Abstract
Objective
GWAS and preclinical studies demonstrated a role of sortilin in lipid metabolism, inflammation, and vascular calcification – all cardiovascular risk factors. We evaluated the association of serum sortilin levels with the risk of major adverse cerebrovascular and cardiovascular events (MACCE) and the severity of abdominal aortic calcification (AAC).
Approach and Results
A cohort of community-dwelling men aged ≥50 (n=830) was assessed. At baseline, sortilin levels were measured by ELISA, and AAC was assessed on lateral spine scans obtained by dual-energy X-ray absorptiometry. Men aged ≥60 (n=745) were followed up prospectively for the incidence of MACCE. During the median follow-up of 7.9 years, 76 MACCE occurred. The unadjusted incidence of MACCE across increasing sortilin quartiles was: 8.0; 7.4; 19.8, and 20.3 per 1000 person-years. In multivariate-adjusted analysis, sortilin associated with increased risk of MACCE (HR=1.70 per SD; 95%CI: 1.30–2.20, p<0.001). The third and fourth quartiles associated with 3.42-fold (95%CI: 1.61–7.25, p<0.005) and 3.82-fold (95%CI: 1.77–8.26, p<0.001) higher risk of MACCE compared to the first quartile. High sortilin also predicted MACCE independent of traditional Framingham risk factors. Higher sortilin associated with higher odds of severe AAC (score>5) after adjustment for confounders (OR=1.43 per SD, 95%CI: 1.10–1.85, p<0.01). The highest sortilin quartile associated with two-fold higher odds of severe AAC (vs. three lower quartiles combined). After adjustment for LDL-cholesterol the odds of severe AAC remained significant.
Conclusions
In older men, higher serum sortilin levels associated with higher MACCE risk and severe AAC independently of relevant confounders, including C-reactive protein and LDL-cholesterol. This finding however, needs to be validated in other cohorts.
Keywords: sortilin, risk factors, cardiovascular calcification, cardiovascular disease
Introduction
Genome wide association studies have shown a strong association between the 1p13 locus and cardiovascular diseases, including myocardial infarction1, abdominal aortic aneurysm2 and aortic stenosis.3 Variations of the 1p13.3 locus were associated with coronary artery4 and aortic valve5 calcification as well as low-density lipoprotein (LDL)-cholesterol levels in several populations.6 The 1p13.3 locus harbors four genes, CELSR2, PSRC1, MYBPHL and SORT1. The functional role of the SORT1 gene, encoding sortilin, has been studied in vitro and in vivo. Sortilin is a multi-ligand sorting receptor with functional characteristics of the vacuolar protein sorting 10 protein domain family.7 Preclinical in vivo evidence suggests a significant role of sortilin in the pathogenesis of cardiovascular and metabolic diseases, including type 2 diabetes by regulating insulin resistance,8 atherosclerosis through arterial wall inflammation and dysregulated lipoprotein metabolism,9, 10 and vascular calcification.11 We have recently reported that sortilin plays a key role in the development of vascular calcification by promoting the calcification potential of smooth muscle cell-derived extracellular vesicles.11 A recent study also reported a correlation of plasma sortilin with C-reactive protein (CRP) and low-density lipoprotein cholesterol (LDL-C) – both cardiovascular risk factors.12
The value of arterial calcification in the cardiovascular risk assessment is well established.13 Coronary artery calcification (CAC)14, 15 and abdominal aortic calcification (AAC)16 have been investigated as tools for cardiovascular risk assessment.17 Severe AAC is associated with higher risk of cardiovascular morbidity and mortality due to heart attack and stroke.18 As sortilin is reported as a proatherogenic and procalcific molecule, its serum level may be associated with cardiovascular morbidity. However, direct clinical evidence of serum sortilin as a marker for cardiovascular risk as well as of the role of LDL cholesterol and vascular calcification as potential confounders of such an association is lacking.12, 19 Therefore, the primary object of the current study was to evaluate the association of serum sortilin levels with the prospectively assessed risk of major adverse cerebro-cardiovascular events (MACCE) and with severe AAC in a community-dwelling cohort of men aged ≥50.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Baseline characteristics
Supplementary Tables I and II show characteristics of the cohort by sortilin quartiles at baseline. Higher sortilin levels were associated with younger age, lower prevalence of self-reported ischemic heart disease, less frequent treatment with statins and angiotensin converting enzyme inhibitors, lower serum phosphorus and osteoprotegerin, as well as higher serum levels of total cholesterol (TC), LDL-C, and C-reactive protein (CRP). After adjustment for age, serum sortilin correlated positively with serum levels of TC, LDL-C, and CRP (Supplementary Table III).
