Abstract
Purpose
To determine whether the change in the retinal nerve fiber layer (RNFL) thickness in a region of interest (ROI) is a better measure of glaucoma progression than the change in average circumpapillary (cp) RNFL thickness.
Methods
Disc cube scans were obtained with frequency domain optical coherence tomography from 60 eyes of 60 patients (age 61.7±12.7 years) with early or suspected glaucoma and controlled intraocular pressure. The average time between two test dates was 3.2±1.8 years. En-face images of the scans from the two tests were aligned based on the blood vessels, and circumpapillary images were derived for an annulus 100 µm wide and 3.4 mm in diameter, centered on the disc. An ROI was defined as the portion of the cpRNFL plot within the temporal disc that extended below the 1% confidence interval for ≥5°. Trend analysis employing multilevel mixed-effects models was used to compare the rates of change between ROI width and average cpRNFL thickness.
Results
26 of the 60 eyes had a total of 33 ROIs. The ROI width significantly increased between the two test dates (median: 4.9°; Q1=1.03°, Q3=10.5°). In comparison, the average cpRNFL thickness did not decrease significantly over the same period (median: −0.7 µm; Q1= −2.7 µm, Q3=2.7 µm). Mixed effects linear models confirmed significant ROI progression (p=0.015), but not average cpRNFL (p=0.878).
Conclusion
In this population, RNFL thinning in a ROI is a better measure of progression than is average cpRNFL thickness change.
Keywords: glaucoma, OCT, retinal nerve fiber layer, progression
INTRODUCTION
Glaucoma is a progressive optic neuropathy and a leading cause of blindness worldwide.1 The goal of therapy is to slow or halt the rate of progression to levels that will be unlikely to affect patients’ vision-related quality of life during their lifespan. Therefore, accurately detecting changes over time is a crucial step in glaucoma management.
We recently described a method to measure structural progression in treated glaucoma using a region-of-interest (ROI) approach.2 In brief, the ROI analysis focuses on a specific region around the optic nerve head that was abnormal at an earlier visit. After en-face images of the frequency domain optical coherence tomography (fdOCT) scans were aligned according to the position of the blood vessels, circumpapillary images were derived, the retinal nerve fiber (RNFL) thickness was determined for the baseline and final visit, and the change in width over time (in degrees) of the ROI (i.e., the abnormal region) was measured. This ROI method was validated on eyes with disc hemorrhages, which are known to progress more rapidly.3, 4, 5 When compared to the within-visit variability measurements, the change in the width of the ROI was significant, while the change in average cpRNFL thickness, commonly used to detect progression in clinical settings and research6, 7, was not.2 Furthermore, while 94% of the ROIs increased in width, only 68% of the eyes showed a decrease in average cpRNFL thickness. These findings suggest that the ROI approach may be more sensitive for detecting progression than the more conventional global indices. However, the sample was small and restricted to eyes with disc hemorrhages.
The purpose here was to extend the ROI approach to eyes with early glaucomatous damage. Specifically, we examined the ROI change in eyes with early or suspected glaucomatous damage over time and compared it to the average cpRNFL change during the same time period. We hypothesized that for detecting progression, the ROI approach would be superior to using average cpRNFL thickness.
METHODS
This prospective study was approved by the Institutional Review Boards of Columbia University and the New York Eye and Ear Infirmary of Mount Sinai and followed the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all eligible patients.
60 eyes of 60 patients (age: 61.7 ± 12.7 years) were selected from a larger group of eyes in our early glaucoma database (EGD). These 60 eyes had fdOCT disc scans (3D-OCT2000, Topcon) on at least 2 visits that were free of eye movement and other artifacts. The EGD includes eyes with suspicious or abnormal discs and 24-2 visual field (VF) mean deviation (MD) values better than -6 dB (SITA-Standard, Humphrey Field Analyzer; Carl Zeiss Meditec, Inc., Dublin, CA). Abnormal discs were defined as described by the American Academy of Ophthalmology Preferred Practice Pattern guidelines8 and included the following: focal or diffuse neuroretinal rim thinning, focal or diffuse RNFL loss, or an intereye vertical cup-to- disc ratio asymmetry greater than 0.2 not explained by differences in disc size. All EGD eyes had a refractive error between ±6 diopters, were free of other disease processes that could affect the VF, and did not have clinically-significant cataracts based upon the Lens Opacities Classification System (III). For the 60 eyes, the time between the baseline scan and final scan averaged 3.2±1.8 years (range: 0.5 to 7.0 years), and their average 24-2 MD on the first visit was −1.67.
