Abstract
Introduction
A neonatal illness severity score, The Score for Neonatal Acute Physiology-II (SNAP-II), predicts neurodevelopmental impairments at two years of age among children born extremely preterm. We sought to evaluate to what extent SNAP-II is predictive of cognitive and other neurodevelopmental impairments at 10 years of age.
Methods
In a cohort of 874 children born before 28 weeks of gestation, we prospectively collected clinical, physiologic and laboratory data to calculate SNAP-II for each infant. When the children were 10 years old, examiners who were unaware of the child's medical history assessed neurodevelopmental outcomes, including neurocognitive, gross motor, social, and communication functions, diagnosis and treatment of seizures or attention deficit hyperactivity disorder (ADHD), academic achievement, and quality of life. We used logistic regression to adjust for potential confounders.
Results
An undesirably high SNAP-II (≥ 30), present in 23% of participants, was associated with an increased risk of cognitive impairment (IQ, executive function, language ability), adverse neurological outcomes (epilepsy, impaired gross motor function), behavioral abnormalities (attention deficit disorder and hyperactivity), social dysfunction (autistic spectrum disorder) and education-related adversities (school achievement and need for educational supports.
In analyses that adjusted for potential confounders, Z-scores ≤ -1 on 11 of 18 cognitive outcomes were associated with SNAP-II in the highest category and 6 of 18 were associated with SNAP-II in the intermediate category. Odds ratios and 95% confidence intervals ranged from 1.4 (1.01, 2.1) to 2.1 (1.4, 3.1). Similarly, 2 of the 8 social dysfunctions were associated with SNAP-II in the highest category, and 3 of 8 were associated with SNAP-II in the intermediate category. Odds ratios and 95% confidence intervals were slightly higher for these assessments, ranging from 1.6 (1.1, 2.4) to 2.3 (1.2, 4.6).
Conclusion
Among very preterm newborns, physiologic derangements present in the first 12 postnatal hours are associated with dysfunctions in several neurodevelopmental domains at 10 years of age. We are unable to make inferences about causality.
Keywords: neurocognitive function, illness-severity scores, physiologic instability, SNAP-II, functional development, brain injury
Introduction
The Score for Neonatal Acute Physiology (SNAP),(1) and a revised version, the SNAP-II,(2) are physiology-based indicators of endogenous mortality risk based on routinely available vital signs and laboratory tests obtained during the first 12 postnatal hours, when clinical/physiologic derangements are less likely to be influenced by medical interventions than derangements that occur later in the hospital course. SNAP-II not only predicts death among very preterm newborns, but also neonatal intraventricular hemorrhage,(3-5) respiratory dysfunction, (3-5) and retinopathy of prematurity.(5-7)
In the ELGAN Study of infants born before 28 weeks gestation, high SNAP-II predicted death, ultrasound-defined morphologic abnormalities of the brain, and low developmental scores at age 2 years.(8, 9) However, neurodevelopmental assessments at age 2 years have limited ability to predict later function, (10-12) and functional abilities at age 10 are qualitatively different and more complex than what can be assessed at age 2 years.(13, 14) Thus, we sought to extend our previous work by examining neurodevelopmental function at age 10 as an outcome associated with high SNAP-II.
The relationship between early indicators of physiologic instability and neurodevelopmental outcomes at school age is not yet known. In this report, we examine the relationship between SNAP-II and dysfunctions at 10 years in a cohort of children born extremely preterm at 14 medical centers in the United States.
Materials and Methods
Participants
The ELGAN study is a multi-center prospective, observational study of the risk of structural and functional neurologic disorders in extremely preterm infants.(15) A total of 1506 infants born before the 28th week of gestation were enrolled during the years 2002-2004 and 1200 survived to 2 years. At age 10 years, 966 of these infants were recruited for an assessment of cognition, executive function, behaviors, and academic achievement. Of these 966 children, 889 (92%) returned for follow up and 874 underwent neurocognitive testing. Enrollment and consent procedures for this follow up study were approved by the institutional review boards of each participating institution. Demographic, pregnancy and newborn variables were examined using a standardized protocol that has been reported by others.(15)
Revised Scores for Neonatal Acute Physiology (SNAP-II)
We collected all physiologic, laboratory and therapy data for the first 12 postnatal hours needed to calculate a SNAP-II.(2) We also identified cut-offs for each week of post-menstrual age at birth that defined the top quartile, top decile, and three categories (<20, 20-29, and ≥30) of SNAP-II.
10 year follow up visit
Families willing to participate were scheduled for one visit during which all of the assessments reported here were administered in 3 to 4 hours, including breaks. The assessments were selected to provide the most comprehensive information about neurocognitive and academic function in one testing session. A summary of the neurocognitive assessments used in this study is included as a supplemental table (Supplemental Tables 1a and 1b).
Examiners who were unaware of the child's medical history assessed neurodevelopment in several clinically important domains. While the child was tested, the parent or caregiver completed questionnaires regarding the child's educational, medical, neurological and behavioral status. Questionnaires were also provided to the child's school teacher to obtain teacher-reported behavioral status, as described below.
Neurocognitive and related outcomes
General cognitive ability was assessed with the School-Age Differential Ability Scales–II (DAS-II) Verbal and Nonverbal Reasoning scales.(16) Expressive and receptive language skills were evaluated with the Oral and Written Language Scales (OWLS. (17) Attention and executive function were assessed with both the DAS-II and NEPSY-II (A Developmental NEuroPSYchological Assessment-II).(18, 19) Speed of processing was assessed with NEPSY-II Inhibition Naming. Visual perception was assessed with NEPSY-II Arrows and Geometric Puzzles, while visual motor function was measured with NEPSY-II Visuomotor Precision and Fingertip Tapping. Academic function was assessed with the Wechsler Individual Achievement Test-III (WIAT-III [C]) which provides standard scores in word recognition and decoding, spelling, and numeric operations.(20) Educational outcomes included receipt of an individual educational plan (IEP), repeating a grade in school, and placement in a remedial class.
Neurological outcomes
Neurological outcomes included the diagnosis of “any” seizures or epilepsy, receipt of anti-epileptic drugs at the time of the assessment, and gross motor function.
Seizure identification was a two stage process. If parents answered “yes” to any of 11 broad questions for possible seizures, they were prompted by the study epileptologist to conduct a structured interview followed by an open-ended interview, both by telephone. The epileptologist then determined whether a reported event was a seizure. A second epileptologist independently reviewed interview responses and similarly rated the event type. When the two physicians' disagreed on the presence of seizures, which occurred in only 3% of the children interviewed, a third epileptologist reviewed the interview responses and made the final determination regarding seizure status.
Gross Motor Function was assessed using the Gross Motor Function Classification System (GMFCS).(21) Children with a GMCFS ≥3 (unable to walk without an assistive mobility device) were considered to have a significant gross motor abnormality.
