Abstract
Objective
Accelerated aging can occur in adult survivors of neurodevelopmental disorders, but has been narrowly studied in spina bifida myelomeningocele (SBM). Since discrete aspects of cognitive control and related neural network macrostructure deteriorate in normal aging, the specificity and trajectory of cognition and neuropathology incurred across adulthood in SBM were examined.
Method
Adults (N = 120) with and without SBM completed working memory span and manipulation tasks, and an inhibitory control task. A subset (n = 53) underwent structural MRI. Effects of group, age, and their interaction on performance and select gray matter volumes were examined.
Results
Adults with SBM had significantly poorer working memory accuracy and overall inhibitory control performance than typical peers. Age negatively predicted inhibitory control. Group × age significantly interacted on span accuracy; advanced age related to diminished performance in typical adults, but not in adults with SBM. SBM related to disproportionately enlarged cortical and putamen and reduced hippocampus volumes. Group × age significantly interacted on cortical, but not subcortical gray matter volumes. Dorsolateral prefrontal, hippocampus, and putamen volumes negatively correlated with cognitive performance.
Conclusions
Supporting previous literature, current findings elucidated a profile of executive impairment in SBM that was maintained in a parallel maturational trajectory to typical aging. Accelerated aging in cognitive control or subcortical gray matter was not supported in SBM. However, reductions in anterior and posterior cortical regions were exacerbated in older adults with SBM compared to typical peers. Overall results supported persistent anomalous neurodevelopmental maturation across the lifespan in SBM that related to diminished cognitive control.
Keywords: Spina bifida myelomeningocele, working memory, inhibitory control, aging, dorsolateral prefrontal circuitry, gray matter
Theories of normal aging suggest that low-level processes such as feature extraction remain relatively intact while supportive processes, like maintaining task representations in working memory and other aspects of cognitive control, become impaired (Belleville, Peretz, & Malenfant, 1996; Dobbs & Rule, 1989; Foos & Wright, 1992; Salthouse, Babcock, & Shaw, 1991; Wingfield, Stine, Lahar, & Aberdeen, 1988). Working memory (WM) and inhibitory control comprise important aspects of cognitive control that are highly dependent on the evolved circuitry of the prefrontal cortex. WM is the ability to maintain and manipulate information online in the absence of incoming sensory stimulation, whereas inhibitory control involves the cognitive inhibition of task-irrelevant stimuli and activation of task-relevant information, (Arnsten, 2011; Corbetta, Kincade, & Shulman, 2002; GoldmanRakic, 1996).
WM and inhibitory control involve connections between the dorsolateral prefrontal cortex (DLPFC) and subcortical gray matter (GM) structures, including the striatum (i.e., caudate and putamen) and thalamus (Alexander, DeLong, & Strick, 1986; Butters & Pandya, 1969; Cummings, 1998; Funahashi, Bruce, & Goldman-Rakic, 1993; Goldman & Rosvold, 1970; Mishkin, 1957; Stewart, 2006). WM tasks also recruit the hippocampus since the functional system between this region and the prefrontal cortex supports encoding of new information (Friedman & Goldman-Rakic, 1988). Additionally, frontoparietal connections of the superior parietal cortex facilitates the manipulation of visual information in WM (Cabeza & Nyberg, 2000; Koenigs, Barbey, Postle, & Grafman, 2009; Mottaghy, 2006; Owen, McMillan, Laird, & Bullmore, 2005). Age-related changes in these cortical and subcortical brain structures (Addis, Giovanello, Vu, & Schacter, 2014; Nyberg et al., 2010; Raz, Ghisletta, Rodrigue, Kennedy, & Lindenberger, 2010; Raz et al., 2005) ostensibly mediate performance declines across a range of cognitive domains in normal aging (D’Esposito, Postle, Ballard, & Lease, 1999).
Aging in Neurodevelopmental Disorders
Medical advances for the treatment of a variety of congenital and acquired neurodevelopmental disorders have resulted in longer lifespans for populations previously considered to be incongruent with a long life. Although cohort effects resulting from evolving medical interventions have yielded a dearth of data on adult survivors of many neurodevelopmental disorders, accelerated cognitive decline is a concern for young adult survivors of some neurogenetic developmental disorders such as Down’s, Turner’s, and Fragile X syndrome (Cornish et al., 2008; Devenny, Krinsky-McHale, Sersen, & Silverman, 2000; Oliver, Holland, Hall, & Crayton, 2005).
Spina bifida myelomeningocele (SBM) is a neurogenetic disorder that is the most common severely disabling congenital birth defect of the central nervous system compatible with survival in North America (Au, Ashley-Koch, & Northrup, 2010; Boulet, Gambrell, Shin, Honein, & Mathews, 2009; Fletcher & Brei, 2010). Yet our knowledge of adult outcomes is limited and fragmentary. Prior to the introduction of the valved shunt during the mid 20th century, the majority of live births with SBM and hydrocephalus died in early infancy (Hunt & Holmes, 1976; Nulsen & Spitz, 1951). Today, however, the majority of individuals with SBM survive beyond early adulthood (Bowman & McLone, 2010). Since the oldest cohort with SBM is currently approaching 60 years of age (Dennis, Spiegler, & Hetherington, 2000), better understanding of age-related changes across adulthood is essential.
Two neuropsychological studies have utilized measures that are sensitive to aging to investigate cognitive decline across adulthood in SBM. Slowed reaction time, a hallmark deficit in SBM (Dennis & Barnes, 2010), increases with typical aging (Ratcliff, Thapar, & McKoon, 2001). Using measures of simple, choice, and cognitive reaction time to assess youth and young and middle-aged adults with SBM, Dennis et al. (2015) observed reduced latency performance across the lifespan relative to typical peers, with no evidence of accelerated aging effects (e.g., no age by group interactive effects). Treble-Barna et al. (2015) found that the prospective and episodic memory deficits typically evidenced by children and young adults with SBM (e.g., Hampton et al., 2013; Iddon, Morgan, Loveday, Sahakian, & Pickard, 2004; Scott et al., 1998; Vachha & Adams, 2005; Yeates, Enrile, Loss, Blumenstein, & Delis, 1995) were similarly worsened in older adulthood in SBM and typical peers. Overall, these limited findings suggest stable as opposed to accelerated cognitive deficits across the lifespan in SBM.
Relatively few cognitive tasks have been studied in relation to the life span in SBM. While cognitive control deficits in youth with SBM are well established, the persistence and presentation of executive impairments in adults is not known. Children and young adults with SBM exhibit impaired WM (Iddon et al., 2004) and inhibitory control (e.g., Burmeister et al., 2005 ; Dennis et al., 2005). However, in contrast to prospective and episodic memory systems and reaction time, the specificity and trajectory of these deficits in SBM is unknown, especially in adults.
Information regarding the neural basis of executive dysfunction in SBM is also lacking in adults. Youth exhibit anomalous cortical patterning and altered subcortical GM volumes and microstructure (Juranek & Salman, 2010; Ware et al., 2014). Of particular relevance is that brain regions comprising the distinct, yet overlapping neural networks that subserve cognitive control processes, such as WM and inhibitory control, are affected by SBM (Juranek & Salman, 2010; Ware et al., 2014) and relate to functional impairments in youth (Ware et al., 2016). For instance, greater parent-rated executive dysfunction is related to structural alterations in specific anterior cortical and subcortical GM (Ware et al., 2014; Ware et al., 2016). Understanding the extent to which deviations in brain structure occur and relate to cognitive outcomes at different maturational stages across the lifespan in SBM is necessary as this population is surpassing middle age.
