Abstract
Sweetpotato (Ipomoea batatas (L.) Lam.) is an outcrossing hexaploid that is cultivated in the tropics and warm-temperate regions of the world. Sweetpotato has played an important role as a famine-relief crop during its long history and has recently been reevaluated as a health-promoting food. In Japan, sweetpotato is used for a wide range of applications, such as table use, processed foods, and alcohol and starch production, and two groups at National Agriculture Research Organization (NARO) undertake the breeding of cultivars for these applications. Sweetpotato breeders utilize breeding processes such as grafting for flower induction and the identification of incompatibility groups before crossing to conquer problems peculiar to sweetpotato. For table use, new cultivars with high sugar content were released recently and have become popular among Japanese consumers. New cultivars with high anthocyanin or β-carotene content were released for processed foods and use as colorants. As raw materials, new cultivars with high alcohol yield were released for the production of shochu spirits. In addition, new cultivars with high starch yield and a cultivar containing starch with excellent cold-storage ability were released for starch production. This review deals with recent progress in sweetpotato breeding and cultivars for diverse applications in Japan.
Keywords: sweetpotato, breeding, hexaploid, cultivar, production, utilization
Introduction
Sweetpotato (Ipomoea batatas (L.) Lam.) is a vegetative propagation crop that belongs to the family Convolvulaceae. This crop is an autohexaploid (2n = 6× = 90) (Shiotani and Kawase 1989). Ipomoea trifida (H. B. K.) G. Don (2n = 2× = 30) is the most likely diploid ancestor of cultivated sweetpotato, and it forms a polyploid complex with a series from diploid to hexaploid (Nishiyama et al. 1975). Most varieties of sweetpotato show self- and cross-incompatibility, low natural flowering ability, and low seed fertility (Fujise 1964, Martin 1965). This outcrossing, polyploidy, and vegetative propagation in sweetpotato, causes high heterozygosity and variability in the genetic background of this crop.
It has been suggested that sweetpotato originated in the tropical lowlands of Central or South America (Austin 1988, Yen 1974). After Columbus arrived at the Caribbean in 1492, this crop spread widely from America to other tropical and warm-temperate regions worldwide. The Spanish introduced the sweetpotato into Europe, and from here it was taken to Africa, India, and China. Sweetpotato was brought from China to Japan between 1597 and 1609, and it spread to various parts of Japan as a famine-relief crop over the 17th and 18th centuries.
Farmers can cultivate the sweetpotato twice a year in tropical regions, but in temperate regions sweetpotato can grow in only summer seasons that have at least 5 months of frost-free conditions. Cultivation of sweetpotato does not require much input from farmers except for occasional weeding. The vines grow rapidly and cover the field to prevent soil erosion (Loebenstein and Thottappilly 2009). Endophytic N2 fixation organisms have been isolated from the aerial parts of sweetpotato (Asis and Adachi 2003, Paula et al. 1991), and Yoneyama et al. (1998) reported the occurrence of N2 fixation in sweetpotato plants based on 15N natural abundance data. Sweetpotato can grow well in infertile soils with a little N fertilizer and is tolerant to severe weather conditions such as typhoons and drought. This crop shows higher productivity per unit area than other food crops such as cereals and has played an important role as a famine-relief crop during its long history (Loebenstein and Thottappilly 2009, Woolfe 1992).
In addition, sweetpotato roots contain high levels of nutrients such as carbohydrates, dietary fiber, vitamins A and C, and minerals, contained in both cereals and vegetables. Sweetpotato roots can be boiled, steamed, baked, or fried and can be used for diverse applications, such as table use, processed foods, alcohol and starch production, and animal feed. In developed countries, sweetpotato has recently been reevaluated as a health-promoting food, because of its high content of nutrients in balance and functional components such as anthocyanins, carotenoids, and phenolic compounds, which have anti-oxidant and other activities (Tanaka et al. 2017).
Sweetpotato is the fourteenth most important food crop worldwide from viewpoint of production, with approximately 103 million tons, produced from an area of about 8 million ha and an average yield of about 12.6 tons/ha (FAOSTAT 2013). Among the root and tuber crops of the world, sweetpotato ranks third after potato and cassava in terms of production. Sweetpotato is mainly grown in developing countries in the tropics and warm-temperate regions. China is the largest sweetpotato producer, and its annual production is about 70 million tons, accounting for 68.6% of total production in the world. The rest of Asia accounts for 7.5%, Africa for 19.5%, America for 3.5%, Oceania for 0.8%, and Europe for 0.1% of total production.
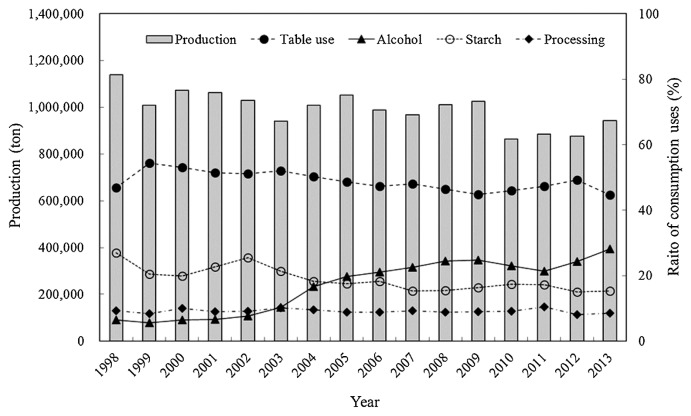
Japan is the fourteenth largest sweetpotato producer, and its production is approximately 942 thousand tons, from a production area of about 38 thousand ha and the average yield is about 24.4 tons/ha (FAOSTAT 2013). In Japan, the production of sweetpotato increased greatly in the period of food shortages immediately after World War II and reached a peak of about 7 million tons in 1955. After that, production declined sharply as the Japanese diet stabilized, and recent production remains around 1 million tons (Fig. 1). Sweetpotato in Japan was a subsistence crop before World War II and it was used mainly as a raw material for starch after the war. Recently, it has mainly been consumed for table use, which accounts for about 45% of total consumption (Fig. 1). Starch use has continued to decrease in the face of competition from inexpensive imported corn and cassava. There was more consumption of alcohol (shochu spirits) produced from sweetpotato than starch in 2005. Food processing use has increased gradually and accounts for around 10% of current total consumption. Sweetpotato breeding in Japan is mainly carried out by two breeding groups at the National Agriculture Research Organization (NARO) for these diverse applications.
Fig. 1.
Change in sweetpotato production and the ratio of consumption uses in Japan.
Previous reviews have reported production and consumption, cultivation and utilization, and breeding and varieties of sweetpotato in Japan up to about 2000 (Komaki 1997, Kumagai 2001, Nakatani 2001, Yamakawa 2000). This review deals with recent approaches to sweetpotato breeding in Japan. In addition, we introduce the cultivars released for diverse applications since 2000.
Breeding procedure in Japan
Sweetpotato breeding at NARO is conducted in two groups: the Institute of Crop Science, NARO (NICS) and Kyushu Okinawa Agricultural Research Center, NARO (KARC/NARO). The laboratory for NICS is located in Tsukuba in the Kanto region, which is the northern limit of commercial production in Japan, and the laboratory for KARC/NARO is located in Miyakonojo in the Kyushu region, which is a southern warm-temperate area in Japan. The group at NICS mainly undertakes the breeding of cultivars for table use, and the group at KARC/NARO predominantly breeds cultivars for processing and raw materials.
Previous studies indicated that sweetpotato is an autohexaploid, and its inheritance mode is hexasomic (Kumagai et al. 1990, Shiotani and Kawase et al. 1989). Frequently, a trait controlled by a single gene can be quantitatively scored by phenotype owing to the specificity of its segregation in autopolyploids (Watanabe 2015). The highly heterozygous, outcrossing polyploidy in sweetpotato complicates its genetic analysis, but theoretical prediction of segregation is very important in sweetpotato breeding.
The two breeding groups at NARO maintain approximately 3,000 accessions of wild species and sweetpotato, which consist of registered cultivars, breeding lines, local varieties from Japan, and introductions from North and South America, Asia, and Oceania (Komaki 1995). About one-third of accessions are duplicated and conserved by both groups. Over 80% of the accessions are maintained using storage roots harvested from the experimental fields. The plants of accessions that produce no storage roots are maintained in pots of greenhouses. Morphological characteristics such as the shapes of leaves and roots, and agricultural characteristics such as disease and pest resistance and quality are evaluated for over 50 accessions every year.