Social Responsiveness
We used the Social Responsiveness Scale-2 (SRS-2) to identify social impairment and to quantify its severity.(22) This 65-item instrument provides a total score reflecting severity of social deficits in the autism spectrum, as well as five subscale scores for: social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behavior.
All children were screened by parent report for Autistic Spectrum Disorder (ASD) with the Social Communication Questionnaire (SCQ).(23) Children who screened positive on the SCQ were assessed with the Autism Diagnostic Interview – Revised (ADI-R), and an in-depth parent interview.(24) Children meeting ADI-R modified criteria for ASD were administered the Autism Diagnostic Observation Schedule-2 (ADOS-2). (25, 26) Finally, all children meeting standardized research criteria for ASD on both the ADI-R and ADOS-2 were classified as having ASD.
Behavioral outcomes
Behavioral outcomes were assessed in two ways, by physician diagnosis or treatment for ADHD, and by parental and teacher report of the behavioral status items included in the Child Symptom Inventory-4 (CSI-4).(27, 28) Teachers and parents did not make any DSM-IV diagnosis. Rather, the CSI-4 functioned as a screening tool for determining a behavioral pattern, based on selected behavioral characteristics.
We operationalized the definition of ADHD, using a convention supported by others, to include any 2 of the following 3 ADHD designations: (1) parent report, (2) teacher report, and (3) physician diagnosis. Parent and teacher reports of a designation of ADHD are reported in Table A. This definition confers a level of agreement sufficient to provide confidence in the child's designation as having ADHD.
Parent-reported Quality of Life
Although health-related quality of life is a complex and sometimes subjective domain, it also conveys information about the biologic impact of exposures on outcomes most important to families. For this reason, we examined five quality of life indicators found in the Pediatric Quality of Life Inventory™ (PedsQL™), including functional assessments of physical, emotional, social, school, and psychological functioning.(29, 30)
Data Analyses
We evaluated the null hypothesis that each measure of neurodevelopmental function at age 10 years was not differentially distributed among children in three SNAP-II categories (< 20, 20-29, and ≥ 30). In the ELGAN cohort, a SNAP-II ≥ 30 correlates roughly with the upper quartile.(8)
To allow for differences in age at the time of the assessment, and to facilitate a comparison to children born at term, we calculated Z-scores using the distributions of values reported in historical normative controls, as described by the authors of the assessments we used.(16, 17, 19, 20)
For assessments of neurocognitive and social function, we created logistic regression models of the risk of a score 1 or more standard deviations below the normative mean for each assessment (i.e., Z-score ≤ -1). These models, which included potential confounders (maternal education, mother's eligibility for government-provided medical insurance, delivery for preeclampsia or fetal indication, gestational age, and birth weight Z-score), allowed us to calculate odds ratios (and 95% confidence intervals) indicating the strength of association between the SNAP-II category and each outcome.
For assessments of educational and neurologic function, behavior, and quality of life, we used a χ2 trend analysis to test the strength of the relationship between SNAP-II and parent and teacher-reported behavioral abnormalities. Similarly, a χ2 trend analysis was used for assessments included in the CSI-4.
Results
Sample characteristics (Table 1)
Table 1.
Sample characteristics among in each Score for Neonatal Physiology (SNAP-II) stratum. These are row percents.
| SNAP-II | Row N | ||||
|---|---|---|---|---|---|
| < 20 | 20-29 | ≥ 30 | |||
| Maternal characteristics | |||||
| Racial identity | White | 53 | 24 | 23 | 546 |
| Black | 50 | 25 | 25 | 222 | |
| Other | 57 | 25 | 18 | 96 | |
| Hispanic | Yes | 55 | 23 | 22 | 86 |
| No | 52 | 25 | 23 | 785 | |
| Age, years | < 21 | 48 | 27 | 26 | 113 |
| 21-35 | 52 | 25 | 23 | 584 | |
| > 35 | 59 | 23 | 17 | 117 | |
| Education, years | ≤ 12 | 49 | 26 | 26 | 353 |
| > 12, < 16 | 50 | 24 | 26 | 196 | |
| ≥ 16 | 59 | 24 | 18 | 299 | |
| Single marital status | Yes | 50 | 25 | 25 | 345 |
| No | 54 | 25 | 21 | 529 | |
| Public insurance | Yes | 50 | 24 | 26 | 299 |
| No | 54 | 25 | 21 | 561 | |
| KBIT Z-score1 | ≤ -1 | 54 | 27 | 19 | 95 |
| > -1 | 52 | 25 | 23 | 730 | |
| Perinatal characteristics | |||||
| Pregnancy complication | PE/FI | 46 | 34 | 19 | 149 |
| Spontaneous | 54 | 23 | 23 | 725 | |
| Fever | Yes | 36 | 28 | 36 | 47 |
| No | 54 | 25 | 21 | 797 | |
| Newborn characteristics | |||||
| Sex | Male | 50 | 26 | 24 | 446 |
| Female | 55 | 23 | 22 | 428 | |
| Gestational age (weeks) | 23-24 | 24 | 28 | 49 | 183 |
| 25-26 | 53 | 26 | 21 | 296 | |
| 27 | 71 | 20 | 9 | 295 | |
| Birth weight, grams | ≤ 750 | 33 | 29 | 38 | 328 |
| 751-1000 | 50 | 24 | 16 | 376 | |
| > 1000 | 71 | 16 | 10 | 170 | |
| Birth weight Z-score | < -2 | 53 | 26 | 21 | 53 |
| ≥ -2, < -1 | 42 | 31 | 28 | 118 | |
| ≥ -1 | 54 | 23 | 22 | 703 | |
| Overall row percent | 53 | 25 | 23 | ||
| Maximum column N | 460 | 215 | 199 | 874 | |
KBIT - Kaufman Brief Intelligence Test used to obtain a quick estimate of intelligence
Of the 874 infants in this sample, 53% (N=460) had a SNAP-II below 20, 25% (N=215) had a SNAP-II between 20 and 29, and the remaining 23% (N=199) had a SNAP-II ≥ 30.
The maternal demographic characteristics associated with a SNAP-II ≥ 30 were younger age at delivery, not having a college education, not being married, and eligibility for government-provided (public) medical insurance. Maternal fever during the delivery admission, lower gestational age at birth and lower birth weight were all associated with a SNAP-II >30; fetal growth restriction, however, was not.
