone M1/70) (BD Pharmingen, San Diego, CA) and incubated on ice for 30 minutes. Cells were washed and resuspended in SM containing propidium iodide to differentiate between live and dead cells. Live cells were gated and the percentages of NK, NKT, CD3, CD4 and CD8 -positive T cells were enumerated. NK cells were characterized as NK1.1+ and CD3−. Various subsets of NK cells were characterized using additional markers such as B220 and CD11b (Mac-1). NKT cells were characterized phenotypically as NK1.1+CD3+ cells. Fluorescence was evaluated using a FACS Calibur analyzer or Accuri C6 and data was processed using CellQuest and FlowJo Software. Lymphocyte gates were designated on the basis of forward and side scatter analysis.
Evaluation of AAD parameters
Total Leukocyte Counts
Total leukocyte counts were obtained from the BAL, lung tissue, and spleen and compared between various groups. Differential cellular analysis of BAL fluid was performed and percentages of eosinophils, macrophages, lymphocytes, and neutrophils were enumerated using cytocentrifuged preparations stained with May-Grunwald/Giemsa.
Determination of pulmonary function
Pulmonary function of OVA-OVA mice was assessed at baseline (after OVA i.p. injections but prior to OVA-aerosol challenge) and 12 hours after 3 days of OVA-aerosol challenge in awake, unrestrained mice by whole-body barometric plethysmography. Briefly, mice were placed in the main chamber of a whole-body plethysmograph (Buxco Electronics, Sharon, CT) and exposed for 2 min to aerosolized saline or increasing concentrations of methacholine from 3 to 300 mg/ml. Respiratory system variables including tidal volume, respiratory frequency, inspiratory/expiratory times, and changes in box pressure were recorded before and during aerosolization and for 4 min after each exposure. The maximal enhanced pause (Penh) value response to methacholine was recorded at each dose.
Histology
Airway inflammation was assessed by performing standard lung histology. Briefly, unmanipulated, noninflated lung tissue was removed from animals at the time of sacrifice, fixed in a 10% buffered formalin solution, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) for general morphology and diastase-periodic acid-Schiff (PAS) for the detection of mucins. Digital images of lung sections were captured using a Carl Zeiss (Thornwood, NY) Axioplan 2 microscope, an Axiocam HR digital camera, and Axiovision software. Pathological scoring for inflammation and mucus production was performed in a blinded manner by five reviewers on a severity scale of 0–3 as previously described [33]. For inflammation scores, a value of ‘0’ was assigned when no inflammation was detectable, ‘1’ for mild peribronchiolar/perivascular inflammation, ‘2’ for significant peribronchiolar/perivascular clustering and ‘3’ for significant clustering and airway remodeling. For mucus scores, a value of ‘0’ was assigned when no mucus was present, ‘1’ for occasional and punctate mucus staining in the airways, ‘2’ for the presence of ring-like mucus structures in <10% of the airways and ‘3’ for the presence of ring-like mucus structures in >10% of the airways.
Antibody Determination
Venous blood samples were obtained by cardiac puncture and analyzed for the presence of OVA-specific IgE antibody. IgE was captured from diluted serum using Costar high binding RIA plates (Corning Inc, Corning, NY) coated with anti-mouse IgE (R35–72; BD Pharmingen, San Diego, CA) at 2 μg/ml in 0.1 mol/L carbonate, pH 9.5. Detection was with an OVA-digoxigenin conjugate followed by horseradish peroxidase-conjugated anti-digoxigenin [9]. Plates were developed with the TMB microwell peroxidase substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, MD). IgE standard was obtained from the supernatant of an IgE producing clone.
Measurement of Cytokines
BAL fluid from control and experimental groups was concentrated 10-fold using an Amicon (Beverly, MA) centriplus YM-10 filtration device and examined by ELISA for the presence of IL-4 and IL-5 (BD Pharmingen) and IL-13 (R & D Systems, Minneapolis, MN). Cytokine concentrations are represented as total amount present in the BAL fluid.
ELISPOT Analysis
Cells secreting IFN-γ and IL-4 in an Ag-specific manner were detected using a standard ELISPOT assay as previously described [6].
Adoptive Transfer Experiments
Mice, including donors, recipients, and controls were sensitized twice with OVA and alum as described above. One day prior to challenge, spleens were harvested from donor WT C57BL/6 mice and CD8+T cells were isolated using negative selection and magnetic beads (Miltenyi Biotec). Approximately, 2.2 million cells were transferred i.v. into recipient WT and IL-15−/− animals. All mice, including recipients and controls were then challenged with 1% nebulized OVA and sacrificed as described above.
Statistical Analysis
Experimental groups were compared to each other by Student’s t test. Multiple group comparisons were done using ANOVA. Values of p equal or < 0.05 were considered significant. All data are expressed as the mean ± SEM.
RESULTS
Enhanced eosinophilic inflammation in IL-15−/− OVA-OVA mice during OVA-AAD
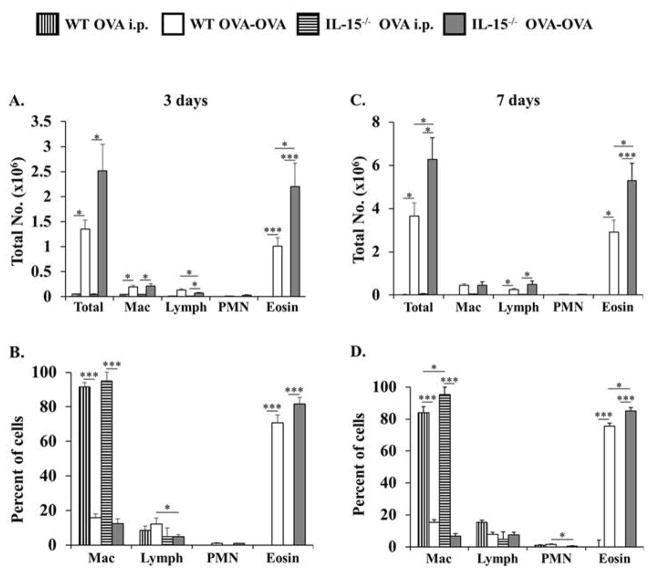
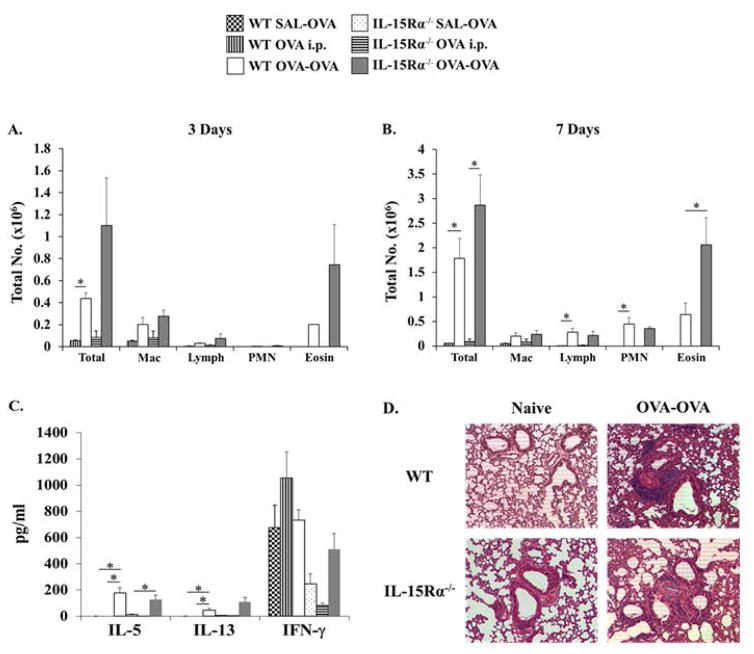
We followed the development of OVA-AAD in IL-15−/− mice after 3 and 7 days of OVA-aerosol challenge. At these time points, BAL collected from naïve, OVA i.p., or SAL-OVA mice showed no inflammation and consisted predominantly of macrophages with less than 2% lymphocytes, neutrophils and eosinophils (data not shown). After 3 days of OVA-aerosol challenge, an acute inflammation was seen in the BAL fluid of both WT and IL-15−/− OVA-OVA mice (Fig. 1A). The inflammatory infiltrate consisted of greater than 80% eosinophils along with lower percentages of macrophages, lymphocytes and neutrophils (Fig. 1B). Interestingly, although there were no differences in the distribution of leukocytes between the two groups, a higher number of inflammatory cells was recovered from the BAL of IL-15−/− OVA-OVA mice as compared with the WT controls (Figs. 1A&B). Similar results were obtained after 7 days of OVA-aerosol challenge. At this time point too, IL-15−/− OVA-OVA mice had a much higher number of inflammatory cells as well as elevated numbers of eosinophils in the BAL as compared with WT controls (Figs. 1C&D). These observations therefore suggested that IL-15−/− mice develop enhanced eosinophilia during OVA-AAD.