Current Study Aims and Hypotheses
The current study examined discrete aspects of cognitive control in adults with SBM and typically developed (TD) peers to further elucidate cognitive and neurodevelopmental trajectories across the lifespan in SBM. Tasks of WM and inhibitory control were utilized given the well-documented sensitivity of these executive processes to frontal lobe function as well as to age-related cognitive decline in both normal aging and in neurodevelopmental disorders. Groups of adults with SBM and TD adults were compared on two visual WM tasks that provided independent assessments of maintenance of information in WM (WM span) and effortful processing of information in WM (WM manipulation). Groups were also compared on a measure of inhibitory control. Furthermore, we examined whether specific cortical and subcortical structures of the cognitive control neural network differed across groups and as a function of age. Finally, brain structure-function relations were examined. The current study address these questions through the following aims:
-
A. Do adults with SBM have impairments in WM span or manipulation? The adults with SBM were hypothesized to have greater difficulty maintaining and manipulating information in WM compared to the TD adults.
B. Do adults with SBM have impaired inhibitory control? We hypothesized that adults with SBM would exhibit poorer inhibitory control than TD peers.
C. Do adults with SBM show accelerated aging on aspects of cognitive control? In line with findings from Treble-Barna et al. (2015) and Dennis et al. (2015), stable deficits in cognitive control were expected across adulthood in the adults with SBM relative to TD adults. Given that general declines in cognitive control occur in normal aging, older participants in both groups were expected to perform more poorly than younger participants (e.g., Wilkinson & Allison, 1989; Williams, Ponesse, Schachar, Logan, & Tannock, 1999).
-
A. Do adults with SBM have volumetric abnormalities in cortical and subcortical GM regions of the visual WM and inhibitory control neural networks? As expected from the published literature in youth, we hypothesized enlarged DLPFC and putamen volumes, similar volumes of the caudate and thalamus, and reduced superior parietal cortex and hippocampal volumes in the adults with SBM compared to the TD adults (Juranek et al., 2008;Treble-Barna et al., 2015; Ware et al., 2014).
B. Do adults with SBM show accelerated aging in cortical and subcortical GM regions of the cognitive control network? Given that younger individuals with SBM typically exhibit disproportionate enlargement of anterior cortices (Juranek et al., 2008), we expected enlarged DLPFC volumes in the adults with SBM compared to TD peers, across age. However, we expected reduced DLPFC volume with older age to occur similarly in both groups (Juranek et al., 2008). Given regional sensitivity of the caudate and hippocampus to aging (Addis et al., 2014; Nyberg et al., 2010; Raz et al., 2010; Raz et al., 2005), reduced volumes with older age were expected to occur similarly in both groups. We had no basis for expecting any age-related changes across groups or any differential group by age effects on volumes of the putamen, thalamus, or superior parietal cortex.
Do volumes of specific components of the visual WM and inhibitory control brain networks differentially predict performance during cognitive control tasks in SBM and TD adults? In accordance with the literature, reduced DLPFC volume was hypothesized to be associated with poorer cognitive control for the adults with SBM and the TD group (D’Esposito et al., 1999). Given previously established relations between executive dysfunction with the caudate (Alexander et al., 1986; Arsalidou, Duerden, & Taylor, 2013; Fryer et al., 2012) and thalamus (Little et al., 2010; Stuss, 2011), volumes of both regions were expected to predict WM manipulation and inhibitory control across groups. Since the putamen is involved in behavioral (motor) regulation and inhibitory control processes (Alexander et al., 1986; Arsalidou et al., 2013), reduced putamen volumes were expected to be related to poorer inhibitory control and WM maintenance in both groups. Hippocampus volume was also expected to be associated with both WM tasks, but to a greater extent in SBM relative to typical adults given findings in adults with SBM (Treble-Barna et al., 2015). Lastly, we expected that superior parietal cortex volume would be positively associated with WM manipulation in both groups (Koenigs et al., 2009).
Method
Participants
Participants (N = 120) included 68 adults with SBM and 52 typically developed adults (the TD group) between the ages of 18 to 56 years, who were retrospectively recruited from clinics associated with the Texas Children’s Hospital and Shriner’s Hospital for Children in Houston, TX, and The Hospital for Sick Children in Toronto, Canada. TD adults were recruited through community advertising in Houston and Toronto. Participants with SBM were also recruited from adult neurosurgical and rehabilitation facilities in Houston, Toronto, Edmonton, and Calgary, and from advertisements as well as from SBM support groups and community contacts. To be included in the current study, participants had to be predominantly English speaking and have verbal and/or nonverbal intelligence quotient (IQ) scores above 70 on an abbreviated Stanford-Binet Intelligence Scale: Fourth Edition (Thorndike, Hagen, & Sattler, 1986). The Full Scale IQ score estimates ranged from 70 to 115 for the group with SBM, and from 77 to 129 for the TD group. Individuals were excluded from participation if they had neurological disorders unrelated to SBM, severe psychiatric disorders that precluded adequate cooperation (autism, psychosis), uncontrolled seizure disorder, or any Chiari or shunt malfunction symptoms at the time of the evaluation.
Participants gave informed consent in accordance with institutional review guidelines. Participants with SBM were born with myelomeningocele, and had arrested (n = 7) or shunted (n = 61) hydrocephalus (verified by medical record review, including pathology and neurosurgical operative reports when available). As expected, the majority of individuals with SBM had a Chiari II malformation of the hindbrain (98%), anomalous corpus callosum (89%), lower level spinal lesions (77%), 0–4 shunt revisions (85%), no history of seizures (83%), and impaired ambulation (77%).
Demographic information for the included sample is presented in Table 1. Groups were similar (ps > .05) on ethnicity, handedness, age, and socioeconomic status per the Four Factor Index of Social Status (Hollingshead, 1975). The group with SBM had significantly more males, χ2 (1) = 7.35, p < .01, and, though expectedly, significantly lower Full Scale IQ scores, t(118) = 9.10, p < .001, relative to the TD adults.
Table 1.
Demographic data for the group of adults with spina bifida myelomeningocele (SBM) and the group of typically developing (TD) controls.
| Variable | SBM (n = 68) | TD (n = 52) |
|---|---|---|
| Sex (n [%Male])* | 35 (51) | 14 (27) |
| Handedness (n [% Right]) | 54 (79) | 47 (90) |
| Ethnicity (n [% Hispanic]) | 6 (9) | 2 (3) |
| Age at MRI (M [SD]) | 28.61 (9.71) | 31.81 (10.48) |
| Socioeconomic status (M [SD]) | 33.56 (15.00) | 39.00 (15.77) |
| Full Scale IQ (M [SD])** | 89.32 (11.57) | 108.67 (11.49) |
Note:
p < .01;
p < .001.
Materials and Procedure
All participants completed a standardized neuropsychological assessment that included computerized measures of working memory and inhibitory control. Fifty-three of the participants with completed behavioral data also underwent structural MRI (SBM, n = 23; TD, n = 30). Participants with SBM and TD who underwent structural MRI were similar (ps > .05) on ethnicity, handedness, and socioeconomic status. The group with SBM had significantly more males, χ2 (1) = 5.05, p < .05, was younger, t(51) = 2.22, p < .05, and, though expectedly, significantly lower Full Scale IQ scores, t(51) = 4.97, p < .001, relative to the TD adults.
Time between behavioral and MRI assessments ranged between 0 to 33 weeks, and was similar for both groups. With the exception of two participants with SBM who had longer between-session intervals up to 33 weeks, most participants completed both assessments within 13 weeks. Exceptions resulted from missed appointments and/or unexpected metallic artifacts. Most participants (57%) underwent neurobehavioral testing and neuroimaging within two weeks, with a total median for both groups equal to 6.5 days. Compared to those who did not undergo MRI, participants who completed MRI were comparable (exceptions listed below) in terms of demographic characteristics and on the neuropsychological measures of interest to the current study, with a few exceptions. Within the group with SBM, participants who completed MRI were younger (M = 24.58, SD = 7.70) relative to the participants who did not complete scanning (M = 30.67, SD = 10.06; t(66) = 2.54, p < .05). In terms of neuropsychological measures, the participants with SBM who completed MRI (M = 407.30, SD = 72.91) were faster on incongruent condition of the inhibitory control task relative to SBM participants who did not complete scanning (M = 467.10, SD = 85.91; t(66) = 2.85, p < .05). Within the TD group, participants who completed MRI had lower IQ scores (M = 105.20, SD = 11.44) relative to the participants who did not undergo MRI (M = 113.50, SD = 9.92; t(50) = 2.73, p < .05). In terms of neuropsychological measures, the TD participants who completed the MRI (M = 1145.20, SD = 214.20) were faster on the working memory manipulation task relative to TD participants who did not complete scanning (M = 1260.90, SD = 167.40; t(50) = 2.11, p < .05).