The initiation of sweetpotato breeding involves the selection of crossing parents from the genetic resources and cross combinations. Most varieties of sweetpotato can produce few or no flower in temperate regions and they show the self- and cross-incompatibility. Therefore, sweetpotato breeders utilize breeding processes such as grafting for flower induction and the identification of incompatibility groups before crossing to overcome these problems. The top grafting method using morning glory stocks is adopted to induce flowering (Reynoso et al. 1999a). A dwarf type of Japanese morning glory, Ipomoea nil (L.) Roth cv. “Kidachi-asagao” is used as a rootstock for two reasons: one has a thick stem, which makes grafting manipulations easier, and it also has a long flowering period that increases the total number of flowers (Kobayashi and Nakanishi 1982). The sweetpotato scions form flower buds in 3 weeks after grafting and continue to flower for more than 2 months. The flowers of sweetpotato and I. trifida possess a strong sporophytic type of self-incompatibility (Kowyama et al. 2000) and 16 cross-incompatibility groups (A–P) have been classified in sweetpotato (Nakanishi and Kobayashi 1979). This is the main barrier to seed production after self-pollination and crossing within the same cross-incompatibility groups. Representative varieties from each group have been identified as “incompatibility testers”, and they are used in crossing between varieties whose incompatibility groups are unknown. Incompatibility is determined by observing the development of pollen tubes stained with aniline blue using a fluorescence microscope (Reynoso et al. 1999b). This approach allows unknown varieties to be identified according to five groups (A, B, C, D, and E) and the other groups. Recently, the laboratory at KARC/NARO has handled 200–300 cross combinations in total twice a year in spring and fall for the two breeding groups of NARO and they harvest approximately 50,000–70,000 seeds each year.
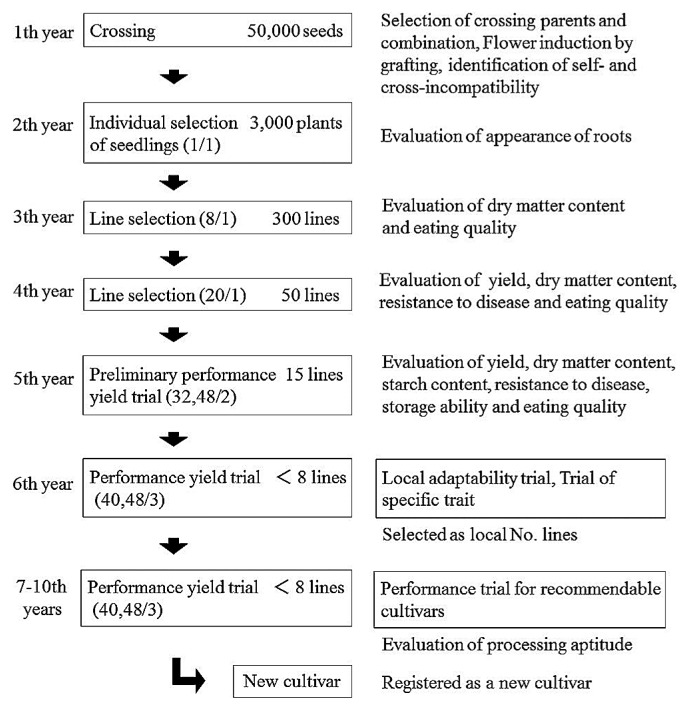
The breeding scheme of sweetpotato at NARO is shown in Fig. 2. These seeds are sown in nursery beds, and each seedling is transplanted into the field. Storage roots of selected plants with a good appearance are harvested and stored over winter. After the second year, they are investigated in various tests for line selection. Breeders decrease the number of selected lines and increase the amount of storage roots of the selected lines every year. In the sixth year, the local number lines are selected in performance yield trials and they are investigated for resistance to diseases and pests, local adaptability, trials of specific traits or performance trials for recommendable cultivars in several prefectures. The laboratory at NICS conducts resistance tests for major diseases and pests, including black rot (Ceratocystis fimbriata), stem rot (Fusarium oxysporum f. sp. batatas), soil rot (Streptomyces ipomoea), and southern root-knot nematodes (RKN, Meloidogyne incognita) (Okada et al. 2017). The laboratory at KARC/NARO investigates resistance to RKN and coffee root-lesion nematodes (RLN, Pratylenchus coffeaceae). Completing a new cultivar takes over 8 years after crossing.
Fig. 2.
Breeding scheme for sweetpotato at the National Agriculture Research Organization (NARO) in Japan. The numbers in parentheses of each square indicate the number of plants and replicates in each trial in NARO. The right column indicates the evaluated traits in each trial and the trials conducted at agricultural experimental stations in some prefectures.
Breeding objectives and cultivars for table use
Recently, table use has accounts for about 45% of total consumption in Japan. Important breeding objectives for table use are good eating quality, good appearance, resistance to diseases and pests, and yield. Eating quality is affected by two main factors, namely sweetness and texture. Sweetpotato has a mechanism whereby β-amylase saccharifies the starches gelatinized by heating to maltose and the sweetness, sugar content, increases with cooking. The maltose content is influenced by β-amylase activity and starch gelatinization temperature (Kitahara et al. 2017). In Japan, the monoculture of sweetpotato for table use has increased rapidly in commercial cultivation, and the continuous cropping of sweetpotato causes an increase in soil-borne diseases, such as soil rot and RKN. Resistance to these diseases is an important breeding objective for table use, too. In this chapter, we review the sweetpotato cultivars bred recently for table use (Table 1).
Table 1.
Characteristics of leading varieties and current cultivars for table use in Japan
| Cultivars | Released year | Ratio for yield of the standard cultivara | Resistance to diseases/pests | Quality of steamed roots and other characteristics |
|---|---|---|---|---|
| Kokei No.14 | 1945 | — | — | Leading variety in west Japan, moderate texture, moderate brix%, moderate taste |
| Beniazuma | 1984 | 104% of Kokei No.14 | Soil rot | Leading variety in east Japan, dry texture, slightly high brix%, slightly good taste |
| Benimasari | 2002 | 115% of Kokei No.14 | Black rot | Slightly moist texture, slightly high brix%, good taste |
| Quick Sweet | 2002 | 107% of Beniazuma | RKN | Slightly moist texture, high brix%, slightly good taste, low pasting temperature starch |
| Purple Sweet Lord | 2002 | 157% of Beniazuma | — | Slightly dry texture, slightly low brix%, moderate taste, anthocyanin-rich |
| Beniharuka | 2008 | 114% of Kokei No.14 | RKN | Slightly moist texture, high brix%, good taste, wide regional adaptability |
| Himeayaka | 2009 | 78% of Beniazuma | — | Slightly moist texture, slightly high brix%, good taste, compact size storage roots |
| Aikomachi | 2012 | 101% of Beniazuma | RKN, black rot | Slightly dry texture, high brix%, slightly good taste, suitable for confectioneries |
Values under standard cultivation conditions.
The leading varieties for table use in Japan are ‘Beniazuma’ and ‘Kokei No.14’. ‘Beniazuma’ was released by the National Agricultural Research Center (now known as NICS) in 1984 and is the progeny of a cross between ‘Kanto No.85’ and ‘Koganesengan’ (Shiga et al. 1985). The storage root is long fusiform with a dark red-purple skin and yellow flesh. This cultivar shows higher yields than ‘Kokei No.14’ and exhibits a better taste and higher brix% than ‘Kokei No.14’ and a dry texture to the steamed root flesh. ‘Beniazuma’ is slightly resistant to soil rot and has high sprouting ability but low storage ability. This cultivar is mainly cultivated in eastern Japan and its cultivation area in 2013 was about 4,800 ha. ‘Kokei No.14’ was released by Kochi prefecture in 1945 and was derived from a cross between ‘Nancy Hall’ and ‘Siam’. The storage root is fusiform with a red-purple skin and yellowish-white flesh. This cultivar exhibits wide regional adaptability, is multipurpose, and shows moderate brix% and texture of the steamed root flesh. However, it is susceptible to soil rot and RKN and has moderate sprouting ability and slightly high storage ability. This cultivar contains some derivative lines, and these lines are mainly cultivated in western Japan. The total cultivation area in 2013 was about 5,200 ha.
During the last two decades, consumers’ preferences for sweetpotato have changed in Japan. Japanese consumers currently prefer sweetpotato cultivars that exhibit strong sweetness and a slightly moist texture to the cooked root flesh. Therefore, the cultivation of new cultivars with high sugar content has increased recently. Baked sweetpotato, called ‘yaki-imo’ in Japanese, which is very sweet, gained in popularity with current Japanese consumers. In addition, Japanese sweetpotato is increasing the volume of exports to Asian countries.
The trendy beginning of high-sugar cultivars was ‘Annou-imo’, such as ‘Annou-beni’ and ‘Annou-kogane’. These cultivars were bred from some landraces on Tanegashima Island by Kagoshima Prefectural Agricultural Experiment Station, Kumage Branch, in 1998 (Kouzuma et al. 2003). The storage root of ‘Annou-beni’ is fusiform with a brown skin and yellow flesh, and the taste is very sweet and smooth with a moist texture. The storage root of ‘Annou-kogane’ is cylindrical with a light-yellow skin and yellow flesh, and the taste is very sweet and smooth with a moist texture. These cultivars are cultivated on Tanegashima Island and their cultivation area is about 500 ha.
