Abstract
Purpose
The Short Physical Performance Battery (SPPB) is a strong predictor for risk of physical disability in older adults. Roughly half of individuals participating in phase II cardiac rehabilitation (CR) are ≥65 years of age, many presenting with low aerobic capacities and may be at increased risk for physical disability.
Methods
The cohort consisted of 196 consecutive patients (136 males), aged ≥65 years, entering CR were prospectively assessed by SPPB. Data were also obtained for age, self-reported physical function (Medical Outcomes Short Form-36), and peak aerobic capacity. Measures were repeated when patients completed CR for those individuals that completed the program.
Results
The average age of patients was 74±0.5 years. At baseline, total SPPB score was 9.7±0.2 (out of 12). Follow-up data was obtained on 133 (68%) patients with a mean improvement of 0.8±0.1 (p<0.0001), which was not clinically significant (≥1 point). Focusing on patients with a low baseline SPPB, 72 subjects scored 9 or below (7.1±0.2) with 45 completing exit measures. Improvements were found in gait speed (0.5±0.1, p<0.0001), chair-stand (1.0±0.1, p<0.0001), and total SPPB (1.6±0.3, p<0.0001) in this more disabled group. Measures of VO2peak was significantly reduced in the low SPPB group (13.5±0.4 vs 17.5±0.4 ml/kg/min, p<0.0001). VO2peak (R2=26%, p<0.0001) and self-reported physical function score (R2= 5%, p=0.02) were the only multivariate predictors of baseline SPPB.
Conclusion
For patients who enter CR with low SPPB scores (37%) significant improvements in physical function are noted, largely explained by improved walking speed and leg strength (chair-stand).
Brief Abstract
The Short Physical Performance Battery (SPPB) is a strong predictor for risk of physical disability in older adults. For older patients who enter cardiac rehabilitation with diminished physical function (37%), significant improvement is noted in walking speed and leg strength (chair-stand) following completion of an exercise program.
Introduction
Despite well documented effects of cardiac rehabilitation (CR) on mortality and aerobic capacity,1,2,3,4,5 little is known regarding its effect on directly measured physical function. The CR demographic often consists of elderly patients with low aerobic fitness,6,7 both significant risk factors for disability with the mean age of participants at many programs approaching 65 years.7 Additionally, rates of frailty in community-dwelling adults with cardiovascular disease range from 10–60%, indicating that many CR participants may be at increased risk of disability.8,9
The Short Physical Performance Battery (SPPB) was developed to assess disability risk through performance in balance, gait speed, and repeated chair-stands.10 Better performance in individual components or total score is associated with lower rates of mortality, disability, hospitalizations, and nursing home admissions.10,11,12,13 The SPPB has been validated in multiple populations12,14 and is sensitive to major clinical events including hospitalization for hip fracture, stroke, congestive heart failure, and myocardial infarction. While structured exercise has been shown to result in modest improvements on the SPPB in a healthy population,15 there is limited information regarding physical function and the SPPB in cardiac patients in a Phase II CR program. Additionally, there are conflicting reports as to whether long-term maintenance programs following CR produce benefits in this domain. One study found no change in SPPB following 1.6 years of a maintenance exercise program16 while another resulted in improvements at 1 year in elderly frail patients who incorporated strength, flexibility, balance, and coordination exercises in addition to aerobic exercise.17
Of studies that addressed the effect of CR on physical function, most employ self-reported questionnaires for their analysis.18,19 Therefore, we sought to directly measure physical function in an older CR population utilizing the SPPB and its response to exercise training. We hypothesize that completion of phase II CR will improve performance on the SPPB.
Methods
Subjects
The study sample included consecutive patient’s aged 65 years or older entering an outpatient phase II CR program. Study data was prospectively collected from January 2011 to October 2013. During an initial visit, data was collected on age, sex, body weight, height, aerobic capacity, handgrip strength (kg), self-reported physical fitness (Medical Outcomes Study Short Form-36 (MOS SF-36)),20 and depression scores (Geriatric Depression Questionnaire).21 The primary cardiac diagnosis at entry into CR was also recorded including: coronary artery bypass graft surgery; myocardial infarction; angina treated with a percutaneous intervention alone (without MI); angina treated medically; congestive heart failure, and heart valve replacement/repair surgery.