Figure 1. IL-15−/− OVA-OVA mice develop enhanced eosinophilia during OVA-AAD.
BAL was obtained 24 hrs. after 3 and 7 days of OVA-aerosol challenge from animals sensitized and challenged with OVA (OVA-OVA) and naïve, SAL-OVA, or OVA i.p. controls. Total numbers of BAL leukocytes were enumerated and the extent of eosinophilic inflammation was assessed by determining relative numbers of macrophages (Mac), lymphocytes (Lymph), polymorphonuclear neutrophils (PMN) and eosinophils (Eosin) using differential analysis. (A&B) Absolute numbers and percentages of cells recovered from BAL of WT and IL-15−/− OVA-OVA and OVA i.p. mice after 3 days of OVA-aerosol challenge are shown. (C&D) Absolute numbers and percentages of cells recovered from BAL of WT and IL-15−/− OVA-OVA and OVA i.p. mice after 7 days of OVA-aerosol challenge are shown. Data is representative of 3 or more experiments. n=14. * = p < 0.05; *** = p < 0.001.
Changes in airway hyperresponsiveness during the development of OVA-AAD
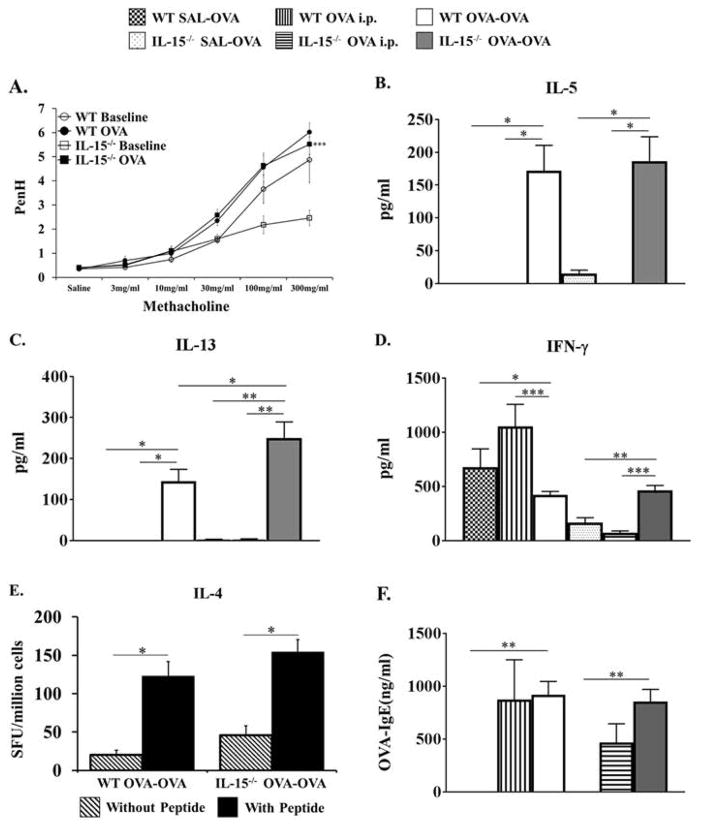
The ability of IL-15−/− OVA-OVA mice to develop enhanced inflammatory responses during OVA-AAD was further evaluated by assessing changes in airway sensitivity to inhaled methacholine (Fig. 2A). Prior to OVA-aerosol challenges, paired WT and IL-15−/− mice both demonstrated similar Penh responses to inhaled methacholine with a slightly enhanced response observed in WT mice at higher doses (Fig. 2A). Following 3 OVA-aerosol challenges, both WT and IL-15−/− animals exhibited enhanced methacholine sensitivity compared with pre-OVA exposure, with similar increases in Penh observed to increasing doses of the bronchoconstrictor (Fig. 2A). These data therefore indicated that there is no difference in airway hyperresponsiveness between OVA-sensitized and challenged WT and IL-15−/− mice in this model, suggesting that endogenous IL-15 is not required for the development of airway hyperresponsiveness during OVA-AAD.
Figure 2. Evaluation of lung function changes, cytokine responses, and OVA-IgE levels in allergic mice.
(A) OVA-sensitized WT and IL-15−/− mice were exposed to increasing doses of methacholine prior to (baseline) and after 3 days of OVA-aerosol challenge (OVA) and airway hyperresponsiveness was assessed by non-invasive whole body plethysmography. Values for PenH were calculated and are shown on the y-axis. ***=p<0.01 by ANOVA between baseline and OVA groups (B–D) Cytokines were measured in the BAL of WT and IL-15−/− mice after 3 days of OVA-aerosol challenge by ELISA. Levels of (B) IL-5, (C) IL-13 and (D) IFN-γ are shown. (E) Systemic production of IL-4 from splenic cells from WT and IL-15−/− OVA-OVA mice was evaluated by ELISPOT after 7 days of OVA-aerosol challenge. Data are represented as spot forming units per million CD4+ T cells. Production of IL-4 from unstimulated (hatched bars) and OVA peptide stimulated cells (solid bars) is shown. (F) Serum OVA-specific IgE as determined by ELISA after 7 days of OVA-aerosol challenge is shown. OVA i.p. control groups are shown and are similar to SAL-OVA mice. Data are representative of 3 or more experiments. n = 7–14. * = p < 0.05; ** = p < 0.01; **=p<0.001.
Increased levels of IL-13 in BAL of IL-15−/− OVA-OVA mice
In order to determine the nature of the BAL inflammation and evaluate systemic responses during OVA-AAD, cytokines were measured in the BAL (Fig. 2B–D) and splenic cells were examined for the presence of IL-4-producing T cells (Fig. 2E). After 3 days of OVA-aerosol challenge, increased levels of the cytokines IL-5, IL-13, and IFN-γ were detected in the BAL of WT and IL-15−/− OVA-OVA mice as compared with OVA i.p. and SAL-OVA controls (Fig. 2B–D). Interestingly, while there were no differences in the levels of IL-5 (Fig. 2B) and IFN-γ (Fig. 2D) between the two groups, the levels of IL-13 were significantly increased in IL-15−/− OVA-OVA mice as compared with WT OVA-OVA mice (Fig. 2C). Elevated levels of IL-13 were also found in the BAL of IL-15−/− OVA-OVA mice after 7 days of OVA-aerosol challenge (data not shown).
The ELISPOT assay was used to determine the systemic nature of the cytokine response during the progression of OVA-AAD. Splenic cells were isolated from OVA-OVA mice and their T cell IL-4 production capacity was evaluated in the presence of specific OVA peptide (Fig. 2E). After 7 days of OVA-aerosol challenge, splenic cells from both WT and IL-15−/− OVA-OVA mice were able to make equivalent levels of IL-4 in response to OVA peptide restimulation. After 3 days of OVA-aerosol challenge, splenic IL-4 production could not be detected in either group examined (data not shown). The production of IFN-γ using the ELISPOT assay was also assessed, but no differences were observed (data not shown).
Systemic Antibody responses during OVA-AAD
The systemic nature of the immune response was further evaluated by enumerating the levels of serum OVA-specific IgE after 3 and 7 days of OVA-aerosol challenge. At these time points, equivalent levels of OVA-specific IgE were recovered from the serum of both WT and IL-15−/− OVA-OVA groups and no significant increases were observed in either group compared to OVA i.p. mice. (Fig. 2F and data not shown). No OVA-IgE was recovered from naïve or SAL-OVA animals from both groups (data not shown).