‘Benimasari’ is a cultivar for table use, developed at KARC/NARO and released in 2001 (Ishiguro et al. 2004). It is the progeny of a cross between ‘Kyushu No.104’ and ‘Kyukei 87010-21’. The storage root is uniformly fusiform with a red skin and yellow flesh. The yield of ‘Benimasari’ is considerably higher than that of ‘Kokei No.14’. The texture of the steamed root is slightly moist and its taste is better than that of ‘Kokei No.14’. The brix% of the steamed root is slightly higher than that of ‘Kokei No.14’. This cultivar is also resistant to black rot. Its sprouting and storage abilities are slightly high. The total cultivation area in 2013 was 195 ha in Ibaraki prefecture.
‘Quick Sweet’ was released by the NICS in 2002 and is the progeny of a cross between ‘Beniazuma’ and ‘Kyushu No.30’ (Katayama et al. 2003). The storage root is fusiform with a dark red-purple skin and yellowish-white flesh. The yield of this cultivar is similar to that of ‘Beniazuma’. The taste is equivalent to ‘Beniazuma’, the brix% is higher than ‘Beniazuma’, and the steamed root flesh has a slightly moist texture. This cultivar is slightly resistant to stem rot and RKN, but its sprouting ability and storage ability are low. The starch of ‘Quick Sweet’ has approximately 20°C lower gelatinization and pasting temperatures, slower retrogradation and higher digestibility of raw starch than ordinary cultivars, and this starch is qualitatively different from those of ordinary cultivars in terms of the chain-length distribution of amylopectin molecules (Katayama et al. 2002, Kitahara et al. 2005). It was reported that inhibition of expression of the starch synthase II gene led to low pasting temperature of sweetpotato starch (Takahata et al. 2010). This trait is a qualitative character controlled by one recessive allele, which is inherited in a hexasomic manner (Katayama et al. 2015). The storage roots of ‘Quick Sweet’ can be cooked quickly and taste good even when cooked rapidly in a microwave oven, because of its low pasting temperature. This cultivar is mainly cultivated in eastern Japan and its cultivation area in 2013 was about 10 ha.
‘Purple Sweet Lord’ was released by the NICS in 2002 and is derived from cross-pollinating ‘Kyushu No.119’ with a mixture of pollen from five lines: ‘Kanto No.85’, ‘Kanto No.99’, ‘Kanto No.103’, Kyushu No.105’, and ‘Beniotome’ (Tamiya et al. 2003). Monden et al. (2013) reported that the pollen parent was ‘Kanto No.99’ as a result of DNA marker analysis. ‘Kyushu No.119’ is an anthocyanin-rich line, and ‘Purple Sweet Lord’ contains anthocyanin. The storage root is fusiform and uniform with a dark red-purple skin and purple flesh. The yield of this cultivar is higher than that of ‘Beniazuma’. The taste is equivalent to ‘Kokei No.14’, the brix% is lower than ‘Beniazuma’, and the steamed root flesh shows a slightly dry texture. This cultivar is slightly resistant to stem rot, and its sprouting ability and storage ability are slightly high. ‘Purple Sweet Lord’ is also used for side dishes, confectioneries, and shochu spirits. This cultivar is grown in various part of Japan and its cultivation area in 2013 was about 30 ha.
‘Beniharuka’ is a cultivar for table use, developed at KARC/NARO and released in 2007 (Kai et al. 2010). It is the progeny of a cross between ‘Kyushu No.121’ and ‘Harukogane’. The storage root is uniformly fusiform with a reddish-purple skin and yellowish-white flesh. The yield of ‘Beniharuka’ is higher than that of ‘Kokei No.14’. The dry matter content is higher than that of ‘Kokei No.14’ and the brix% of steamed root is higher than ‘Kokei No.14’. The steamed root texture is slightly dry soon after harvesting but it becomes slightly moister after about 1 month in storage, and its taste becomes sweeter. ‘Beniharuka’ is typical of the high-sugar cultivar and exhibits wide regional adaptability. It is slightly resistant to RLN, and is resistant to RKN. ‘Beniharuka’ exhibits moderate sprouting ability and high storage ability. Total cultivation area in 2013 was 2,012 ha, mainly in Ibaraki, Chiba, Kumamoto, Oita, and Kagoshima prefectures.
‘Himeayaka’ was released by the NICS in 2009 and is derived from a cross between ‘Kyushu No.127’ and ‘Kanto No.115’ (Ohara-Takada et al. 2011). ‘Himeayaka’ has compact size roots suitable for consumption in a single meal and the storage root is average weight about 140 g, which is 60% of that of ‘Beniazuma’ or ‘Kokei No.14’. The storage roots are short fusiform with a red-purple skin and yellow flesh. The yield of this cultivar is lower than those of ‘Beniazuma’ or ‘Kokei No.14’, but this has higher yield than both cultivars with roots less than 200 g. The taste is good and brix% is higher than both cultivars. In addition, the steamed root flesh shows a slightly moist texture and is bright yellow, and the incidence of blackening after cooking is low. This cultivar is slightly resistant to soil rot, stem rot, and black rot, and its sprouting ability is slightly high and storage ability is moderate. ‘Himeayaka’ is suitable for yaki-imo and is mainly cultivated in parts of the Kanto region.
‘Aikomachi’ was released by the NICS in 2012 and is derived from a cross between ‘Quick Sweet’ and ‘Kankei 107’ (Ohara-Takada et al. 2016). The storage root has a good appearance and is fusiform with a dark red-purple skin and light-yellow flesh. The average weight of storage roots is slightly lower than that of ‘Beniazuma’, but the yield is similar. The taste is good, its brix% is higher than ‘Beniazuma’, and the steamed root flesh shows a slightly dry texture. Because this cultivar shows little blackening after cooking and high dry matter content, it is suitable for processing for imo-yokan, solidified sweetpotato paste, and daigaku-imo, sugar-glazed fried sweetpotato, which are traditional Japanese confectioneries. This cultivar is resistant to RKN and black rot, and although its sprouting ability is slightly low, its storage ability is moderate. ‘Aikomachi’ is mainly expected to be cultivated in parts of the Kanto region.
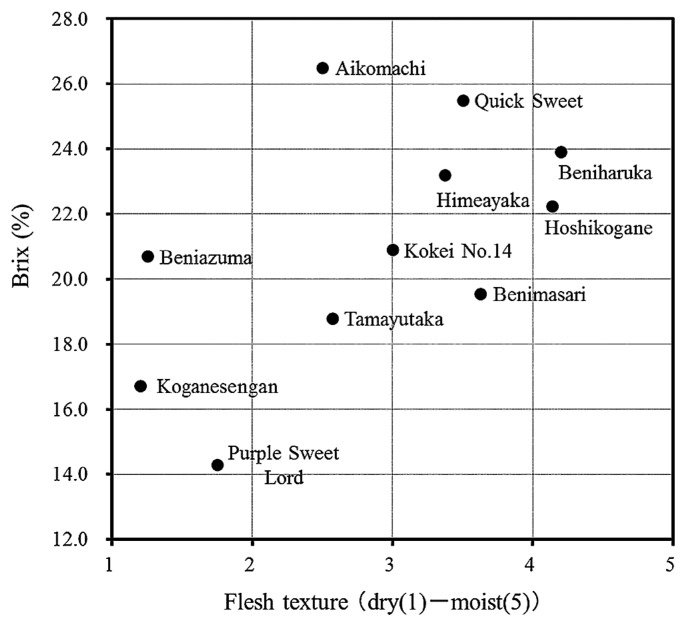
Fig. 3 shows the classification of Japanese cultivars according to the relationship between brix% and the flesh texture of the steamed roots. Most of new cultivars bred recently in Japan have high brix% and a slightly moist texture of the steamed roots (Katayama et al. 2014). In addition, these cultivars with high sugar content exhibit higher β-amylase activity and/or lower starch pasting temperature than those of ordinary cultivars. These results suggest that β-amylase activity and starch pasting temperature are important characteristics for breeding the cultivars with high sugar content.
Fig. 3.
Classification of Japanese cultivars according to the relationship between brix% and flesh texture of steamed roots. Average value in 2006–2013 of cultivars that were cultivated at the Institute of Crop Science, NARO (NICS).
Breeding objectives and cultivars for processed foods
Since the latter half of the 1980s, breeders have actively promoted programs for developing cultivars adaptable for food processing in order to replace cultivars for starch production, which was decreased gradually in their area of cultivation in southern Kyushu. As a result, breeders have developed cultivars that have a high content of β-carotene and anthocyanin, which are suitable as natural food colorants, pastes, flours, juices, and precooked foods.
It was reported that β-carotene, which is the origin of orange flesh color, can change into vitamin A in the human body, so it has antioxidative functions and immunomodulating effects (Tanaka et al. 2017). Breeders have used a landrace, ‘Hayatoimo’, which has a lower β-carotene content, and American cultivars ‘Centennial’ or ‘86J-6’, which have higher β-carotene content as crossing parents, and have developed ‘Benihayato’ for processed foods and ‘J-Red’ for juices. In recent years, to focus on higher β-carotene content and taste, ‘Ayakomachi’ with its good taste and ‘Tamaakane’ with higher than 50 mg/100 g dry weight (DW) of β-carotene content have been developed (Kai et al. 2004, Sakai et al. 2011).