Serum sortilin levels and incident of MACCE
Men aged 60 or older (n=745) were followed up prospectively. As 15 men dropped out after the first visit, prospective information was available in 730 men. Overall, 76 MACCE occurred over the median of 7.9 years of follow up [IQ range: 7.0; 8.0 years]. The MACCE incidence rate was 14.2 per 1000 person-years. The unadjusted incidence of MACCE across increasing sortilin quartiles was 8.0; 7.4; 19.8, and 20.3 per 1000 person-years, respectively (Table 1).
Table 1.
Multivariate Cox proportional hazard regression analysis for MACCE per sortilin quartiles
| n/N (%) | Incidence* | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|---|
| Per SD (17.6 ng/ml) | 1.58 (1.24 – 2.01)b | 1.64 (1.28 – 2.10)b | 1.67 (1.29 – 2.16)b | 1.70 (1.30 – 2.20)b | ||
| Sortilin quartile | ||||||
| Quartile 1 | 12/178 (6) | 8.0 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 12/180 (6) | 7.4 | 1.15 (0.49 – 2.72) | 1.17 (0.50 – 2.75) | 1.37 (0.57 – 3.26) | 1.44 (0.60 – 3.46) |
| Quartile 3 | 26/187 (13) | 19.8 | 3.04 (1.46 – 6.30)a | 3.19 (1.53 – 6.65)a | 3.44 (1.64 – 7.20)a | 3.42 (1.61 – 7.25)a |
| Quartile 4 | 24/185 (14) | 20.3 | 3.23 (1.54 – 6.76)a | 3.41 (1.62 – 7.19)a | 3.64 (1.71 – 7.76)b | 3.82 (1.77 – 8.26)b |
| Sortilin ≥ vs <median | ||||||
| ≤71 ng/ml | 24/358 (6) | 7.8 | 1.00 | 1.00 | 1.00 | 1.00 |
| >71 ng/ml | 50/372 (13) | 20.0 | 2.90 (1.74 – 4.84)b | 3.05 (1.82 – 5.10)b | 3.03 (1.80 – 5.09)b | 3.00 (1.77 – 5.08)b |
| Sortilin – threshold obtained by the ROC curve | ||||||
| ≤76 ng/ml | 33/466 (6) | 7.9 | 1.00 | 1.00 | 1.00 | 1.00 |
| >76 ng/ml | 41/264 (15) | 21.4 | 2.77 (1.72 – 4.45)b | 2.96 (1.83 – 4.79)b | 3.01 (1.84 – 4.92)b | 3.08 (1.89 – 5.03)b |
p<0.005,
p<0.001;
number of events per 1000 person-years.
Quartile 1, <60 ng/mL; Quartile 2, 60–71 ng/mL; Quartile 3, >71–84 ng/mL; Quartile 4, >84–125 ng/mL.
MACCE is defined as composite of cardiovascular death, non-fatal ST-elevation or non-ST-elevation myocardial infarction, unstable angina, and non-fatal stroke.
Model 1 is adjusted for age, fat mass.
Model 2, adjusted for age, fat mass, smoking (current, former, never), alcohol intake and physical activity.
Model 3, adjusted for age and model 2 variables plus health status (self-reported pharmacologically treated diabetes mellitus, systolic blood pressure, AAC) and medications (vitamin K antagonist, angiotensin-converting enzyme inhibitors, diuretics, fibrates).
Model 4, adjusted for age and model 3 variables plus biological measurements (testosterone, osteoprotegerin, LDL-C, C-reactive protein).