Cognitive Control Measures
Participants completed two WM tasks, the Working Memory Span Task (WMST) and the Working Memory Manipulation Task (WMMT), and the Inhibitory Control Task (ICT), which varied in cognitive control demands. The WMST and the WMMT respectively measured the maintenance and manipulation of visual information in WM. The WMST and the ICT were based on the paradigms utilized by Diamond, et al. (2002) and Roncadin, Guger, Archibald, Barnes, & Dennis (2004), respectively. The WMST used was modified in the current study to reduce the likelihood of verbal encoding by replacing the original shapes with different abstract designs (Diamond, Kirkham, & Amso, 2002). In the current study, the ICT was adapted from the Directional Stroop Task (Roncadin, Guger, Archibald, Barnes, & Dennis, 2004). The WMMT was developed for the present study. All participants completed the ICT first, followed by the WMST and, lastly, the WMMT.
Internal task conditions were counterbalanced across participants to control for response bias to one side as well as for stimulus response bias. Reaction time and performance accuracy data from each task were used as final outcomes variables for the current study. Both the latency and accuracy data derived from each task were adjusted to account for performance during correct trials only. For all tasks, participants were instructed to respond as quickly and as accurately as possible. The stimulus exposure time and the interstimulus interval were set at 750 ms for all tasks. To attract participants’ attention, stimuli were preceded by a cross that lasted for 750 ms for the ICT and 500 ms for the WMST and (before stimuli sets) the WMMT.
Working Memory Span Task (WMST)
The WMST required participants to learn associated responses (i.e., either a left or right button press) to a random presentation of six abstract shapes. During an initial training set, associations were established between visual stimuli (i.e., abstract shape), presented individually in the middle of the computer screen, and manual responses (left or right button press). After the training set, participants completed one set of practice trials with help from the examiner and three additional practice runs of the six shapes, one in the same order and two in a different order from the initial practice set, on the laptop. A test set of 30 randomized trials that were counterbalanced across participants was then performed. Each shape was presented once in a random order in each set of 6 trials across a total of 5 sets.
Working Memory Manipulation Task (WMMT)
The WMMT involved serial learning of sets of shapes. On a given trial, between two to six shapes (4 trials of each) were presented individually and were followed by a forced-choice recency judgment response. During the initial serial learning, sequences of two to six shapes from a subset of eight possible shapes were presented in the middle of the screen. Subsequently, two of the shapes were presented simultaneously (on the left and right side of the computer screen) and participants were instructed to press the button (i.e., left or right) indicating which of the shapes had been presented earliest in the sequence.
Prior to practice trials, the examiner used cards to provide a demonstration. Following a repetition of this trial on the laptop and three more practice trials with two shapes, the administrator repeated the demonstrations with a sequence of three shapes. Three additional practice runs of three shapes were administered, followed by 4 unprompted practice runs (two with two shapes and two with three shapes). The task was stopped and participants (not included in the current study: SBM, n = 16; TD, n = 4) were excluded from further participation after 3 failed practice sets. For the test, the particular shapes used and the order in which they were presented in a given sequence and on simultaneous presentation were randomized once and then fixed across participants. Six trials were administered during each sequence length for a total of 30 trials. The stimulus exposure time and interstimulus interval were set at 1000 ms for the serial learning component and at 2000 ms for the recency judgment component.
Inhibitory Control Task (ICT)
The ICT has 64 trials across three conditions: 16 congruent trials (congruent condition), 16 incongruent trials (incongruent condition), and 32 mixed congruent and incongruent trials (inhibitory control condition).
First, for the congruent condition, an association between a visual stimulus (i.e., dot type, counterbalanced across participants) and a congruent motor response was established. Participants were instructed to press the right button when the visual stimulus (e.g., gray dot) was presented on the right side of the computer screen and, conversely, to press the left button when the stimulus was presented on the left side of the computer screen. Next, for the incongruent trials, incongruent motor responses were established in association with a second stimulus type (e.g., striped dot). Instructions were to press the left button when the visual stimulus was presented on the right side of the computer screen and, conversely, the right button when the stimulus was presented on the left side of the computer screen. The incongruent condition builds upon the Simon effect, in which a propensity to respond on the same side as perceptual cues produces slower, less accurate performance when the required response does not match the location of the stimulus (Umilta, Rubichi, & Nicoletti, 1999). Finally, a set of mixed stimuli (half congruent, half incongruent) was presented to measure inhibitory control. For the inhibitory control condition, efficient performance is dependent upon both effortful activation of the associated response requirement (congruent or incongruent) for each stimulus (i.e., gray vs. striped dot) in WM while withholding prepotent response tendencies through inhibitory control. Performance accuracy and reaction time from the inhibitory control condition were used as the measure of inhibitory control. Participants completed 4 practice trials (two right and two left responses) prior to each condition.
Magnetic Resonance Imaging
Image Acquisition
High-resolution brain MR images were acquired on a Philips 3T scanner with SENSE (Sensitivity Encoding) technology (described in Juranek et al., 2008). Whole-brain coverage was obtained through a 3D T1-weighted sequence following conventional sagittal scout and coronal T2-weighted sequences. Acquisition parameters of the 3D turbo fast spin-echo sequence were as follows: repetition time/echo time = 6.5–6.7/3.04–3.14; flip angle = 8°; field of view = 240 mm; matrix = 256 × 256; slice thickness = 1.5 mm; in-plane pixel dimensions (x, y) = 0.94, 0.94 mm; number of excitations (NEX) = 2.
Image Processing
More details regarding neuroimaging data preprocessing and processing can be found in Ware, et al. (2014). Briefly, all scans were analyzed by a rater blind to IQ scores, age, and gender (Juranek et al., 2008). Image segmentation and morphometric analyses were conducted in FreeSurfer v4.0.5 (www.surfer.nmr.mgh.harvard.edu; Fischl, 2012). Subsequent to skull stripping and global segmentation of GM, white matter, and cerebrospinal fluid, FreeSurfer labeled deep GM structures (i.e., caudate, putamen, thalamus). Due to the large variability in dysmorphology of anatomical structures across the adults with SBM, segmentation edits were manually performed by an expert (JJ) to accurately label each region of interest (ROI). Additionally, as part of the surface-based processing stream, the cortical ribbon was parcellated and labeled based on the Destrieux cortical annotation nomenclature (Destrieux, Fischl, Dale, & Halgren, 2010). The DLPFC label was created by merging together three labels from the Destrieux annotation file associated with each individual brain (G_frontal_middle, S_frontal_middle, and S_frontal_inferior). Subsequently, volumetric data for each label of interest were quantified within FreeSurfer (Fischl & Dale, 2000) and exported to an external statistical package for subsequent analyses.
Data Analysis
The statistical analyses were completed using the PROC REG procedure in SAS 9.4 software (SAS, Inc., Cary, NC). For Aim 1, multiple regression analyses were used to examine the effects of group (SBM, TD), age, and the two-way group × age interaction on performance accuracy and reaction time for the WMST, WMMT, and ICT. Group × age interactions were estimated to determine whether accelerated aging occurred in the group with SBM relative to the TD group. A similar, multiple regression approach was taken to address Aim 2. Multiple regression analyses were used to examine the effects of group, age, and the two-way group × age interaction on cortical and subcortical volumes of select GM regions of the cognitive control neural network. Specifically, cortical ROIs included the DLPFC and superior parietal cortex, and subcortical ROIs included the caudate, putamen, thalamus, and hippocampus. For Aims 1 and 2, only the models for which an overall, omnibus effect of p < .05 was detected were considered to be statistically significant.