Neurocognitive and related outcomes (Supplemental Table 2 and Figures 1 and 2)
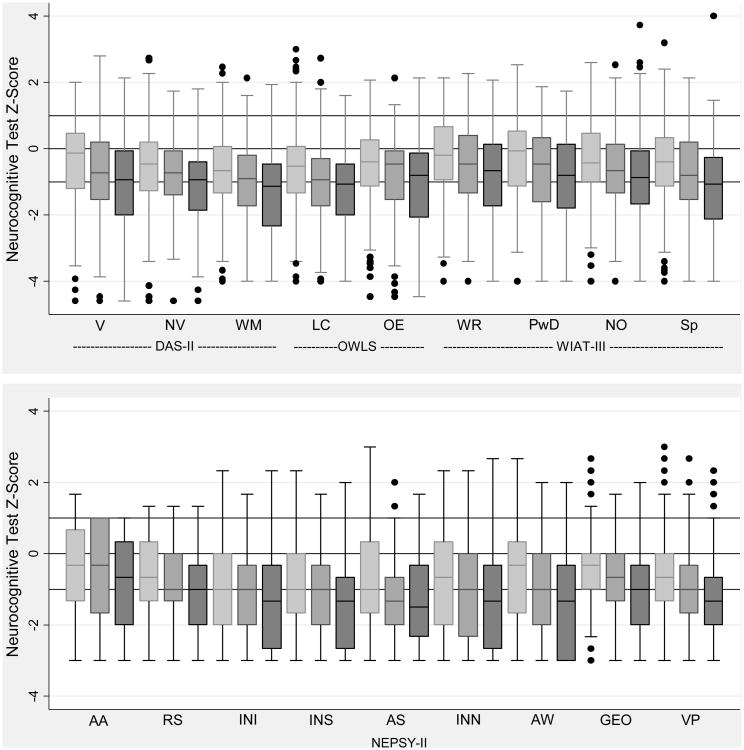
Figure 1.
1. Box-and-whisker plots of each neurocognitive subtest by SNAP-II. All subtest Z-scores are adjusted to population norms. Key: light gray is < 25, medium gray is ≥ 25, < 30, dark gray is ≥ 30. The central line in the box indicates the median (50th centile), while the top of the box indicates the 75th centile and the bottom of the box indicates the 25th centile. V=Verbal, NV=Nonverbal reasoning, WM=Working memory, LC=Listening comprehension, OE=Oral expression, WR=Word reading, PwD=Pseudoword decoding, NO=Numerical operations, Sp=Spelling, AA=Auditory attention, RS= Auditory response set, INI= Inhibition inhibition, INS= Inhibition switching, AS= Animal sorting, INN= Inhibition naming, AW=Arrows, GEO=Geometric puzzles, VP=Visuomotor precision.
1Odds ratios whose lower bound is to the right of the 1.0 vertical line are statistically significant at the p < 0.05 level.
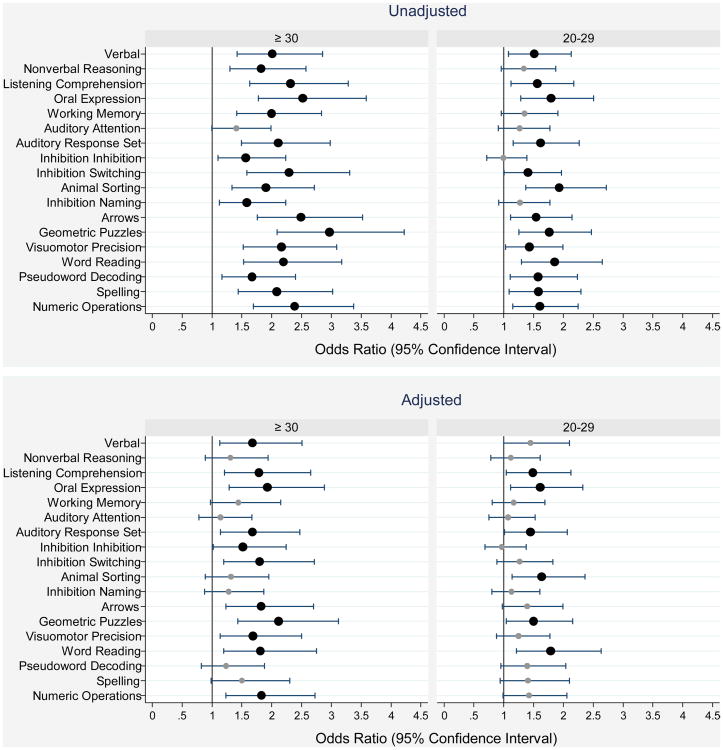
Figure 2.
1. Forest plots of odds ratios (ORs) and 95% confidence intervals of a Z-score ≤ -1 on each DAS-II and NEPSY-II neurocognitive assessment at age 10 associated with a SNAP-II ≥ 30 or a SNAP-II between 20 and 29. Odds ratios in the top panel are unadjusted while those in the bottom panel are adjusted for maternal education (≤ 12 and > 12, < 16 years), public insurance, delivery for preeclampsia or fetal indication, gestational age (23-24 and 25-26 weeks) and birth weight Z-score (< -2 and ≥ -2, < -1).
1Odds ratios whose lower bound is to the right of the 1.0 vertical line are statistically significant at the p < 0.05 level.
Roughly one quarter of all children who had a SNAP-II ≥ 30 had a score 2 or more standard deviations below the normative mean on the DAS-II Verbal, OWLS Listening Comprehension, and OWLS Oral Expression, and WIAT-III Numeric operations assessments. The strength of the association between SNAP-II and both verbal IQ and OWLS, both of which are measures of language function, is strong. Almost one-third of all children with a SNAP-II ≥ 30 had measures of executive function (NEPSY-II) 2 or more standard deviations below the normative mean. In general, the higher the SNAP-II category, the lower the neurocognitive score.
The box and whisker plots (Figure 1) display the distribution of scores on each assessment separately, for each SNAP-II group. The central line in the box indicates the median (50th centile), while the top of the box indicates the 75th centile and the bottom of the box indicates the 25th centile. Children with higher SNAP-IIs had consistently lower scores on the DAS-II, OWLS, WIAT–III and NEPSY-II.
Odds ratios and 95% confidence intervals of a Z-score ≤ 1 displayed in forest plots (Figure 2, top panel) indicate that children with a high SNAP-II were at significantly increased risk of scores one or more standard deviation below the normative mean on almost every cognitive test. In analyses that adjusted for potential confounders, Z-scores ≤ -1 on 11 of 18 cognitive outcomes were associated with SNAP-II in the highest category, and Z-scores ≤ -1 on 6 of 18 were associated with SNAP-II in the intermediate category. Odds ratio's and 95% confidence intervals ranged from 1.4 (1.01, 2.1) to 2.1 (1.4, 3.1) (Figure 2, bottom panel).