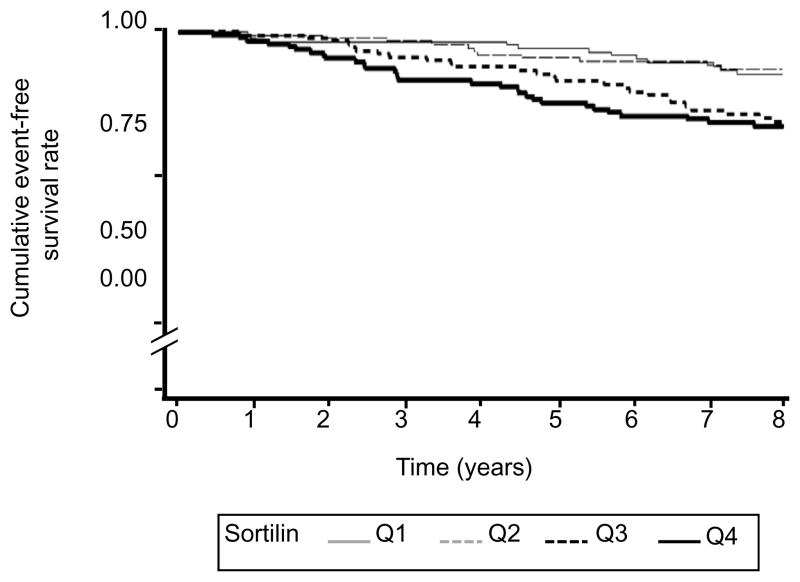
Figure 1 shows the unadjusted Kaplan-Meier curves for event-free survival by sortilin quartiles over 8 years follow-up. After adjustment for age, MACCE incidence increased with elevated sortilin concentration (HR= 1.52 per SD (17.6 ng/ml), 95%CI: 1.20–1.94, p<0.001). The third and fourth quartiles of sortilin associated with more than two-fold higher MACCE risk compared to the first quartile. In the fully multivariate-adjusted analysis the third and fourth quartiles of sortilin associated with a significant 3.3-fold higher MACCE risk compared to the first quartile, respectively (Table 1).
Figure 1.
Kaplan-Meier analyses for MACCE per sortilin quartiles (unadjusted). p<0.001: Q1, Q2 vs. Q3, Q4.
MACCE is defined as composite of cardiovascular death, non-fatal ST-elevation or non ST-elevation myocardial infarction, unstable angina, and non-fatal stroke.
Given the higher risk of MACCE in the third and fourth sortilin quartiles, we categorized men into low and high risk groups using the median value of sortilin as an appropiate cut-off. The incidence of MACCE was 20.0/1000 person-years (95%CI: 15.6–25.8) in men with sortilin above the median (>71ng/mL) and 8.8/1000 person-years (95%CI: 5.7–13.1) in men with lower sortilin levels. After adjustment for other risk factors, the risk of MACCE was 3-fold higher in men with sortilin above the median vs. below the median.
We additionally performed two different analyses to determine an optimal binary cut-off for the sortilin levels. First, we determined the sortilin threshold using the Youden index on the ROC curve analysis. The threshold of 76 ng/mL has a sensitivity of 52% and specificity of 65%. In the fully adjusted model, men with sortilin above the 76 ng/mL associated with 3.1-fold higher MACCE risk compared below the threshold (Table 1). We then explored the sortilin-MACCE relationship by unadjusted spline polynomials that indicated three sortilin groups with low, intermediate and high cardiovascular risk (Supplementary Figure I). The unadjusted incidence of MACCE increased across the sortilin levels (Supplementary Table IV). In the fully adjusted model, highest sortilin levels (>80 ng/mL) associated with 4-fold higher MACCE risk compared to the reference group (<60 ng/mL). Overall, the binary cut-off determined by different approaches gave very similar results regarding the MACCE risk.
Adjustment for LDL-C had no effect in the multi-variate model, suggesting that the observed association of sortilin and MACCE is LDL-C independent (Table 1). When we substitute TC for LDL-C or combined TC and LDL-C, we obtained similar results regardless of using LDL-C or TC as the confounding variable (Supplementary Table V).
Higher sortilin levels were associated with higher risk of MACE (n=43) and stroke (n=33) analyzed separately. In multivariate-adjusted analysis, the HR per SD sortilin was 1.43 for MACE (95%CI: 1.05–1.96, p<0.05) and 1.69 for stroke (95%CI: 1.18–2.41, p<0.005). Men with sortilin levels above the median had higher risk of MACE (HR= 2.76, 95%: 1.45–5.22, p<0.005) and of stroke (HR= 2.37, 95%CI: 1.15–4.88, p<0.05).
