Abstract
Background
Most studies of brain white matter (WM) in posttraumatic stress disorder (PTSD) have focused on combat trauma, and often were confounded by neurological and substance dependence comorbidity. This study used tract-based spatial statistics (TBSS) and probabilistic tractography to characterize WM microstructure in a mixed-sex community sample of PTSD patients exposed to diverse and multiple traumas, and in trauma-exposed normal comparison (TENC) subjects.
Methods
TBSS compared diffusion measures between 20 adults with DSM-IV PTSD and 17 TENC, using a whole-brain voxel-wise approach. Probabilistic tractography using Freesurfer’s TRACULA was employed to measure diffusion tensor imaging (DTI) metrics within anatomically defined pathways. DTI metrics were compared between groups and correlated with PTSD symptom severity and trauma load.
Results
Controlling for age, sex, and motion, PTSD subjects had significantly reduced fractional anisotropy (FA) in a left frontal lobe cluster compared with TENC, at p < 0.05, family-wise error corrected. Tractography identified significant group differences in the inferior longitudinal fasciculus (ILF), including lower FA and higher radial diffusivity in PTSD compared with TENC. Within the PTSD group, FA values were not correlated with symptom severity or trauma load. Results remained significant after removing participants using psychotropic medication or those with comorbid major depression.
Conclusions
PTSD patients had reduced WM integrity in left hemisphere frontal WM and temporal-occipital WM tracts, compared to trauma-exposed controls. Reduced frontal FA is consistent with compromised top-down attentional control and emotion regulation in PTSD, while reduced ILF FA may be related to sensory processing and gating abnormalities in this disorder.
Keywords: Posttraumatic stress disorder, diffusion tensor imaging, probabilistic tractography, anterior cingulate cortex, inferior longitudinal fasciculus
1. Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric illness that can develop following emotionally traumatic experiences, including combat, childhood maltreatment, sexual assault, and motor vehicle accidents (Solomon, Gerrity, & Muff, 1992). PTSD is associated with reduced gray matter volume and density in a number of brain regions (Daniels, Lamke, Gaebler, Walter, & Scheel, 2013; Li et al., 2014; Rauch et al., 2003), including the anterior cingulate cortex (ACC) (Bryant et al., 2008; Thomaes et al., 2010), medial-orbital frontal cortex (Hakamata et al., 2007; Thomaes et al., 2010), hippocampus (Woon, Sood, & Hedges, 2010), and insula (Corbo, Clément, Armony, Pruessner, & Brunet, 2005). These findings are generally consistent with neurocircuitry models of PTSD (Rauch, Shin, & Phelps, 2006; Shin & Liberzon, 2010), which propose that compromised medial prefrontal regions result in insufficient control over limbic and subcortical structures.
While the bulk of neuroimaging research in PTSD has focused on gray matter morphology and functional abnormalities, there has been a recent surge in research on brain white matter (WM) pathology, particularly using diffusion tensor imaging (DTI), a magnetic resonance imaging (MRI) method that characterizes WM connections (Alexander, Lee, Lazar, & Field, 2007; Basser, Mattiello, & LeBihan, 1994; Hagmann et al., 2006). To date, the DTI literature in PTSD has been dominated by studies focused on combat-related trauma, which may have limited generalizability to community samples. Combat trauma occurs in less than 2% of the general population; other forms of assaultive trauma are more prevalent and more strongly associated with PTSD in community samples (Breslau et al., 1998). Combat samples tend to be predominantly male, often have neurological comorbidities such as traumatic brain injury (TBI), and frequently include a high proportion of treatment-seeking patients, which limits generalizability to female patients and the general community. Reports in civilian samples have been dominated by studies of single mass-trauma disasters (Abe et al., 2006; M. J. Kim et al., 2005; Zhang et al., 2011). Although the etiology of PTSD is complex, the risk for the disorder is particularly elevated following exposure to multiple compared with single trauma (Kessler, 2000). Overall, there is a need for DTI studies in community-based samples that are not complicated by neurologic confounds and that represent exposures to multiple trauma types.
Many previous DTI studies in PTSD have employed tract-based statistics (TBSS: Smith et al., 2006), which allows for whole-brain analysis within a core white-matter ‘skeleton’. A TBSS study in veterans with PTSD found evidence for lower fractional anisotropy (FA; i.e. less coherent organization of WM tracts) near the ACC, prefrontal cortex, posterior central gyrus, angular gyrus, and posterior internal capsule (Schuff et al., 2011), though other TBSS studies found no significant group differences (Bierer et al., 2015; Durkee, Sarlls, Hommer, & Momenan, 2013). Several voxel-based analysis (VBA) and region-of-interest (ROI) studies of PTSD have corroborated evidence of lower FA in the ACC (Kim et al. 2005, 2006; Zhang et al. 2011; Sanjuan et al. 2013), medial orbitofrontal cortex (Sun et al. 2013), and temporal lobe (Sun et al., 2013). There are a few conflicting reports that PTSD samples show higher FA in the ACC (Abe et al., 2006) and temporal cortex (Zhang et al. 2011), and higher generalized fractional anisotropy in right frontotemporal pathways (Davenport, Lim, & Sponheim, 2015). In one study of a highly traumatized community sample, PTSD patients had lower FA in the posterior cingulum bundle, which connects the cingulate to entorhinal cortex; however, this finding did not survive family-wise error correction (Fani et al., 2012). To summarize, there is evidence of FA alterations in PTSD patients compared with control samples, which may be particularly robust for pathways near the ACC and temporal lobe cortices.