Social outcomes (Supplemental Table 3 and Figure 3)
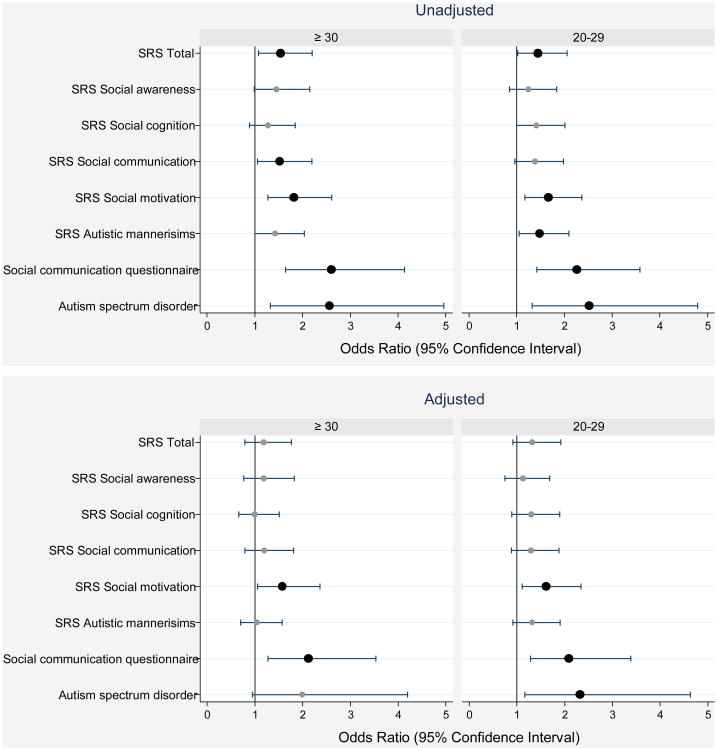
Figure 3.
1. Forest plots of odds ratios (ORs) and 95% confidence intervals of a T score ≥ 60 on the Social Responsiveness Scale (SRS-2) subtests, and of documented characteristics of ASD based on the Autism Diagnostic Observation Schedule-2 (ADOS-2) at age 10 associated with a SNAP-II ≥ 30 or a SNAP-II between 20 and 29. Odds ratios in the top panel are unadjusted while those in the bottom panel are adjusted for gestational age (23-24 and 25-6 weeks), birth weight Z-score (< -2 and ≥ -2, < -1), delivery for maternal or fetal indications, and maternal fever with 48 hours of delivery.
1Odds ratios whose lower bound is to the right of the 1.0 vertical line are statistically significant at the p < 0.05 level.
Infants with an undesirable SNAP-II (≥ 20) had modestly increased total and component scores on the SRS, with higher scores reflecting increased social impairment. Fully 10% of boys in both the middle and high SNAP-II groups were considered to have ASD based on a positive Autism Diagnostic Observation Schedule - 2 (ADOS - 2) assessment compared to 4% in the lowest SNAP-II group.
Infants in the middle and high SNAP-II groups were at significantly increased risk on the social motivation subscale of the SRS and a “positive” Social Communication Questionnaire (SCQ), which screens for ASD. In analyses that adjusted for potential confounders, 2 of the 8 social outcomes were associated with SNAP-II in the highest category, and 3 of 8 social outcomes were associated with SNAP-II in the intermediate category. Odds ratio's and 95% confidence intervals ranged from 1.6 (1.1, 2.4) to 2.3 (1.2, 4.6) (Figure 3, bottom panel).
Educational, neurologic, behavioral and quality of life outcomes (Table 2)
Table 2.
Educational, neurologic, behavioral and quality of life characteristics. The percent of children in each Score for Neonatal Physiology (SNAP-II) stratum who also had the characteristics listed on the left at 10 years. Column percents.
| Characteristic | SNAP-II | Row N | p-ValueI | |||
|---|---|---|---|---|---|---|
| < 20 | 20-29 | ≥ 30 | ||||
| Educational characteristics | ||||||
| Had an IEP | Yes | 44 | 60 | 70 | 470 | < 0.001 |
| Repeated a grade | Yes | 16 | 21 | 22 | 161 | 0.053 |
| Placed in a remedial class | Yes | 15 | 24 | 31 | 184 | < 0.001 |
| Physician diagnoses | ||||||
| Any seizure | Yes | 10 | 14 | 13 | 102 | 0.14 |
| Epilepsy | Yes | 6 | 9 | 11 | 65 | 0.03 |
| ADHD | Yes | 19 | 27 | 30 | 204 | 0.002 |
| Currently receiving medication for: | ||||||
| Seizures | Yes | 3 | 5 | 11 | 45 | 0.03 |
| ADHD | Yes | 13 | 20 | 19 | 144 | < 0.001 |
| Attention deficit hyperactivity disorder | ||||||
| Operational definition of ADHD | Any 2 of 3 | 14 | 19 | 21 | 148 | 0.02 |
| Gross Motor function derangement | ||||||
| GMFCSII | ≥ 3 | 3 | 6 | 8 | 44 | .007 |
| Peds QL© inventory | ||||||
| Physical functioning | < 70 | 14 | 22 | 23 | 153 | < 0.001 |
| ≥ 70, < 85 | 13 | 15 | 19 | 124 | ||
| Emotional functioning | < 70 | 25 | 32 | 26 | 229 | 0.62 |
| ≥ 70, < 85 | 26 | 22 | 25 | 212 | ||
| Social functioning | < 70 | 20 | 28 | 36 | 221 | < 0.001 |
| ≥ 70, < 85 | 18 | 20 | 15 | 155 | ||
| School functioning | < 70 | 31 | 47 | 48 | 345 | < 0.001 |
| ≥ 70, < 85 | 24 | 22 | 25 | 203 | ||
| Psychosocial functioning | < 70 | 25 | 36 | 38 | 264 | < 0.001 |
| ≥ 70, < 85 | 30 | 27 | 30 | 249 | ||
| Maximum column N | 460 | 215 | 199 | 874 | ||
χ2trend
Gross Motor Function Classification System
Whereas approximately 40% of children with a SNAP-II in the lowest SNAP-II category required an individual education plan (IEP), 70% had an IEP if their SNAP-II was ≥ 30, and twice as many children with a SNAP-II ≥ 30 required remedial education, compared to those in the lowest SNAP-II category (Table 2). A high SNAP-II was significantly associated with receipt of an IEP (p < 0.001) and placement in a remedial class (p < 0.001).
The rate of epilepsy increased significantly with increasing SNAP-II category (p = 0.03) as did the use of seizure medication (p = 0.03) at the time of the assessment.
A high SNAP-II was also associated with a GMFCS ≥3. Almost three times as many infants (8%) with a SNAP-II ≥ 30 and twice as many (6%) with a SNAP-II 20-29 had a GMFCS ≥ 3, compared to those with a score < 20 (3%) (p= 0.007).
By and large, children with high SNAP-IIs had lower quality of life scores on the PedsQL™ inventory. A high SNAP-II was associated with adverse outcomes in 4 out of 5 domains, including physical, social, school, and psychosocial functioning (all p < 0.001).