SPPB
The SPPB was performed as previously described.10 The SPPB is composed of three tasks assessing an individual’s balance, gait speed, and ability to stand from a chair (chair-stand). The balance component required subjects to hold a standing position (side-by-side, semi-tandem, tandem) for 10 seconds each. Gait speed was measured over a 4 meter course at a usual walking pace. Chair-stand was the time required to rise from a seated position 5 times. Scores for each domain range from of 0–4 for a total score of 0 to 12. Scores reflect quartiles of the original study population, i.e. 1<25th percentile, 4>75th percentile. A higher score indicates better physical function with lower scores sequentially increasing the likelihood of future disability. The initial SPPB was performed within the time frame of the first 4 exercise sessions and the exit analysis was obtained during the final exercise session or prior to an exit ETT.
Exercise Tolerance Test
At entry and exit from the CR program, patients performed a symptom limited treadmill exercise tolerance test (ETT) to determine peak aerobic capacity (VO2peak) in mL O2·kg−1·min−1. Treadmill protocols included the Bruce, modified-Balke, and modified-Naughton, depending on an initial estimation of fitness, and continued until volitional exhaustion, progressive angina, or other untoward findings that necessitated termination. Individuals performed the same exercise protocol at baseline and exit from CR. Expired gas analysis was measured continuously throughout the ETT with a Medgraphics Ultima CPX (St. Paul, Minnesota). The highest average 30-second value for VO2 was defined as the VO2peak. Calibration was performed for each test as described previously.6 Quality control is performed according to published guidelines.22
For patients completing an entry ETT without expired gas analysis (i.e. at referring private cardiologist office), VO2peak was estimated using the Ades nomogram.6 The regression equation utilizes age and treadmill time and has been validated in clinical cardiovascular populations entering CR as more accurate than estimating oxygen consumption based on treadmill time alone.23
Exercise Protocol
The exercise training program generally consisted of 3 sessions per week of aerobic and strength training to a total of 36 sessions. Patients were monitored during training sessions, and exercise intensity was adjusted to maintain participant heart rate (HR) in the range of 70% to 85% of the peak HR obtained on the entry ETT and/or a Borg scale for rating of perceived exertion between “fairly light” and “hard” (11–15 on a scale of 6–20). Typically, exercise training sessions comprised 25 minutes on the treadmill; and 8 minutes on 3 other implements including elliptical trainers; upright and seated steppers; and cycle, arm, and rowing ergometers. Resistance training included both upper and lower extremity exercises with most patients performing 1 set of 10 repetitions with a subjective rating of “hard.” Patients were encouraged to exercise 1–2 times per week on their own to a total of 3–5 exercise sessions per week.
Statistical Analysis
The cohort was stratified into a higher (≥10 total SPPB) and lower (≤9 total SPPB) performing group for further analysis according to baseline performance. Between-group comparisons at baseline and following CR were performed with ANOVA and chi2 tests (IBM SPSS Statistics version 22). Paired t-tests were used to compare changes following CR. Stepwise linear regression was utilized for prediction of baseline SPPB scores. Statistical significance was set at the level of p<0.05. All data are reported as mean ± SEM.
Results
Baseline Demographics
Baseline demographics were obtained for 196 subjects (Table 1) and these characteristics did not differ by index diagnosis (not shown, p=NS).
Table 1.
High vs Low SPPB Group Comparisons.