Unlike TBSS, diffusion tensor tractography uses diffusion data to identify and trace WM pathways. This fiber-based method therefore can be used as a complement to the more local TBSS approach (Roine et al., 2015), because it is not limited to voxels with the highest FA at the center of the WM skeleton. There have been a handful of tractography studies in trauma-exposed populations, mainly focused on patients with combat trauma. Several investigations examined DTI metrics within particular tracts of a priori interest, including the uncinate fasciculus (Admon et al., 2013; Isaac et al., 2015), cingulum (Bierer et al., 2015; Isaac et al., 2015), stria terminalis (Kennis et al., 2015), corticospinal tracts (Levin et al., 2010), and fornix (Kennis et al., 2015; Levin et al., 2010). Some of these studies demonstrated that lower FA is related to negative clinical outcomes following combat trauma (in the uncinate fasciculus: Admon et al., 2013; left uncinate and right cingulum: Isaac et al., 2015; and corpus callosum: Levin et al., 2010), while others found that higher FA is associated with greater severity or persistence of PTSD symptoms (in the right cingulum: Bierer et al., 2015; dorsal and hippocampal cingulum: Kennis et al., 2015). With the exception of the study by Admon and colleagues (2013), these prior investigations involved predominantly male participants, many of whom had sustained TBI during combat. In one study of a civilian sample, Long et al. (2013) examined individuals with PTSD following motor vehicle accidents. They found alterations in graph theoretical parameters relating to structural connectivity, predominantly in the bilateral ACC, pallidum, and hippocampus/parahippocampal gyrus. This points to involvement of fibers in the medial frontal and temporal-limbic cortices, although a single study in a community based sample cannot be taken as conclusive. Thus, additional studies applying tractography in community samples without comorbid TBI are needed.
In the present study, we used TBSS and probabilistic tractography in a mixed-sex, community sample of adults with PTSD and trauma-exposed normal comparison (TENC) subjects without a history of psychiatric illness. By using both TBSS and probabilistic tractography, we examined group differences in DTI metrics within the WM skeleton, and also within anatomically defined WM pathways. We tested the hypothesis that PTSD patients would show significantly lower FA than TENC subjects in frontal and temporal cortex WM. Based on prior evidence that some PTSD neuropathology tracks with illness severity (De Bellis et al., 2015; Herringa, Phillips, Almeida, Insana, & Germain, 2012; Sanjuan, Thoma, Claus, Mays, & Caprihan, 2013) and repeated trauma exposure/trauma load (C. Catani, Adenauer, Keil, Aichinger, & Neuner, 2009; Nardo et al., 2010), we also examined whether DTI metrics that differed between groups were correlated with symptom severity or lifetime trauma load.
2. Materials and Methods
2.1. Participants
This study recruited 21 right-handed PTSD patients and 25 matched trauma-exposed normal comparison (TENC) adult subjects, who responded to advertisements in the Boston area. All subjects provided written informed consent after a full explanation of study procedures and were paid for their participation. Study procedures were approved by the Institutional Review Board of McLean Hospital, and complied with the ethical standards of the Declaration of Helsinki.
Participants were excluded from the study according to pre-specified criteria (Supplement 1). Three participants (one PTSD and two TENC) were removed from the analysis because of DTI data quality problems. All participants completed the Traumatic Life Events Questionnaire (TLEQ: Kubany et al., 2000), a 23-item self-report questionnaire assessing lifetime exposure to a variety of potentially traumatic events. Healthy participants who reported no lifetime exposure to any TLEQ event were excluded from the analyses (n = 6), resulting in a final sample of 20 PTSD and 17 TENC participants (Table 1).
Table 1.
Demographic and clinical characteristics of PTSD and trauma-exposed normal control (TENC) participants (Mean ± SD or N(%))
| PTSD N = 20 |
TENC N = 17 |
Group difference | |
|---|---|---|---|
| Sex (female) | 12 (60.0%) | 11 (64.7%) | X2(1) = 0.09, p = 0.77 |
| Age Range |
33.84 ± 11.06 21–53 |
37.93 ± 12.54 22–56 |
t(35) = −1.06, p = 0.30 |
| Total Motion Index (TMI) | 0.94 ± 1.97 | 0.25 ± 1.67 | t(35) = 1.15, p = 0.26 |
| Duration of illness (years) | 14.84 ± 12.60 | —— | |
| CAPS, total | 56.85 ± 21.44 | —— | |
| CAPS, reexperiencing | 17.65 ± 7.03 | —— | |
| CAPS, avoidance | 23.50 ± 10.79 | —— | |
| CAPS, hyperarousal | 15.70 ± 8.29 | —— | |
| TLEQ, types of Crit A1 events | 7.60 ± 3.95 | 2.18 ± 1.22* | t(23.145) = 5.883, p < 0.001 |
PTSD: posttraumatic stress disorder; CAPS: Clinician-Administered PTSD Scale.