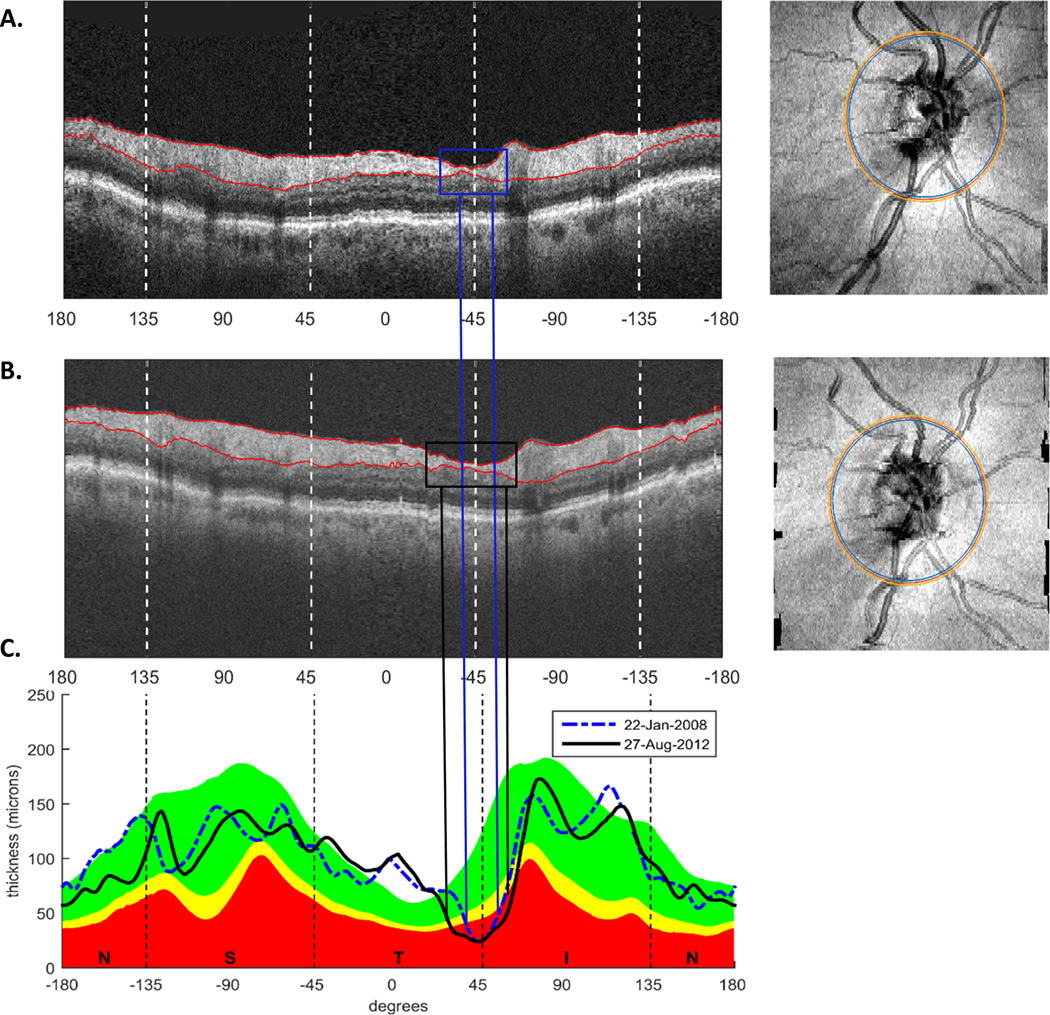
Figure 1 illustrates the ROI method on one example eye. On each OCT scan, the RNFL borders were defined by commercial software and the segmentation was corrected by hand, if necessary. En-face images of the disc cube scans on the baseline (Fig. 1A, right panel) and final visit (Fig. 1A, right panel) were aligned based on the position of the main blood vessels using custom software.2 Annuli centered on the optic disc were derived with a diameter of 3.4 mm and a width of 100 µm (Fig 1A,B, right panel, yellow and blue circles), and circumpapillary images (Fig. 1A,B, left panel) were derived. Finally, cpRNFL thickness as a function of distance around the disc (Fig. 1C) was generated for both visits.2 In Fig. 1C, these cpRNFL thickness profiles are shown for the baseline visit (dashed blue line) and the final visit (black solid line) for the example eye.