| Total | Low | High | P value | |
|---|---|---|---|---|
| n (M/F) | 196 (136/60) | 72 (43/29) | 124 (93/31) | |
| Sessions | 28 ± 1 | 28 ± 1 | 28 ± 1 | 0.78 |
| Age (yr) | 74 ± 1 | 77 ± 1 | 73 ± 1 | <0.0001 |
| Body mass (kg) | 80.7 ± 1.1 | 80.0 ± 1.8 | 81.4 ± 1.4 | 0.60 |
| Body mass index (kg/m2) | 28.3 ± 0.4 | 29.1 ± 0.8 | 27.8 ± 0.4 | 0.12 |
| Handgrip Strength (kg) | 30 ± 1 | 26 ± 1 | 32 ± 1 | <0.01 |
| MOS SF-36 Physical Function | 61 ± 2 | 52 ± 3 | 67 ± 3 | <0.01 |
| Geriatric Depression Score | 2.7 ± 0.2 | 2.8 ±0.3 | 2.6 ± 0.3 | 0.57 |
| Peak VO2 (mLO2*kg−1*min−1) | 16.3 ± 0.3 | 13.5 ± 0.4 | 17.5 ± 0.4 | <0.0001 |
| Index Diagnosis | ||||
| Coronary Artery Bypass Grafting | 26% (50) | 22% (16) | 27% (34) | 0.51 |
| Myocardial Infarction | 31% (61) | 29% (21) | 32% (40) | |
| Percutaneous Coronary Intervention | 27% (52) | 25% (18) | 27% (34) | |
| Medical Therapy/Stable Angina | 1% (2) | 1% (1) | 1% (1) | |
| Congestive Heart Failure | 2% (3) | 1% (1) | 2% (2) | |
| Valvular Heart Disease | 14% (28) | 21% (15) | 10% (13) |
Data are mean ± SEM. Depression score >5 demonstrates significant symptoms of depression.
Baseline SPPB scores
Average total SPPB score at entry to CR was 9.7±0.2 (out of 12) and multiple regression revealed a significant correlation between SPPB and VO2peak (r=0.508). Further analysis found 124 (63%) patients scored 10 or higher for total SPPB, thus indicating these patients were unlikely to have significant near term risk of disability and may display a ceiling effect in terms of changes with exercise training.
Training Effect
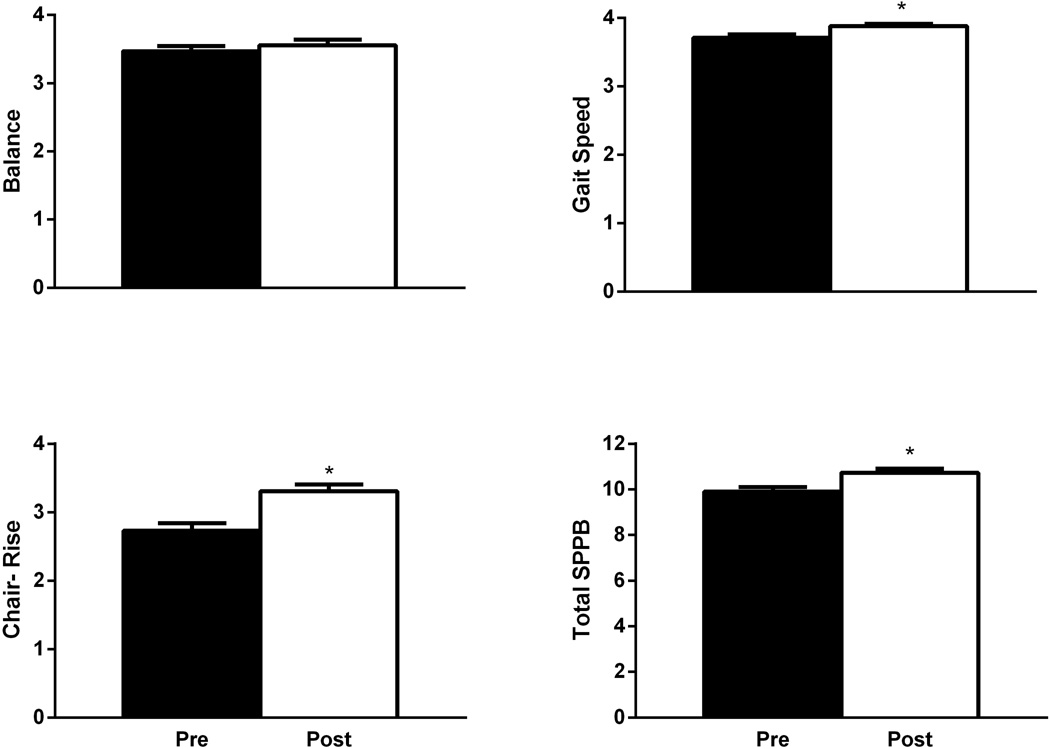
Following CR, 133 subjects completed follow-up measures with no change noted for balance (3.5±0.1vs3.6±0.1, p=0.25). Statistically significant improvements were observed for gait speed (3.7±0.1vs3.9±0.0), chair-stand (2.7±0.1vs3.3±0.1), and total SPPB (9.9±0.2vs10.7±0.2) (all p<0.0001) though none of these overall changes were clinically significant (≥1 unit) as determined by previous studies (Figure 1).