Increased CD4+T cells and B cells in IL-15−/− OVA-OVA mice during OVA-AAD
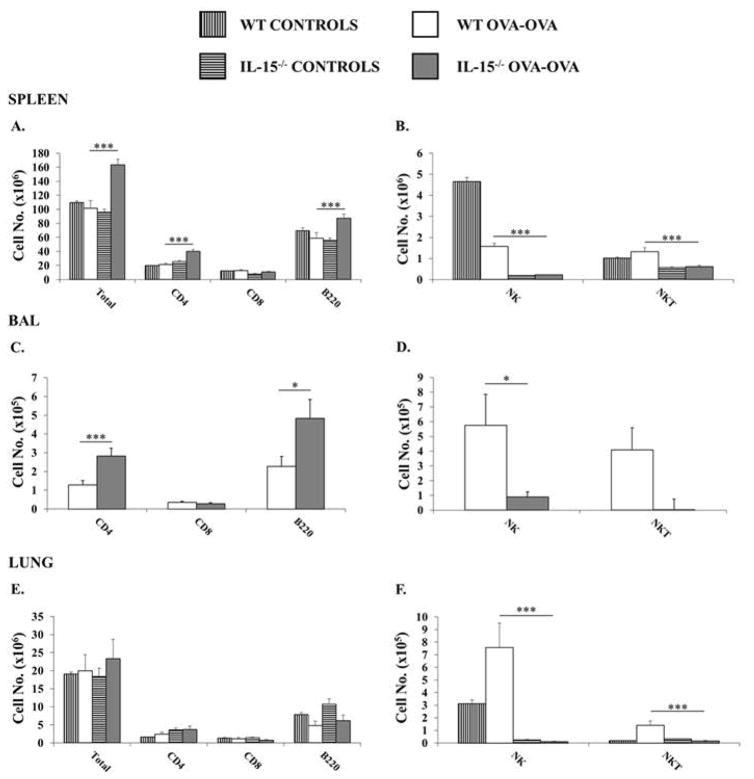
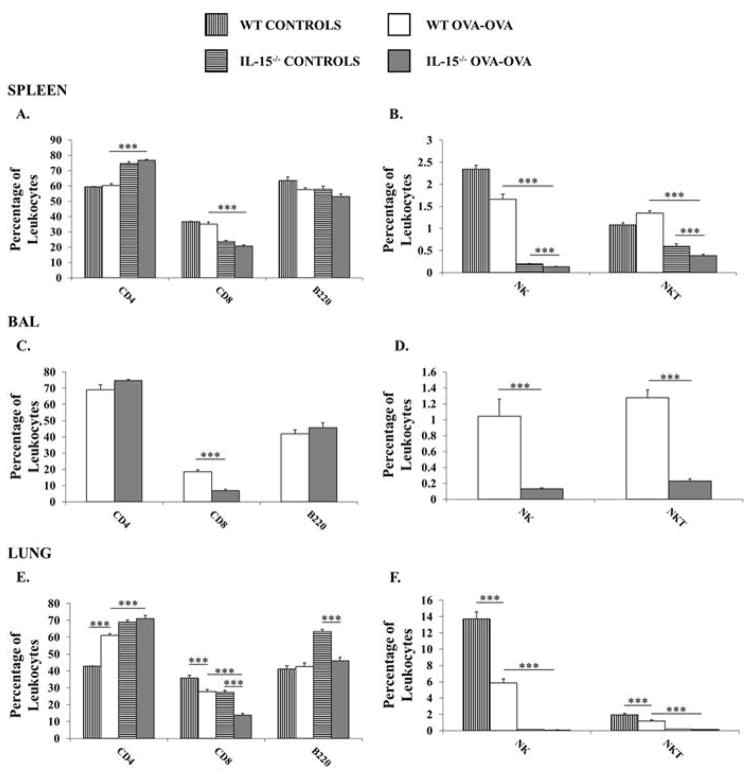
Since IL-15−/− mice are deficient in certain lymphocyte sub-types, we evaluated the lymphocyte compartment in the spleen, lung, and BAL to determine if there were any changes in the absolute numbers (Fig. 3) and percentages (Fig. 4) of lymphocyte populations during the development of OVA-AAD. Examination of naïve, SAL-OVA or OVA i.p. mice revealed profound deficiencies in the percentages of NK and NKT cells in the spleens and lungs of IL-15−/− mice (Supplementary Fig. 1). Very few cells were recovered from the BAL of control animals to allow accurate enumeration by flow cytometry. After 7 days of OVA-aerosol challenge, increased total numbers of cells were found in the spleens of IL-15−/− OVA-OVA mice in comparison to both WT OVA-OVA mice and saline-sensitized or unchallenged controls (naïve, SAL-OVA and OVA i.p. mice are represented as one group since they exhibited a similar phenotype in both animal strains) (Fig. 3A). The increase in spleen total numbers in IL-15−/− OVA-OVA mice was also accompanied by an increase in the absolute number of CD4+T and B cells, along with significantly decreased numbers and percentages of CD8+T cells as well as NK (1.66±0.1 vs. 0.135±0.01 %) and NKT (1.34±0.05 vs. 0.38±0.03 %) cells in comparison with similarly treated WT animals and corresponding controls (Fig. 3A–B & Fig. 4A–B). A similar phenotype was observed in the BAL of IL-15−/− OVA-OVA mice with increased numbers of CD4+T cells and B cells observed along with decreased numbers and percentages of NK and NKT cells in comparison with WT OVA-OVA mice. (Fig. 3C–D & Fig. 4C–D). In the lungs, equivalent numbers of total cells were seen in both WT and IL-15−/− OVA-OVA mice (Fig. 3E). As observed in the spleen and as expected, CD8+T cells, NK (5.85±0.5 vs. 0.08±0.004 %) and NKT (1.2±0.1 vs. 0.14±0.02 %) cells were also decreased in the lungs of IL-15−/− OVA-OVA mice as compared with WT OVA-OVA mice (Fig. 3E–F & Fig. 4E–F). Lastly, although the percentages of CD4+T cells were increased in the lungs of IL-15−/− OVA-OVA mice, this did not correlate with an increase in absolute numbers of CD4 T cells (Fig. 4E–F). These data therefore suggested that the dramatic increase in eosinophilia in OVA-challenged IL-15−/− mice was also accompanied by an increase in CD4+T cells and B cells in the spleen and BAL of these animals.
Figure 3. IL-15−/− OVA-OVA mice exhibit increased numbers of CD4+T cells and B cells in the spleens and BAL during OVA-AAD.
Leukocytes were obtained from spleens, collagenase digested lungs, and BAL of experimental animals and total numbers of T, B, NK and NKT cells were enumerated by flow cytometry. Total numbers of live (propidium iodide-negative) CD3+CD4+, CD3+ CD8+, B220+, NK1.1+ and NK1.1+CD3+ cells in the (A&B) spleen (C&D) BAL and (E&F) lung are shown. Controls represent naïve, SAL-OVA and OVA i.p. animals. Data are representative of 3 or more experiments. n =5. * = p < 0.05; *** = p < 0.001.
Figure 4. Distribution of lymphocytes in IL-15−/− mice during OVA-AAD.
Leukocytes were obtained from spleens, collagenase digested lungs, and BAL of experimental animals and percentages of T, B, NK and NKT cells were enumerated by flow cytometry. Percentages of live (propidium iodide-negative) CD3+CD4+, CD3+ CD8+, B220+, NK1.1+ and NK1.1+CD3+ cells in the (A&B) spleen (C&D) BAL and (E&F) lung are shown. Controls represent naïve, SAL-OVA and OVA i.p. animals. Data are representative of 3 or more experiments. n =5. *** = p < 0.001.