Figure 1.
A,B. Circumpapillary images (left panels) derived from the OCT cube scans at baseline (A) and final visit (B) after the en-face images (right panels in A and B) were aligned for one example eye. C. cpRNFL thickness profiles for the same example eye on the baseline (blue) and final (black) visits.
An ROI was defined as the portion of the cpRNFL thickness profile in the baseline scan that extended below the 1% confidence interval (red region in Fig. 1C) of the manufacturer’s normative database for at least 5°. Only the temporal half of the disc (between −90 and 90 degrees for a right eye orientation) was included in order to correspond to the major portion of the 24-2 VF test locations.
Because the goal was to evaluate progression, an ROI on the final scan was measured only if there was an ROI present on the baseline scan. Also, if an eye had more than one ROI, each was recorded as a separate defect. Adjustments for using more than one ROI per eye and both eyes of the same patient are described below. Furthermore, to avoid splitting a continuous defect into two, if two defects fell within 12° of each other, they were recorded as the same defect by using the starting point of the first ROI and the ending point of the final ROI.
In addition to the width of each ROI, the average cpRNFL thickness was also calculated for the 60 eyes on each visit by taking the average of the values in the cpRNFL thickness profile in Fig. 1C.
Statistical analysis
The ROI width and average cpRNFL thickness progression were evaluated using a trend-based approach and multilevel mixed-effects models. These models included 3 levels: patient, eye, and ROI. This statistical method involves both random intercepts and random slopes associated with these levels of observations. Hence, it takes into account the correlation between ROIs within the same eye, when they existed, as well as inter-eye correlations. Additionally, mixed-effects models allow the analysis of repeated measurements of individuals over time, despite the correlation of errors between time-points, for which ordinary least squares models are not recommended.9
Two models were built using the following dependent variables: (i) ROI width and (ii) average cpRNFL thickness. Time was set as independent variable, whereas random effects related to the subject (i.e., each eye nested within each subject, and each ROI nested within each eye) were included to measure changes over time. The coefficient associated with the variable ‘Time’ describes the slope (or rate of change) per year of the dependent variables and its p-value reflects whether this change was significant compared to a flat slope (i.e.: no progression). Statistical significance was defined at P<5%. Statistical analyses were carried out with commercially available software (STATA, version 14; StataCorp LP, College Station, TX).
RESULTS
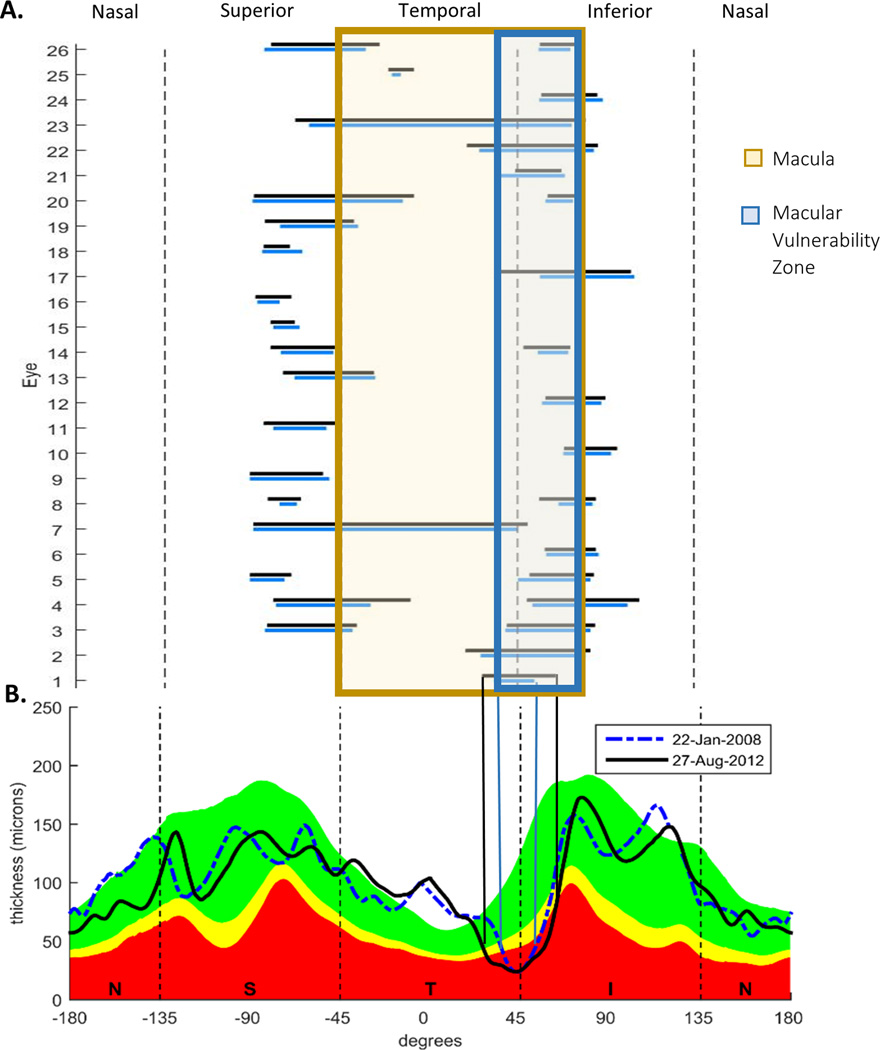