Figure 1.
Short Physical Performance Battery (SPPB) scores at entry to and exit from cardiac rehabilitation. Data are mean ± SEM. * indicates statistical significance between pre and post scores at p<0.0001.
Follow-up performance measures were not obtained for 63 patients due to discontinuation of CR for medical or personal reasons. When compared to those who completed exit evaluations, baseline measures for non-completers were lower for VO2peak (15.0±3.2vs16.8±4.7mL/kg/min, p=0.015), MOS SF-36 (54±23vs64±23, p=0.05), and total SPPB (9.2±2.4vs9.9±2.3, p=0.034) with trends towards poorer chair-stand performance (2.4±1.2vs2.7±1.3, p=0.061) and higher weight (185±39vs174±33lbs, p=0.052). Additionally, non-completers attended significantly fewer CR sessions (19±13vs32±8, p<0.0001). Of the 63 non-completers, 36 (57%) scored 10 or above and 27 (43%) 9 or below for total SPPB, making it unlikely that SPPB scores influenced CR participation.
Focus on Low SPPB Scores
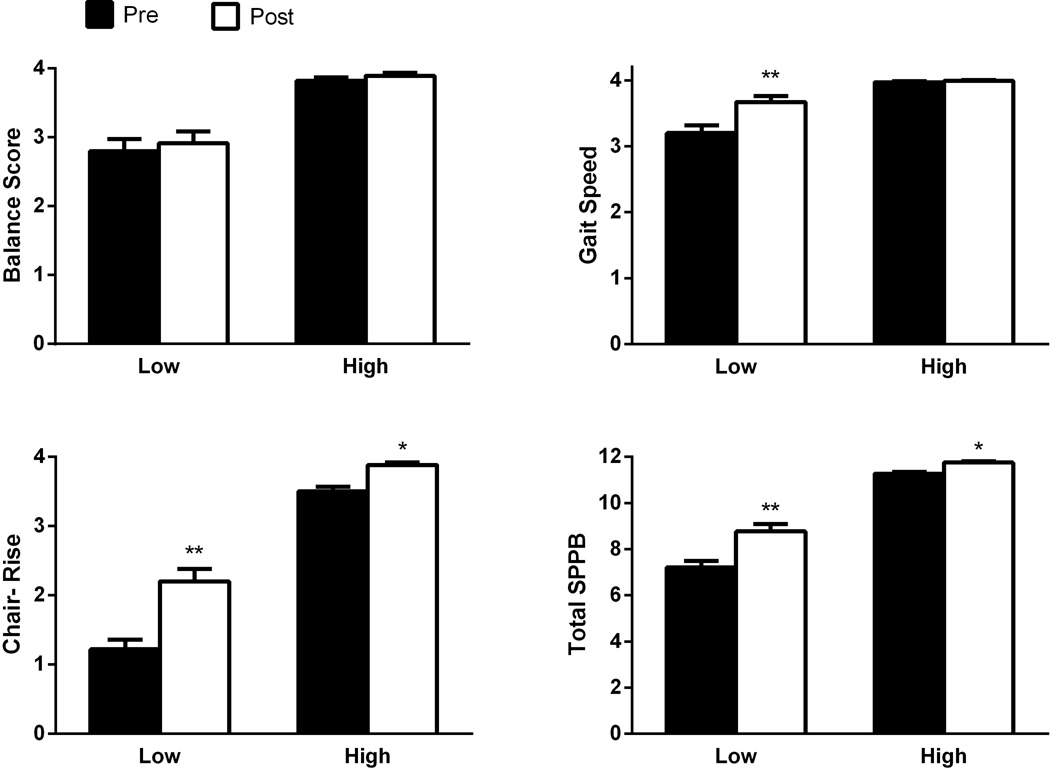
When patients were stratified by baseline SPPB, 72 subjects scored at 9 or less (low group, 7.1±0.2) with 45 completing exit measures. Improvements were found for gait speed (+0.5±0.1, p<0.0001) and chair-stand (+1.0±0.1, p<0.0001) in addition to a clinically significant improvement in total SPPB (+1.6±0.3, p<0.0001) in this higher risk group. 124 patients scored 10 or greater at baseline (high group, 11.2±0.1), suggesting a low risk of future physical disability, with no meaningful improvements found at exit in this group (n=88).