To address Aim 3, Pearson correlations were examined separately within each group (SBM, TD) to determine whether ROI volumes related to performance (i.e., accuracy and reaction time) during the WMST, WMMT, and ICT. Structure-function findings were discussed in terms of the magnitude of correlational coefficients across the estimators rather than statistical significance given concerns of limited power. Because criteria for interpreting magnitude of correlation coefficients are somewhat arbitrary and vary depending on the context (Cohen, 1998), the following set of criteria was used in the current study for structure-function relations: (a) the magnitude of correlation coefficients below |.20| were considered weakly related (i.e., small effect), (b) the magnitude of correlation coefficients between |.20| and |.40| were considered moderately related (i.e., moderate effect), and (c) the magnitude of correlation coefficients exceeding |.40| being considered highly (i.e., large effect) related. Only the brain structure-function associations with large effect sizes (r > |.40|) were considered statistically robust. As follow-up, the magnitudes of within-group structure-function correlation coefficients (with large effect sizes) were examined using Fisher r-to-z score transformations to assess for any differential, between groups effects.
Preliminary Analyses
The initial models for Aims 1 and 2 included sex, handedness, and socioeconomic status as covariates as well as the two-way group × age interaction. However, covariates and group × age interactions that did not yield statically significant results were trimmed from the final models.
For Aim 2, group differences in total brain volume were examined to determine the most appropriate correction method for the ROI volumes. Given that enlarged ventricles can inherently be enlarged in individuals with SBM as the result of early hydrocephalus (Juranek & Salman, 2010), groups were compared on total brain volume excluding ventricular (i.e., cerebrospinal fluid) volume. However, no significant group differences were detected (p = .388). Therefore, volumes of each ROI were corrected for heterogeneity in individual differences in total brain volume by calculating the proportion of each raw ROI volume to total brain volume (excluding ventricular volume). Corrected proportions were used for all subsequent analyses.
To examine whether hemispheric differences existed between groups, separate 2 (group: SBM, TD) × 2 (hemisphere: left, right) repeated measures ANOVAs were conducted on volumes of each corrected GM ROI. None of the group × hemisphere interaction terms reached statistical significance (p > .05). Across groups, significant hemispheric effects were found for the corrected volume of the DLPFC, however the non-significant group × hemisphere interaction term indicated similar lateralization effects across groups. In addition, the three-way group × age × hemisphere interaction, F(50) = 5.43, p = .024, and the two-way age × hemisphere interaction, F(50) = 7.28, p = .010, terms were significant for corrected putamen volume. However, follow-up analyses, which did not yield statistically significant effects, indicated that the relation between age and corrected putamen volume was negatively associated in both groups [TD: r(30) = −.14, p = .456; SBM: r(23) = −.14, p = .518] in the right hemisphere, but positively associated in the group with SBM, r(23) = .20, p = .359, and negatively associated in the TD group, r(30) = −.12, p = .545, in the left hemisphere. Therefore, the average of corrected (for total brain volume excluding cerebrospinal fluid) left and right hemisphere volumes for each GM ROI was included in subsequent analyses.
Results
Aim 1: Working Memory and Inhibitory Control
Average performance, i.e., accuracy and reaction time, during the Working Memory Span Task, Working Memory Manipulation Task, and Inhibitory Control Task for each group are shown in Table 2.
Table 2.
Mean (standard deviation) task performance for the group of adults with spina bifida myelomeningocele (SBM) and the group of typically developing (TD) controls.
| SBM (n = 68) | TD (n = 52) | |
|---|---|---|
| WMST RT | 614.48 (125.39) | 636.16 (79.79) |
| WMST AC | 0.62 (0.19) | 0.80 (0.19) |
| WMMT RT | 1121.13 (226.47) | 1194.12 (202.35) |
| WMMT AC** | 0.67 (0.15) | 0.75 (0.12) |
| ICT Inhibitory Control RT*** | 624.72 (113.73) | 575.83 (99.51) |
| ICT Inhibitory Control AC*** | 0.76 (0.18) | 0.89 (0.07) |
Note:
p < .05;
p < .01;
p < .001;
WMST = Working Memory Span Task; WMMT = Working Memory Manipulation Task; ICT = Inhibitory Control Task; RT = Reaction time; AC = Accuracy.
Working Memory Span Task (WMST)
Performance accuracy
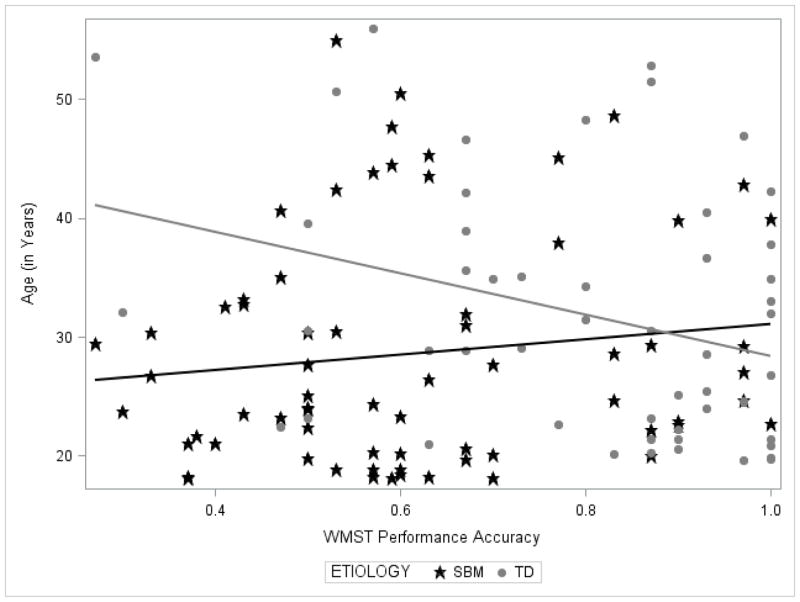
The final model for WMST performance accuracy, which included group, age and the two-way group × age interaction as predictors, reached overall statistical significance, F(3, 116) = 11.71, p < .001. As expected, the group with SBM had significantly lower accuracy during the WMST compared to the TD group, t(116) = −4.01, p < .001, controlling for age. As expected, age was significantly associated with WMST accuracy, t(116) = −2.31, p = .023, across groups; older adults were less accurate than younger adults. As depicted in Figure 1, the two-way group × age interaction was significant for performance accuracy during the WMST t(116) = 2.41, p = .018. Follow-up analyses indicated that older adults had significantly poorer accuracy than younger adults in the TD group, r(52) = −0.318, p < .05, but that older and younger adults performed similarly in the group with SBM, r(68) = 0.127, p = .301.
Figure 1.
A line plot demonstrating the relation between age and performance accuracy during the Working Memory Span Task (WMST) for adults with spina bifida myelomeningocele (SBM) and typically developing (TD) adults.
Reaction time
The final model for reaction time during the WMST reached overall significance, F(2, 117) = 16.08, p < .001, with group and age as predictors. Reaction time during the WMST was similar for both groups, t(117) = --0.33, p = .740. As expected, age was significantly associated with WMST reaction time, t(117) = 5.54, p < .001, across groups; older adults performed more slowly (i.e., greater reaction time) than younger adults.
Working Memory Manipulation Task (WMMT)
Performance accuracy
For WMMT performance accuracy, the final model reached overall significance, F(2, 117) = 4.95, p = .009, with group and age included as predictors. As expected, WMMT accuracy was significantly lower in the group with SBM relative to the TD group, t(117) = −3.11, p = .002, controlling for the non-significant effect of age, t(117) = −0.97, p = .335.
Reaction time
The final model for reaction time during the WMMT did not reach overall significance, F(2, 117) = 2.90, p > .05.