Twenty-six of the 60 eyes had ROIs. Figure 2 shows the location and extent of the ROIs for each of these eyes. Seven of these 26 eyes had 2 ROIs, for a total of 33 ROIs. The horizontal bars in Fig. 2 indicate the extent of the ROIs on the baseline (blue) and final (black) visit. 25 of the 33 ROIs included part of the region associated with the macula (pale orange: between –45° to 69°) and 17 of these included at least part of the macular vulnerability zone (MVZ, blue, 38° to 69°), the region of the disc most often associated with arcuate damage of the macula.10, 11
Figure 2.
A. Locations and extent of the between-visit ROIs on the first (blue) and last (black) visits. B. The cpRNFL profiles for the baseline (dashed blue) and final visit (solid black) visits.
For the ROIs, 85% (28/33) showed an increase in width between visits, with 88% (23/26) of the eyes showing at least one ROI that increased. The median ROI value on the first visit was 31.5 ° (range: 5.1 ° to 168.9 °), and the median ROI value on the second visit was 25.1 ° (range: 4.7° to 164.7 °). The mean ROI change between visits was 6.35° and the median was 4.9 ° (inter-quartiles, Q1: 1.03°, Q3: 10.5°).
In comparison, only 58% (35/60) of the average cpRNFL thickness measurements showed a decrease between visits. The median cpRNFL value on the first visit was 91.9 µm (range: 49.8 µm to 114.7 µm), and the median cpRNFL value on the second visit was 89.9 µm (range: 48.8 µm to 115.1 µm). The mean cpRNFL change between visits was −0.18 µm and the median was −0.7 µm (Q1: −2.7 µm, Q3: 2.7 µm). In addition, there was no relationship between change in average cpRNFL thickness and change in ROI (R2 = 0.003, p=0.78).
To overcome the fact that not all eyes had an ROI, we compared the ROI and cpRNFL measurements for the subset of eyes with ROIs (26 eyes, 33 ROIs). For eyes with multiple ROIs, only the largest ROI based on the initial visit was considered. For this subset, 77% (20/26) of the eyes showed positive ROI change between visits (ie. progression), while only 35% (9/26) showed cpRNFL thinning between visits.
The results of the multilevel mixed effects linear models are shown in Table 1. The ROIs were classified as being in the macula or not (i.e.: projecting to the central 8 degrees around the fovea). Overall ROI change and macular ROI change between visits had statistically significant p values (1.4 degrees increase/year, p=0.015 and 1.69 degrees increase/year, p=0.011, respectively), while cpRNFL change did not (p=0.878). The residual plots did not indicate any deviations from normality or heteroscedasticity, supporting the assumptions of the models.
Table 1.
Mixed effects linear model assessing the significance of ROI change, macular ROI change, and cpRNFL change between visits
| Method | Beta coefficient | 95% Confidence Interval |
P value |
|---|---|---|---|
| ROI over time | 1.409 | 0.269 to 2.550 | 0.015 |
| Macular ROI over time | 1.693 | 0.395 to 2.990 | 0.011 |
| cpRNFL over time | −0.036 | −0.502 to 0.429 | 0.878 |
DISCUSSION
Global metrics such as average cpRNFL thickness are commonly used to track progression in patients with early or suspected glaucoma. In this study, we tested the hypothesis that a ROI approach would be a more robust method for evaluating the progression of local, glaucomatous damage. Our results support this hypothesis in eyes with early glaucoma. The ROI change over time was significant, but the average cpRNFL thickness was not.