Comparisons Between High and Low Scoring Groups
When comparing changes in physical function between the high and low scoring groups, greater improvements occurred in the low scorers for all measures except balance, which was not affected by exercise (Figure 2). VO2peak was significantly reduced in the low scoring subgroup at baseline (13.5±0.4 vs 17.5±0.4 mL/kg/min, p<0.0001). Additionally, low total SPPB scores were associated with older age and decreased handgrip strength and self-reported physical function (Table 1). Incidence of diagnoses did not vary between groups indicating that baseline differences were not the result of cardiac status (i.e. surgery).
Figure 2.
Change in Short Physical Performance Battery (SPPB) scores with cardiac rehabilitation participants stratified into lower (≤9) and higher (≥10) scoring groups according to baseline performance. Data are mean ± SEM. * indicates within group statistical significance at p<0.0001. ** indicates both within and between group differences in response to exercise training at p<0.0001.
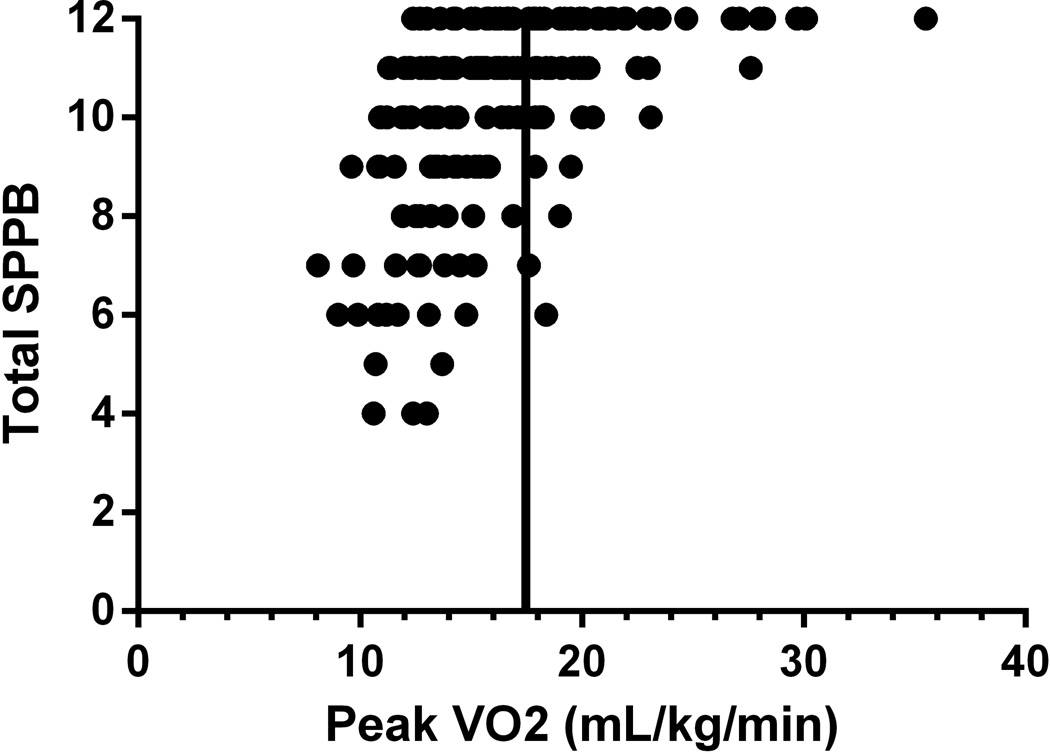
When analyzing patients with a VO2peak greater than 5 metabolic equivalents (METs, approximately 17.5mL/kg/min) to those less than 5 METs, total SPPB scores were 11.1±0.2 vs 9.5±0.2 respectively (p<0.0001). Furthermore, balance (p<0.0001), gait speed (p=0.028), chair-stand (p<0.0001), and total SPPB (p<0.0001) were significantly higher in the high fitness group. VO2peak (R2= 26%) and sf-36 (R2= 5%) were the only significant independent predictors of baseline SPPB by multiple regression. Additionally, 91% of patients with a baseline aerobic fitness greater than 5 METs had total SPPB scores of 10 or above as compared to 58% of patients below 5 METs (Figure 3).