Sweetpotato cultivars with purple flesh color containing anthocyanin for processed foods are recognized broadly as the health-promoting foods. At the same time, it was also supported widely as a foodstuff. It is well-known that ‘Bise’ from Okinawa prefecture and ‘Tanegashimamurasaki’ from Kagoshima prefecture are landraces that contain anthocyanin. However, because of their low anthocyanin content, they cannot be used as the natural food colorant materials, which needs the factors of high anthocyanin content, sufficient supply and high stability during processing, storing and displaying. In the middle of the 1980s, ‘Yamagawamurasaki’ was found in Kagoshima prefecture and shown to contain higher anthocyanin than the other landraces. This cultivar mainly contains eight anthocyanins, identified as acylated glycosides of cyanidin and peonidin (Goda et al. 1997, Terahara et al. 1999), so that it has stability of a similar level to red cabbage in response to stimulation of light and heat (Odake et al. 1994). The expression of anthocyanin is a dominant trait (Mano et al. 2007), and its content has a high arithmetic effect, so that it is easy to select offspring lines containing higher anthocyanin levels than the parents. In fact, the anthocyanin production per unit area of ‘Ayamurasaki’, which is a second-generation offspring of ‘Yamagawamurasaki’, can be ten-times higher than ‘Yamagawamurasaki’, and there is no unpleasant odor unlike with other anthocyanin-rich crop, red cabbage. This market scale is estimated as being the same as or somewhat higher than that of red cabbage colorants. Except for applications as a natural food colorant, it is widely used in confectionary, juices, jams, vinegars, and bread. In addition, a lot of physiological functions have been clarified, for example, radical-scavenging activity, reduction of liver injury, anti-hyperglycemic effect, and blood fluidity improving effects (Tanaka et al. 2017).
Nowadays, breeders are focusing on processing aptitude, increasing pigment content, and high competitiveness by adding high value in the colorants. New lines with high anthocyanin and low dry matter content were selected for reduction in the production cost. Manufacturers seek improvements of the stability for light and heat stimulation. The color is mainly dependent upon the structure (Hong and Ronald 1990), existence ratio (Yoshinaga et al. 1999), and/or coexisting substances (Takeda 1988) of each anthocyanin. Therefore, breeders have tried to make the line mass with various anthocyanin components. The mechanism of anthocyanin synthesis has been clarified in the gene level (Mano et al. 2007). It can be expected that efficient selection techniques will lead to the development of anthocyanin-rich lines in the future.
Steamed and dried sweetpotato cakes, called ‘hoshi-imo’ in Japanese, are an important local agricultural product in Japan. Recently, shirota, which is a white-opaque defect that occurs frequently in hoshi-imo as a result of hot summer, has become a more frequent problem. This defect greatly reduces the commodity value of hoshi-imo, and the breeding of cultivars with lower occurrence rates of shirota is becoming important aim. Moreover, expanding the variety of colors has been requested by farmers and consumers. It is also important to breed new cultivars with excellent taste for hoshi-imo. In this chapter, we review the sweetpotato cultivars bred recently for processed foods (Table 2).
Table 2.
Characteristics of leading varieties and current cultivars for processed foods in Japan
| Cultivars | Released year | Ratio for yield of the standard cultivara | Resistance to diseases/pests | Quality of processed foods and other characteristics |
|---|---|---|---|---|
| Tamayutaka | 1960 | — | Black rot | Leading variety until recently for hoshi-imo, high yield, slightly good taste |
| Ayamurasaki | 1995 | — | — | Leading variety for purple food colorant, high anthocyanin content |
| Murasakimasari | 2001 | 107% of Ayamurasaki | RKN, RLN | High anthocyanin content, shochu with a sweet and wine-like flavor |
| Ayakomachi | 2003 | 100% of Sunny Red | RKN | High β-carotene content, suitable for cooking materials |
| Okikogane | 2004 | 112% of Koganesengan | — | The steamed root is not sweet, high yield, suitable for cooking materials |
| Akemurasaki | 2005 | 105% of Ayamurasaki | RKN, RLN | High anthocyanin content, suitable for food colorant |
| Tamaakane | 2009 | 92% of J-Red | RKN, RLN | High β-carotene content, shochu with a tropical fruit flavor |
| Hoshikogane | 2012 | 90% of Tamayutaka | RKN | Hoshi-imo with high quality, high yield |
Values under standard cultivation conditions.
‘Tamayutaka’ was bred by the Kanto-Tosan agriculture experiment station in 1960 and is the progeny of a cross between ‘Kanto No.33’ and ‘Kuroshirazu’ (Onoda et al. 1970). ‘Tamayutaka’ is suitable for hoshi-imo. The storage roots are short fusiform with a light-yellow-white with partially light crimson skin and white-yellow flesh. The yield of this cultivar is high, and the mean weight of a root is more than 300 g. Shirota defects occur frequently in hoshi-imo made from ‘Tamayutaka’. This cultivar is resistant to black rot and its sprouting ability and storage ability are slightly high. This cultivar has been a leading variety for hoshi-imo processing for a long time, and the total cultivation area in 2013 was 127 ha in the Kanto region.
‘Ayamurasaki’ is a cultivar for processed foods with high anthocyanin content, developed at KARC/NARO and released in 1995 (Yamakawa et al. 1997). It is derived from a cross between ‘Kyushu No.109’ and ‘Satsumahikari’. The storage root is elongated fusiform with deep-purple skin and flesh. The yield of ‘Ayamurasaki’ is higher than that of ‘Kokei No.14’. The anthocyanin content in storage roots is very high. The color value, which is based on the absorption coefficient and is an indication of anthocyanin content, is about five times that of ‘Yamagawamurasaki’. Anthocyanin-rich extracts from storage roots are used in various foods and beverages. This cultivar is slightly resistant to RKN. ‘Ayamurasaki’ exhibits moderate sprouting ability and slightly high storage ability. The total cultivation area in 2015 was about 100 ha in southern Kyushu.
‘Murasakimasari’ is a cultivar with high anthocyanin content used for shochu spirits and processed foods. It was developed at KARC/NARO and released in 2001 (Kumagai et al. 2002a) and is the progeny of a cross between ‘Ayamurasaki’ and ‘Siroyutaka’. The storage root is uniformly fusiform with a good shape and deep purple skin. The flesh color is deep purple, and the color value is the same as that of ‘Ayamurasaki’. The yield, dry matter content, and starch content of ‘Murasakimasari’ are higher than those of ‘Ayamurasaki’. ‘Murasakimasari’ is suitable for direct-planting methods, which reduces labor and production costs for management of nurseries and transplanting. The alcohol yield is similar to that of ‘Koganesengan’ in the brewing of shochu, and spirits made from ‘Murasakimasari’ have a slightly sweet, wine-like flavor. This cultivar is resistant to RLN and RKN, and it is slightly resistant to black rot. ‘Murasakimasari’ exhibits moderate sprouting ability and high storage ability. The total cultivation area in 2015 was about 300 ha in southern Kyushu.
‘Ayakomachi’ is a cultivar for cooking materials and table use that was developed at KARC/NARO and released in 2003 (Kai et al. 2004). It is derived from a cross between ‘Sunny Red’ and ‘Hamakomachi’. The storage root is uniformly fusiform with a red skin and orange flesh. The yield of ‘Ayakomachi’ is similar to that of ‘Sunny Red’ and slightly less than that of ‘Kokei No.14’. The dry matter content is less than that of ‘Sunny Red’ and ‘Kokei No.14’, and the β-carotene content is 30 mg/100 g DW. This cultivar is slightly resistant to RLN and is resistant to RKN. ‘Ayakomachi’ exhibits moderate sprouting ability and high storage ability, and is cultivated in Chiba prefecture.
‘Okikogane’, a cultivar for cooking materials and food processing, was developed at KARC/NARO and released in 2004 (Kai et al. 2005). It is the progeny of a cross between ‘Beniwase’ and ‘Satsumahikari’. ‘Satsumahikari’ is a non-sweet cultivar lacking β-amylase activity for food processing. The storage root is uniformly short fusiform with a pale-yellowish-brown skin and pale-yellowish-white flesh. Although the yield of ‘Okikogane’ is higher than that of ‘Koganesengan’, its starch content is lower. The steamed root texture is slightly dry with a slightly unpleasant taste because it is not sweet. ‘Okikogane’ is suitable for wide variety of dishes, such as croquettes or French-fries like an Irish potato. This cultivar is slightly resistant to RLN, and it exhibits moderate sprouting ability and high storage ability. ‘Okikogane’ is mainly cultivated in Kyushu and Okinawa region.
‘Akemurasaki’ is a cultivar for processed foods with high anthocyanin content that was developed at KARC/NARO and released in 2005 (Sakai et al. 2010). It is derived from a cross between ‘Ayamurasaki’ and ‘Kyukei 174’. The storage root is elongated fusiform with a deep red-purple skin and deep purple flesh. The yield, dry matter content, and starch content of ‘Akemurasaki’ are all similar to those of ‘Ayamurasaki’. The anthocyanin content of ‘Akemurasaki’ is 15–30% higher than that of ‘Ayamurasaki’, and the ratio of cyanidin-based anthocyanins is higher than that of ‘Ayamurasaki’, and thus the paste and powder colors of ‘Akemurasaki’ are a slightly strong shade of blue. This cultivar is resistant to RLN and RKN. ‘Akemurasaki’ exhibits moderate sprouting ability and slightly high storage ability, and it is cultivated in southern Kyushu.