2.2. Clinical Interviews and Measures
All participants were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P) (First et al. 2002). PTSD symptom severity was rated with the Clinician Administered PTSD Scale (CAPS; (Blake et al., 1995)), administered by a clinical psychologist (IMR) to those endorsing one or more DSM-IV Criterion A events on the SCID-I/P. The CAPS yields total symptom scores as well as subscale scores for re-experiencing, avoidance and numbing, and hyperarousal symptoms (Table 1). On the SCID-I/P, all participants in the PTSD group met full criteria for current DSM-IV PTSD, except for one who met for lifetime PTSD, in partial remission. Sixteen PTSD patients were unmedicated, and the remaining 4 patients were taking a selective serotonin reuptake inhibitor (SSRI; stable dose for greater than 8 weeks). Additional clinical information is presented in Supplement 1.
The TLEQ was used to derive a measure of lifetime trauma load (number of different types of lifetime trauma endorsed). On average, PTSD participants reported exposure to multiple types of DSM-IV Criterion A1 traumas, with many PTSD group participants also reporting having experienced multiple events within each category. All participants in the TENC group also reported exposure to at least one type of DSM-IV Criterion A1 trauma, though lifetime trauma load was significantly lower than in the PTSD group (Table 1). TENC participants never met full criteria for lifetime PTSD.
2.3. MR image acquisition
Scans were collected on a 3.0 Tesla Siemens Tim Trio scanner (Siemens, Erlangen, Germany), using a 12-channel head coil. Structural T1-weighted 3D magnetization-prepared rapid gradient-echo (MPRAGE) images and whole brain DTI images were acquired (for parameters, see Supplement 1).
2.4. Tract-based spatial statistics (TBSS)
For voxel-wise diffusion analyses, whole brain DTI data were processed with the FSL 5.0.4 Diffusion Toolbox (FDT, http://www.fmrib.ox.ac.uk/fsl/fdt/) (Smith et al., 2004), using a standard TBSS processing stream (Supplement 1). Two-group unpaired t-tests were conducted with permutation-based nonparametric inference on FA, mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) using FSL Randomise with threshold-free cluster enhancement (TFCE: Smith & Nichols, 2009) and 5000 permutations per analysis to assess group differences between PTSD and TENC. Age, sex, and the total motion index (TMI) derived from TRACULA (see Supplement 1) were de-meaned and entered as nuisance covariates. The statistical threshold was set at p<0.05 with family-wise error (FWE) whole-brain multiple comparison correction.
2.5. Reconstruction of white matter tracts (TRACULA)
For tractography analyses, diffusion data were processed using Freesurfer’s TRActs Constrained by UnderLying Anatomy (TRACULA) tool (Yendiki et al., 2011), using a standard pipeline (Supplement 1). FA, MD, RD, and AD were computed for each of 10 available tracts.
2.6. Statistical Analyses
Statistical analyses were performed using SPSS Statistics 20 (IBM Corporation, Armonk, New York, USA). TRACULA results were analyzed using SPSS’s General Linear Models routine. Analyses were univariate for the forceps major and forceps minor (which are midline structures), and multivariate for the remaining tracts, with values from the left and right hemispheres entered as dependent variables. Sex and group (PTSD vs TENC) were entered as fixed factors, and age and TMI (Yendiki, Koldewyn, Kakunoori, Kanwisher, & Fischl, 2014) were entered as covariates (though for results eliminating selected nuisance covariates, see Supplement 1). Results were Bonferroni-corrected for comparison across 10 tracts (significant at p<0.005).
Because FA is the dominant metric for examining group differences in the existing literature, and to reduce multiple comparisons, the group comparison analyses were initially restricted to FA. Where significant group differences were found in FA, the other metrics were probed to clarify the nature of the group difference. Relationships with symptom severity and trauma load were assessed by correlating significant imaging findings with CAPS current total scores or TLEQ trauma load, controlling for age, sex, and TMI.
3. Results
3.1. Tract-based spatial statistics
TBSS analyses identified one cluster of significantly lower FA in PTSD versus TENC subjects (Figure 1). This was a left frontal cluster involving fibers from the left anterior corona radiata/anterior thalamic radiation/forceps minor/uncinate fasciculus/inferior fronto-occipital fasciculus/cingulate gyrus (cluster size k = 148 voxels, p = 0.039, MNI: x= −17, y = 38, z = 3). There were no clusters with significantly higher FA in PTSD than TENC participants, and there were no significant differences in MD, AD or RD between groups.
Figure 1.