Figure 3.
Relationship between Short Physical Performance Battery (SPPB) and VO2peak at baseline. The vertical bar indicates an aerobic capacity of 5 METs (approximately 17.5 mLO2*kg−1*min−1)
Discussion
A significant percentage (37%) of participants ≥65 years entering phase II CR score low on the SPPB (≤9), presumably placing them at higher risk of disability over four years.10 While these patient’s baseline SPPB scores were lower, they experienced significant improvement following participation in a phase II CR exercise program. The gains observed in physical function are explained primarily by improvements in walking speed and leg strength (chair-stand), both of which are impacted through aerobic and resistance training in the CR setting whereas balance did not improve.
The majority of older patients entering CR do so with relatively higher levels of physical function as measured by the SPPB and, likely, with a relatively low risk of disability. Previous research indicates a substantial change in physical function is indicated by increases in gait speed (0.10m/s) and total SPPB (>1point).24 While we found statistically significant improvement in overall scores, the changes in gait speed, chair-stand, and total SPPB were not clinically significant in our overall population. Therefore, there is likely a ceiling effect for high functioning patients where no improvements can be seen when utilizing the SPPB. This could explain why Mandic et al. found no improvements in their follow-up of CR patients as the average SPPB of their population was 11.5 points at baseline.16 Additionally, their participants may have experienced significant improvements during CR, similar to our population, which was then maintained through a long-term exercise program. Focusing on lower functioning patients (≤9) demonstrated significant improvements in gait speed, chair-stand and total SPPB but not balance. Although performance on the balance section remained unaffected by CR, the result is consistent with previous literature specifying that the SPPB balance component is less sensitive to change than chair-stand, gait speed, and handgrip strength after an exercise program25,26 although it may improve after a longer duration and more intensive strength training program.27 It should be noted that while there were baseline differences between the high and low groups in age (73vs77 years) and handgrip strength (32vs26kg), no differences were found in weight or BMI. It is therefore difficult to determine the degree to which age or age-related muscle loss influenced baseline SPPB scores as neither was a significant predictor in the multiple regression model.
An aerobic capacity less than 5 METs (approximately 17.5mLO2*kg−1*min−1) classifies CR participants at moderate risk for cardiac events during exercise participation.28 Therefore, the differences found in VO2peak between the high and low scoring groups provide evidence that lower physical function may place CR patients not only at an increased risk of disability, but may be indirectly associated with increased risk of cardiac events as well. The SPPB appears to be less useful in CR participants with better aerobic fitness as higher VO2peak was associated with high SPPB scores (11.1 points). We found that 91% of patients with aerobic capacities greater than 5 METs scored high on the SPPB, which corresponds with completion of 3 minutes on the Bruce, 6 minutes on the modified Balke, or 10 minutes on the modified Naughton ETT protocols. As aerobic consumption measures incorporate components of walking speed, muscle mass, and muscular performance (strength and endurance) among other factors, the ETT may provide similarly useful measures of physical function as the SPPB. In support of this concept, the 6 minute walk test was deemed highly reliable and capable of discriminating between good and high functioning individuals25 even though it is a submaximal test for aerobic function. Therefore, an alternative to measuring SPPB to identify low physical function could be utilizing an ETT to prescreen for further testing. Additional benefits of employing an ETT include objectively determining aerobic capacity, peak heart rate, and target heart rate zones as well as screening for ECG changes and cardiovascular symptoms.
Our observations have the potential to be applied to additional frail cardiovascular patients. Frailty, as defined by gait speed, is associated with a higher 6-month mortality in multivessel acute coronary syndrome (<0.62m/s)29 as well as a 3-fold increase in post-operative mortality following cardiac surgery (<0.83m/s).30 Therefore CR may serve to facilitate surgical recovery in older patients and improve long term outcomes.26 Our data supports this view as surgical patients SPPB scores improved similarly to other diagnosis following CR. Furthermore, we have previously demonstrated that surgical patients display 18–20% and 5–10% improvements in VO2peak and handgrip strength respectively.31,32 Congestive heart failure can lead to cardiac cachexia33,34 and is known to alter skeletal muscle function through changes in myosin cross-bridge interaction.35,36 This skeletal muscle dysfunction can be mitigated through strength training,34 underscoring the importance of including resistance training in CR for older patients in general.27 Because our population only included 3 primary congestive heart failure patients, we cannot speak to the efficacy of CR to improve SPPB in this cohort.