The associations remained significant after adjustment for the Framingham risk factors (Supplementary Table VI). After adjustment for these risk factors, men with sortilin levels above the median had higher risk of MACCE (HR= 2.83, p<0.001), MACE (HR= 2.48, p<0.005) and stroke (HR= 3.04, p<0.005).
Next, we analyzed interactions and joint effects of sortilin levels and cardiovascular risk factors associated with the outcomes. High sortilin (>71 vs. ≤71 ng/mL) levels were associated with significant 4.8-, 3.2- and 2.8-fold higher risk of MACCE independent of the presence of hypertension, diabetes and ischemic heart disease, respectively at baseline (Table 2). The MACCE risk further increased when both high sortilin levels and diabetes (HR= 5.5, p<0.001) or high sortilin levels and hypertension (HR= 7.9, p<0.001) were present at baseline. High sortilin levels and ischemic heart disease had no joint effect on MACCE. Low testosterone levels (<11.4 nmol/L) were associated with 2.9-fold higher risk of MACCE (p<0.05). Men with higher sortilin levels had a 4.8-fold increased risk of MACCE (p<0.001), independent of testosterone levels.
Table 2.
The risk of MACCE according to the presence of elevated sortilin* and other confounders
| Sortilin | Co-variable | n/N (%) | HR (95%CI) | p-value |
|---|---|---|---|---|
| Systolic hypertension | ||||
| ≤71 ng/ml | No (<140 mm Hg) | 13/278 (4.7%) | 1.00 | |
| Yes (≥140 mm Hg) | 11/80 (13.7%) | 3.89 (1.49 – 10.18) | <0.01 | |
| >71 ng/ml | No (<140 mm Hg) | 33/275 (12.0%) | 5.45 (2.14 – 13.20) | <0.001 |
| Yes (≥140 mm Hg) | 17/97 (17.5%) | 7.92 (3.22 – 19.49) | <0.001 | |
| Diabetes mellitus | ||||
| ≤71 ng/ml | No | 16/312 (5.1 %) | 1.00 | |
| Yes | 8/46 (17.4 %) | 2.33 (0.89 – 6.13) | 0.08 | |
| >71 ng/ml | No | 43/332 (13.0 %) | 3.39 (1.82 – 6.30) | <0.001 |
| Yes | 7/40 (17.5 %) | 4.96 (2.08 – 11.85) | <0.001 | |
| Ischemic heart disease | ||||
| ≤71 ng/ml | No | 16/289 (5.5 %) | 1.00 | |
| Yes | 10/69 (14.5 %) | 1.03 (0.34 – 3.11) | 0.96 | |
| >71 ng/ml | No | 39/328 (11.9 %) | 2.98 (1.64 – 5.42) | <0.001 |
| Yes | 9/44 (20.4 %) | 3.18 (1.38 – 7.35) | <0.01 | |
| Testosterone | ||||
| ≤71 ng/ml | ≥11.4 nmol/L | 6/178 (3.4 %) | 1.00 | |
| <11.4 nmol/L | 18/180 (9.5 %) | 2.94 (1.06 – 8.16) | <0.05 | |
| >71 ng/ml | ≥11.4 nmol/L | 24/183 (13.1 %) | 5.78 (2.19 – 15.26) | <0.001 |
| <11.4 nmol/L | 26/189 (13.8 %) | 5.98 (2.24 – 15.98) | <0.001 |
> vs. ≤median; n/N (%), number of MACCE per number quartile of serum sortilin level.
MACCE is defined as composite of cardiovascular death, non-fatal ST-elevation or non-ST-elevation myocardial infarction, unstable angina, and non-fatal stroke.
All the models adjusted for age, fat mass, smoking (current, former, never) and physical activity, vitamin K antagonist, angiotensin-converting enzyme inhibitors, diuretics, fibrates, osteoprotegerin, LDL-C, C-reactive protein, and mutually exclusively for testosterone, self-reported pharmacologically treated diabetes mellitus, and systolic blood pressure.