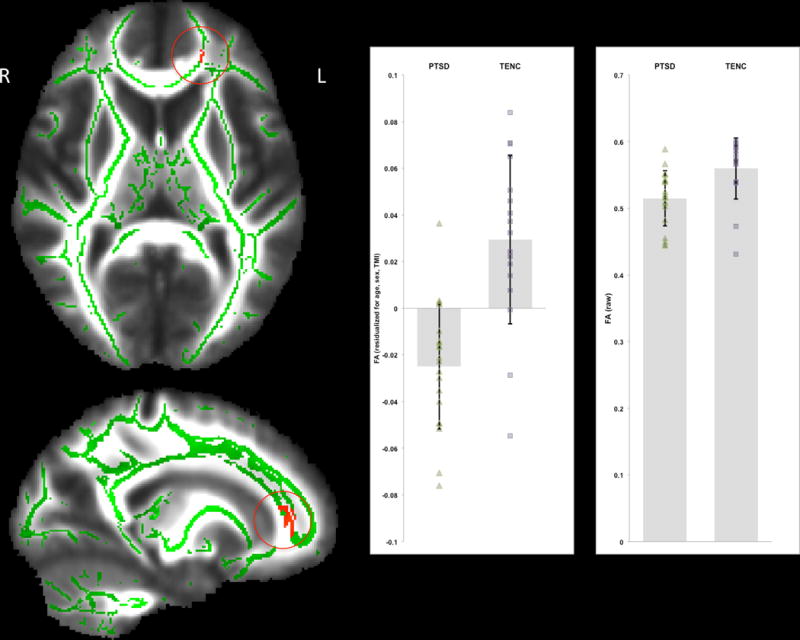
Results of tract-based spatial statistics (TBSS) using a whole-brain analysis with TFCE and FWE correction at p < 0.05. There was one cluster with significantly lower FA values in PTSD compared to TENC (PTSD < TENC). This left frontal cluster involved fibers from the left anterior corona radiata/anterior thalamic radiation/forceps minor/uncinate fasciculus/inferior fronto-occipital fasciculus/cingulate gyrus. The significant cluster is shown in red, superimposed on the mean_FA_skeleton (in green) and overlaid on the mean_FA image in the MNI standard space. FA values residualized for age, sex, and TMI (at left) as well as raw FA values (at right) are displayed for each group.
FA values were extracted from the left frontal cluster to examine clinical correlates. Within the PTSD group, CAPS total scores were not significantly associated with mean FA values (partial r(15)= −0.002, p = 0.993), or with TLEQ trauma load (partial r(15)= −0.051, p = 0.845). Separate whole-brain TBSS analyses performed in FSL also found no significant relationships of FA with either total CAPS scores or trauma load, controlling for group status. (Exploratory correlations with CAPS symptom subscales also were not significant: see Supplement 1).
3.2. Probabilistic tractography
As shown in Table 2, after correcting for multiple comparisons, there was a significant between-group difference in FA in the ILF (F(2,30) = 6.654, p = 0.004 partial eta squared = 0.307). This effect was driven by the left hemisphere: FA was significantly lower in the PTSD group in the left ILF (F(1,31) = 10.684, p = 0.003, partial eta squared = 0.256), but not the right ILF (F(1,31) = 1.708, p = 0.201, partial eta squared = 0.052). CAPS total scores were not significantly associated with left ILF mean FA in the PTSD group (partial r(15) = −0.361, p = 0.154,), nor was trauma load (partial r(15)= −0.109, p = 0.676).
Table 2.
Group comparison of fractional anisotropy in ten reconstructed pathways
| PTSD | TENC | F* | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Forceps major | 0.625 | 0.027 | 0.644 | 0.028 | 7.329 | 0.011 |
| Forceps minor | 0.521 | 0.045 | 0.531 | 0.041 | 1.730 | 0.198 |
| ATR | 0.417 (l) 0.410 (r) |
0.034 (l) 0.031 (r) |
0.431 (l) 0.421 (r) |
0.034 (l) 0.035 (r) |
2.641 | 0.088 |
| Cingulum (angular) | 0.330 (l) 0.342 (r) |
0.032 (l) 0.042 (r) |
0.348 (l) 0.340 (r) |
0.039 (l) 0.044 (r) |
1.735 | 0.194 |
| Cingulum (c. gyrus) | 0.494 (l) 0.418 (r) |
0.040 (l) 0.043 (r) |
0.509 (l) 0.447 (r) |
0.067 (l) 0.049 (r) |
2.766 | 0.079 |
| CST | 0.517 (l) 0.486 (r) |
0.027 (l) 0.026 (r) |
0.520 (l) 0.479 (r) |
0.031 (r) 0.026 (r) |
0.732 | 0.489 |
| ILF | 0.487 (l) 0.483 (r) |
0.047 (l) 0.045 (r) |
0.521 (l) 0.490 (r) |
0.037 (l) 0.034 (r) |
6.654 | 0.004** |
| SLF (parietal) | 0.423 (l) 0.405 (r) |
0.034 (l) 0.036 (r) |
0.444 (l) 0.420 (r) |
0.038 (l) 0.033 (r) |
2.510 | 0.098 |
| SLF (temporal) | 0.461 (l) 0.432 (r) |
0.034 (l) 0.027 (r) |
0.474 (l) 0.439 (r) |
0.029 (l) 0.031 (r) |
2.443 | 0.104 |
| Uncinate | 0.403 (l) 0.412 (r) |
0.038 (l) 0.025 (r) |
0.422 (l) 0.422 (r) |
0.023 (l) 0.024 (r) |
2.713 | 0.083 |
F value for effect of group in univariate (f. major, f. minor) or multivariate (all others, predicting both hemispheres) analysis controlling for age, sex, and TMI.