Our study was limited by its nonrandomized observational nature in a single clinical setting. Additionally, aerobic capacity was indirectly determined in 43% of the subjects. The method of measurement is not likely important as our main outcome was SPPB, the majority of patients underwent expired gas analysis testing, and the Ades equation has been confirmed to be accurate in the CR population.23 Furthermore, aerobic capacities were similar regardless of direct or indirect measurement (15.8vs16.4). Nonetheless, we found that most older patients enter CR with high SPPB scores and exercise training clearly improves physical function in patients with low baseline values. Additional follow up is required to determine if increases seen in SPPB scores lead to lower rates of physical disability, nursing home admissions, or mortality in an older CR population.
In summary, measurement of the SPPB in older CR patients entering CR was moderately useful from a clinical point of view although a baseline ETT was a useful proxy for SPPB score and thus, risk of disability. After conditioning, when older subjects were stratified by baseline SPPB, CR resulted in an improvement in walking speed, chair stand and total SPPB score in subjects who scored 9 or less when entering CR. Stratifying by fitness, 42% of older patients with aerobic capacities below 5 METs exhibited lower performance (≤9 points) on the SPPB. Rather than screening all older patients, it may be advantageous to target those with a functional capacity below 5 METs for additional testing with the SPPB.
Acknowledgments
Funding for this study was provided in part by:
National Institutes of Health Center of Biomedical Research Excellence award from the National Institute of General Medical Sciences: P20GM103644 (Dr. Ades)
Footnotes
Disclosure: There are no relationships with industry to report.
All authors have read and approved submission of the manuscript and the manuscript has not been published and is not being considered for publication elsewhere in whole or part in any language except as an abstract.
References
- 1.Vanhees L, Fagard R, Thijs L, Amery A. Prognostic value of training-induced change in peak exercise capacity in patients with myocardial infarcts and patients with coronary bypass surgery. Am J Cardiol. 1995;76(14):1014–1019. doi: 10.1016/s0002-9149(99)80287-2. [DOI] [PubMed] [Google Scholar]
- 2.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121(1):63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev(7) 2011 doi: 10.1002/14651858.CD001800.pub2. Cd001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin BJ, Hauer T, Arena R, Austford LD, Galbraith PD, Lewin AM, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012;126(6):677–687. doi: 10.1161/CIRCULATIONAHA.111.066738. [DOI] [PubMed] [Google Scholar]
- 5.Anderson L, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev(12) 2014 doi: 10.1002/14651858.CD011273.pub2. Cd011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ades PA, Savage PD, Brawner CA, Lyon CE, Ehrman JK, Bunn JY, et al. Aerobic capacity in patients entering cardiac rehabilitation. Circulation. 2006;113(23):2706–2712. doi: 10.1161/CIRCULATIONAHA.105.606624. [DOI] [PubMed] [Google Scholar]
- 7.Audelin MC, Savage PD, Ades PA. Changing clinical profile of patients entering cardiac rehabilitation/secondary prevention programs: 1996 to 2006. J Cardiopulm Rehabil Prev. 2008;28(5):299–306. doi: 10.1097/01.HCR.0000336139.48698.26. [DOI] [PubMed] [Google Scholar]
- 8.Pinsky JL, Jette AM, Branch LG, Kannel WB, Feinleib M. The Framingham Disability Study: relationship of various coronary heart disease manifestations to disability in older persons living in the community. Am J Public Health. 1990;80(11):1363–1367. doi: 10.2105/ajph.80.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of Frailty in Patients With Cardiovascular Disease. The American Journal of Cardiology. 2009;103(11):1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 11.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 14.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55(9):916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 15.Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 16.Mandic S, Hodge C, Stevens E, Walker R, Nye ER, Body D, et al. Effects of community-based cardiac rehabilitation on body composition and physical function in individuals with stable coronary artery disease: 1.6-year followup. Biomed Res Int. 2013 doi: 10.1155/2013/903604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molino-Lova R, Pasquini G, Vannetti F, Paperini A, Forconi T, Polcaro P, et al. Effects of a structured physical activity intervention on measures of physical performance in frail elderly patients after cardiac rehabilitation: a pilot study with 1-year follow-up. Intern Emerg Med. 2013;8(7):581–589. doi: 10.1007/s11739-011-0654-z. [DOI] [PubMed] [Google Scholar]