Association between serum sortilin levels and severe abdominal aortic calcification
As expected, prevalence of self-reported ischemic heart disease and AAC severity increased with age (Supplementary Table VII). Men self-reporting ischemic heart disease had more severe AAC, also after adjustment for age, weight, smoking, diabetes and hypertension (Supplementary Table VIII). In the continuous model, serum sortilin and AAC were not significantlly associated. In the unadjusted model, sortilin levels did not differ across the categories of AAC severity (p=0.41) (Supplementary Table IX).
The unadjusted and age-adjusted association between sortilin quartiles and severe AAC (AAC>5) was not significant. However, after adjustment for confounders, odds of severe AAC were higher for elevated serum sortilin levels (OR = 1.43 per SD, 95%CI: 1.10–1.85, p<0.01) (Table 3). Odds of severe AAC were higher in the highest sortilin quartile vs. the lowest quartile (OR=2.19, 95%Cl: 1.12–4.30, p<0.01). Furthermore, the analysis across the quartiles suggested that there was a critical threshold at the level of the third/fourth quartile limit. Odds ratio of severe AAC was higher in the upper sortilin quartile vs. the three lower quartiles combined (OR = 1.94, 95%CI: 1.12–3.36, p<0.05). Adjustment for TC or LDL-C did not have a major impact on the association of serum sortilin and severe AAC. There was no interaction between sortilin, TC and/or LDL-C levels.
Table 3.
Impact of TC and LDL-C on the odds ratio of severe AAC (AAC score >5 vs 0–5) per sortilin quartiles
| Model 1 | Model 1 + TC | Model 1 + LDL-C | Model 1 + TC and LDL-C# | |
|---|---|---|---|---|
| Per 1SD (17.6ng/ml) | 1.43 (1.10 – 1.85)b | 1.42 (1.09 – 1.85)a | 1.46 (1.12 – 1.89)b | 1.42 (1.09 – 1.85)a |
| Sortilin quartile | ||||
| Quartile 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.02 (0.54 – 1.95) | 1.01 (0.53 – 1.93) | 1.08 (0.56 – 2.07) | 1.08 (0.56 – 2.07) |
| Quartile 3 | 1.40 (0.72 – 2.70) | 1.39 (0.72 – 2.68) | 1.42 (0.74 – 2.75) | 1.41 (0.73 – 2.74) |
| Quartile 4 | 2.19 (1.12 – 4.30)b | 2.14 (1.08 – 4.24)a | 2.29 (1.16 – 4.53)b | 2.14 (1.08 – 4.26)a |
| Quartile 1–3 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 4 | 1.94 (1.12 – 3.36)a | 1.91 (1.09 – 3.32)a | 1.99 (1.14 – 3.45)a | 1.86 (1.06 – 3.25)a |
p<0.05;
p<0.01;
the interaction was not significant.
Quartile 1, <60 ng/mL; Quartile 2, >60 – 72 ng/mL; Quartile 3, >72 – 85 ng/mL; Quartile 4, >85 ng/ml.
TC; total cholesterol, LDL-C; low density lipoprotein cholesterol.
Model 1, adjusted for age, fat mass, body height, current smoking, self-reported coronary heart disease, blood pressure, self-reported pharmacologically treated diabetes mellitus, calcium intake, vitamin D and calcium supplements, vitamin K-antagonists, calcium channel blockers, phosphorus, calcium and interaction between calcium and phosphorus.
Sensitivity analysis
Additionally, the analysis was carried out in the entire cohort (i.e. including men with sortilin levels >125 ng/mL). In 769 men aged 60 and over, 76 men had MACCE. Higher sortilin levels (>71 ng/mL) remained significantly associated with higher risk of MACCE (HR= 3.09, 95%CI: 1.86–5.14, p<0.001), MACE (HR= 2.89, 95%CI: 1.52–5.49, p<0.005) and stroke (HR= 2.83, 95%CI: 1.47–5.42, p<0.005). TC, introduced in the model instead of LDL-C, was not significant and not retained in the final model. By contrast, all associations between sortilin and AAC lost significance.
Discussion
The present study demonstrates that in older community-dwelling men, high serum sortilin levels associate with increased MACCE risk (both MACE and stroke) after adjustment for multiple relevant confounders including CRP, TC and LDL-C.
Sortilin is best known for its genetic relationship to LDL-C6 and for its role in controlling hepatic lipoprotein secretion.20, 21 However, emerging evidence suggests a cholesterol-independent role of sortilin in cardiovascular disease.2, 9, 11 Our data support this notion.