Because parent and teacher-reported outcomes are less reliable than standardized neurocognitive testing, we did not include adjustments for potential confounders in these analyses. Nonetheless, we view these outcomes as meaningful clinical markers of the derangements we found in neurocognitive testing (Figures 2 and 3).
Behavioral outcomes (Supplemental Table 4)
The association between the diagnosis of ADHD and high SNAP-II appears to be stronger than that of treatment for ADHD and a high SNAP-II. The diagnosis of ADHD was more common among children with higher SNAP-IIs than among children who had lower SNAP-IIs, both by parent report (p=0.03) and teacher report (p=0.003). Associations with other CSI-4-identified behaviors presented in Supplemental Table 4, are viewed largely as exploratory.
Discussion
In this sample of 10-year-old children born before the 28th week of gestation, those who had a SNAP-II ≥ 30 were at increased risk of neurocognitive, behavioral, and social dysfunctions. Children who had a high SNAP-II were also more likely than those with a low SNAP-II to have an IEP, to repeat a grade, to be placed in a remedial class, to be diagnosed or treated for ADHD, ASD, or epilepsy, to need an assistive device to ambulate, and to have diminished quality of life.
The characteristic most strongly associated with a high SNAP-II is gestational age, but within each gestational age group those with a SNAP-II ≥ 30 were at even greater risk of these dysfunctions than those with normal physiologic status.(31) Thus, high SNAPs convey risk information that supplements the risk information conveyed by low gestational age.
Most surprising, however, was the multitude of dysfunctions we found at 10 years among children with early physiologic derangements, even after adjusting for potential confounders. Arguing from Occam's razor, we seek a cohesive explanation for these observations. What characteristic or exposure that differentiates extremely preterm from term infants also differentiates those extremely preterm infants with a high SNAP-II from those with a lower SNAP-II?
Possible explanations for our findings
We offer four explanations for the link between SNAP scores and brain injury in children born extremely preterm. Immaturity may contribute to physiologic instability, increase the risk for neonatal complications, or result in a paucity of endogenous neuroprotectors normally provided by placenta, all of which may be associated with brain injury. In addition, prenatal infection and/or inflammation associated with preterm birth may contribute to brain injury as well.
First, physiologic instability may be in the causal chain between immaturity (and its correlates) and brain injury. Accordingly, SNAP-II could be viewed as a marker or indicator for such risk. While both hypoxemia and hypotension, examples of physiologic instability, have been invoked to account for brain damage in very preterm newborns,(32-39) sufficient support for these claims has yet to be provided.(40-44) Further, despite efforts to improve physiologic stability,(41, 45-52) the rate of neurodevelopmental derangements among extremely preterm infants remains high in numerous studies.(43, 44, 53-57)
Second, high SNAP-II scores are associated with postnatal events such as bacteremia/sepsis, necrotizing enterocolitis, and chronic lung disease,(4, 58) which are associated with adverse brain-related outcomes.(59-62) In this way, SNAP-II could be viewed as a marker for subsequent neonatal adversities. Because these intervening disorders might be in the causal path between high SNAP-II and 10-year outcomes, they are not confounders. Therefore, we did not adjust for them in any of our analyses.
Third, elevated SNAP-II scores may convey information about immaturity/vulnerability, such as that attributable to a paucity of placenta-provided endogenous protectors (63), which are known to have beneficial neurotrophic effects on development. Consider that all babies of the same gestational age are not equally mature or vulnerable. From this perspective, SNAP-II provides additional information about physiologic maturation, serving as a marker for processes that are developmentally regulated, including the ability to synthesize growth factors and other proteins capable of protecting the brain.(63) SNAP-II has been correlated with corticospinal tract development, independent of both gestational age and postnatal risk factors, lending support to the theory argument that SNAP-II provides information about neurotrophic effects on brain maturation.(64)
Finally, systemic inflammation, which may be developmentally-regulated, puts the newborn brain at increased risk of multiple disturbances.(65-68) Although systemic inflammation differentiates very preterm from term newborns,(69) early physiologic derangements and first day of life elevations of circulatory inflammation-related proteins, in general, were not associated with systemic inflammation in the ELGAN Study.(70) The rate of maternal fever, which is associated with both chorioamnionitis and early-onset sepsis,(71, 72) however, was increased among those with a SNAP-II ≥ 30. Nonetheless, while preterm infants exposed to chorioamnionitis tend to have higher SNAP-IIs than children not so exposed,(73) the evidence that chorioamnionitis contributes to brain damage in very preterm newborn is mixed.(74-77).
Strengths and limitations
Our study has several strengths. First, we included a large number of infants, making it unlikely that we have missed important associations due to lack of statistical power, or that we claimed associations that might reflect the instability of small numbers. Second, we selected infants based on gestational age, not birth weight, in order to minimize confounding due to factors related to fetal growth restriction.(78) Third, we collected all of our data prospectively. Fourth, attrition at neurocognitive assessment was only modest. The weaknesses of our study are those of all observational studies. We are unable to distinguish between causation and association as explanation for what we found.
Conclusion
SNAP-II provides information that supplements the risk information conveyed by gestational age, and conveys important information about infants' vulnerability to neurodevelopmental adversities 10 years later. We view the multiplicity of neurodevelopmental dysfunctions associated with a high SNAP-II as support for SNAP-II as a marker for immaturity/vulnerability. Support for or against this view could come from studies that evaluate the relationship between SNAP-II and developmentally-regulated biomarkers.
Because no other group has evaluated the relationship between SNAP during the first 12 postnatal hours in very preterm newborns and their function 10 years later, we view our assessments as exploratory. We offer 95% confidence intervals of odds ratios to illustrate the range of values that might be expected when attempts are made to replicate our findings, or test associations between SNAPs and later function in somewhat different ways.
Supplementary Material
Supplemental Tables 1a and 1b are tables provide a summary of the neurocognitive and academic achievement test measures used in the present study, and are available on the Journal of Perinatology website.
Supplemental Table 2, which includes the distribution of neurocognitive test scores for each Score for Neonatal Physiology (SNAP-II) stratum, is available on the Journal of Perinatology website.
Supplemental Table 3, which includes the percent of children in each Score for Neonatal Physiology (SNAP-II) stratum who also had high scores on the Social Responsiveness Scale (SRS-2), both total and subscales, the Social Communication Questionnaire (SCQ), and the autism spectrum disorder (ASD) [based on a positive Autism Diagnostic Observation Schedule (ADOS) assessment], is available on the Journal of Perinatology website.
Supplemental Table 4, which includes the percent of children who had the behavioral characteristic listed on left as identified using the parent and teacher-reported versions of the Child Symptom Inventory-4 (based on the symptom count score) in each Score for Neonatal Physiology (SNAP-II) stratum, and is available on the Journal of Perinatology website.