Enhanced Lung Histopathology in IL-15−/− OVA-OVA mice
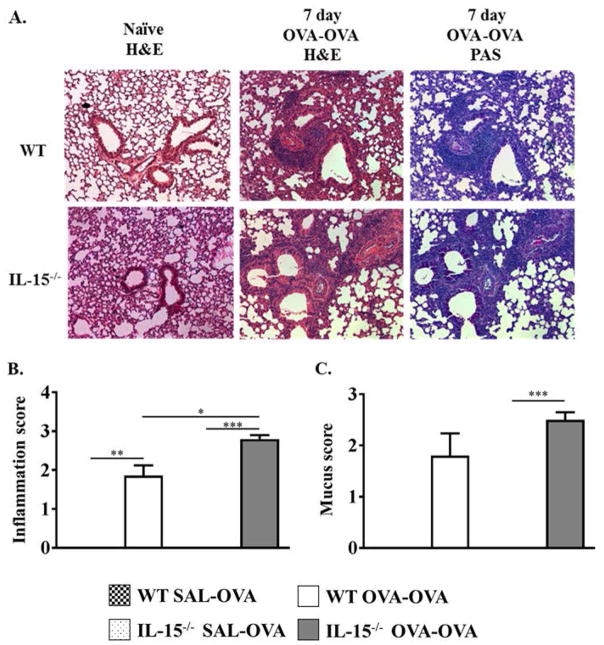
To further assess whether the dramatic increase in eosinophilia in IL-15−/− mice translated to severe changes in lung pathology in OVA-exposed animals, qualitative histological analysis of lung tissue was performed. The lungs from naïve non-OVA exposed mice appeared normal without any remarkable pathologic changes (Fig. 5A, Top Left Panel). As observed previously [32], after 7 days of OVA-aerosol challenge, WT OVA-OVA mice had significant peribronchial and perivascular inflammation in the lung tissue characterized by an infiltration of lymphocytes and eosinophils (Fig. 5A, Top Middle Panel). Similarly, IL-15−/− OVA-OVA mice also developed extensive peribronchial and perivascular inflammation which appeared to be significantly enhanced as compared with WT OVA-OVA mice (Fig. 5A, Bottom Middle Panel). Lungs from IL-15−/− naïve mice appeared normal for the most part without any remarkable pathologic changes (Fig. 5A, Bottom Left Panel). However, there were noticeable small focal areas of inflammation suggesting some minor level of alveolar inflammation. IL-15−/− OVA-OVA mice also showed the presence of mucus secreting cells by PAS stain and this also appeared to be enhanced to that observed in WT OVA-OVA controls (Fig. 5, Top and Bottom Right Panels). Pathological scoring of histological sections by blinded, independent observers confirmed these observations with increased lung damage observed in IL-15−/− OVA-OVA mice as compared to WT OVA-OVA animals (Fig. 5B). In contrast, quantitative scoring of the degree of mucus staining suggested that both groups exhibited similar levels of mucus staining (Fig. 5C). These observations therefore confirm the results obtained from BAL differential analysis demonstrating that IL-15−/− mice have enhanced airway inflammation during the development of OVA-AAD.
Figure 5. Comparison of OVA-AAD by Qualitative Histological Analysis.
(A) Lung histology of normal naïve mice (Left) and OVA-OVA mice 7 days after OVA-aerosol challenge (Middle and Right). The lungs were not inflated, formalin fixed and stained with hematoxylin and eosin (H&E) stain and Periodic Acid Schiff (PAS) stain. The Top Panel represents WT mice, and the Bottom Panel IL-15−/− mice. (B–C) Histological samples were analyzed blind by 3–5 separate persons and scored from 0 to 3+ depending on the severity of the inflammation observed. Statistical analysis of raw scores obtained was used to derive the (B) Inflammation score as well as (C) Mucus score, which are shown. n = 5. * = p < 0.05; ** = p < 0.01; **=p<0.001.
IL-15Rα−/− animals also exhibit enhanced airway eosinophilia during OVA-AAD
The secretion of IL-15 in the serum is tightly regulated and the homeostasis of IL-15-dependent cell types such as NK and memory CD8+T cells is thought to be regulated by transpresentation of IL-15 by IL-15Rα on accessory cells. [12, 18, 19, 22, 34–39]. Since IL-15−/− mice exhibited enhanced airway responses to OVA challenge, we wondered whether a similar phenotype would be observed in IL-15Rα−/− mice, which are deficient in IL-15Rα, the specific private receptor for IL-15. Like IL-15−/− animals, these mice also exhibit profound deficiencies in NK, NKT, and memory CD8+T cells as described above. OVA-sensitization and challenge of IL-15Rα−/− mice resulted in enhanced bronchial inflammation at both 3 and 7 days after OVA-aerosol challenge. Differential analysis of the BAL fluid revealed the presence of infiltrates comprising of a majority of eosinophils accompanied by lymphocytes and some macrophages (Fig. 6A&B). This suggested that OVA-challenged IL-15Rα−/− animals also develop enhanced eosinophilia as compared to WT OVA-OVA mice and as observed in their IL-15−/− counterparts. Evaluation of other parameters of AAD such as airway hyperresponsiveness and BAL Th2 cytokine production levels also yielded similar results to those observed in IL-15−/− animals. These included a tendency to develop elevated BAL IL-13 in comparison to allergic WT mice (Fig. 6C) as well as a comparable degree of sensitivity to increasing doses of inhaled methacholine exposure (data not shown). Lastly, examination of lung histological sections revealed the presence of severe lung inflammation in OVA-challenged IL-15Rα−/− mice. However, the degree of inflammation was comparable to that observed in WT OVA-OVA mice in terms of both the intensity and type of cellular inflammatory response as well as the presence of mucus in the airways (Fig. 6D). These data therefore suggested that IL-15Rα−/− mice also exhibit enhanced airway eosinophilia and develop severe lung inflammation during OVA-AAD.
Figure 6. IL-15Rα−/− OVA-OVA mice develop enhanced airway eosinophilia during OVA-AAD.
(A–B) BAL was obtained from experimental animals after (A) 3 and (B) 7 OVA-aerosol challenges and differential analysis was performed. Total numbers of BAL cells including macrophages, lymphocytes, neutrophils and eosinophils in OVA i.p. and OVA-OVA mice are shown. (C) Cytokines were evaluated in the BAL of experimental mice 3 days after OVA-aerosol challenge by ELISA. Levels of IL-5, IL-13, and IFN-γ are shown. (D) H&E-stained lung histological sections were evaluated qualitatively for assessment of lung damage. (E) Histological samples were scored blind by 3 separate persons. Statistical analysis of raw scores is depicted as Inflammation score. n = 5–7. * = p < 0.05.
Partial suppression of eosinophilia in IL-15−/− OVA-OVA mice by OVA-sensitized WT CD8+T cells
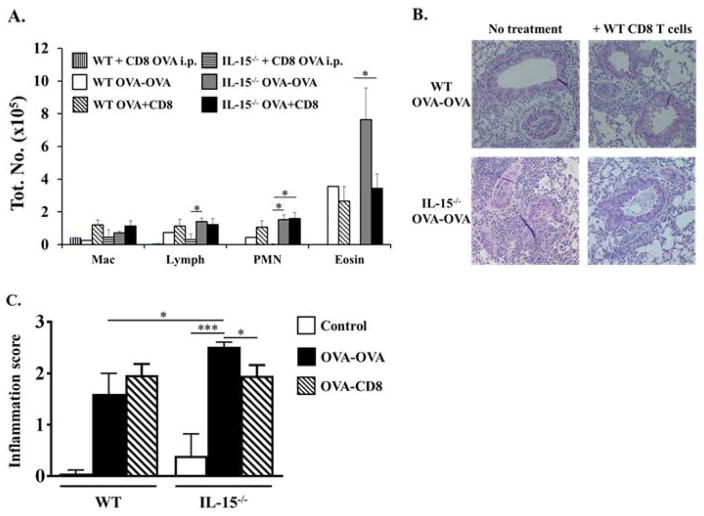
Since we observed decreased percentages of CD8+T cells in IL-15−/− mice during OVA-AAD and these mice are known to be deficient in populations of memory CD8+T cells, we wondered whether the allergic phenotype observed in these animals may be due to a lack of immunoregulation by CD8+T cells. The role of CD8+T cells during AAD is controversial with several studies suggesting both pro- and anti-inflammatory effects on airway inflammation and airway hyperresponsiveness [40–47]. To determine whether the increased airway eosinophilia observed in IL-15−/− OVA-OVA mice is regulated by CD8+T cells in this model, we assessed whether the adoptive transfer of splenic CD8+T cells from OVA-sensitized WT mice would attenuate the development of allergic inflammation in IL-15−/− animals. Splenic CD8+T cells were isolated from OVA-sensitized WT mice and transferred i.v. into both OVA-sensitized WT and IL-15−/− recipients one day prior to the commencement of OVA challenges. At this time, an increased number of OVA-specific CD8+T cells are present in the spleens of OVA-sensitized WT mice as we have previously shown [48].
Seven days after OVA-challenge, elevated numbers of eosinophils were observed in the BAL of WT OVA-OVA mice as compared to unchallenged controls (Fig. 7A). Furthermore, comparably elevated numbers of eosinophils were also observed in the BAL of WT OVA-OVA recipients of CD8+T cells, suggesting that the adoptive transfer of CD8+T cells has no significant effects on the development of BAL eosinophilia in these mice (Fig. 7A). In contrast, while IL-15−/− OVA-OVA mice developed enhanced airway inflammation and eosinophilia in comparison with WT OVA-OVA mice, adoptive transfer of WT CD8+T cells suppressed the development of BAL eosinophilia in IL-15−/− OVA-OVA recipients to levels observed in WT OVA-OVA mice (Fig. 7A). Qualitative evaluation as well as pathological scoring of lung histological sections similarly revealed increased peribrionchial and perivascular airway inflammation in both untreated and CD8+T cell-treated WT OVA-OVA mice, with enhanced overall inflammation observed in some of the WT CD8+T cell recipients (Fig. 7B&C). In contrast, while IL-15−/− OVA-OVA mice exhibited severe lung histopathology compared with WT OVA-OVA mice, the degree of lung inflammation was slightly reduced in IL-15−/− recipient mice receiving WT CD8+T cells (Fig. 7B&C). These data therefore suggest that antigen-specific CD8+T cells are able to regulate the development of airway eosinophilia in IL-15−/− mice. However, this regulation is incomplete, since Th2 responses and eosinophilia can still be induced in IL-15−/− mice receiving exogenous WT CD8+T cells and in WT mice with a full complement of endogenous CD8+T cells.