We tested the possible confounding effect of serum TC and LDL-C levels on the association between high serum sortilin and AAC. In our cohort, sortilin serum levels were positively correlated with CRP, TC and LDL-C levels, the data consistent with observations in a previously published study.12 Nevertheless, when CRP, TC or LDL-C levels were added to the multivariate model, along with other known AAC risk factors, the sortilin-AAC association remained significant. A recent report showed that statin therapy in 90 statin-naive patients with CAD reduced sortilin serum levels by 12%.19 In our study, treatment with statins or fibrates did not alter the association between serum sortilin and AAC, suggesting that the observed association of high serum sortilin with severe AAC might be independent of lipid abnormalities. This is in line with the cholesterol-independent role of sortilin in the development of experimental atherosclerosis9 and vascular calcification,11 and its genetic association with abdominal aortic aneurysm predisposition.2 We previously reported reduced atherosclerotic vascular calcification in mice lacking sortilin.11 We demonstrated using human smooth muscle cells that sortilin is a key component of extracellular vesicles required for their calcification propensity.11 Therefore, serum sortilin levels are likely to reflect the susceptibility to aortic calcification.
In this study, high serum sortilin levels associated with 3-fold higher risk of MACCE, after adjustment for potential confounders including CRP, TC and LDL-C. AAC is an independent predictor of cardiovascular morbidity and mortality.17 Further, AAC can predict CAC22- one of the strongest predictors of MACCE.14, 23 However, the association between sortilin and MACCE remained significant after adjustment for AAC, suggesting that this association cannot be solely explained by the cofounding effect of AAC visible on a DXA scan. The previous observation that the density of calcification present within the arterial plaque inversely correlates with cardiovascular morbidity showed that the pattern/density of calcification is an important variable in addition to the calcification volume in predicting cardiac events.24 Low-density calcification, so called spotty calcifiation or microcalcification, in the fibrous cap promotes atherosclerotic plaque instability and rupture — the leading cause of myocardial infarction.25 However, whether this represents a causal relationship or large atherosclerotic calcification adds protective features to plaques and serves as a surrogate marker for disease is still of debate. Recently, we found that vascular smooth muscle cell-derived calcifying extracellular vesicles are precursors of microcalcifications in the atherosclerotic plaque26 and that sortilin regulates their calcification competence.11 In mice and humans, high-resolution imaging techniques identified small microcalcifications that may contribute to plaque rupture risk.27, 28 These microcalcifications are smaller than the detection limit of dual-energy x-ray absorptiometry scans, which may explain the remaining association between sortilin and MACCE. Therefore, future studies are required to determine if calcification located in the tunica media of the abdominal aorta reflects the overall cardiovascular calcium burden and the presence of microcalcification.
Importantly, experimental data reveals a role for sortilin in type 2 diabetes by regulating insulin resistance8 as well as in the development of atherosclerotic lesions by controling the interleukin-6 release from inflammartory cells9, LDL-uptake into macrophages,10 or involvement in platelet activation12 - the risk factors for cardiovascular events that may contribute to the sortilin association with MACCE risk. In our cohort, high serum sortilin was associated with MACCE independently of hypertension, diabetes mellitus, or decreased testosterone levels - all cardiovascular risk factors.29–31 Thus, sortilin seems to be associated to MACCE risk even in individuals without these cardiovascular risk factors, suggesting that it may act through independent mechanisms.