Inhibitory Control Task (ICT)
To determine whether group differences in inhibitory control performance occurred above and beyond observed WM span impairments in the group with SBM (reported above), WMST performance accuracy and reaction time were respectively included as predictors of the inhibitory control performance accuracy and reaction time.
Performance accuracy
The final model for inhibitory control performance accuracy included group, age, and WMST accuracy, and reached overall statistical significance, F(3, 116) = 14.26, p < .001. As expected, the group with SBM had significantly poorer accuracy during inhibitory control compared to the TD group, t(116) = −3.32, p = .001, controlling for age and WMST accuracy. Contrary to expectations, age was not significantly related to inhibitory control performance accuracy, t(116) = −0.20, p = .843, over the effects of group and WMST accuracy. However, as hypothesized, performance accuracy during the WMST was significantly positively associated with inhibitory control performance accuracy, t(116) = 3.59, p < .001, across groups and age.
Reaction time
The final model for reaction time during inhibitory control, which included group, age, sex and WMST reaction time as predictors, reached overall statistical significance, F(3, 115) = 23.74, p = < .001. As expected, the group with SBM has significantly longer reaction time during inhibitory control than the TD group, t(115) = 4.94, p < .001, controlling for age, sex, and WMST reaction time. As expected, age was significantly associated with reaction time during inhibitory control, t(115) = 4.91, p < .001, across groups, sex, and WMST reaction time; older participants were slower than younger participants. Sex was also significantly associated with inhibitory control reaction time, t(115) = 2.34, p = .021, above the effects of group, age, and WMST reaction time; females had slower reaction time than males. Expectedly, reaction time during the WMST was significantly, positively associated with reaction time during inhibitory control, t(115) = 4.01, p < .001, across group, age, and sex.
Aim 2: Structures of the Working Memory and Inhibitory Control Neural Networks
Average (i.e., collapsed across left and right hemisphere) corrected volumes for each cortical and subcortical ROI are presented for each group in Table 3. The final models for corrected caudate, F(2, 51) = 1.46, p = .243, and thalamus, F(2, 51) = 2.44, p = .098, volumes did not reach overall significance and are therefore not discussed below.
Table 3.
Mean (standard deviation) volume for corrected cortical and subcortical gray matter structures for the adults with spina bifida myelomeningocele (SBM) and the typically developing (TD) adults.
| Variable | SBM (n = 68) | TD (n = 52) |
|---|---|---|
| Dorsolateral prefrontal cortex (DLPFC)*** | 0.086 (0.010) | 0.076 (0.006) |
| Superior parietal cortex** | 0.024 (0.003) | 0.021 (0.002) |
| Putamen* | 0.010 (0.001) | 0.009 (0.001) |
| Hippocampus*** | 0.005 (0.001) | 0.006 (0.001) |
| Caudate | 0.006 (0.001) | 0.006 (0.001) |
| Thalamus | 0.014 (0.002) | 0.013 (0.001) |
Note:
p < .05;
p < .01;
p < .001.
Dorsolateral Prefrontal Cortex (DLPFC)
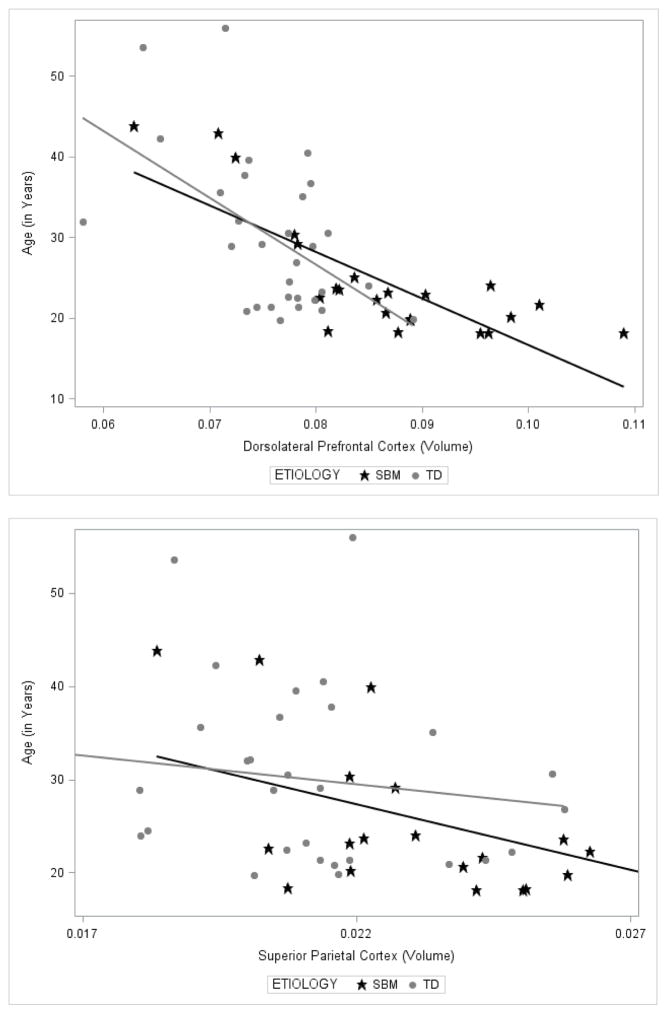
The overall model for corrected DLPFC volume, which included group, age, and the two-way group × age interaction, reached overall statistical significance, F(3, 49) = 30.50, p < .001. As expected, DLPFC volume was significantly, disproportionately enlarged in the group with SBM compared to the TD group, t(49) = 4.78, p < .001, controlling for age. As expected, age was significantly negatively associated with corrected DLPFC volume, t(49) = −2.95, p < .01, across groups. The two-way group × age interaction was also statistically significant, t(49) = −3.67, p < .001. As shown in Figure 2, corrected DLPFC volume reduction in the older adults compared to younger adults occurred to a greater extent in the group with SBM, r(23) = −.79, p < .001, compared to the TD group, r(30) = −.53, p = .003.
Figure 2.
Line graphs demonstrating the relations between age and corrected volumes of the a) dorsolateral prefrontal cortex (DLPFC) (upper panel) and b) superior parietal cortex (lower panel) for adults with spina bifida myelomeningocele (SBM) and typically developing (TD) adults.
Superior Parietal Cortex
The overall model for corrected volume of the superior parietal cortex included group, age, and the two-way group × age interaction, and reached overall statistical significance, F(3, 49) = 9.89, p < .001. Contrary to hypotheses, superior parietal volume was significantly, disproportionately enlarged in the group with SBM compared to the TD group, F(49) = 5.26, p = .026, across age. As expected, age was not significantly associated with corrected superior parietal cortex volume, F(49) = −0.72, p = .477, across groups. Additionally, the significant group × age interaction effect on corrected volume of the superior parietal cortex was unexpected, F(49) = 5.26, p = .026. As shown in Figure 2, corrected volume of the superior parietal cortex was significantly reduced in older relative to younger adults in the group with SBM, r(23) = −.55, p = .006, but was not associated with age in the TD group, r(30) = −.14, p = .451.
Putamen
The overall model for corrected volume of the putamen included group and age, and reached overall statistical significance, F(2, 51) = 3.40, p = .041. As expected, putamen volume was significantly, disproportionately enlarged in the group with SBM compared to the TD group, t(51) = 2.44, p < .05, controlling for the expectedly, nonsignificant effect of age, t(51) = −0.37, p = .710.
Hippocampus
The overall model for corrected volume of the hippocampus included group and age, and reached overall statistical significance, F(2, 51) = 10.83, p < .001. As expected, hippocampus volume was significantly, disproportionately reduced in the group with SBM compared to the TD group, t(51) = −4.17, p < .001, after controlling for the nonsignificant effect of age, t(51) = 1.13, p = .136.
Aim 3: Brain-Behavior Relations
Table 4 presents all of the brain-behavior relations for the group with SBM and the TD group separately. In the interest of space, only relations with large effect sizes (r > |.40|) are discussed below.
Table 4.