p < 0.005. PTSD: posttraumatic stress disorder; TENC: trauma-exposed normal control; ATR: anterior thalamic radiation; CST: corticospinal tract; ILF: inferior longitudinal fasciculus; SLF: superior longitudinal fasciculus
To follow up on the significant group difference in FA, the additional DTI metrics were also examined for group differences in the left ILF. There was no significant group difference in left ILF MD (F(1,31) = 0.624, p = 0.435) or AD (F(1,31) = 3.615, p = 0.067). However, there was a significant group difference in RD (F(1,31) = 6.374, p = 0.017, partial eta squared = 0.171), which was higher in PTSD (M = 0.000538, SD = 0.0000425) than TENC (M = 0.000509, SD = 0.0000355). Within the PTSD group, left ILF RD values were not significantly associated with CAPS total scores (partial r(15) = 0.256, p = 0.322) or with trauma load (partial r(15) = 0.091, p = 0.727).
3.3. Possible clinical confounds
After removing one participant in the PTSD group with sub-threshold current PTSD and re-running the TBSS analysis, the left frontal cluster continued to demonstrate significantly reduced FA in the PTSD group. Similarly, after removing that participant and re-running the tractography analysis, the difference in ILF FA remained statistically significant (F(2,29) = 6.907, p = 0.004). All results therefore are reported for the full PTSD sample (n = 37).
In the TBSS analysis, the group difference in left frontal lobe FA persisted when the four PTSD participants taking SSRIs were excluded (t(28) = 5.086, p < 0.001). The frontal lobe FA finding also remained significant after removing the six PTSD participants with current or lifetime MDD from the analysis (t(26) = 5.327, p < 0.001). Similarly, the tractography finding in the left ILF remained significant after removing the four medicated participants, (F(2,26) = 6.280, p = 0.006), or after excluding the six participants with MDD (F(2,24) = 6.292, p = 0.006). For additional results related to the possible confound of head injury status, see Supplement 1.
4. Discussion
This study used TBSS and probabilistic tractography to examine group differences in brain WM structure in a community-based sample of adults with PTSD and matched trauma-exposed controls. TBSS revealed significantly lower FA in a left frontal lobe cluster in the PTSD group. In addition, tractography showed that PTSD patients had significantly lower FA and higher RD in the left ILF. These results suggest that PTSD is associated with compromised structural integrity of WM pathways that connect the medial frontal cortex to cortical and subcortical targets, and of fibers that connect the temporal and occipital cortices. These findings were not confounded by psychotropic medication use or depression comorbidity. To our knowledge, this is the first study to use both TBSS and probabilistic tractography to examine WM microstructural abnormalities in a community-based PTSD sample.
Our voxelwise TBSS analysis demonstrated that PTSD patients had lower FA in a left frontal cluster involving fibers near the rostral ACC. This finding is consistent with findings of two prior studies using VBA and ROI-based methods that identified lower FA in the left ACC in civilian PTSD (Kim et al. 2005, 2006). TBSS studies in PTSD have found bilateral decreases in ACC FA (Fani et al., 2012; Sanjuan et al., 2013; Schuff et al., 2011; but for null results see Bierer et al., 2015; Durkee et al., 2013). A finding of lower FA aligns with other evidence for structural compromise of the ACC in PTSD, including decreased gray matter volume (O’Doherty, Chitty, Saddiqui, Bennett, & Lagopoulos, 2015; Rauch et al., 2003). Emerging evidence suggests that the rACC may be involved in downregulating fear circuitry during fear extinction recall (Helpman et al., 2016), a process believed to contribute to overgeneralization of fear responses in PTSD (Milad et al., 2009).
Using tractography, we found that PTSD patients had significantly lower FA in the ILF, a long-association visual-limbic pathway that connects the occipital and anterior temporal lobes (Catani, Jones, Donato, & Ffytche, 2003). In addition, RD was significantly higher in the ILF, a pattern that may reflect lesser myelination of WM fibers (Song et al., 2003). We are aware of no previous reports of reduced WM integrity of the ILF in PTSD. One study reported lower FA in the ILF of adults who witnessed domestic violence in childhood (Choi, Jeong, Polcari, Rohan, & Teicher, 2012). The ILF supports information transfer between primary sensory (visual and auditory) and association cortices (Catani et al. 2003; Choi et al. 2012). PTSD may involve atypical sensory processing, including reduced activity in areas of the ventral visual stream (including the ILF) (Adenauer et al., 2011; Mueller-Pfeiffer et al., 2013) and auditory processing abnormalities (Clark et al., 2009; Neylan et al., 1999). Individuals with PTSD demonstrate insufficient suppression of irrelevant sensory stimuli, including abnormal P50 suppression (Neylan et al., 1999) and prepulse inhibition (Pineles et al., 2016). Although speculative, we suggest that compromise of the ILF could be relevant to these sensory filtering abnormalities. These sensory phenomena are not fully captured by DSM PTSD symptoms (Mueller-Pfeiffer et al., 2013), which may help explain the lack of correlation between ILF DTI measures and CAPS scores in our study.