‘Tamaakane’ is a cultivar with high β-carotene content used for shochu spirits, and it was developed at KARC/NARO and released in 2009 (Sakai et al. 2011). It is the progeny of a cross between ‘Resisto’ and ‘Kyukei 179’. The storage root is uniformly short fusiform with a light-brown skin and bright-orange flesh. The yield of ‘Tamaakane’ is higher than that of ‘Koganesengan’, but the dry matter and starch contents are lower. The alcohol yield is less than that of ‘Koganesengan’ in the brewing of shochu, and spirits made from ‘Tamaakane’ have a tropical fruit-like flavor derived from β-ionone, which is a decomposition product of β-carotene during the distillation process. ‘Tamaakane’ can be adapted for direct-planting methods because the ‘seed’ storage root of this cultivar disappears during the growth process, as is the case with the Irish potato. This cultivar is resistant to RLN and RKN. ‘Tamaakane’ exhibits slightly low sprouting ability and high storage ability. The total cultivation area in 2015 was about 40 ha in southern Kyushu.
‘Hoshikogane’ was released by NICS in 2012 and is derived from a cross between ‘Kanto No.120’ and ‘Quick Sweet’ (Kuranouchi et al. 2015). ‘Hoshikogane’ is suitable for hoshi-imo processing. The storage roots are short fusiform with a red-purple skin and light-yellow flesh. The yield of this cultivar is about 90% of that of ‘Tamayutaka’, and the mean weight of a storage root is slightly lower than that of ‘Tamayutaka’. Shirota hardly occurs in this cultivar. The brix% of hoshi-imo made from this cultivar exceeds that of ‘Tamayutaka’ from early winter, and the taste of ‘Hoshikogane’ is almost the same or slightly exceeds that of ‘Tamayutaka’. This cultivar is resistant to RKN and is slightly resistant to stem rot and black rot. Its sprouting ability is slightly high, but its storage ability is low. ‘Hoshikogane’ is cultivated in Ibaraki prefecture.
Breeding objectives and cultivars for raw materials of alcohol and starch
Imo-shochu is a traditional spirit made from the sweetpotato and is popular with consumers in Japan. The leading variety used as a raw material of alcohol production is ‘Koganesengan’ (Sakai et al. 1967). This cultivar exhibits high yield, wide regional adaptability, and is multipurpose. In addition, shochu made from ‘Koganesengan’ has a good flavor and sweetness. Thus ‘Koganesengan’ has gained a strong reputation from users as a raw material for shochu. However, ‘Koganesengan’ has some disadvantages including limitations in disease resistance, storage ability, and shape of the storage root. Therefore, the breeding objectives for alcohol are to provide not only high yield and high brewing aptitude but also disease resistance, high storage ability, and superior shape of the storage root. Moreover, to expand the consumption of shochu, there is a desire to develop new products more-favored by young people and woman, who are currently not major consumers of shochu. It is thought that the characteristic flavor of conventional shochu comes from monoterpene alcohols, especially linalool, and β-damascenone as the main flavor ingredients (Kamiwatari et al. 2006, Kamiwatari and Setoguchi 2011). Currently, many characteristic shochu products have been developed such as that made from purple-fleshed cultivars, which have wine- or yogurt-like flavors characterized by diacetyl and orange-fleshed cultivars with fruity tastes characterized by β-ionon. These shochu products contribute to the development of the shochu industry, thus breeding of cultivars corresponding to the diversification of flavors is also desirable.
Sweetpotato starch does not have excellent characteristics as a food material, thus approximately 70–80% of starch obtained from sweetpotato is used as a raw material for saccharification, and the starch is competing with cheaper imported corn and tapioca starches. Therefore, a reduction in production costs is a key requirement, and the most important breeding objectives for starch production are high yielding ability and high starch content. Moreover, to extend use as a food raw material, improvement of the quality of the starch becomes an important object. For adding value to sweetpotato starch, a new cultivar ‘Konamizuki’ has been developed, which provides starch with an extremely low degree of retrogradation compared with conventional starch (Katayama et al. 2012). In this chapter, we review the sweetpotato cultivars bred recently as raw materials of alcohol and starch (Table 3).
Table 3.
Characteristics of leading varieties and current cultivars for raw materials in Japan
| Cultivars | Released year | Ratio for yield of the standard cultivara | Resistance to diseases/pests | Quality of products and other characteristics |
|---|---|---|---|---|
| Koganesengan | 1966 | — | — | Leading variety for shochu spirits, good flavor and taste |
| Siroyutaka | 1985 | 124% of Koganesengan | RKN, black rot | Leading variety for starch production, high yield |
| Konahomare | 2000 | 106% of Shiroyutaka | — | Starch production, high yield, high starch content, high starch yield |
| Daichinoyume | 2003 | 109% of Shiroyutaka | RKN | Starch production, high yield, high starch content, high starch yield |
| Tokimasari | 2008 | 99% of Koganesengan | — | Shochu with full-dodied and rich flavor, high alcohol yield |
| Satsumamasari | 2010 | 108% of Koganesengan | RKN | Shochu with sweet and fruity flavor, and refreshing taste, high alcohol yield |
| Konamizuki | 2010 | 99% of Shiroyutaka | RKN | Starch production, low pasting temperature starch, cold storage stability |
| Koganemasari | 2012 | 102% of Koganesengan | RKN, RLN | Shochu with flavor similar to Koganesengan, high alcohol yield |
Values under standard cultivation conditions.
‘Koganesengan’ was developed at Kyushu National Agricultural Experiment Station (now known as KARC/NARO) and released in 1966 for starch production (Sakai et al. 1967). It is the progeny of a cross between ‘Kakei 7–120’ and ‘L-4–5 (Pelican Processor, cultivar from the USA)’. The storage root is short and fusiform with yellowish-white skin and flesh. The yield of ‘Koganesengan’ is higher than that of ‘Norin No.2’, a predecessor cultivar of starch production. The starch content shows a range of 24–26% and the starch yield is higher than that of ‘Norin No.2’, but ‘Koganesengan’ is inferior in resistance to RLN and RKN. This cultivar exhibits slightly high sprouting ability but slightly low storage ability. The steamed roots have a favorable taste. ‘Koganesengan’ is a main cultivar grown in southern Kyushu and used for shochu, table use, and processing. Shochu made from this cultivar has a strong flavor of sweetpotato and is superior in terms of the balance between flavor and sweetness. Therefore, ‘Koganesengan’ is regarded as the most suitable cultivar for shochu. The total cultivation area in 2013 was about 8,300 ha in southern Kyushu.
‘Shiroyutaka’ was developed at Kyushu National Agricultural Experiment Station (now KARC/NARO) and released in 1985 for starch production (Sakamoto et al. 1987). It is derived from a cross between ‘Kyukei 708–13’ and ‘S684–6’. The storage root is fusiform with a white skin, faintly red colored at the top and tail and white flesh. The yield of ‘Shiroyutaka’ is 124% of that of ‘Koganesengan’ under standard cultivation conditions. The starch content is 24.0%, which is similar to that of ‘Koganesengan’, but starch yield is 120% of that of ‘Koganesengan’. ‘Siroyutaka’ exhibits resistance to RKN and black rot and slight resistance to RLN. This cultivar exhibits high sprouting ability but slightly low storage ability. ‘Shiroyutaka’ is a leading variety for starch production in southern Kyushu and the total cultivation area in 2013 was about 4,100 ha.
‘Konahomare’ is a cultivar for starch production, developed at KARC/NARO and released in 2000 (Kumagai et al. 2002b). It is the progeny of a cross between ‘Hi-Starch’ and ‘Kyukei 82124-1’. The storage root is short and fusiform with light brown skin and light-yellowish-white flesh. The yield of ‘Konahomare’ is 106% of that of ‘Shiroyutaka’ under standard cultivation conditions. The starch content is 27.3%, which is 2.2% higher than that of ‘Siroyutaka’. Depending on the cultivation conditions, the starch yield of ‘Konahomare’ is 15–29% higher than that of ‘Siroyutaka’. This cultivar is slightly resistant to RKN, and it exhibits moderate sprouting ability but slightly low storage ability. The total cultivation area in 2013 was 92 ha in southern Kyushu.
‘Daichinoyume’ is a cultivar for starch production, developed at KARC/NARO and released in 2003 (Katayama et al. 2004). It is derived from a cross between ‘Kyukei 117’ and ‘Hi-starch’. The storage root is uniformly fusiform with white skin and light yellowish-white flesh. The yield of ‘Daichinoyume’ is 109% of that of ‘Shiroyutaka’ under standard cultivation conditions. The starch content is 26.9%, which is 1.9% higher than that of ‘Shiroyutaka’, and the starch yield is 17–31% higher depending on the cultivation conditions. This cultivar is resistant to RKN and slightly resistant to RLN. ‘Daichinoyume’ exhibits slightly high sprouting and storage abilities, which are superior to those of ‘Siroyutaka’. The total cultivation area in 2013 was 371 ha in southern Kyushu.