Brain-behavior relations for the adults with spina bifida myelomeningocele (SBM) and the typically developing (TD) adults.
| DLPFC | SPC | Hippocampus | Putamen | Caudate | Thalamus | |
|---|---|---|---|---|---|---|
| TD (n = 30) | ||||||
|
| ||||||
| WMST RT | −.444* | −.190 | .092 | .101 | .042 | .033 |
| WMST AC | .216 | .098 | −.247 | −.298 | −.009 | −.210 |
| WMMT RT | −.212 | −.147 | −.210 | .204 | .019 | .020 |
| WMMT AC | .106 | .156 | −.101 | −.471* | −.277 | −.207 |
| ICT Inhibitory Control RT | −.515* | −.157 | .135 | .074 | .157 | −.095 |
| ICT Inhibitory Control AC | −.179 | .121 | .150 | −.260 | −.082 | .081 |
|
| ||||||
| SBM (n = 23) | ||||||
|
| ||||||
| WMST RT | −.003 | −.080 | −.421* | .104 | −.173 | .167 |
| WMST AC | −.329 | −.112 | .179 | .008 | −.096 | .093 |
| WMMT RT | −.234 | .015 | −.145 | .038 | −.105 | .001 |
| WMMT AC | −.277 | −.052 | .391 | −.113 | .159 | −.102 |
| ICT Inhibitory Control RT | −.060 | −.165 | −.047 | .123 | .054 | .269 |
| ICT Inhibitory Control AC | −.413* | −.092 | .177 | −.282 | −.044 | −.058 |
Note:
magnitude of correlation coefficient > |.40|;
DLPFC = Dorsolateral prefrontal cortex; SPC = Superior parietal cortex; WMST = Working Memory Span Task; WMMT = Working Memory Manipulation Task; ICT = Inhibitory Control Task; RT = Reaction time; AC = Accuracy.
In the group with SBM, corrected hippocampus volume was negatively associated with reaction time during the WMST, r(23) = −.42 and corrected DLPFC volume was negatively associated with performance accuracy during inhibitory control, r(23) = −.41. As expected, larger corrected hippocampus volume was associated with faster (i.e., better) WMST reaction time in the adults with SBM. Contrary to expectations, however, larger corrected DLPFC volume was associated with less accurate inhibitory control performance in the group with SBM.
In the TD group, corrected DLPFC volume was negatively associated with WMST, r(30) = −.44, and inhibitory control, r(30) = −.52, reaction time. As expected, larger corrected DLPFC volume was associated with faster reaction time performance during the WMST and inhibitory control in the TD group. In addition, corrected putamen volume was negatively associated with WMMT performance accuracy, r(30) = −.47, in the TD group; unexpectedly, smaller corrected putamen volume in the TD adults was associated with better WM manipulation performance accuracy.
Follow-up analyses indicated that the magnitude of the association between corrected hippocampus volume and the WMST was significantly larger in the group with SBM compared to the TD group, Z = 1.83, p = .034. The magnitude of the association between corrected DLPFC volume and inhibitory control accuracy did not statistically differ between groups, Z = 0.88, p = .196. The magnitude of the associations between corrected volume of the DLPFC with performance accuracy during inhibitory control was significantly greater in the TD group compared to the group with SBM, Z = −1.73, p = .042. The associations between corrected DLPFC volume with reaction time during the WMST, Z = −1.61, p = .054, trended towards a larger magnitude in the TD group compared to the group with SBM. However, the magnitude of the association between corrected putamen volume with WMMT performance accuracy, Z = −1.35, p = .089, did not statistically differ between groups.
Discussion
Current results extend the limited knowledge of cognitive deficits in adults with SBM to include aspects of cognitive control. Despite similar reaction times during WM maintenance (i.e., span) and manipulation, adults with SBM showed significantly poorer response accuracy during both WM tasks than the typical adults. During inhibitory control, the adults with SBM exhibited less accurate and more sluggish inhibition of prepotent response tendencies, which were maintained after accounting for WM span performance. Together, results indicated that executive deficits in adults with SBM include WM and inhibitory control that, though related, occur independently.
It is difficult to interpret the current results as being indicative of global executive dysfunction in adults with SBM. The overall research literature in SBM has primarily supported that deficits in higher-order cognitive processes, including aspects of cognitive control, relate more to disruption of lower-level, or bottom-up, cognitive processes than to deficient higher-level or top-down processes per se (greater review provided in: Dennis and Barnes, 2010). This has lead to the formulation of a modal profile of cognitive processing in SBM that includes relatively spared associative processing abilities, such as the activation and categorization of information related to stimuli that is based on formed associations, and relatively disrupted assembled processing or integration of information, particularly to exogenous stimuli. Although the conceptualization of this profile is predominantly based on studies of younger individuals and youth, the two known studies of adults with SBM supported assembled processing deficits of impaired prospective and verbal episodic memory and response (processing) speed (Dennis et al., 2015; Treble-Barna et al., 2015). In the current study, difficulty responding to learned associations of visually presented stimuli supported deficient capacity for maintaining information of exogenous stimuli in the adults with SBM. Therefore, the current results extend our knowledge of assembled processing deficits in adults with SBM to include visual WM and inhibitory control.
As expected from our previous findings, the adults with SBM exhibited parallel deterioration of cognitive control abilities to that in normal aging (Dennis et al., 2015; Treble-Barna et al., 2015). With the exception of performance accuracy during the WMST, none of the group by age interactions significantly related to cognitive performance. However, the interaction supported reduced WM span with older age in normal aging was not supported in the adults with SBM. The lack of differential aging effects on performance accuracy across groups supports a control deficiency during executive cognitive processing in older adults compared to younger adults that does not appear to be exacerbated by SBM (e.g., Dywan & Murphy, 1996). When considered within the larger literature, these results support relative stability of cognitive impairments in SBM across the lifespan. However future research utilizing a longitudinal approach in a single cohort could be useful in confirming this assumption.
Although our findings did not identify accelerated cognitive aging in SBM, age differentially related to cortical brain volume alterations in SBM compared to typical adults. Expected reduction of the DLPFC volume with advanced age was exacerbated in SBM (Nyberg et al., 2010; Raz et al., 2010; Raz et al., 2005). Therefore, in spite of having disproportionately enlarged DLPFC volumes on average, volumetric reduction occurred more rapidly in older adults with SBM than in the typical adults. Surprisingly, volume of the superior parietal cortex was also reduced in older aged adults with SBM compared to younger adults, an effect that did not occur in the typical adults. Reduced posterior cortex volume with advanced age was unexpected since normal age-related changes are typically observed in anterior regions (Nyberg et al., 2010), as exhibited in our current sample of typical adults. The neuropathological consequences of hydrocephalus could explain this finding. Deleterious effects of congenital hydrocephalus in pre-clinical animal models include altered myelination and cortical thinning in later development (Del Bigio, 2010; Juranek & Salman, 2010; Sellin et al., 2014). It is possible that posterior brain regions are particularly affected. However, the generally disproportionately enlarged volume of the superior parietal cortex across adulthood in the adults with SBM challenges this interpretation. Another possible explanation could include effects of shunting and shunt revision(s) that are frequently performed to treat and manage hydrocephalus in SBM. However, the lack of hemispheric differences in parietal volume offers limited support for this explanation, as this procedure was typically performed in the right hemisphere in the current sample.
Results indicated that subcortical brain regions may be relatively less sensitive to the effects of age than cortical regions in SBM. Consistent with Treble-Barna, et al. (2015), no age-related alterations were observed in the subcortical structures in SBM or the typical adults. However, results are restricted to the highly specific and limited number of brain structures included in the current study and may not extent to all brain regions. The adults with SBM also exhibited subcortical GM structural abnormalities that generally resembled findings in youth (Ware et al., 2014). These results suggest that proportionate thalamus and caudate volumes along with disproportionate reduction and enlargement of the hippocampus and putamen, respectively, may persist across the lifespan in SBM. A longitudinal investigation is warranted and could elucidate the validity of this assumed pattern.