Acknowledgments
Funding: This study was supported by grants from the National Institute of Neurologic Disorders and Stroke (5U01NS040069-05, 2R01NS040069-06A2), The National Eye Institute (1-R01-EY021820-01) and the National Institute of Child Health and Human Development (5P30HD018655-34).
The authors would like to thank the parents, families and collaborators who contributed to this project, without whom, this project would not have been possible. The primary author would also like to acknowledge Dr. Leif Nelin and colleagues at Nationwide Children's Hospital for their ongoing support of his academic interests.
Footnotes
Disclosures: The authors have no relevant conflicts of interest to disclose.
References
- 1.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91(3):617–23. Epub 1993/03/01. [PubMed] [Google Scholar]
- 2.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 3.Escobar GJ, Shaheen SM, Breed EM, Botas C, Greene JD, Yoshida CK, et al. Richardson score predicts short-term adverse respiratory outcomes in newborns >/=34 weeks gestation. J Pediatr. 2004;145(6):754–60. doi: 10.1016/j.jpeds.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 4.Chien LY, Whyte R, Thiessen P, Walker R, Brabyn D, Lee SK. Snap-II predicts severe intraventricular hemorrhage and chronic lung disease in the neonatal intensive care unit. Journal of perinatology. 2002;22(1):26–30. doi: 10.1038/sj.jp.7210585. Epub 2002/02/13. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho PR, Moreira ME, Sa RA, Lopes LM. SNAPPE-II application in newborns with very low birth weight: evaluation of adverse outcomes in severe placental dysfunction. Journal of perinatal medicine. 2011;39(3):343–7. doi: 10.1515/jpm.2010.141. Epub 2011/02/15. [DOI] [PubMed] [Google Scholar]
- 6.Hagadorn JI, Richardson DK, Schmid CH, Cole CH. Cumulative illness severity and progression from moderate to severe retinopathy of prematurity. J Perinatol. 2007;27(8):502–9. doi: 10.1038/sj.jp.7211780. [DOI] [PubMed] [Google Scholar]
- 7.Fortes Filho JB, Dill JC, Ishizaki A, Aguiar WW, Silveira RC, Procianoy RS. Score for Neonatal Acute Physiology and Perinatal Extension II as a predictor of retinopathy of prematurity: study in 304 very-low-birth-weight preterm infants. Ophthalmologica. 2009;223(3):177–82. doi: 10.1159/000197114. Epub 2009/01/29. [DOI] [PubMed] [Google Scholar]
- 8.Dammann O, Naples M, Bednarek F, Shah B, Kuban KC, O'Shea TM, et al. SNAP-II and SNAPPE-II and the risk of structural and functional brain disorders in extremely low gestational age newborns: the ELGAN study. Neonatology. 2010;97(2):71–82. doi: 10.1159/000232588. Epub 2009/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dammann O, Shah B, Naples M, Bednarek F, Zupancic J, Allred EN, et al. Interinstitutional variation in prediction of death by SNAP-II and SNAPPE-II among extremely preterm infants. Pediatrics. 2009;124(5):e1001–6. doi: 10.1542/peds.2008-3233. Epub 2009/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–41. doi: 10.1542/peds.2005-0173. Epub 2005/08/03. [DOI] [PubMed] [Google Scholar]
- 11.Potharst ES, Houtzager BA, van Sonderen L, Tamminga P, Kok JH, Last BF, et al. Prediction of cognitive abilities at the age of 5 years using developmental follow-up assessments at the age of 2 and 3 years in very preterm children. Developmental medicine and child neurology. 2012;54(3):240–6. doi: 10.1111/j.1469-8749.2011.04181.x. Epub 2011/12/23. [DOI] [PubMed] [Google Scholar]
- 12.Roberts G, Anderson PJ, Doyle LW. The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Archives of disease in childhood. 2010;95(10):786–90. doi: 10.1136/adc.2009.160283. Epub 2009/10/16. [DOI] [PubMed] [Google Scholar]
- 13.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Journal of developmental and behavioral pediatrics: JDBP. 2014;35(6):394–407. doi: 10.1097/01.DBP.0000452240.39511.d4. Epub 2014/07/10. [DOI] [PubMed] [Google Scholar]
- 14.Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Mental retardation and developmental disabilities research reviews. 2002;8(4):234–40. doi: 10.1002/mrdd.10043. Epub 2002/11/28. [DOI] [PubMed] [Google Scholar]
- 15.O'Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–25. doi: 10.1016/j.earlhumdev.2009.08.060. Epub 2009/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott CD. Differential Ability Scales. 2nd. San Antonio, TX: Pearson; 2007. [Google Scholar]
- 17.Carrow-Woolfolk E. Oral and Written Language Scales: Written Expression Scale Manual. Circle Pines, MN: American Guidance Service; 1996. [Google Scholar]
- 18.Korkman M, Kemp S. NEPSY: A Developmental Neuropsychological Assessment New York. New York: The Psychological Corporation; 1998. [Google Scholar]
- 19.Korkman K, KU, Kemp S. NEPSY II: Clinical and interpretative manual. 2007b. San Antonio, TX: Psychological Corporation; 2007. [Google Scholar]
- 20.Wechsler D. The Wechsler Individual Achievement Test-III. Oxford, UK: Pearson Assessment; 2009. [Google Scholar]
- 21.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Developmental medicine and child neurology. 2008;50(10):744–50. doi: 10.1111/j.1469-8749.2008.03089.x. Epub 2008/10/07. [DOI] [PubMed] [Google Scholar]
- 22.Constantino JN. GC Social Responsiveness Scale, Second Edition (SRS-2) Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- 23.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire – Manual. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 24.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview – Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 25.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(9):1094–103. doi: 10.1097/01.chi.0000227880.42780.0e. Epub 2006/08/24. [DOI] [PubMed] [Google Scholar]
- 26.Lord CRM, DiLavore PC, et al. ADOS™-2. second. Torrance: Western Psychological Services; 2012. Autism diagnostic observation schedule™. [Google Scholar]
- 27.Gadow KD. SJ Child Symptom Inventory–4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- 28.Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. Journal of clinical child and adolescent psychology. 4. Vol. 31. American Psychological Association, Division 53; 2002. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys; pp. 513–24. Epub 2002/10/31. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda E, Hinckson E, Krageloh C. Assessment of quality of life in children and youth with autism spectrum disorder: a critical review. Quality of life research. 2014;23(4):1069–85. doi: 10.1007/s11136-013-0591-6. Epub 2013/12/07. [DOI] [PubMed] [Google Scholar]
- 30.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Medical care. 1999;37(2):126–39. doi: 10.1097/00005650-199902000-00003. Epub 1999/02/19. [DOI] [PubMed] [Google Scholar]