‘Tokimasari’ is a cultivar for shochu spirits, developed at KARC/NARO and released in 2008 (Katayama et al. 2009). It is the progeny of a cross between ‘Starch Queen’ and ‘Konahomare’. The storage root is short and fusiform with light-pink skin and light-yellowish-white flesh. The yield of ‘Tokimasari’ is similar to that of ‘Koganesengan’ under standard cultivation conditions. The starch content is 25.5%, which is 1.5% higher than that of ‘Koganesengan’, and the starch yield is 106%. The alcohol yield is higher than that of ‘Koganesengan’ in the brewing process. Shochu made from ‘Tokimasari’ is full-bodied with light sweetness and characterized by a rich flavor of steamed storage roots. The monoterpene alcohol concentration in ‘Tokimasari’ shochu is four times higher than that made from ‘Koganesengan’. This cultivar is slightly resistant to RKN. Its sprouting ability and storage ability are slightly high. The total cultivation area in 2013 was 5 ha in southern Kyushu.
‘Satsumamasari’ is a cultivar for shochu spirits, developed at KARC/NARO and released in 2010 (Katayama et al. 2013). It is derived from a cross between ‘Tokimasari’ and ‘Kyushu No.102’. The storage root is short and fusiform with yellowish-white skin and flesh. The yield of ‘Satsumamasari’ is 108% of that of ‘Koganesengan’ under standard cultivation conditions, and the starch content is 25.2%, which is 1.6% higher than that of ‘Koganesengan’. The starch yield is 114% of that of ‘Koganesengan’, and the alcohol yield is higher. Shochu made from ‘Satsumamasari’ has a sweet and fruity flavor, and a refreshing taste. This cultivar is resistant to RKN. Its sprouting and storage abilities are slightly high. The total cultivation area in 2014 was 12 ha in Kagoshima prefecture.
‘Konamizuki’ is a cultivar for starch production, developed at KARC/NARO and released in 2010 (Katayama et al. 2012). It is the progeny of a cross between ‘99L04-3’ and ‘Kyukei 236’. The storage root is long and fusiform with white skin and flesh. The yield of ‘Konamizuki’ is similar to that of ‘Shiroyutaka’ under standard cultivation conditions. On the other hand, the yield was 79% of that of ‘Shiroyutaka’ in long-term mulching conditions. The starch content was 24.5–24.6%, which is 0.1–1.0% higher than that of ‘Shiroyutaka’. The starch yield is similar to that of ‘Shiroyutaka’ under standard cultivation conditions but 21% lower in long-term mulching cultivation conditions. The chain-length distribution of the amylopectin molecules shows that ‘Konamizuki’ starch has a higher proportion of short chains (DP 6–10) than the starch of ordinary cultivars (Kitahara et al. 2014). The starch pasting temperature is approximately 20°C lower than that of ‘Shiroyutaka’. The percentage of leaked water and hardness of starch gel after cold storage revealed that the starch of ‘Konamizuki’ retrogrades extremely slowly compared with that of ‘Shiroyutaka’ and has excellent cold storage stability. The raw starch of ‘Konamizuki’ has higher digestibility than ‘Shiroyutaka’, and ‘Konamizuki’ starch is of naturally high quality and is beneficial in keeping the texture of processed products soft. This cultivar is slightly resistant to black rot and RLN and resistant to RKN. ‘Konamizuki’ exhibits slightly high sprouting ability and moderate storage ability. The total cultivation area in 2015 was 36 ha in Kagoshima prefecture.
‘Koganemasari’ is a cultivar for shochu spirits, developed at KARC/NARO and released in 2012 (Sakai et al. 2013). It is derived from a cross between ‘Starch Queen’ and ‘Kyukei 236’. The storage root is short and fusiform with a yellowish-white skin and white flesh. The yield of ‘Koganemasari’ is similar to that of ‘Koganesengan’ under standard cultivation conditions. The starch content is 26.9%, which is 4.1% higher than that of ‘Koganesengan’, and the starch yield is 120% of that of ‘Koganesengan’. The alcohol yield is higher than that of ‘Koganesengan’ in the brewing of shochu. The flavor of shochu made from ‘Koganemasari’ is similar to that of ‘Koganesengan’. This cultivar is resistant to RLN and RKN. ‘Koganemasari’ exhibits slightly high sprouting ability and high storage ability, and is mainly cultivated in Miyazaki prefecture.
Future prospects
There are many remaining problems to be resolved thorough future sweetpotato breeding programs. The major problems focus on the reduction of production costs, stable production and supply, climate change measures, high added value, and developments to meet new demands. The mechanization of sweetpotato cultivation has not advanced greatly, and working hours for management of nurseries and transplanting are higher than those of other crops. In order to reduce the working hours and production costs, it is important to improve seedling plant types that are suitable for transplanting and cultivating using machinery (Kuranouchi et al. 2016). In addition, direct planting methods of ‘seed’ storage roots are considered to remove the need for transplanting work, and new cultivars with high direct planting suitability are expected for raw materials of starch and alcohol, following ‘Tamaakane’ and ‘Murasakimasari’ for processing use. In order to secure stable production and supply, it is also necessary to improve yields, resistance to diseases, and storage ability. The production of sweetpotato is gradually decreasing year by year in Japan, and new cultivars with high yield and multiple disease resistances are being sought by the sweetpotato farmers. Sweetpotato storage roots do not exhibit dormancy and are not suitable for a long-term storage, such as for more than 6 or 7 months. In Japan, the storage roots are harvested in autumn and are stored until the next summer. The storage roots saccharify during storage and their quality is easy to change during long-term storage. The farmers use a curing technic (4–7 days at 30–33°C and 90–100% relative humidity) to aid in wound healing and reduce loss due to disease during storage. Because the supply shows a slight lack in summer, new cultivars with high storage ability or early thickening ability are desired to improve the annual supply.
The influence of climate change and global warming is of concern. The invasion of tropical pests, such as sweetpotato weevils, may become a new threat, but the northernmost limit of sweetpotato cultivation area is advancing. Sweetpotato weevils are already endemic to the South-West Islands and Ogasawara Islands in Japan. Research on resistance to these new pests is considered important and is already being undertaken at KARC/NARO (Okada et al. 2017). On the other hand, sweetpotato cultivation has recently spread to colder climate areas that were not suitable for production in the past. Therefore, it is also necessary to breed new cultivars suitable for cultivation in colder areas, and NICS is developing new lines with better low soil temperature tolerance.
Japanese consumers are highly discerning with regard to food quality, and Japanese sweetpotato cultivars for table use have the highest quality levels in the world. Consumers are also very conscious of health functionality as well as high quality for future sweetpotato production. In order to enhance the health functionality for consumers and increase consumption, it is important to improve eating quality and the levels of constituents related to health function, such as anthocyanins, β-carotenes, and dietary fibers (Tanaka et al. 2017). In addition, the manufacturers of processed foods, Shochu spirits, or starch are keen to achieve reduced costs and to develop new demand for sweetpotato products. We evaluate the quality and processing aptitude of new lines, in which the manufactures collaborate. It is necessary to breed new cultivars containing specific pigments or starches with new properties in order to meet these new demands (Kitahara et al. 2017, Tanaka et al. 2017).
Because sweetpotato is a highly heterozygous, outcrossing hexaploid with 90 chromosomes, this complicates genetic and linkage analysis, and the accumulation of genome sequence information for this crop had been slower than in selfing diploid crops. However, whole genome sequencing in the diploid ancestor, Ipomoea trifida (H. B. K.) Don was reported recently (Hirakawa et al. 2015). Additionally, whole genome sequencing and genetic linkage mapping in hexaploid sweetpotato is progressing now (Isobe et al. 2017, Monden and Tahara 2017). In the near future, DNA makers for sweetpotato will be developed, and the DNA maker selection will be possible in sweetpotato breeding programs. Marker-assisted breeding may also offer new targets through the modification of pigments or starches by accumulating recessive alleles.
Sweetpotato has played various important roles as a famine-relief crop, biomass crop, commercial crop, and health promoting crop in its long history. These diverse applications will also support the cultivation and use of this crop in various regions and situations. It will be necessary to continue to improve sweetpotato for a wide range of applications in the future.