Regional brain volumes were significantly associated with aspects of WM and inhibitory control in both groups. Hippocampal involvement during information encoding (Friedman & Goldman-Rakic, 1988) was supported through significant relations among hippocampus volume and working memory capacity. In SBM but not the typical adults, proportionately reduced hippocampus volume was related to slower responses to stimuli tapping information maintained within WM. In accordance with the literature, DLPFC volume was inversely associated with inhibitory control capacity in the typical adults (Table 4) (Chambers, Garavan, & Bellgrove, 2009). Volume of the DLPFC was also inversely related to response speed for information maintained in WM, although this relation was only marginally stronger in the typical adults compared to the adults with SBM (D’Esposito et al., 1999; Wager & Smith, 2003).
Limitations
As briefly stated above, the mechanical effects of hydrocephalus and associated shunting likely complicate the protracted development of brain maturation in SBM. In the current sample, the majority of adults with SBM had hydrocephalus and had histories of shunt surgery. Although brain volumes and cognition were unrelated to shunting, it is possible that this treatment disrupts surrounding brain tissue (Ware et al., 2014). Factors related to shunt intervention and associated shunt revisions(s) could also account for the different developmental trajectories of anterior and posterior cortical regions across the lifespan in SBM, although understanding is limited and requires further confirmation (Bowman & McLone, 2010).
The current study examined gross neuroanatomy and did not account for microstructural integrity of gray or white matter. Findings of aberrant volumes of the DLPFC and subcortical GM structures, and, as previously indicated in youth, altered microstructural integrity of subcortical GM structures (Ware et al., 2014), support deviant cellular microstructure in GM as well as in white matter pathways. Given that cognitive control is associated with efficient white matter connections between GM regions in other neurodevelopmental populations (e.g., Cubillo, Halari, Smith, Taylor, & Rubia, 2012), periventricular and cortical-subcortical white matter dysgenesis may be particularly related to higher order cognitive control processes in SBM. Future research would benefit from a general examination of white matter integrity in SBM.
Conclusion and Future Directions
The cognitive effects of normal aging vary in frequency, direction, and extent. Functional implications of our current findings elucidated a profile of general impairment that appears to be maintained in a parallel maturational trajectory across aging in adults with SBM. Though accelerated aging was not noted in aspects of cognitive control or in any of the selected subcortical brain structures, it occurred in both the anterior and posterior cortical regions (i.e., DLPFC and superior parietal cortex). However, the mechanisms leading to volumetric declines with age in cortical brain regions needs further investigation. Though current results indicated that adults with SBM show stable deficits that parallel typical developmental trajectories with older age, as is supported in other recent studies of adults with SBM, it is unclear whether the currently utilized paradigms are sensitive to accelerated aging in this population. Therefore, general understanding of aging in SBM could benefit from examinations using a broader number of measures of discrete cognitive domains. Longitudinal studies of cognition as well as of brain structure and function could also promote our understanding of neurodevelopment and maturation across the lifespan in SBM.
Public significance statements.
The current study addressed literature gaps regarding adult outcomes of the neurogenetic disorder spina bifida myelomeningocele through an assessment of cognitive performance and specific brain volumes. Unlike the accelerated aging effects of some other neurodevelopmental disorders like Down’s syndrome, the adults with spina bifida myelomeningocele exhibited stable deficits in working memory or inhibitory control with age relative to controls. However, volumes of cortical, but not subcortical, brain regions demonstrated greater alteration with older age in the adults with spina bifida myelomeningocele compared to controls, although specific brain volumes in cortical and subcortical regions correlated with cognition.
Acknowledgments
Conflict of interests: None to report. Preparation of this paper was supported in part by grant 5 P01 HD35946 awarded from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
References
- Addis DR, Giovanello KS, Vu MA, Schacter DL. Age-related changes in prefrontal and hippocampal contributions to relational encoding. Neuroimage. 2014;84:19–26. doi: 10.1016/j.neuroimage.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. International Journal of Developmental Neuroscience. 2011;29(3):215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Duerden EG, Taylor MJ. The Centre of the Brain: Topographical Model of Motor, Cognitive, Affective, and Somatosensory Functions of the Basal Ganglia. Hum Brain Mapp. 2013;34(11):3031–3054. doi: 10.1002/Hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16(1):6–15. doi: 10.1002/ddrr.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Peretz I, Malenfant D. Examination of the working memory components in normal aging and in dementia of the Alzheimer type. Neuropsychologia. 1996;34(3):195–207. doi: 10.1016/0028-3932(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Gambrell D, Shin M, Honein MA, Mathews TJ. Racial/Ethnic Differences in the Birth Prevalence of Spina Bifida-United States, 1995–2005 (Reprinted from MMWR, vol 57, pg 1409–1413, 2009) Jama-Journal of the American Medical Association. 2009;301(21):2203–2204. [Google Scholar]
- Bowman RM, McLone DG. Neurosurgical management of spina bifida: research issues. Dev Disabil Res Rev. 2010;16(1):82–87. doi: 10.1002/ddrr.100. [DOI] [PubMed] [Google Scholar]
- Brookshire BL, Fletcher JM, Bohan TP, Landry SH, Davidson KC, Francis DJ. Verbal and nonverbal skill discrepancies in children with hydrocephalus: a five-year longitudinal follow-up. J Pediatr Psychol. 1995;20(6):785–800. doi: 10.1093/jpepsy/20.6.785. [DOI] [PubMed] [Google Scholar]
- Burmeister R, Hannay HJ, Copeland K, Fletcher JM, Boudousquie A, Dennis M. Attention problems and executive functions in children with spina bifida and hydrocephalus. Child Neuropsychol. 2005;11(3):265–283. doi: 10.1080/092970490911324. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya D. Retention of Delayed-Alternation - Effect of Selective Lesions of Sulcus Principalis. Science. 1969;165(3899):1271. doi: 10.1126/science.165.3899.1271. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33(5):631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2 1988. [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, … Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44(6):628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crayton L, Oliver C, Holland A, Bradbury J, Hall S. The neuropsychological assessment of age related cognitive deficits in adults with Down’s syndrome. Journal of Applied Research in Intellectual Disabilities. 1998;11(3):255–272. [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48(2):194–215. doi: 10.1016/J.Cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. J Psychosom Res. 1998;44(6):627–628. doi: 10.1016/s0022-3999(98)00034-8. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16(1):16–22. doi: 10.1002/ddrr.94. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes MA. The cognitive phenotype of spina bifida meningomyelocele. Dev Disabil Res Rev. 2010;16(1):31–39. doi: 10.1002/ddrr.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Cirino PT, Simic N, Juranek J, Taylor WP, Fletcher JM. White and grey matter relations to simple, choice, and cognitive reaction time in spina bifida. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9388-2. [DOI] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Copeland K, Frederick JA, Francis DJ, Hetherington R, … Fletcher JM. Space-based inhibition of return in children with spina bifida. Neuropsychology. 2005;19(4):456–465. doi: 10.1037/0894-4105.19.4.456. [DOI] [PubMed] [Google Scholar]