- 31.Anderson PJ. Neuropsychological outcomes of children born very preterm. Semin Fetal Neonatal Med. 2014;19(2):90–6. doi: 10.1016/j.siny.2013.11.012. Epub 2013/12/24. [DOI] [PubMed] [Google Scholar]
- 32.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Early blood pressure, antihypotensive therapy and outcomes at 18-22 months' corrected age in extremely preterm infants. Archives of disease in childhood Fetal and neonatal edition. 2016;101(3):F201–6. doi: 10.1136/archdischild-2015-308899. Epub 2015/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuint J, Barak M, Morag I, Maayan-Metzger A. Early treated hypotension and outcome in very low birth weight infants. Neonatology. 2009;95(4):311–6. doi: 10.1159/000180113. Epub 2008/12/05. [DOI] [PubMed] [Google Scholar]
- 34.du Plessis AJ. The role of systemic hemodynamic disturbances in prematurity-related brain injury. J Child Neurol. 2009;24(9):1127–40. doi: 10.1177/0883073809339361. Epub 2009/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arduini A, Escobar J, Vento M, Escrig R, Quintas G, Sastre J, et al. Metabolic adaptation and neuroprotection differ in the retina and choroid in a piglet model of acute postnatal hypoxia. Pediatric research. 2014;76(2):127–34. doi: 10.1038/pr.2014.70. Epub 2014/05/14. [DOI] [PubMed] [Google Scholar]
- 36.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106(4):659–71. doi: 10.1542/peds.106.4.659. Epub 2000/10/04. [DOI] [PubMed] [Google Scholar]
- 37.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. The New England journal of medicine. 2000;343(6):378–84. doi: 10.1056/NEJM200008103430601. Epub 2000/08/10. [DOI] [PubMed] [Google Scholar]
- 38.Back SA, Craig A, Kayton RJ, Luo NL, Meshul CK, Allcock N, et al. Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. Journal of cerebral blood flow and metabolism. 2007;27(2):334–47. doi: 10.1038/sj.jcbfm.9600344. Epub 2006/06/08. [DOI] [PubMed] [Google Scholar]
- 39.Greisen G, Vannucci RC. Is periventricular leucomalacia a result of hypoxic-ischaemic injury? Hypocapnia and the preterm brain. Biol Neonate. 2001;79(3-4):194–200. doi: 10.1159/000047090. Epub 2001/03/29. [DOI] [PubMed] [Google Scholar]
- 40.Limperopoulos C, Bassan H, Kalish LA, Ringer SA, Eichenwald EC, Walter G, et al. Current definitions of hypotension do not predict abnormal cranial ultrasound findings in preterm infants. Pediatrics. 2007;120(5):966–77. doi: 10.1542/peds.2007-0075. Epub 2007/11/03. [DOI] [PubMed] [Google Scholar]
- 41.Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014;105(1):55–63. doi: 10.1159/000356561. Epub 2013/11/20. [DOI] [PubMed] [Google Scholar]
- 42.Manja V, Lakshminrusimha S, Cook DJ. Oxygen saturation target range for extremely preterm infants: a systematic review and meta-analysis. JAMA pediatrics. 2015;169(4):332–40. doi: 10.1001/jamapediatrics.2014.3307. Epub 2015/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Logan JW, O'Shea TM, Allred EN, Laughon MM, Bose CL, Dammann O, et al. Early postnatal hypotension and developmental delay at 24 months of age among extremely low gestational age newborns. Archives of disease in childhood Fetal and neonatal edition. 2011;96(5):F321–8. doi: 10.1136/adc.2010.183335. Epub 2010/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logan JW, O'Shea TM, Allred EN, Laughon MM, Bose CL, Dammann O, et al. Early postnatal hypotension is not associated with indicators of white matter damage or cerebral palsy in extremely low gestational age newborns. Journal of perinatology. 2011;31(8):524–34. doi: 10.1038/jp.2010.201. Epub 2011/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrington KJ. Management during the first 72 h of age of the periviable infant: an evidence-based review. Seminars in perinatology. 2014;38(1):17–24. doi: 10.1053/j.semperi.2013.07.004. Epub 2014/01/29. [DOI] [PubMed] [Google Scholar]
- 46.Carlo WA. Gentle ventilation: the new evidence from the SUPPORT, COIN, VON, CURPAP, Colombian Network, and Neocosur Network trials. Early human development. 2012;88(2):S81–3. doi: 10.1016/S0378-3782(12)70022-1. Epub 2012/05/29. [DOI] [PubMed] [Google Scholar]
- 47.Dempsey E, Pammi M, Ryan AC, Barrington KJ. Standardised formal resuscitation training programmes for reducing mortality and morbidity in newborn infants. The Cochrane database of systematic reviews. 2015;9:CD009106. doi: 10.1002/14651858.CD009106.pub2. Epub 2015/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapadia VS, Chalak LF, Sparks JE, Allen JR, Savani RC, Wyckoff MH. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics. 2013;132(6):e1488–96. doi: 10.1542/peds.2013-0978. Epub 2013/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laptook AR, Watkinson M. Temperature management in the delivery room. Seminars in fetal & neonatal medicine. 2008;13(6):383–91. doi: 10.1016/j.siny.2008.04.003. Epub 2008/05/27. [DOI] [PubMed] [Google Scholar]
- 50.Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126(5):e1319–44. doi: 10.1542/peds.2010-2972B. Epub 2010/10/20. [DOI] [PubMed] [Google Scholar]
- 51.Laughon M, Bose C, Allred E, O'Shea TM, Van Marter LJ, Bednarek F, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119(2):273–80. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith PB, Ambalavanan N, Li L, Cotten CM, Laughon M, Walsh MC, et al. Approach to infants born at 22 to 24 weeks' gestation: relationship to outcomes of more-mature infants. Pediatrics. 2012;129(6):e1508–16. doi: 10.1542/peds.2011-2216. Epub 2012/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph RM, O'Shea TM, Allred EN, Heeren T, Hirtz D, Jara H, et al. Neurocognitive and Academic Outcomes at Age 10 Years of Extremely Preterm Newborns. Pediatrics. 2016 doi: 10.1542/peds.2015-4343. Epub 2016/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson S, Hennessy E, Smith R, Trikic R, Wolke D, Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Archives of disease in childhood Fetal and neonatal edition. 2009;94(4):F283–9. doi: 10.1136/adc.2008.152793. Epub 2009/03/14. [DOI] [PubMed] [Google Scholar]
- 55.Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. The New England Journal of Medicine. 2015;372(19):1801–11. doi: 10.1056/NEJMoa1410689. Epub 2015/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. The New England Journal of Medicine. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. Epub 2005/01/07. [DOI] [PubMed] [Google Scholar]