Literature Cited
- Asis, C.A.Jr. and Adachi, K. (2003) Isolation of endophytic diazotroph Pantoea agglomerans and nondiazotroph Enterobacter asburiae from sweetpotato stem in Japan. Lett. Appl. Microbiol. 38: 19–23. [DOI] [PubMed] [Google Scholar]
- Austin, D.F. (1988) The taxonomy, evolution and genetic diversity of sweet potatoes and related wild species. In: Exploration, Maintenance, and Utilization of Sweet Potato Genetic Resources: Report of the First Sweet Potato Planning Conference 1987, International Potato Center, Lima, pp. 27–59. [Google Scholar]
- FAOSTAT (2013) URL: http://faostat3.fao.org/home/E
- Fujise, K. (1964) Studies on flowering, seeds setting and self- and cross-incompatibility in the varieties of sweet potato. Bull. Kyushu Agr. Exp. Sta. 9: 123–246. [Google Scholar]
- Goda, Y., Shimizu, T., Kato, Y., Nakamura, M., Maitani, T., Yamada, T., Terahara, N. and Yamaguchi, M. (1997) Two acylated anthocyanins from purple sweet potato. Phytochemistry 44: 183–186. [DOI] [PubMed] [Google Scholar]
- Hirakawa, H., Okada, Y., Tabuchi, H., Shirasawa, K., Watanabe, A., Tsuruoka, H., Minami, C., Nakayama, S., Sasamoto, S., Kohara, M.et al. (2015) Survey of genome sequences in a wild sweet potato, Ipomoea trifida (H. B. K.) G. Don. DNA Res. 22: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, V. and Ronald, E. (1990) Wrolstad Use of HPLC separation/photodiode array detection for characterization of anthocyanins. J. Agric. Food Chem. 38: 708–715. [Google Scholar]
- Ishiguro, K., Yamakawa, O., Kumagai, T., Yoshinaga, M., Kai, Y. and Hidaka, M. (2004) Benimasari: new sweetpotato cultivar for table use. Bull. Natl. Agric. Res. Cent. Kyushu Okinawa Reg. 43: 59–85. [Google Scholar]
- Isobe, S., Shirasawa, K. and Hirakawa, H. (2017) Challenges to genome sequence dissection in sweetpotato. Breed. Sci. 67: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai, Y., Katayama, K., Sakai, T. and Yoshinaga, M. (2004) Ayakomachi: New sweetpotato cultivar for cooking material and table use. Sweetpotato Research Front 17: 4. [Google Scholar]
- Kai, Y., Katayama, K., Sakai, T. and Yoshinaga, M. (2005) Okikogane: New sweetpotato cultivar for cooking material and food processing. Sweetpotato Research Front 19: 4. [Google Scholar]
- Kai, Y., Katayama, K., Sakai, T. and Yoshinaga, M. (2010) Beniharuka: A new sweetpotato cultivar for table use. Sweetpotato Research Front 23: 2. [Google Scholar]
- Kamiwatari, T., Setoguchi, S., Kanda, J., Setoguchi, T. and Ogata, S. (2006) Effects of a sweetpotato cultivar on the quality of Imo-shochu with references to the characteristic flavor. J. Brew. Soc. Japan 101: 437–445. [Google Scholar]
- Kamiwatari, T. and Setoguchi, T. (2011) Effects of a sweetpotato cultivar on the flavor of Imo-shochu. Seibutsu-Kogaku Kaishi 89: 724–727. [Google Scholar]
- Katayama, K., Komae, K., Kohyama, K., Kato, T., Tamiya, S. and Komaki, K. (2002) New sweet potato line having low gelatinization temperature and altered starch structure. Starch/Starke 54: 51–57. [Google Scholar]
- Katayama, K., Tamiya, S., Kuranouchi, T., Komaki, K. and Nakatani, M. (2003) New sweet potato cultivar “Quick Sweet”. Bull. Natl. Inst. Crop. Sci. 3: 35–52. [Google Scholar]
- Katayama, K., Kumagai, T., Kai, Y., Ishiguro, K., Yoshinaga, M. and Nakazawa, Y. (2004) Daichinoyume: New sweetpotato cultivar for starch production. Sweetpotato Research Front 18: 4. [Google Scholar]
- Katayama, K., Kumagai, T., Yamakawa, O., Kai, Y., Yoshinaga, M., Ishiguro, K., Sakai, T. and Nakazawa, Y. (2009) “Tokimasari”: A new sweetpotato cultivar”. Bull. Natl. Agric. Res. Cent. Kyushu Okinawa Reg. 50: 111–129. [Google Scholar]
- Katayama, K., Sakai, T., Kai, Y., Nakazawa, Y. and Yoshinaga, M. (2012) “Konamizuki”: a new sweetpotato cultivar”. Bull. NARO Kyushu Okinawa Agric. Res. Cent. 58: 15–36. [Google Scholar]
- Katayama, K., Kai, Y., Sakai, T., Kumagai, T., Ishiguro, K., Nakazawa, Y., Yamakawa, O. and Yoshinaga, M. (2013) “Satsumamasari”: A new sweetpotato cultivar”. Bull. NARO Kyushu Okinawa Agric. Res. Cent. 60: 39–60. [Google Scholar]
- Katayama, K., Ohara-Takada, A., Kuranouchi, T., Nakamura, Y., Kai, Y., Kumagai, T. and Yoshinaga, M. (2014) New sweetpotato cultivars bred for food recently in Japan. In: Proceedings of NARO International Symposium 2014, New Era of Sweetpotato Research in East Asia (6th Japan – China – Korea Sweetpotato Workshop), November 28–30, 2014, Kagoshima, Japan, pp. 14–15. [Google Scholar]
- Katayama, K., Tamiya, S., Sakai, T., Kai, Y., Ohara-Takada, A., Kuranouchi, T. and Yoshinaga, M. (2015) Inheritance of low pasting temperature in sweetpotato starch and the dosage effect of wild-type alleles. Breed. Sci. 65: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara, K., Fukunaga, S., Katayama, K., Takahata, Y., Nakazawa, Y., Yoshinaga, M. and Suganuma, T. (2005) Physicochemical properties of sweetpotato starches with different gelatinization temperatures. Starch/Starke 57: 473–479. [Google Scholar]
- Kitahara, K., Yamasaki, T., Fujita, K. and Suganuma, T. (2014) Physicochemical properties of starches from recently bred sweetpotatoes in Japan. J. Appl. Glycosci. 61: 81–88. [Google Scholar]
- Kitahara, K., Nakamura, Y., Otani, M., Hamada, T., Nakayachi, O. and Takahata, Y. (2017) Carbohydrate components in sweetpotato storage roots: their diversities and genetic improvement. Breed. Sci. 67: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, M. and Nakanishi, T. (1982) Flower induction by top-grafting in sweet potato. In: Proceedings of the Fifth International Symposium on Tropical Root and Tuber Crops, 17–21 September 1979, Philippine Council for Agriculture and Resources Research; Los Baños, Laguna, Philippines, pp. 49–55. [Google Scholar]
- Komaki, K. (1995) Sweetpotato genetic resources and breeding in Japan. In: Root and tuber crops: MAFF International Workshop on Genetic Resources 1995, Research Council Secretariat of Ministry of Agriculture, Forestry and Fisheries, Tsukuba, pp. 115–120. [Google Scholar]
- Komaki, K. (1997) Present status and future prospect of sweetpotato production and consumption in Japan. In: Proceedings of International Workshop on Sweetpotato Production System toward the 21st Century, 9–10 December 1997, Miyakonojo, Japan, pp. 149–158. [Google Scholar]
- Kouzuma, M., Uchimura, C., Yasuniwa, M., Kamikado, T., Satou, M. and Yoshida, N. (2003) New sweetpotato cultivars bred in Tanegashima: ‘Annou-beni’, ‘Annou-kogane’ and ‘Tanegashima-roman’, ‘Tanegashima-gold’. Bull. Kagoshima Agri. Exp. Sta. 31: 1–15. [Google Scholar]
- Kowyama, Y., Tsuchiya, T. and Kakeda, K. (2000) Sporophytic self-incompatibility in Ipomoea trifida, a close relative of sweetpotato. Ann. Bot. 85: 191–196. [Google Scholar]
- Kumagai, T., Umemura, Y., Baba, T. and Iwanaga, M. (1990) The inheritance of β-amylase null in storage roots of sweet potato, Ipomoea batatas (L.) Lam. Theor. Appl. Genet. 79: 369–376. [DOI] [PubMed] [Google Scholar]
- Kumagai, T. (2001) Breeding and varieties of sweetpotato. Farming Japan 35: 11–17. [Google Scholar]
- Kumagai, T., Yamakawa, O., Kai, Y. and Ishiguro, K. (2002a) Murasakimasari: New sweetpotato cultivar for processing. Sweetpotato Research Front 13: 3. [Google Scholar]
- Kumagai, T., Yamakawa, O., Yoshinaga, M., Ishiguro, K., Hidaka, M. and Kai, Y. (2002b) “Konahomare”: A new sweetpotato cultivar for starch production”. Bull. Natl. Agric. Res Cent. Kyushu Okinawa Reg. 40: 1–16. [Google Scholar]