Figure 7. Evaluation of airway inflammation in IL-15−/− OVA-OVA mice treated with WT CD8+T cells.
WT and IL-15−/− OVA-OVA mice were treated with OVA-sensitized WT CD8+T cells as described in the Materials and Methods. (A) BAL was obtained from experimental animals 24 h after 7 OVA challenges and differential analysis was performed. Data from OVA-OVA and OVA i.p. animals is shown. (B–C) Lung sections were evaluated histologically to assess the extent of airway inflammation and lung damage. (B) H&E-stained sections and (C) Inflammation score are shown. n=4. * = p < 0.05; **=p<0.001.
DISCUSSION
The present study addressed the role of endogenous IL-15 in the regulation of AAD in a murine model of allergic asthma. During the development of OVA-AAD, IL-15−/− mice showed enhanced allergic responses after both 3 and 7 days of OVA-aerosol challenge, as compared to WT mice. These enhanced responses included increased airway eosinophilia, more severe histopathologic changes, and increased production of the Th2 cytokine IL-13. In addition, IL-15−/− mice also had greater numbers of CD4+T cells and B cells in the spleen and BAL as compared with WT mice during AAD. IL-15Rα−/− mice also developed enhanced airway inflammation in response to OVA-aerosol challenge resulting in increased airway eosinophilia and the production of Th2-specific cytokines. These results indicate that endogenous IL-15 may have anti-inflammatory effects in the development of AAD, and that the activation of IL-15-dependent cell types such as NK cells and NKT cells is not essential for the development of allergic inflammation in IL-15−/− mice.
IL-15 is a pleiotropic cytokine that has multiple effects on various cell types. Although it was initially found to mimic IL-2 due to the shared binding receptor utilized by both cytokines, several studies demonstrate that IL-15 has distinct effects on many different cell types in vivo and makes distinct contributions to the life and death of lymphocytes [24, 49, 50]. Mice lacking IL-15 or its private receptor IL-15Rα have selective defects in the generation of NK and NKT cells, subsets of γδT cells, intestinal intraepithelial lymphocytes and memory CD8T cells [30, 31]. Moreover, through its effects on the activation of NK cells and DCs and its regulation of the homeostasis and survival of peripheral pools of memory cells, IL-15 has critical impacts on the development and regulation of innate and adaptive immune responses [12, 13, 19, 21, 23, 24, 26, 28, 29, 36, 37, 51–53]
We and others have previously demonstrated a proinflammatory role for NK cells in the development of AAD [6]. Since endogenous IL-15 plays a critical role in the expansion and survival of peripheral pools of NK cells, we initially hypothesized that induction of AAD in IL-15−/− animals would result in an attenuation of allergic disease and confirm our previously observed results. Furthermore, IL-15−/− mice also have defects in NKT cells, memory and intraepithelial CD8+T cells, and γδ T cells, each of which has been shown to play critical roles in AAD. Thus, we expected that a combined deficiency of these cell types would prevent the induction of allergic disease in IL-15−/− mice. Our data demonstrate that endogenous IL-15 is not required for the development of asthma, but also suggests that allergic inflammation accompanied by a strong Th2 cell response can develop in the absence of functional populations of IL-15-dependent cell types such as NK, NKT, γδ, and intraepithelial and memory CD8+ T cells. While several of these cell types have been shown to drive distinct aspects of allergic inflammation (including airway eosinophilia as well as airway hyperresponsiveness), our data suggests that allergen-specific CD4+T cells can develop in their absence, and it is likely that the proinflammatory contribution of these cell types to airway inflammation and airway hyperreactivity is dependent on the immune microenvironment including the type of allergen, the route of exposure, the host genetic background, other variables such as viral infections, as well as the appropriate cytokine stimulus. The host genetic background is an especially important consideration since evidence from several studies suggests that the proinflammatory contributions of innate cells can differ depending on the priming regimen and type of mouse strain. For example, while iNKT cells play critical roles in the development of airway inflammation and airway hyperresponsiveness in Th2-prone BALB/c mice [8], they have sometimes been found to be dispensable in an environment that favors Th1 responses, as in C57BL/6 mice [5]. Furthermore, despite profound deficiencies of various cell types in IL-15−/− mice, it is important to be careful in interpreting their respective contributions, since IL-15-independent subsets of functional NK and CD8+T cells have been recently observed in these animals during specific experimental conditions [54, 55]. Lastly, other pro-allergic cell types may also be involved and several recent studies now suggest that the initial cytokine stimulus required to induce allergen-specific Th2 responses is provided by innate cells such as ILC2s [4, 56, 57].
The exacerbation of allergic inflammation due to IL-15 deficiency has been observed in other models as well. Aoi et al. demonstrated the presence of aggravated allergic rhinitis in IL-15−/− mice [58] and Laza-Stanca et al. have shown that IL-15 deficiency in humans is associated with virus-induced asthma exacerbations [59]. Similarly, Ong et al. showed that decreased levels of IL-15 may contribute to elevated IgE and inflammation in subjects with atopic dermatitis, and that the addition of IL-15 to in vitro cultures of peripheral mononuclear cells from atopic dermatitis patients suppresses their production of IgE [60]. Furthermore, haplotype analysis and investigation of IL-15 polymorphisms provide evidence both for and against association of IL-15 gene variants with asthma [61, 62]. In contrast to these observations as well as our own findings in IL-15−/− and IL-15Rα−/− mice, Ruckert et. al. reported that treatment with soluble IL-15Rα prevents the induction of allergen-specific T cells and allergic inflammation, suggesting that the blocking of IL-15 prevents the development of asthma [63]. One reason for this apparent discrepancy may be that these investigators used Th2-biased BALB/c mice in their studies as opposed our C57BL/6 animals, which require a more robust immunization regimen with OVA and alum. Additionally, the use of different model systems such as soluble IL-15Rα versus immunodeficient mice must also be taken into account, since IL-15−/− mice lack not only IL-15, but also several IL-15-dependent cell types which may impact the observed results. Furthermore, occasional naïve IL-15−/− animals exhibited minor alveolar inflammation which could also potentially impact our observations. Taken together, these data therefore suggest a regulatory role for IL-15 in allergic disease.
IL-15 is widely expressed by many cells including epithelial cells, macrophages and DCs, and is produced early during immune responses [10, 11]. IL-15 is also a known inducer of IL-12 and IFN-γ production and is upregulated in many Th1-type inflammatory diseases [64, 65]. Furthermore, IL-15-dependent cell types such as NK and NKT cells as well as CD8+T cells are also major producers of IFN-γ during immune responses. As such, IL-15 deficiency as well as deficiencies in IFN-γ-producing IL-15-dependent cells during AAD, may result in the removal of critical signals that are needed for immune regulation, leading to the development of an exaggerated Th2 inflammatory response. In this regard, Ohteki et al. showed that macrophages and DCs from γC subunit deficient, IL-2/IL-15Rβ deficient or IL-15−/− mice were severely impaired in their capacity to induce microbicidal activity due to impaired production of IL-12, IFN-γ and nitric oxide [23]. They also had defective upregulation of MHC class II and CD40, thus affecting their antigen presenting function. However, in contrast to these results observed in the context of Th1-mediated responses, our data demonstrated that both local lung levels as well as systemic levels of IFN-γ were comparable in WT and IL-15−/− OVA-OVA mice, suggesting that the production of IFN-γ is not affected during Th2-mediated OVA-AAD responses in these animals, and that other IFN-γ producing cells such as subsets of effector CD8+T cells may be present in the lung compartment.