This is not to say that cpRNFL thickness should not be used in addition to a ROI approach. It is plausible that the average cpRNFL thickness may pick up global decreases in RNFL due to widespread thinning of the RNFL over time. Progression of the ROI may be overlooked using cpRNFL thickness due to spatial averaging, which inherently reduces sensitivity to detect local change.
Various alternative strategies to detect progression of glaucomatous damage have been proposed. Of the studies that investigate changes in OCT cpRNFL thickness measurements, some12, 13 favor average cpRNFL thickness change, while others14, 15 emphasize quadrant or clock hour analyses. Additionally, recent reviews16, 17 encourage analyzing the RNFL thickness maps of the entire region around the disc. Our approach also measures RNFL thickness from optic disc scans, but we specifically focus on the damaged region. In particular, our ROI method is part of a general approach18 that examines OCT scans in a manner similar to that used by a radiologist when analyzing an MRI or CAT scan.
Limitations
The ROI approach was designed for identifying changes in local defects over time. Consequently, it may not be suitable for patients with early diffuse damage;19–21 advanced glaucoma, where the damage is often widespread;22 or eyes with extensive peripapillary atrophy (in which cpRNFL segmentation errors are common). On the other hand, in many eyes with advanced glaucoma, there is a region of relatively healthy cpRNFL tissue, often associated with the papillomacular bundle. In these cases, the ROI method could be modified to track the preserved region as opposed to the affected region. That is, we suggest reversing our present definition of an ROI to measure the portion of the circumpapillary scan that exceeds the 99% (or 95%) confidence interval for healthy norms.
In addition, in this study, we defined progression based on an increase in width of a localized cpRNFL defect, which, unlike a thickness measure, does not have a clear floor effect. Although expansion of an existing defect is the most common pattern of progression23, there are other types of progression, such as the deepening of a defect and the development of a new defect at a different location. Thus, there may have been eyes in our study that exemplified progression in ways that we did not consider.
Moreover, although we showed a higher sensitivity of the ROI approach to detect progression, we did not investigate its specificity in the present study. This would require either (i) following healthy subjects over a similar follow-up time (3.2 years) or (ii) following glaucoma patients with short test-retest intervals (e.g., days or weeks). The former is not plausible as healthy eyes do not have a ROI. The latter would then be ideal, although we did not collect data using that protocol. Nonetheless, when looking at the short-term variability of our dataset (i.e.: test-retest within the same day) the standardized difference between ROI width measurements on the same day was negligible (−0.02; 95% CI=−0.55 to 0.50, P=0.927), and the same was true for cpRNFL thickness (0.008; 95% CI=−0.52 to 0.53, P=0.975).
Lastly, the ROI method calls for certain requirements that limit the number of eyes that can be analyzed. First, the eye must have good quality scans with accurate segmentation and without significant vitreous floaters obscuring the image. Second, it is not always possible to perfectly align the en-face images. In other words, 2D rotation and manual alignment do not adjust for tilting of the eye/head. Finally, as of today, this approach cannot be done with commercially available devices.
CONCLUSION
For eyes with early glaucoma, tracking the change in RNFL thinning in a region of interest over time is a more sensitive measure of progression than is tracking change in the average cpRNFL thickness. Combining both approaches may be the best way to track progression of both local and widespread RNFL damage over time.