Our study limitation is the exclusive examination of men. Elderly men are the population with the highest risk for atherosclerosis and cardiovascular diseases. The high incidence of cardiovascular emergencies evaluated in our study is a major public health problem in the developed countries. The study in a high-risk group provides a foundation for the future studies to explore sortilin as a biomarker in other populations. In addition, two genetic studies found that only male carriers of the minor (protective) allele of rs646776 within the Sort locus had significant changes in LDL-C levels while female subjects were unaffected.32, 33 Genetic reports, however, do not provide causality and do not allow a conclusion about an association with serum sortilin levels. In addition, our work does not suggest the impact of sex hormones, since the association between sortilin and testosterone levels was not significant. A previous report on serum sortilin levels and depression did not identify sex-dependent differences in serum sortilin.34 Therefore it is unlikely that serum sortilin levels in male and female would differ. The study limitations further include a relatively small sample size from a single center that represents a homogenous group in terms of ethnicity and geography. Older volunteers for an epidemiological study may be a healthier group and not representative of their age range. Chronic diseases were self-reported and no formal adjudication was performed. Blood was taken under non-fasting conditions. As sortilin is mainly secreted by the liver,6 it cannot be excluded that sortilin serum levels are affected by food intake. Men above 65 are at risk for abdominal aortic aneurysm, which was genetically linked to sortilin2 and hence should be an adjusting variable in our study. Dual-energy x-ray absorptiometry scannot detect abdominal aortic aneurysm, therefore a correction for it was not possible. Our definition of sudden cardiac death does not exclude death from intracerebral bleeding, aortic dissection, or fulminant pulmonary embolism. Although our analyses were assessed for potential confounding effect of various factors and the final models adjusted for relevant confounders, a significant association due to the residual variability cannot be excluded.
In summary, we showed that, in older men, high serum sortilin levels are associated with increased MACCE risk after adjustment for multiple confounders including CRP and LDL-C. Many genetic studies focus on possible relationship between variations in the sort locus and cardiovascular disease; however clinical implications of results from genom wide association studies are rare. Our study therefore, provides valuable information about the potential use of sortilin as a new cardiovascular risk marker. Nonetheless, before a clinical value of sortilin levels can be proposed, our findings must be validated in the de novo independent cohorts and a potential predictive value of sortilin has to be compared with other widely recognized and validated cardiovascular markers.
Future studies are needed to connect the prospective clinical observations to the mechanistic findings on sortilin in cardiovascular calcification, lipid metabolism and inflammation. A limitation of epidemiological studies is that the specific mechanisms cannot be dissected. These studies may instead explore clinically relevant risk factors and provide valuable information for the future mechanistic studies. Our recent study reported specific molecular mechanisms for sortilin’s action11 and the present study has linked serum sortilin levels and cardiovascular risk. Future investigations are warranted to probe the mechanistic relationship between circulating sortilin and cardiovascular events. If a causal relationship is found, sortilin may serve as both a diagnostic biomarker and a therapeutic target for cardiovascular disease.
Our experimental data associated sortilin with microcalcifications in vitro and in vivo.11 A correlation of serum sortilin levels with coronary artery calcification and valve calcification, however, should be validated via high resolution imaging of subclinical calcification. The potential promise for clinical applications must await large scale and multi-center, prospective follow-up studies involving men and women that can both validate the findings of our study and demonstrate potential clinical utility of serum sortilin in cardiovascular risk prediction in particular for the identification of patients at high MACCE risk.
Supplementary Material
Highlights.
Sortilin serum levels and abdominal aortic calcification were assessed in a cohort of community-dwelling men aged ≥50 (n=830).
Follow up for the incidence of MACCE was for 7.9 median years.
High sortilin levels associated with 3-fold increased MACCE risk independent of traditional Framingham risk factors.
Higher sortilin levels associated with higher odds of severe AAC (score>5) after adjustment for confounders independent of LDL-C and CRP.
Acknowledgments
Funding Sources
This study was supported by a research grant from Kowa Company, Ltd. (M.A.). Dr. Elena Aikawa laboratory is supported by the National Institutes of Health grants R01HL114805 and R01HL109506. Masanori Aikawa is supported by R01HL107550 and R01HL126901.
The STRAMBO study was supported by grants from the Roche pharmaceutical company, Basel, Switzerland, Agence Nationale de la Recherche (ANR-07-PHYSIO-023-01), Hospices Civils de Lyon (50564) and Abondement ANVAR (E1482.042) to Dr. Pawel Szulc.
Non-standard abbreviations
- AAC
Abdominal aortic calcification
- CAC
Coronary artery calcification
- GWAS
Genome wide association studies
- LDL-C
Low density lipoprotein cholesterol
- MACE
Major adverse cardiovascular events
- MACCE
Major adverse cerebro-cardiovascular events
- TC
Total cholesterol
Footnotes
Disclosures
None.