Brain alterations in PTSD, including WM compromise, can represent the influence(s) of predisposing factors, severity of trauma exposure, or disease severity and progression (De Bellis et al., 2015; Jovanovic & Ressler, 2010; Karl et al., 2006; Nardo et al., 2010). We did not find significant associations of ACC or ILF FA decrements with PTSD symptom scores, suggesting that they do not track with illness severity. Additionally, FA was not significantly associated with trauma load within the PTSD group. For the frontal TBSS cluster, there was no relationship with broad measures of symptom severity (CAPS scores). There was some evidence for a relationship between left ILF FA and CAPS scores, corresponding to a medium-sized effect that we were underpowered to detect (partial r = −0.361). It would be helpful to replicate these findings in a larger sample that is characterized in terms of underlying dimensional features such as fear extinction and visual/auditory sensory gating that may more closely track with alterations in WM organization.
This study has a number of strengths. By using both TBSS and probabilistic tractography, we evaluated group differences in diffusion properties within the WM skeleton and also within anatomically verified WM pathways. TBSS tends to be more sensitive to focal alterations in WM integrity, whereas tractography-based analysis can better detect alterations that are diffusely present along the length of a fiber tract (Roine et al., 2015). Differences in localized sections of WM with TBSS may not be detected using the mean FA of the whole tract (Sarica et al., 2014; Yeatman, Dougherty, Myall, Wandell, & Feldman, 2012), and conversely diffuse changes picked up by tractography may not cross the threshold of detection using TBSS. Additionally, our sample was comprised of a community-based, mixed-sex population with diverse trauma histories, a demographic profile that is underrepresented in the trauma literature as a whole and the PTSD DTI literature in particular. Lastly, our results were robust to medication status and depression comorbidity.
Our findings should be interpreted in the context of several limitations. Replication in groups matched on trauma load will be an important future step in verifying whether the effects are entirely related to PTSD diagnosis or whether there is a contribution of trauma exposure. As discussed above, in this sample it was not possible to determine whether the observed reductions in FA are associated with PTSD diagnosis or with exposure to childhood trauma. Additionally, our sample size may have limited our statistical power to detect associations of WM measures with variability in clinical symptoms. Future studies using multimodal neuroimaging will be helpful in identifying how alterations in structural connectivity are related to altered brain functioning. Finally, our PTSD sample included a lower rate of comorbid depression than is seen in some clinical samples, which may be because the sample was a community-based, non-treatment-seeking sample.
4.2. Summary
PTSD patients had lower FA than trauma-exposed adults in brain WM pathways that course through the left ACC. We also found lower FA in the left ILF, a temporal-occipital pathway that has not been considered central to PTSD pathophysiology, but which has been implicated in sensory processing abnormalities. Taken together, our results underscore the importance of characterizing disruptions of WM tracts in PTSD, as these may help elucidate or provide context to functional brain and behavioral deficits.
Supplementary Material
Figure 2.
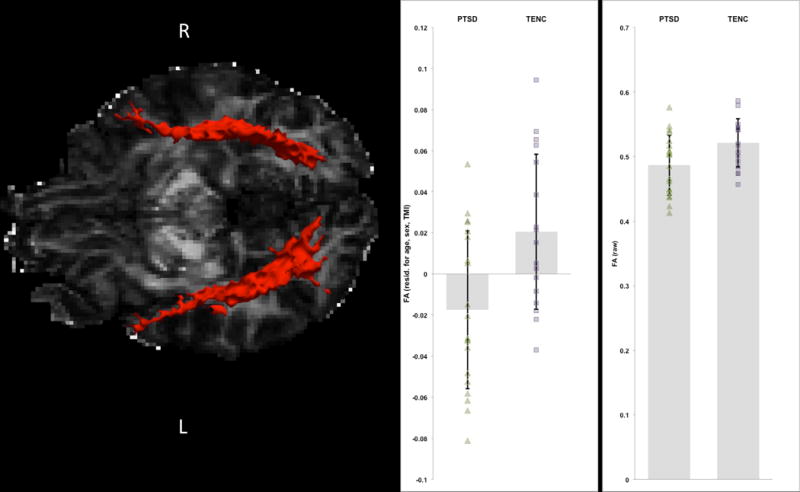
Illustration of the posterior probability distribution of the left and right ILF for one representative PTSD participant. FA values residualized for age, sex, and TMI (at left) as well as raw FA values (at right) are displayed for each group.