Acknowledgments
Supported by National Institutes of Health Grant R01-EY-02115 (DCH), R01-EY-025253 (CGDM), and Lary Stromfeld Research Fund of NYEEI (RR and RJ)
Footnotes
Disclosures: A. Thenappan, None; C.G. de Moraes, None; D.L. Wang, None; D. Xin, None; R. Jarukasetphon, None; R. Ritch, None; D.C. Hood, Topcon Medical Systems, Inc. (F, C)
REFERENCES
- 1.Susanna R, De Moraes CG, Cioffi GA, et al. Why do people (still) go blind from glaucoma? Trans Vis Sci Technol. 2015:1–1. doi: 10.1167/tvst.4.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood DC, Xin D, Wang D, et al. A region-of-interest approach for detecting progression of glaucomatous damage with optical coherence tomography. JAMA Ophthalmol. 2015:1–7. doi: 10.1001/jamaophthalmol.2015.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernest PJ, Schouten JS, Beckers HJ, et al. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013:512–519. doi: 10.1016/j.ophtha.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Suh MH, Park KH, Kim H, et al. Glaucoma progression after the first-detected optic disc hemorrhage by optical coherence tomography. J Glaucoma. 2012:358–366. doi: 10.1097/IJG.0b013e3182120700. [DOI] [PubMed] [Google Scholar]
- 5.De Moraes CG, Demirel S, Gardiner SK, et al. Rate of visual field progression in eyes with optic disc hemorrhages in the ocular hypertension treatment study. Arch Ophthalmol. 2012:1541–1546. doi: 10.1001/jamaophthalmol.2013.1137. [DOI] [PubMed] [Google Scholar]
- 6.Suda K, Hangai M, Akagi T, et al. Comparison of Longitudinal Changes in Functional and Structural Measures for Evaluating Progression of Glaucomatous Optic Neuropathy. Invest Ophthalmol Vis Sci. 2015;56(9):5477–5484. doi: 10.1167/iovs.15-16704. [DOI] [PubMed] [Google Scholar]
- 7.Abe RY, Diniz-Filho A, Zangwill LM, et al. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9) doi: 10.1167/iovs.15-18940. OCT421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prum BE, Jr, Rosenberg LF, Gedde SJ, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern Guidelines. Ophthalmology. 2016;123(1):P41–P111. doi: 10.1016/j.ophtha.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 9.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982:963–974. [PubMed] [Google Scholar]
- 10.Hood DC, Raza AS, De Moraes, et al. Glaucomatous damage of the macula. Prog Retinal Rye Res. 2013:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood DC, Raza AS, De Moraes CG, et al. The nature of macular damage in glaucoma as revealed by averaging optical coherence tomography data. Trans Vis Sci Technol. 2012:1–15. doi: 10.1167/tvst.1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014 doi: 10.1136/bjophthalmol-2013-304326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wessel JM, Horn FK, Tornow RP, et al. Longitudinal analysis of progression in glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013:3613–3620. doi: 10.1167/iovs.12-9786. [DOI] [PubMed] [Google Scholar]
- 14.Lee EJ, Kim TW, Weinreb RN, et al. Trend-based analysis of retinal nerve fiber layer thickness measured by optical coherence tomography in eyes with localized nerve fiber layer defects. Invest Ophthalmol Vis Sci. 2011:1138–1144. doi: 10.1167/iovs.10-5975. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, Kim TW, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: A study on optical coherence tomography guided progression analysis. Invest Ophthalmol Vis Sci. 2010:217–222. doi: 10.1167/iovs.09-3468. [DOI] [PubMed] [Google Scholar]
- 16.Leung CK. Diagnosing glaucoma progression with optical coherence tomography. Curr Opin Ophthalmol. 2014:104–111. doi: 10.1097/ICU.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 17.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: Patterns of retinal nerve fiber layer progression. Ophthalmology. 2012:1858–1866. doi: 10.1016/j.ophtha.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Hood DC, Raza AS. On improving the use of oct imaging for detecting glaucomatous damage. Br J Ophthalmol. 2014:ii1–ii9. doi: 10.1136/bjophthalmol-2014-305156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artes PH, Chauhan BC, Keltner JL, et al. Longitudinal and cross-sectional analyses of visual field progression in participants of the ocular hypertension treatment study. Arch ophthalmol. 2010:1528–1532. doi: 10.1001/archophthalmol.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henson DB, Artes PH, Chauhan BC. Diffuse loss of sensitivity in early glaucoma. Invest Ophthalmol Vis Sci. 1999:3147–3151. [PubMed] [Google Scholar]
- 21.Hood DC, Slobodnick A, Raza AS, et al. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest Ophthalmol Vis Sci. 2014:632–649. doi: 10.1167/iovs.13-13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong JS, Kang MG, Kim CY, et al. Pattern of macular ganglion cell-inner plexiform layer defect generated by spectral-domain oct in glaucoma patients and normal subjects. J Glaucoma. 2015:583–590. doi: 10.1097/IJG.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 23.Leung CK, Yung WH, Ng AC, et al. Evalution of scanning resolution on retinal nerve fiber layer measurement using optical coherence tomography in normal and glaucomatous eyes. J glaucoma. 2004:479–485. doi: 10.1097/01.ijg.0000138205.99424.24. [DOI] [PubMed] [Google Scholar]