OVA-challenged IL-15−/− mice in this model showed increased levels of the Th2 cytokine IL-13 in BAL during OVA-AAD relative to WT OVA-OVA mice. An inverse correlation between IL-15 and IL-13 has also been observed in other experimental systems [66] and in humans with asthma [67]. These investigators showed that the sputum of asthmatic patients contained higher levels of IL-13 and lower levels of IL-15. However, inhaled corticosteroid treatment of affected subjects resulted in the reverse with increased levels of IL-15 and decreased levels of IL-13[67]. The increased production of IL-13 during Th2 responses has been specifically correlated with both increased airway hyperresponsiveness, as well as, increased mucus production [68]. As such, the increased levels of IL-13 observed during AAD in our IL-15−/− OVA-OVA mice may thus also explain their increased mucus production. In this context, the elucidation of the cellular source of IL-13 production during OVA-AAD is an important consideration and merits further investigation.
During AAD, IL-15−/− mice also showed increased numbers of CD4+T cells and B cells in the spleen and BAL, and decreased numbers of CD8+T cells in the spleen and lung, relative to WT controls. IL-15Rα is a negative regulator of proliferation of CD4+T cells [69]. In the absence of IL-15 signaling, CD4+T cells hyperproliferate when stimulated via the T cell receptor. However, addition of exogenous IL-15 attenuates this proliferation. Accordingly, the exaggerated AAD phenotype in IL-15−/− mice may be due to hyperproliferation of CD4+T cells in our model. Alternately, this finding may also be due to impaired generation or maintenance of CD8+T lymphocytes in the absence of IL-15 [30, 31].
IL-15 transgenic mice overexpress CD8+T cells and preferentially develop Tc1 responses. An attempt to induce OVA-AAD in these transgenic animals resulted in attenuated eosinophilia and Th2 cytokine responses [70]. Depletion of CD8+T cells with an anti-CD8 monoclonal antibody enhanced the development of AAD in these animals, and adoptive transfer of these CD8+T cells to OVA-sensitized WT animals suppressed their eosinophilia [70]. Thus, it is possible that our IL-15-deficient mice failed to receive certain cues that induce Tc1 responses that are important for immune regulation. Similarly, Aoi et al. demonstrated that IL-15−/− mice develop an aggravated form of allergic rhinitis and showed that transfer of OVA-sensitized WT CD8+T cells could suppress inflammation in WT mice but not IL-15−/− mice. They therefore concluded that IL-15 prevents the development of allergic rhinitis through reactivation of antigen-specific CD8+T cells in this model. In contrast, several other studies including our own, have shown that specific subsets of effector CD8+T cells, possibly of the Tc2 phenotype, may have critical roles in asthma pathogenesis [40, 43, 46–48, 71–75]. In a biphasic model of OVA-AAD, we showed that increased numbers of OVA-specific proinflammatory CD8+T cells, expressing high levels of CD11a and increased functional expression of granzyme B and IFN-γ, can be found in the pulmonary compartment during the acute phase of OVA-AAD, but not during the local inhalational tolerance phase, during which they acquire inhibitory markers instead [48]. Furthermore, in a discontinuous model, we showed that antigen-specific CD8+T cells could be recalled on re-exposure to OVA, demonstrating that these cells had acquired memory and were responsive to antigen re-challenge. This may account for our observations that transfer of WT CD8+T cells does not significantly attenuate BAL eosinophilia in WT recipients, and instead may have exacerbated lung inflammation in a couple animals. In IL-15−/− mice however, we observed partial suppression of airway eosinophilia resulting in levels comparable to those observed in WT mice, suggesting that the enhancement of eosinophilia observed in these animals can be regulated by CD8+T cells.
A number of reasons may account for the dichotomous behavior of WT CD8+T cells with regards to suppression versus no suppression post-transfer into IL-15−/− and WT recipients. Since the adoptive transfer of WT CD8+T cells resulted in suppression of eosinophilia only to a certain degree and comparable with WT levels, it is possible that IL-15−/− mice lack subsets of inhibitory CD8+T cells that were restored post-adoptive transfer with WT CD8+T cells. In this case, while transfer of WT cells to IL-15−/− mice would confer suppressive effects of CD8+T cells during allergic inflammation, they would have no further effects in WT recipients that have endogenous populations of pro- and anti-inflammatory CD8+T cells. Furthermore, the effects of such suppressive CD8+T cells would potentially be even more prominent if the enhancement of airway inflammation in IL-15−/− mice were due to a corresponding increase in proinflammatory subsets of CD8+T cells. Previous studies including our own have demonstrated that CD8+T cells in different organ compartments can exhibit distinct cytokine profiles which can influence the trajectory of the allergic response [48, 71]. For example, Stock et al. demonstrated that while CD8+T cells in the pulmonary compartment exhibit Th2-like profiles producing high levels of IL-5 and IL-13, those in the spleen exhibit a Th1-like profile producing high levels of IFN-γ [71]. Since our adoptive transfer experiments involved the transfer of splenic CD8+T cells, it is possible that these may have produced significantly more IFN-γ than normally present in IL-15-deficient mice, which may have consequently suppressed the development of eosinophilia. Indeed, the examination of BAL fluid of IL-15−/− OVA-OVA mice receiving WT CD8+T cells demonstrated increased overall levels of IFN-γ compared with untreated mice (data not shown). Furthermore, assessment of the effects of systemic and intranasal IFN-γ treatment both prior to and during OVA challenges demonstrated the suppression of airway eosinophilia in both WT and IL-15−/− mice (data not shown), suggesting that the regulatory effects of CD8+T cells maybe dependent on IFN-γ as has been previously shown [42, 76]. Other possibilities for the dichotomous behavior of CD8+T cells may also exist. For example, the potential effects of IL-15-dependent innate cell types such as NK and NKT cells in modulating the functional behavior of effector and memory CD8+T cell populations has not been well-examined. Several recent studies suggest that innate cells can influence the proliferation and functions of CD8+T cells [77–79], and their absence in IL-15−/− animals may thus also potentially modulate the homeostasis and functions of distinct CD8+T cell subsets during allergic inflammation. Similarly, the specific effects of IL-15 in modulating the survival, function, and maintenance of diverse populations of CD8+T cells, including naïve, effector, and memory subsets, must also be considered. Depending on the antigen, the type of inflammation, and the specific immune compartment, IL-15 can have distinct effects on the proliferation, migration and functions of CD8+T cell populations [80], which could potentially impact the behavior of transferred WT CD8+T cells in an IL-15-deficient environment. Lastly, several populations of IL-15-independent CD8+T cells, including tissue resident memory CD8+T cells also exist in mucosal tissues, and may have the potential to influence the development of inflammation. [81–85].
Our findings are different from those observed by Aoi. et al. in the model of allergic rhinitis wherein they reported that CD8+T cells suppressed inflammation in WT but not IL-15−/− mice. However, it is important to consider that subsets of IL-15-dependent CD8+T cells may exert distinct effects during distinct manifestations of allergic inflammation such as allergic rhinitis and allergic asthma. Furthermore, the use of different types of adjuvants may also account for this discrepancy. Our model system uses an OVA/alum sensitization regimen resulting in Th2 type responses, whereas the model system used by Aoi et. al relies on OVA/CFA sensitization resulting in a Th1/Tc1 and Th2 response as described by the authors [58]. The precise mechanisms by which IL-15-dependent CD8+T cells modulate the enhancement of eosinophilia during OVA-AAD need to be further delineated and further insight may also be gained from comparing the effects of adoptive transfer of CD8+T cells from both OVA-sensitized WT and IL-15−/− mice into IL-15−/− recipients, and examining their cytokine production. Taken together, these data suggest that different subsets of CD8+T cells can promote both pro- and anti-inflammatory effects during OVA-AAD. However, the lack of complete suppression of airway eosinophilia argues against this being the sole mechanism by which AAD is regulated in these animals.
Lastly, it is also important to consider that the IL-15 receptor system utilizes a unique means of generating IL-15 signaling [12]. We and others have shown that IL-15Rα is not required on responding lymphocytes for IL-15-mediated signals [86–88]. Instead, IL-15 binds IL-15Rα with high affinity on accessory cells, resulting in endosomal recycling of IL-15 and trans-presentation to the IL-2/IL-15Rβ and γC chains on responding lymphocyes [34, 89], thus ensuring a continuous pool of IL-15 for IL-15 signaling. Our findings demonstrating that OVA-challenged IL-15Rα−/− mice also develop enhanced eosinophilic airway inflammation and exhibit similar pathologic changes to those observed in OVA-challenged IL-15−/− mice not only confirm the role of IL-15, but also elucidate a novel role for IL-15Rα-mediated signaling events during OVA-AAD. Future studies investigating the specific functions of IL-15Rα as well as the cellular source of IL-15 and its trans-presentation may shed further light on the mechanisms by which IL-15-mediated signaling modulates the allergic response in this model system.