References
- 1.Kathiresan S, Voight BF, Purcell S, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–41. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones GT, Bown MJ, Gretarsdottir S, et al. A sequence variant associated with sortilin-1 (SORT1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet. 2013;22:2941–7. doi: 10.1093/hmg/ddt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muendlein A, Geller-Rhomberg S, Saely CH, Winder T, Sonderegger G, Rein P, Beer S, Vonbank A, Drexel H. Significant impact of chromosomal locus 1p13.3 on serum LDL cholesterol and on angiographically characterized coronary atherosclerosis. Atherosclerosis. 2009;206:494–9. doi: 10.1016/j.atherosclerosis.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell CJ, Kavousi M, Smith AV, et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation. 2011;124:2855–64. doi: 10.1161/CIRCULATIONAHA.110.974899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JG, Luk K, Schulz CA, et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312:1764–71. doi: 10.1001/jama.2014.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–9. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–6. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell. 2005;9:99–108. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Mortensen MB, Kjolby M, Gunnersen S, Larsen JV, Palmfeldt J, Falk E, Nykjaer A, Bentzon JF. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J Clin Invest. 2014;124:5317–22. doi: 10.1172/JCI76002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, Millar J, Kruth H, Rader DJ. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116:789–96. doi: 10.1161/CIRCRESAHA.116.305811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016 doi: 10.1172/JCI80851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa K, Ueno T, Iwasaki T, Kujiraoka T, Ishihara M, Kunimoto S, Takayama T, Kanai T, Hirayama A, Hattori H. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis. 2016;249:110–115. doi: 10.1016/j.atherosclerosis.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 14.Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–7. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 16.Levitzky YS, Cupples LA, Murabito JM, Kannel WB, Kiel DP, Wilson PW, Wolf PA, O’Donnell CJ. Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs. Am J Cardiol. 2008;101:326–31. doi: 10.1016/j.amjcard.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann U, Massaro JM, D’Agostino RB, Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 19.Nozue T, Hattori H, Ogawa K, Kujiraoka T, Iwasaki T, Michishita I. Effects of Statin Therapy on Plasma Proprotein Convertase Subtilisin/kexin Type 9 and Sortilin Levels in Statin-Naive Patients with Coronary Artery Disease. J Atheroscler Thromb. 2016 doi: 10.5551/jat.33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12:213–23. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Strong A, Ding Q, Edmondson AC, et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J Clin Invest. 2012;122:2807–16. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, Cohoon KP, Srikanthan P, Watson KE, Wong ND. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1574–9. doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 24.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–8. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheson JD, Goettsch C, Rogers MA, Aikawa E. Revisiting cardiovascular calcification: A multifaceted disease requiring a multidisciplinary approach. Semin Cell Dev Biol. 2015 doi: 10.1016/j.semcdb.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, Yabusaki K, Faits T, Bouten C, Franck G, Quillard T, Libby P, Aikawa M, Weinbaum S, Aikawa E. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–43. doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–50. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 28.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daka B, Langer RD, Larsson CA, Rosen T, Jansson PA, Rastam L, Lindblad U. Low concentrations of serum testosterone predict acute myocardial infarction in men with type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:35. doi: 10.1186/s12902-015-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275:1571–6. [PubMed] [Google Scholar]
- 31.Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96:1821–5. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- 32.Klein MS, Connors KE, Shearer J, Vogel HJ, Hittel DS. Metabolomics reveals the sex-specific effects of the SORT1 low-density lipoprotein cholesterol locus in healthy young adults. J Proteome Res. 2014;13:5063–70. doi: 10.1021/pr500659r. [DOI] [PubMed] [Google Scholar]
- 33.Karlos A, Shearer J, Gnatiuk E, Onyewu C, Many G, Hoffman EP, Hittel DS. Effect of the SORT1 low-density lipoprotein cholesterol locus is sex-specific in a fit, Canadian young-adult population. Appl Physiol Nutr Metab. 2013;38:188–93. doi: 10.1139/apnm-2012-0231. [DOI] [PubMed] [Google Scholar]
- 34.Buttenschon HN, Demontis D, Kaas M, Elfving B, Molgaard S, Gustafsen C, Kaerlev L, Petersen CM, Borglum AD, Mors O, Glerup S. Increased serum levels of sortilin are associated with depression and correlated with BDNF and VEGF. Transl Psychiatry. 2015;5:e677. doi: 10.1038/tp.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.