- Dennis M, Sinopoli KJ, Fletcher JM, Schachar R. Puppets, robots, critics, and actors within a taxonomy of attention for developmental disorders. J Int Neuropsychol Soc. 2008;14(5):673–690. doi: 10.1017/S1355617708080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Hetherington R. New survivors for the new millennium: Cognitive risk and reserve in adults with childhood brain insults. Brain Cogn. 2000;42(1):102–105. doi: 10.1006/brcg.1999.1174. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenny DA, Krinsky-McHale SJ, Sersen G, Silverman WP. Sequence of cognitive decline in dementia in adults with Down’s syndrome. J Intellect Disabil Res. 2000;44(Pt 6):654–665. doi: 10.1046/j.1365-2788.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- Devenny DA, Wegiel J, Schupf N, Jenkins E, Zigman W, Krinsky-McHale SJ, Silverman WP. Dementia of the Alzheimer’s type and accelerated aging in Down syndrome. Sci Aging Knowledge Environ. 2005;2005(14):dn1. doi: 10.1126/sageke.2005.14.dn1. [DOI] [PubMed] [Google Scholar]
- Diamond A, Kirkham N, Amso D. Conditions under which young children can hold two rules in mind and inhibit a prepotent response. Dev Psychol. 2002;38(3):352–362. [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult Age-Differences in Working Memory. Psychol Aging. 1989;4(4):500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Dywan J, Murphy WE. Aging and inhibitory control in text comprehension. Psychol Aging. 1996;11(2):199–206. doi: 10.1037//0882-7974.11.2.199. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Brei TJ. Introduction: Spina bifida--a multidisciplinary perspective. Dev Disabil Res Rev. 2010;16(1):1–5. doi: 10.1002/ddrr.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foos PW, Wright L. Adult Age-Differences in the Storage of Information in Working Memory. Experimental Aging Research. 1992;18(1–2):51–57. doi: 10.1080/03610739208253911. [DOI] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988;8(12):4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Mattson SN, Jernigan TL, Archibald SL, Jones KL, Riley EP. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2012;36(11):1932–1941. doi: 10.1111/j.1530-0277.2012.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of Function within Dorsolateral Prefrontal Cortex of Rhesus Monkey. Exp Neurol. 1970;27(2):291. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- GoldmanRakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1996;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Hampton LE, Fletcher JM, Cirino P, Blaser S, Kramer LA, Dennis M. Neuropsychological profiles of children with aqueductal stenosis and Spina Bifida myelomeningocele. J Int Neuropsychol Soc. 2013;19(2):127–136. doi: 10.1017/S1355617712001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton LE, Fletcher JM, Cirino PT, Blaser S, Kramer LA, Drake J, Dennis M. Hydrocephalus status in spina bifida: an evaluation of variations in neuropsychological outcomes. J Neurosurg Pediatr. 2011;8(3):289–298. doi: 10.3171/2011.6.PEDS10584. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975. Unpublished working paper. [Google Scholar]
- Hunt GM, Holmes AE. Factors relating to intelligence in treated cases of spina bifida cystica. Am J Dis Child. 1976;130(8):823–827. doi: 10.1001/archpedi.1976.02120090033006. [DOI] [PubMed] [Google Scholar]
- Iddon JL, Morgan DJ, Loveday C, Sahakian BJ, Pickard JD. Neuropsychological profile of young adults with spina bifida with or without hydrocephalus. J Neurol Neurosurg Psychiatry. 2004;75(8):1112–1118. doi: 10.1136/jnnp.2003.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Fletcher JM, Hasan KM, Breier JI, Cirino PT, Pazo-Alvarez P, … Papanicolaou AC. Neocortical reorganization in spina bifida. Neuroimage. 2008;40(4):1516–1522. doi: 10.1016/j.neuroimage.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Salman MS. Anomalous development of brain structure and function in spina bifida myelomeningocele. Dev Disabil Res Rev. 2010;16(1):23–30. doi: 10.1002/ddrr.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior Parietal Cortex Is Critical for the Manipulation of Information in Working Memory. Journal of Neuroscience. 2009;29(47):14980–14986. doi: 10.1523/Jneurosci.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little DM, Kraus MF, Joseph J, Geary EK, Susmaras T, Zhou XJ, … Gorelick PB. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74(7):558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M. Effects of Small Frontal Lesions on Delayed Alternation in Monkeys. J Neurophysiol. 1957;20(6):615–622. doi: 10.1152/jn.1957.20.6.615. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM. Interfering with working memory in humans. Neuroscience. 2006;139(1):85–90. doi: 10.1016/j.neuroscience.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vain. Surg Forum. 1951:399–403. [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, … Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A. 2010;107(52):22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C, Holland T, Hall S, Crayton L. Effects of increasing task load on memory impairment in adults with Down syndrome. Am J Ment Retard. 2005;110(5):339–345. doi: 10.1352/0895-8017(2005)110[339:EOITLO]2.0.CO2. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore ET. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. The effects of aging on reaction time in a signal detection task. Psychol Aging. 2001;16(2):323–341. doi: 10.1037//0882-7974.16.2.323. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51(2):501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Roncadin C, Guger S, Archibald J, Barnes M, Dennis M. Working memory after mild, moderate, or severe childhood closed head injury. Dev Neuropsychol. 2004;25(1–2):21–36. doi: 10.1080/87565641.2004.9651920. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL, Shaw RJ. Effects of Adult Age on Structural and Operational Capacities in Working Memory. Psychol Aging. 1991;6(1):118–127. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- Scott MA, Fletcher JM, Brookshire BL, Davidson KC, Landry SH, Bohan TC, … Francis DJ. Memory functions in children with early hydrocephalus. Neuropsychology. 1998;12(4):578–589. doi: 10.1037//0894-4105.12.4.578. [DOI] [PubMed] [Google Scholar]
- Sellin JN, Cherian J, Barry JM, Ryan SL, Luerssen TG, Jea A. Utility of computed tomography or magnetic resonance imaging evaluation of ventricular morphology in suspected cerebrospinal fluid shunt malfunction. J Neurosurg Pediatr. 2014;14(2):160–166. doi: 10.3171/2014.4.PEDS13451. [DOI] [PubMed] [Google Scholar]
- Stewart JT. The frontal/subcortical dementias: common dementing illnesses associated with prominent and disturbing behavioral changes. Geriatrics. 2006;61(8):23–27. [PubMed] [Google Scholar]
- Stuss DT. Functions of the Frontal Lobes: Relation to Executive Functions. Journal of the International Neuropsychological Society. 2011;17(5):759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Swartwout MD, Cirino PT, Hampson AW, Fletcher JM, Brandt ME, Dennis M. Sustained attention in children with two etiologies of early hydrocephalus. Neuropsychology. 2008;22(6):765–775. doi: 10.1037/a0013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike R, Hagen E, Sattler J. The Stanford-Binet intelligence scale. Itasca, IL: Riverside; 1986. [Google Scholar]
- Treble-Barna A, Juranek J, Stuebing KK, Cirino PT, Dennis M, Fletcher JM. Prospective and episodic memory in relation to hippocampal volume in adults with spina bifida myelomeningocele. Neuropsychology. 2015;29(1):92–101. doi: 10.1037/neu0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umilta C, Rubichi S, Nicoletti R. Facilitation and interference components in the Simon effect. Arch Ital Biol. 1999;137(2–3):139–149. [PubMed] [Google Scholar]
- Vachha B, Adams RC. Memory and selective learning in children with spina bifida-myelomeningocele and shunted hydrocephalus: a preliminary study. Cerebrospinal Fluid Res. 2005;2:10. doi: 10.1186/1743-8454-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Ware AL, Juranek J, Williams VJ, Cirino PT, Dennis M, Fletcher JM. Anatomical and diffusion MRI of deep gray matter in pediatric spina bifida. Neuroimage Clin. 2014;5:120–127. doi: 10.1016/j.nicl.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, Kulesz PA, Williams VJ, Juranek J, Cirino PT, Fletcher JM. Gray matter integrity within regions of the dorsolateral prefrontal cortical-subcortical network predicts executive function and fine motor dexterity in spina bifida. Neuropsychology. 2016;30(2) doi: 10.1037/neu0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wilkinson RT, Allison S. Age and simple reaction time: decade differences for 5,325 subjects. J Gerontol. 1989;44(2):P29–35. doi: 10.1093/geronj/44.2.p29. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Stine EAL, Lahar CJ, Aberdeen JS. Does the Capacity of Working Memory Change with Age. Experimental Aging Research. 1988;14(2–3):103–107. doi: 10.1080/03610738808259731. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17(3):278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Enrile BG, Loss N, Blumenstein E, Delis DC. Verbal learning and memory in children with myelomeningocele. J Pediatr Psychol. 1995;20(6):801–815. doi: 10.1093/jpepsy/20.6.801. [DOI] [PubMed] [Google Scholar]