In summary, our results show that endogenous IL-15 production is not essential for the development of murine OVA-induced AAD. The allergic phenotype was not only established but was also enhanced in IL-15−/− animals. These findings were associated with increased eosinophilia and enhanced numbers of CD4+ T cells, B cells, and IL-13 levels relative to WT controls. The specific roles of NK cells (or NKT and γδ T cells) in AAD cannot be accurately determined in our model, since IL-15−/− mice exhibit defects in many different cell types and the role of IL-15-independent subsets of both NK and CD8+T cells must also be considered [54, 55]. Nevertheless, the deficiencies in function of these and other cell types in IL-15−/− mice suggested that these animals would demonstrate attenuated AAD inflammatory responses and thus confirm our previous observations regarding the role of NK cells. We found the opposite: IL-15−/− mice developed enhanced AAD relative to WT animals. These novel observations suggest that IL-15 may exert additional, as yet unidentified, forms of immune regulation that are anti-inflammatory in this asthma model.
Supplementary Material
WT and IL-15−/− mice were sensitized with saline or OVA as described in Materials and Methods and either challenged or not challenged with 1% OVA aerosol. Spleen, lung, and BAL leukocytes were isolated and the percentages of live NK and NKT cells were enumerated.
Acknowledgments
Grants: This work was supported by grants from the American Asthma Foundation, NIH-AI46078, NIH-HL068692, NIH-AI43573 and NIAID-R15AI107668.
The authors would like to thank Dr. Elizabeth C. Nowak, Dr. Amanda Marzo, and Ms. Alexandra Feldenzer for expert technical assistance, Dr. Kathleen Arcaro for equipment support, Drs. Leo Lefrancois and Sara Colpitts for discussion and comments, and Dr. Adam Matson for assistance with scoring of histological samples.
ABBREVIATIONS USED
- AAD
allergic airway disease
- OVA-AAD
ovalbumin-induced model of allergic airway disease
References
- 1.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–83. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 2.DeKruyff RH, Yu S, Kim HY, Umetsu DT. Innate immunity in the lung regulates the development of asthma. Immunol Rev. 2014;260:235–48. doi: 10.1111/imr.12187. [DOI] [PubMed] [Google Scholar]
- 3.Kugelberg E. Innate lymphoid cells: breathing into allergic inflammation. Nat Rev Immunol. 2014;14:281. doi: 10.1038/nri3668. [DOI] [PubMed] [Google Scholar]
- 4.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korsgren M, Persson CG, Sundler F, Bjerke T, Hansson T, Chambers BJ, Hong S, Van Kaer L, Ljunggren HG, Korsgren O. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J Exp Med. 1999;189:553–62. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias CB, Guernsey LA, Zammit D, Brammer C, Wu CA, Thrall RS, Aguila HL. Pro-inflammatory role of natural killer cells in the development of allergic airway disease. Clin Exp Allergy. 2014;44:589–601. doi: 10.1111/cea.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathias CB. Natural killer cells in the development of asthma. Curr Allergy Asthma Rep. 2015;15:500. doi: 10.1007/s11882-014-0500-2. [DOI] [PubMed] [Google Scholar]
- 8.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 9.Schramm CM, Puddington L, Yiamouyiannis CA, Lingenheld EG, Whiteley HE, Wolyniec WW, Noonan TC, Thrall RS. Proinflammatory roles of T-cell receptor (TCR)gammadelta and TCRalphabeta lymphocytes in a murine model of asthma. Am J Respir Cell Mol Biol. 2000;22:218–25. doi: 10.1165/ajrcmb.22.2.3620. [DOI] [PubMed] [Google Scholar]
- 10.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine. 2012;59:479–90. doi: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 15.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–76. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003;100:2663–8. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–93. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 18.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee GA, Liou YH, Wang SW, Ko KL, Jiang ST, Liao NS. Different NK cell developmental events require different levels of IL-15 trans-presentation. J Immunol. 2011;187:1212–21. doi: 10.4049/jimmunol.1100331. [DOI] [PubMed] [Google Scholar]
- 20.Zhao YM, French AR. Two-compartment model of NK cell proliferation: insights from population response to IL-15 stimulation. J Immunol. 2012;188:2981–90. doi: 10.4049/jimmunol.1102989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol Cell Biol. 2014;92:210–3. doi: 10.1038/icb.2014.1. [DOI] [PubMed] [Google Scholar]
- 22.Tamzalit F, Barbieux I, Plet A, Heim J, Nedellec S, Morisseau S, Jacques Y, Mortier E. IL-15. IL-15Ralpha complex shedding following trans-presentation is essential for the survival of IL-15 responding NK and T cells. Proc Natl Acad Sci U S A. 2014;111:8565–70. doi: 10.1073/pnas.1405514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol. 2001;2:1138–43. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 24.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 25.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–46. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 27.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prlic M, Lefrancois L, Jameson SC. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J Exp Med. 2002;195:F49–52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol. 2010;184:6719–30. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 32.Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, Whiteley HE, Thrall RS. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–21. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bracken SJ, Adami AJ, Szczepanek SM, Ehsan M, Natarajan P, Guernsey LA, Shahriari N, Rafti E, Matson AP, Schramm CM, Thrall RS. Long-Term Exposure to House Dust Mite Leads to the Suppression of Allergic Airway Disease Despite Persistent Lung Inflammation. Int Arch Allergy Immunol. 2015;166:243–58. doi: 10.1159/000381058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–34. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–25. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009;183:4948–56. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–34. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castillo EF, Acero LF, Stonier SW, Zhou D, Schluns KS. Thymic and peripheral microenvironments differentially mediate development and maturation of iNKT cells by IL-15 transpresentation. Blood. 2010;116:2494–503. doi: 10.1182/blood-2010-03-277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, Larsen GL, Gelfand EW. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a marine model of airway sensitization. J Exp Med. 1996;183:1719–29. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Sullivan S, Cormican L, Faul JL, Ichinohe S, Johnston SL, Burke CM, Poulter LW. Activated, cytotoxic CD8(+) T lymphocytes contribute to the pathology of asthma death. Am J Respir Crit Care Med. 2001;164:560–4. doi: 10.1164/ajrccm.164.4.2102018. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Maghni K, Molet S, Shimbara A, Hamid QA, Martin JG. IFN-gamma secretion by CD8T cells inhibits allergen-induced airway eosinophilia but not late airway responses. J Allergy Clin Immunol. 2002;109:803–9. doi: 10.1067/mai.2002.123233. [DOI] [PubMed] [Google Scholar]
- 43.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand EW. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10:865–9. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 44.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, Miyahara S, Balhorn A, Dakhama A, Gelfand EW. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol. 2004;172:2549–58. doi: 10.4049/jimmunol.172.4.2549. [DOI] [PubMed] [Google Scholar]
- 45.Tang Y, Guan SP, Chua BY, Zhou Q, Ho AW, Wong KH, Wong KL, Wong WS, Kemeny DM. Antigen-specific effector CD8 T cells regulate allergic responses via IFN-gamma and dendritic cell function. J Allergy Clin Immunol. 2012;129:1611–20. e4. doi: 10.1016/j.jaci.2011.12.976. [DOI] [PubMed] [Google Scholar]
- 46.Dakhama A, Collins ML, Ohnishi H, Goleva E, Leung DY, Alam R, Sutherland ER, Martin RJ, Gelfand EW. IL-13-producing BLT1-positive CD8 cells are increased in asthma and are associated with airway obstruction. Allergy. 2013;68:666–73. doi: 10.1111/all.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia Y, Takeda K, Han J, Joetham A, Marcus RA, Lucas JJ, O’Connor BP, Gelfand EW. Stepwise epigenetic and phenotypic alterations poise CD8+ T cells to mediate airway hyperresponsiveness and inflammation. J Immunol. 2013;190:4056–65. doi: 10.4049/jimmunol.1202640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNamara JT, Schramm CM, Singh A, Secor ER, Jr, Guernsey LA, Lefrancois L, Thrall RS. Phenotypic changes to the endogenous antigen-specific CD8+ T cell response correlates with the development and resolution of allergic airway disease. Am J Pathol. 2012;180:1991–2000. doi: 10.1016/j.ajpath.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma A, Boone DL, Lodolce JP. The pleiotropic functions of interleukin 15: not so interleukin 2-like after all. J Exp Med. 2000;191:753–6. doi: 10.1084/jem.191.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–10. [PubMed] [Google Scholar]