Acknowledgments
Funding: This work was supported by the Dana Foundation (IMR) and the National Institute of Mental Health (5R01MH096987[IMR]).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare in relation to this work.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, Ohtomo K. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Research. 2006;146(3):231–42. doi: 10.1016/j.pscychresns.2006.01.004. http://doi.org/10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Adenauer H, Catani C, Gola H, Keil J, Ruf M, Schauer M, Neuner F. Narrative exposure therapy for PTSD increases top-down processing of aversive stimuli - evidence from a randomized controlled treatment trial. BMC Neuroscience. 2011 doi: 10.1186/1471-2202-12-127. http://doi.org/10.1186/1471-2202-12-127. [DOI] [PMC free article] [PubMed]
- Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, Hendler T. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Human Brain Mapping. 2013;34(11):2808–16. doi: 10.1002/hbm.22100. http://doi.org/10.1002/hbm.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics : The Journal of the American Society for Experimental NeuroTherapeutics. 2007;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. http://doi.org/10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. http://doi.org/10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer LM, Ivanov I, Carpenter DM, Wong EW, Golier Ja, Tang CY, Yehuda R. White matter abnormalities in Gulf War veterans with posttraumatic stress disorder : A pilot study. Psychoneuroendocrinology. 2015;51:567–576. doi: 10.1016/j.psyneuen.2014.11.007. http://doi.org/10.1016/j.psyneuen.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. http://doi.org/10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Arch Gen Psychiatry. 1998;55(7):626–632. doi: 10.1001/archpsyc.55.7.626. http://doi.org/10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Whitford TJ, Kemp A, Hughes G, Peduto A, Williams LM. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. Journal of Psychiatry and Neuroscience. 2008;33(2):142–146. [PMC free article] [PubMed] [Google Scholar]
- Catani C, Adenauer H, Keil J, Aichinger H, Neuner F. Pattern of cortical activation during processing of aversive stimuli in traumatized survivors of war and torture. European Archives of Psychiatry and Clinical Neuroscience. 2009;259(6):340–351. doi: 10.1007/s00406-009-0006-4. http://doi.org/10.1007/s00406-009-0006-4. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–107. doi: 10.1093/brain/awg203. http://doi.org/10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage. 2012;59(2):1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. http://doi.org/10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CR, Galletly Ca, Ash DJ, Moores Ka, Penrose Ra, McFarlane AC. Evidence-based medicine evaluation of electrophysiological studies of the anxiety disorders. Clinical EEG and Neuroscience. 2009;40(2):84–112. doi: 10.1177/155005940904000208. http://doi.org/10.1177/155005940904000208. [DOI] [PubMed] [Google Scholar]
- Corbo V, Clément MH, Armony JL, Pruessner JC, Brunet A. Size versus shape differences: Contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biological Psychiatry. 2005;58(2):119–124. doi: 10.1016/j.biopsych.2005.02.032. http://doi.org/10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Lamke J-P, Gaebler M, Walter H, Scheel M. White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depression and Anxiety. 2013;30(3):207–16. doi: 10.1002/da.22044. http://doi.org/10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, Sponheim SR. White matter abnormalities associated with military PTSD in the context of blast TBI. Human Brain Mapping. 2015;36(3):1053–64. doi: 10.1002/hbm.22685. http://doi.org/10.1002/hbm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE, Woolley DP. Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Development and Psychopathology. 2015;27(4pt2):1555–1576. doi: 10.1017/S0954579415000942. http://doi.org/10.1017/S0954579415000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee Ca, Sarlls JE, Hommer DW, Momenan R. White matter microstructure alterations: a study of alcoholics with and without post-traumatic stress disorder. PloS One. 2013;8(11):e80952. doi: 10.1371/journal.pone.0080952. http://doi.org/10.1371/journal.pone.0080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ressler KJ. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(12):2740–2746. doi: 10.1038/npp.2012.146. http://doi.org/10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P) New York: New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Hagmann P, Jonasson L, Maeder P, Thiran J, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: From scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. RadioGraphics. 2006;26:205–223. doi: 10.1148/rg.26si065510. http://doi.org/10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Matsuoka Y, Inagaki M, Nagamine M, Hara E, Imoto S, Uchitomi Y. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neuroscience Research. 2007;59(4):383–389. doi: 10.1016/j.neures.2007.08.012. http://doi.org/10.1016/j.neures.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier F, Neria Y. Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. NeuroImage: Clinical. 2016;12:715–723. doi: 10.1016/j.nicl.2016.10.007. http://doi.org/10.1016/j.nicl.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Research. 2012;203(2–3):139–45. doi: 10.1016/j.pscychresns.2012.02.005. http://doi.org/10.1016/j.pscychresns.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac L, Main KL, Soman S, Gotlib IH, Furst AJ, Kinoshita LM, Adamson MM. The impact of depression on veterans with PTSD and traumatic brain Injury: A diffusion tensor imaging study. Biological Psychology. 2015;105:20–28. doi: 10.1016/j.biopsycho.2014.12.011. http://doi.org/10.1016/j.biopsycho.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry. 2010 doi: 10.1176/appi.ajp.2009.09071074. http://doi.org/10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006 doi: 10.1016/j.neubiorev.2006.03.004. http://doi.org/10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed]
- Kennis M, van Rooij SJH, Tromp DPM, Fox AS, Rademaker AR, Kahn RS, Geuze E. Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology. 2015;40(10):2434–32. doi: 10.1038/npp.2015.94. http://doi.org/10.1038/npp.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61 http://doi.org/http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-4679. [PubMed] [Google Scholar]