- 18.Ades PA, Maloney A, Savage P, Carhart RL., Jr Determinants of physical functioning in coronary patients: response to cardiac rehabilitation. Arch Intern Med. 1999;159(19):2357–2360. doi: 10.1001/archinte.159.19.2357. [DOI] [PubMed] [Google Scholar]
- 19.Johnston M, MacDonald K, Manns P, Senaratne M, Rodgers W, Haennel RG. Impact of cardiac rehabilitation on the ability of elderly cardiac patients to perform common household tasks. J Cardiopulm Rehabil Prev. 2011;31(2):100–104. doi: 10.1097/HCR.0b013e3181f1fd8c. [DOI] [PubMed] [Google Scholar]
- 20.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Brawner CA, Ehrman JK, Aldred H, Schairer JR, Keteyian SJ. Quality assurance and cardiopulmonary exercise testing in clinical trials. J Card Fail. 2008;14(4):283–289. doi: 10.1016/j.cardfail.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Pierson LM, Pierson M, Herbert W, Cook J. Validation of Nomogram to Predict Exercise Capacity from Treadmill Speed and Grade in Cardiac Patients. American College of Sports Medicine 57th Annual Meeting. 2010 doi: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=e87147ea-90f0-4131-8c60-e9ba47b67390&cKey=ba205f02-b625-4f3d-a8bb-616cb88f5bf2&mKey={24A58842-A6E4-47C5-889B-B8D603BBBA25} [Google Scholar]
- 24.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 25.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54(5):737–742. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 26.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, et al. Frailty Assessment in the Cardiovascular Care of Older Adults. Journal of the American College of Cardiology. 2014;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brochu M, Savage P, Lee M, Dee J, Cress ME, Poehlman ET, et al. Effects of resistance training on physical function in older disabled women with coronary heart disease. J Appl Physiol. 2002;92(2):672–678. doi: 10.1152/japplphysiol.00804.2001. [DOI] [PubMed] [Google Scholar]
- 28.American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th. Champaign, IL: Human Kinetics; 2013. [Google Scholar]
- 29.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying Frailty in Hospitalized Older Adults with Significant Coronary Artery Disease. J Am Geriatr Soc. 2006;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 30.Afilalo J, Eisenberg MJ, Morin J-F, Bergman H, Monette J, Noiseux N, et al. Gait Speed as an Incremental Predictor of Mortality and Major Morbidity in Elderly Patients Undergoing Cardiac Surgery. Journal of the American College of Cardiology. 2010;56(20):1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 31.Mroszczyk-McDonald A, Savage PD, Ades PA. Handgrip strength in cardiac rehabilitation: normative values, interaction with physical function, and response to training. J Cardiopulm Rehabil Prev. 2007;27(5):298–302. doi: 10.1097/01.HCR.0000291297.70517.9a. [DOI] [PubMed] [Google Scholar]
- 32.Savage PD, Rengo JL, Menzies KE, Ades PA. Cardiac Rehabilitation After Heart Valve Surgery: Comparison with Coronary Artery Bypass Graft Patients. J Cardiopulm Rehabil Prev. 2015;35(4):231–237. doi: 10.1097/HCR.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittman JG, Cohen P. The Pathogenesis of Cardiac Cachexia. New England Journal of Medicine. 1964;271(9):453–460. doi: 10.1056/NEJM196408272710908. [DOI] [PubMed] [Google Scholar]
- 34.Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, et al. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol. 2012;590(Pt 5):1243–1259. doi: 10.1113/jphysiol.2011.219659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller MS, Vanburen P, Lewinter MM, Lecker SH, Selby DE, Palmer BM, et al. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail. 2009;2(6):700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller MS, VanBuren P, LeWinter MM, Braddock JM, Ades PA, Maughan DW, et al. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J Physiol. 2010;588(Pt 20):4039–4053. doi: 10.1113/jphysiol.2010.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]