- 51.Lodolce J, Burkett P, Koka R, Boone D, Chien M, Chan F, Madonia M, Chai S, Ma A. Interleukin-15 and the regulation of lymphoid homeostasis. Mol Immunol. 2002;39:537–44. doi: 10.1016/s0161-5890(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 52.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–80. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-Cell immunity in health and disease. Crit Rev Immunol. 2008;28:325–39. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 54.Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009;183:2911–4. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verbist KC, Field MB, Klonowski KD. Cutting edge: IL-15-independent maintenance of mucosally generated memory CD8 T cells. J Immunol. 2011;186:6667–71. doi: 10.4049/jimmunol.1004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin J, Nair P, Sehmi R. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86. e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 57.Kim HY, Umetsu DT, Dekruyff RH. Innate lymphoid cells in asthma: Will they take your breath away? Eur J Immunol. 2016;46:795–806. doi: 10.1002/eji.201444557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aoi N, Masuda T, Murakami D, Yajima T, Mizubuchi H, Yamada H, Kawauchi H, Yoshikai Y. IL-15 prevents allergic rhinitis through reactivation of antigen-specific CD8+ cells. J Allergy Clin Immunol. 2006;117:1359–66. doi: 10.1016/j.jaci.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Laza-Stanca V, Message SD, Edwards MR, Parker HL, Zdrenghea MT, Kebadze T, Kon OM, Mallia P, Stanciu LA, Johnston SL. The role of IL-15 deficiency in the pathogenesis of virus-induced asthma exacerbations. PLoS Pathog. 2011;7:e1002114. doi: 10.1371/journal.ppat.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong PY, Hamid QA, Travers JB, Strickland I, Al Kerithy M, Boguniewicz M, Leung DY. Decreased IL-15 may contribute to elevated IgE and acute inflammation in atopic dermatitis. J Immunol. 2002;168:505–10. doi: 10.4049/jimmunol.168.1.505. [DOI] [PubMed] [Google Scholar]
- 61.Bierbaum S, Nickel R, Zitnik S, Ahlert I, Lau S, Deichmann KA, Wahn U, Heinzmann A. Confirmation of association of IL-15 with pediatric asthma and comparison of different controls. Allergy. 2006;61:576–80. doi: 10.1111/j.1398-9995.2006.01059.x. [DOI] [PubMed] [Google Scholar]
- 62.Pinto LA, Depner M, Steudemann L, Klopp N, Illig T, von Mutius E, Kabesch M. IL15 gene variants are not associated with asthma and atopy. Allergy. 2009;64:643–6. doi: 10.1111/j.1398-9995.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 63.Ruckert R, Brandt K, Braun A, Hoymann HG, Herz U, Budagian V, Durkop H, Renz H, Bulfone-Paus S. Blocking IL-15 prevents the induction of allergen-specific T cells and allergic inflammation in vivo. J Immunol. 2005;174:5507–15. doi: 10.4049/jimmunol.174.9.5507. [DOI] [PubMed] [Google Scholar]
- 64.Kirman I, Vainer B, Nielsen OH. Interleukin-15 and its role in chronic inflammatory diseases. Inflamm Res. 1998;47:285–9. doi: 10.1007/s000110050331. [DOI] [PubMed] [Google Scholar]
- 65.Muro S, Taha R, Tsicopoulos A, Olivenstein R, Tonnel AB, Christodoulopoulos P, Wallaert B, Hamid Q. Expression of IL-15 in inflammatory pulmonary diseases. J Allergy Clin Immunol. 2001;108:970–5. doi: 10.1067/mai.2001.119556. [DOI] [PubMed] [Google Scholar]
- 66.Ripley D, Shoup B, Majewski A, Chegini N. Differential expression of interleukins IL-13 and IL-15 in normal ovarian tissue and ovarian carcinomas. Gynecol Oncol. 2004;92:761–8. doi: 10.1016/j.ygyno.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 67.Komai-Koma M, McKay A, Thomson L, McSharry C, Chalmers GW, Liew FY, Thomson NC. Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clin Exp Allergy. 2001;31:1441–8. doi: 10.1046/j.1365-2222.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 68.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 69.Lee JM, Chung CY, Chiang WW, Liou YH, Chen CF, Liao NS. IL-15Ralpha is a negative regulator of TCR-activated proliferation in CD4+ T cells. J Immunol. 2004;173:3155–64. doi: 10.4049/jimmunol.173.5.3155. [DOI] [PubMed] [Google Scholar]
- 70.Ishimitsu R, Nishimura H, Yajima T, Watase T, Kawauchi H, Yoshikai Y. Overexpression of IL-15 in vivo enhances Tc1 response, which inhibits allergic inflammation in a murine model of asthma. J Immunol. 2001;166:1991–2001. doi: 10.4049/jimmunol.166.3.1991. [DOI] [PubMed] [Google Scholar]
- 71.Stock P, Kallinich T, Akbari O, Quarcoo D, Gerhold K, Wahn U, Umetsu DT, Hamelmann E. CD8(+) T cells regulate immune responses in a murine model of allergen-induced sensitization and airway inflammation. Eur J Immunol. 2004;34:1817–27. doi: 10.1002/eji.200324623. [DOI] [PubMed] [Google Scholar]
- 72.Isogai S, Taha R, Tamaoka M, Yoshizawa Y, Hamid Q, Martin JG. CD8+ alphabeta T cells can mediate late airway responses and airway eosinophilia in rats. J Allergy Clin Immunol. 2004;114:1345–52. doi: 10.1016/j.jaci.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 73.Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, Matsubara S, Dakhama A, Tager AM, Luster AD, Gelfand EW. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005;174:4979–84. doi: 10.4049/jimmunol.174.8.4979. [DOI] [PubMed] [Google Scholar]
- 74.Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader J, Shultz LD, Tager AM, Luster AD, Dakhama A, Gelfand EW. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006;176:3157–64. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 75.Koya T, Miyahara N, Takeda K, Matsubara S, Matsuda H, Swasey C, Balhorn A, Dakhama A, Gelfand EW. CD8+ T cell-mediated airway hyperresponsiveness and inflammation is dependent on CD4+IL-4+ T cells. J Immunol. 2007;179:2787–96. doi: 10.4049/jimmunol.179.5.2787. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell C, Provost K, Niu N, Homer R, Cohn L. IFN-gamma acts on the airway epithelium to inhibit local and systemic pathology in allergic airway disease. J Immunol. 2011;187:3815–20. doi: 10.4049/jimmunol.1100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leggat JA, Gibbons DL, Haque SF, Smith AL, Wells JW, Choy K, Lloyd CM, Hayday AC, Noble A. Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J Allergy Clin Immunol. 2008;122:1014–21. e4. doi: 10.1016/j.jaci.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morandi B, Mortara L, Carrega P, Cantoni C, Costa G, Accolla RS, Mingari MC, Ferrini S, Moretta L, Ferlazzo G. NK cells provide helper signal for CD8+ T cells by inducing the expression of membrane-bound IL-15 on DCs. Int Immunol. 2009;21:599–606. doi: 10.1093/intimm/dxp029. [DOI] [PubMed] [Google Scholar]
- 79.Tripathi P, Morris SC, Perkins C, Sholl A, Finkelman FD, Hildeman DA. IL-4 and IL-15 promotion of virtual memory CD8+ T cells is determined by genetic background. Eur J Immunol. 2016;46:2333–39. doi: 10.1002/eji.201646404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anthony SMS, KS Emerging roles for IL-15 in the activation and function of T-cells during immune stimulation. Research and Reports in Biology. 2015;2015:25–37. [Google Scholar]
- 81.Anderson KG, Masopust D. Editorial: Pulmonary resident memory CD8 T cells: here today, gone tomorrow. J Leukoc Biol. 2014;95:199–201. doi: 10.1189/jlb.0913493. [DOI] [PubMed] [Google Scholar]
- 82.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, Masopust D. IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J Immunol. 2016;196:3920–6. doi: 10.4049/jimmunol.1502337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–97. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wijeyesinghe S, Masopust D. Resident memory T cells are a Notch above the rest. Nat Immunol. 2016;17:1337–38. doi: 10.1038/ni.3617. [DOI] [PubMed] [Google Scholar]
- 86.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–21. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci U S A. 2003;100:4724–9. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–84. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT and IL-15−/− mice were sensitized with saline or OVA as described in Materials and Methods and either challenged or not challenged with 1% OVA aerosol. Spleen, lung, and BAL leukocytes were isolated and the percentages of live NK and NKT cells were enumerated.