- Kim MJ, Lyoo IK, Kim SJ, Sim M, Kim N, Choi N, Renshaw PF. Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport. 2005;16(10):1049–53. doi: 10.1097/00001756-200507130-00004. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeong D-U, Sim ME, Bae SC, Chung A, Kim MJ, Lyoo IK. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54(2):120–5. doi: 10.1159/000098262. http://doi.org/10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12(2):210–24. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. Journal of Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. 1557–9042 (Electronic) http://doi.org/10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Li L, Wu M, Liao Y, Ouyang L, Du M, Lei D, Gong Q. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neuroscience and Biobehavioral Reviews. 2014;43:163–172. doi: 10.1016/j.neubiorev.2014.04.003. http://doi.org/10.1016/j.neubiorev.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Long Z, Duan X, Xie B, Du H, Li R, Xu Q, Chen H. Altered brain structural connectivity in post-traumatic stress disorder: A diffusion tensor imaging tractography study. Journal of Affective Disorders. 2013 doi: 10.1016/j.jad.2013.03.004. http://doi.org/10.1016/j.jad.2013.03.004. [DOI] [PubMed]
- Milad M, Pitman R, Ellis C, Gold A, Shin L, Lasko N, Rauch S. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. http://doi.org/10.1016/j.biopsych.2009.06.026.Neurobiological. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Pfeiffer C, Schick M, Schulte-Vels T, O’Gorman R, Michels L, Martin-Soelch C, Hasler G. Atypical visual processing in posttraumatic stress disorder. NeuroImage: Clinical. 2013;3:531–8. doi: 10.1016/j.nicl.2013.08.009. http://doi.org/10.1016/j.nicl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo D, Hogberg G, Looi JCL, Larsson S, Hollstrom T, Pagani M. Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. Journal of Psychiatric Research. 2010;44(7):477–485. doi: 10.1016/j.jpsychires.2009.10.014. http://doi.org/10.1016/j.jpsychires.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Fletcher DJ, Lenoci M, McCallin K, Weiss DS, Schoenfeld FB, Fein G. Sensory gating in chronic posttraumatic stress disorder: reduced auditory P50 suppression in combat veterans. Biological Psychiatry. 1999;46(12):1656–1664. doi: 10.1016/s0006-3223(99)00047-5. http://doi.org/S0006-3223(99)00047-5 [pii] [DOI] [PubMed] [Google Scholar]
- O’Doherty DCMD, Chitty KKM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2015;232(1):1–33. doi: 10.1016/j.pscychresns.2015.01.002. http://doi.org/10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Blumenthal TD, Curreri AJ, Nillni YI, Putnam KM, Resick PA, Orr SP. Prepulse inhibition deficits in women with PTSD. Psychophysiology. 2016;53(9):1377–85. doi: 10.1111/psyp.12679. http://doi.org/10.1111/psyp.12679. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research-Past, Present, and Future. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.06.004. http://doi.org/10.1016/j.biopsych.2006.06.004. [DOI] [PubMed]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson Ma, McMullin K, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14(7):913–916. doi: 10.1097/01.wnr.0000071767.24455.10. http://doi.org/10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Roine U, Salmi J, Roine T, Wendt TN, Leppamaki S, Rintahaka P, Sams M. Constrained spherical deconvolution-based tractography and tract-based spatial statistics show abnormal microstructural organization in Asperger syndrome. Mol Autism. 2015;6:4. doi: 10.1186/2040-2392-6-4. http://doi.org/10.1186/2040-2392-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Research. 2013;214(3):260–8. doi: 10.1016/j.pscychresns.2013.09.002. http://doi.org/10.1016/j.pscychresns.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarica A, Cerasa A, Vasta R, Perrotta P, Valentino P, Mangone G, Quattrone A. Tractography in amyotrophic lateral sclerosis using a novel probabilistic tool: A study with tract-based reconstruction compared to voxel-based approach. Journal of Neuroscience Methods. 2014;224:79–87. doi: 10.1016/j.jneumeth.2013.12.014. http://doi.org/10.1016/j.jneumeth.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, Neylan TC. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage. 2011;54(Suppl 1):S62–8. doi: 10.1016/j.neuroimage.2010.05.024. http://doi.org/10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. http://doi.org/10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. http://doi.org/10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. http://doi.org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. http://doi.org/10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Gerrity ET, Muff AM. Efficacy of Treatments for Posttraumatic Stress Disorder: An Empirical Review. JAMA: The Journal of the American Medical Association. 1992;268(5):633–638. http://doi.org/10.1001/jama.1992.03490050081031. [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. http://doi.org/10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Z, Ding W, Wan J, Zhuang Z, Zhang Y, Xu J. Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PLoS ONE. 2013;8(12):e83473. doi: 10.1371/journal.pone.0083473. http://doi.org/10.1371/journal.pone.0083473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, De Ruiter MB, Van Balkom AJ, Smit JH, Veltman DJ. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. Journal of Clinical Psychiatry. 2010;71:1636–1644. doi: 10.4088/JCP.08m04754blu. http://doi.org/10.4088/JCP.08m04754blu. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: A meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. http://doi.org/10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049790. http://doi.org/10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. http://doi.org/10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, Fischl B. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics. 2011 Oct;5:23. doi: 10.3389/fninf.2011.00023. http://doi.org/10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Li LJ, Li ZX, Li WH, Ma N, Lu GM. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. Journal of Affective Disorders. 2011;133:294–299. doi: 10.1016/j.jad.2011.03.040. http://doi.org/DOI10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