- 57.Plomgaard AM, Hagmann C, Alderliesten T, Austin T, van Bel F, Claris O, et al. Brain injury in the international multicenter randomized SafeBoosC phase II feasibility trial: cranial ultrasound and magnetic resonance imaging assessments. Pediatric research. 2016;79(3):466–72. doi: 10.1038/pr.2015.239. Epub 2015/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gray JE, Richardson DK, McCormick MC, Goldmann DA. Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use, and outcome. Pediatrics. 1995;95(2):225–30. Epub 1995/02/01. [PubMed] [Google Scholar]
- 59.Barton SK, Tolcos M, Miller SL, Christoph-Roehr C, Schmolzer GM, Moss TJ, et al. Ventilation-Induced Brain Injury in Preterm Neonates: A Review of Potential Therapies. Neonatology. 2016;110(2):155–62. doi: 10.1159/000444918. Epub 2016/04/23. [DOI] [PubMed] [Google Scholar]
- 60.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. The Journal of Pediatrics. 2008;153(2):170–5. 5 e1. doi: 10.1016/j.jpeds.2008.02.033. Epub 2008/06/07. [DOI] [PubMed] [Google Scholar]
- 61.Lee I, Neil JJ, Huettner PC, Smyser CD, Rogers CE, Shimony JS, et al. The impact of prenatal and neonatal infection on neurodevelopmental outcomes in very preterm infants. Journal of Perinatology. 2014;34(10):741–7. doi: 10.1038/jp.2014.79. Epub 2014/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alshaikh B, Yee W, Lodha A, Henderson E, Yusuf K, Sauve R. Coagulase-negative staphylococcus sepsis in preterm infants and long-term neurodevelopmental outcome. Journal of Perinatology : official journal of the California Perinatal Association. 2014;34(2):125–9. doi: 10.1038/jp.2013.155. Epub 2013/12/21. [DOI] [PubMed] [Google Scholar]
- 63.Dammann O, Leviton A. Brain damage in preterm newborns: might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics. 1999;104(3 Pt 1):541–50. doi: 10.1542/peds.104.3.541. Epub 1999/09/02. [DOI] [PubMed] [Google Scholar]
- 64.Zwicker JG, Grunau RE, Adams E, Chau V, Brant R, Poskitt KJ, et al. Score for neonatal acute physiology-II and neonatal pain predict corticospinal tract development in premature newborns. Pediatric Neurology. 2013;48(2):123–9 e1. doi: 10.1016/j.pediatrneurol.2012.10.016. Epub 2013/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leviton A, Kuban KC, Allred EN, Fichorova RN, O'Shea TM, Paneth N. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Human Development. 2011;87(5):325–30. doi: 10.1016/j.earlhumdev.2011.01.043. Epub 2011/02/22. [DOI] [PubMed] [Google Scholar]
- 66.O'Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. The Journal of Pediatrics. 2012;160(3):395–401 e4. doi: 10.1016/j.jpeds.2011.08.069. Epub 2011/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuban KC, O'Shea TM, Allred EN, Paneth N, Hirtz D, Fichorova RN, et al. Systemic Inflammation and Cerebral Palsy Risk in Extremely Preterm Infants. Journal of Child Neurology. 2014;29(12):1692–8. doi: 10.1177/0883073813513335. Epub 2014/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Shea TM, Joseph RM, Kuban KC, Allred EN, Ware J, Coster T, et al. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatric research. 2014;75(6):781–7. doi: 10.1038/pr.2014.41. Epub 2014/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brochu ME, Girard S, Lavoie K, Sebire G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J Neuroinflammation. 2011;8:55. doi: 10.1186/1742-2094-8-55. Epub 2011/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leviton A, Allred EN, Kuban KC, Dammann O, Fichorova RN, O'Shea TM, et al. Blood protein concentrations in the first two postnatal weeks associated with early postnatal blood gas derangements among infants born before the 28th week of gestation. The ELGAN Study. Cytokine. 2011;56(2):392–8. doi: 10.1016/j.cyto.2011.07.014. Epub 2011/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avila C, Willins JL, Jackson M, Mathai J, Jabsky M, Kong A, et al. Usefulness of two clinical chorioamnionitis definitions in predicting neonatal infectious outcomes: a systematic review. American journal of perinatology. 2015;32(11):1001–9. doi: 10.1055/s-0035-1547325. Epub 2015/05/27. [DOI] [PubMed] [Google Scholar]
- 72.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–26. doi: 10.1542/peds.2010-2217. Epub 2011/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Felice C, Toti P, Parrini S, Del Vecchio A, Bagnoli F, Latini G, et al. Histologic chorioamnionitis and severity of illness in very low birth weight newborns. Pediatric critical care medicine. 2005;6(3):298–302. doi: 10.1097/01.PCC.0000160658.35437.65. Epub 2005/04/29. [DOI] [PubMed] [Google Scholar]
- 74.Bersani I, Thomas W, Speer CP. Chorioamnionitis--the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med. 2012;25(1):12–6. doi: 10.3109/14767058.2012.663161. Epub 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 75.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41(1):83–103. doi: 10.1016/j.clp.2013.10.009. Epub 2014/02/15. [DOI] [PubMed] [Google Scholar]
- 76.Chau V, Poskitt KJ, McFadden DE, Bowen-Roberts T, Synnes A, Brant R, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Annals of neurology. 2009;66(2):155–64. doi: 10.1002/ana.21713. Epub 2009/09/11. [DOI] [PubMed] [Google Scholar]
- 77.Roescher AM, Timmer A, Erwich JJ, Bos AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PloS one. 2014;9(2):e89419. doi: 10.1371/journal.pone.0089419. Epub 2014/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. American journal of epidemiology. 1991;134(6):604–13. doi: 10.1093/oxfordjournals.aje.a116133. Epub 1991/09/15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables 1a and 1b are tables provide a summary of the neurocognitive and academic achievement test measures used in the present study, and are available on the Journal of Perinatology website.
Supplemental Table 2, which includes the distribution of neurocognitive test scores for each Score for Neonatal Physiology (SNAP-II) stratum, is available on the Journal of Perinatology website.
Supplemental Table 3, which includes the percent of children in each Score for Neonatal Physiology (SNAP-II) stratum who also had high scores on the Social Responsiveness Scale (SRS-2), both total and subscales, the Social Communication Questionnaire (SCQ), and the autism spectrum disorder (ASD) [based on a positive Autism Diagnostic Observation Schedule (ADOS) assessment], is available on the Journal of Perinatology website.
Supplemental Table 4, which includes the percent of children who had the behavioral characteristic listed on left as identified using the parent and teacher-reported versions of the Child Symptom Inventory-4 (based on the symptom count score) in each Score for Neonatal Physiology (SNAP-II) stratum, and is available on the Journal of Perinatology website.