- Kuranouchi, T., Takada, A., Nakamura, Y., Fujita, T., Nakatani, M., Kumagai, T. and Katayama, K. (2015) Breeding of a new sweetpotato variety ‘Hoshikogane’ suitable for steamed and cured sweetpotato slices (‘Hoshi-imo’) with high yield and good quality. Bull. NARO Inst. Crop. Sci. 15: 1–28. [Google Scholar]
- Kuranouchi, T., Kumazaki, T., Kumagi, T. and Nakatani, M. (2016) Breeding erect plant type sweetpotato lines using cross breeding and gamma-ray irradiation. Breed. Sci. 66: 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebenstein, G. and Thottappilly, G. Editors (2009) The Sweetpotato, Springer Science+Business Media, p. 522. [Google Scholar]
- Mano, H., Ogasawara, F., Sato, K., Higo, H. and Minobe, Y. (2007) Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol. 143: 1252–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, F.W. (1965) Incompatibility in the sweet potato. A review. Econ. Bot. 19: 406–415. [Google Scholar]
- Monden, Y., Tahara, M. and Yamamoto, A. (2013) Development of DNA markers for anthocyanin content purple sweetpotato using active retrotransposon insertion polymorphism. DNA polymorphism 21: 47–54. [Google Scholar]
- Monden, Y. and Tahara, M. (2017) Genetic linkage analysis using DNA makers in sweetpotato. Breed. Sci. 67: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, T. and Kobayashi, M. (1979) Geographic distribution of cross incompatibility group in sweetpotato. Incompatibility Newsletter 11: 72–75. [Google Scholar]
- Nakatani, M. (2001) Cultivation technology of sweetpotato. Farming Japan 35: 18–21. [Google Scholar]
- Nishiyama, I., Miyazaki, T. and Sakamoto, S. (1975) Evolutionary autoploidy in the sweet potato (Ipomoea batatas (L.) Lam) and its progenitors. Euphytica 24: 197–208. [Google Scholar]
- Odake, K., Hatanaka, A., Kajiwara, T., Muroi, T., Nishiyama, K., Yamakawa, O., Terahara, N. and Yamaguchi, M. (1994) Evaluation method and breeding of purple sweet potato ‘Yamagawamurasaki’ (Ipomoea batatas Poir.) for raw material of food colorants. J. Jpn. Soc. Food. Sci. Technol. 41: 287–293. [Google Scholar]
- Ohara-Takada, A., Kuranouchi, T., Nakamura, Y., Katayama, K., Nakatani, M., Tamiya, S., Komaki, K. and Kumagai, T. (2011) “Himeayaka”, a new sweetpotato cultivar with good taste and compact size storage root”. Bull. Natl. Inst. Crop Sci. 12: 103–122. [Google Scholar]
- Ohara-Takada, A., Kumagai, T., Kuranouchi, T., Nakamura, Y., Fujita, T., Nakatani, M., Tamiya, S. and Katayama, K. (2016) “Aikomachi”, a new sweetpotato cultivar with good appearance and high confectionery quality”. Bull. NARO Inst. Crop Sci. 16: 35–56. [Google Scholar]
- Okada, Y., Kobayashi, A., Tabuchi, H. and Kuranouchi, T. (2017) Review of major sweetpotato pests in Japan, with information on resistance breeding programs. Breed. Sci. 67: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda, M., Fukuda, T., Ota, Y., Chishiki, T., Toyoda, Y., Suzuki, S., Ishikawa, H. and Takemata, T. (1970) On the three new sweet potato varieties ‘Kurimasari’ ‘Tamayutaka’ ‘Konasengan’. Jour. of the Cent. Agric. Exp. Stn. 14: 167–194. [Google Scholar]
- Paula, M.A., Reis, V.M. and Dobereiner, J. (1991) Interactions of Glomus clarum with Acetobacter diazotrophicus in infection of sweet potato (Ipomoea batatas), sugarcane (Saccharum spp.), and sweet sorghum (Sorghum vulgate). Biol. Fertil. Soils 11: 111–115. [Google Scholar]
- Reynoso, D., Huaman, Z. and Aguilar, C. (1999a) Methods to induce flowering in sweetpotato. In: Huaman, Z. (ed.) Sweetpotato Germplasm Management Training Manual, International Potato Center, Lima, Section 2.6, 1–7. [Google Scholar]
- Reynoso, D., Huaman, Z. and Aguilar, C. (1999b) Methods to determine the fertility and compatibility of sweetpotato. In: Huaman, Z. (ed.) Sweetpotato Germplasm Management Training Manual, International Potato Center, Lima, Section 2.7, 1–8. [Google Scholar]
- Sakai, K., Marumine, S., Hirosaki, S., Kikukawa, S., Ide, Y. and Shirasaka, S. (1967) On the new variety of sweet potato “Koganesengan”. Bull. Kyushu Agric. Exp. Stn. 13: 55–68. [Google Scholar]
- Sakai, T., Kumagai, T., Kai, Y., Ishiguro, K., Yamakawa, O., Katayama, K., Nakazawa, Y. and Yoshinaga, M. (2010) ‘Akemurasaki’: A new sweetpotato cultivar. Bull. Natl. Agric. Res. Cent. Kyushu Okinawa Reg. 53: 1–24. [Google Scholar]
- Sakai, T., Katayama, K., Kai, Y. and Yoshinaga, M. (2011) New sweetpotato cultivar ‘Tamaakane’ suitable for brewing and direct planting. Sweetpotato Research Front 25: 3. [Google Scholar]
- Sakai, T., Katayama, K., Kobayashi, A., Kai, Y., Kumagai, T., Nakazawa, Y. and Yoshinaga, M. (2013) Koganemasari: New sweetpotato cultivar for sweetpotato shochu (spirits). Sweetpotato Research Front 29: 3. [Google Scholar]
- Sakamoto, S., Marumine, S., Ide, Y., Yamakawa, O., Kukimura, H., Yoshida, T. and Tabuchi, S. (1987) “Shiroyutaka”: A new sweet potato cultivar registered”. Bull. Kyushu Agric. Exp. Stn. 24: 279–305. [Google Scholar]
- Shiga, T., Sakamoto, S., Ando, T., Ishikawa, H., Kato, S., Takemata, T. and Umehara, M. (1985) On a new sweetpotato cultivar ‘Beniazuma’. Bull. Natl. Agric. Res. Cent. 3: 73–84. [Google Scholar]
- Shiotani, I. and Kawase, T. (1989) Genomic structure of the sweet potato and hexaploids in Ipomoea trifida (H. B. K.) Don. Japan. J. Breed. 39: 57–66. [Google Scholar]
- Takahata, Y., Tanaka, M., Otani, M., Katayama, K., Kitahara, K., Nakayachi, O., Nakayama, H. and Yoshinaga, M. (2010) Inhibition of the expression of the starch synthase II gene leads to lower pasting temperature in sweetpotato starch. Plant Cell Rep. 29: 535–543. [DOI] [PubMed] [Google Scholar]
- Takeda, K. (1988) Expression of flower color by anthocyanin. In: Hayashi, K. (ed.) Plant pigment, Yokendo, Tokyo, pp. 284–299. [Google Scholar]
- Tamiya, S., Nakatani, M., Komaki, K., Katayama, K. and Kuranouchi, T. (2003) New sweet potato cultivar “Purple Sweet Lord”. Bull. Natl. Inst. Crop. Sci. 4: 29–43. [Google Scholar]
- Tanaka, M., Ishiguro, K., Oki, T. and Okuno, S. (2017) Functional components in sweetpotato and their genetic improvement. Breed. Sci. 67: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terahara, N., Shimizu, T., Kato, Y., Nakamura, M., Maitani, T., Yamaguchi, M. and Goda, Y. (1999) Six Diacylated Anthocyanins from the Storage Roots of Purple Sweet Potato, Ipomoea batatas. Biosci. Biotechnol. Biochem. 63: 1420–1424. [DOI] [PubMed] [Google Scholar]
- Yamakawa, O., Yoshinaga, M., Hidaka, M., Kumagai, T. and Komaki, K. (1997) “Ayamurasaki”: a new sweetpotato cultivar”. Bull. Kyushu Natl. Agric. Exp. Stn. 31: 1–22. [Google Scholar]
- Yamakawa, O. (2000) New cultivation and utilization system for sweetpotato in Japan. In: Potential of root crops for food and industrial resources: Twelfth Symposium of the International Society for Tropical and Root Crops, 10–16 September 2000, Tsukuba, Japan, pp. 17–20. [Google Scholar]
- Yen, D.E. (1974) The Sweet Potato in Oceania: An Essay in Ethnobotany, Bishop Museum Press, Honolulu, p. 389. [Google Scholar]
- Yoneyama, T., Terakado, J. and Masuda, T. (1998) Natural abundance of 15N in sweet potato, pumpkin, sorghum and castor bean: possible input of N2-derived nitrogen in sweet potato. Biol. Fertil. Soils 26: 152–154. [Google Scholar]
- Yoshinaga, M., Yamakawa, O. and Nakatani, M. (1999) Genotypic diversity of anthocyanin content and composition in purple-fleshed sweet potato (Ipomoea batatas (L.) Lam). Breed. Sci. 49: 43–47. [Google Scholar]
- Watanabe, K. (2015) Potato genetics, genomics, and applications. Breed. Sci. 65: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfe, J.A. (1992) Sweetpotato an untapped food resource, Cambridge University Press, Cambridge, p. 643. [Google Scholar]