Abstract
Among disadvantaged persons living with HIV/AIDS (PLHIV), patient-provider engagement, which has been defined as patient-provider relationships that promote the use of health care services and are characterized by active listening and supportive decision making, has been associated with antiretroviral therapy (ART) maintenance and viral suppression. However, chronic pain, depression, and substance use, all of which are prevalent in this population, can reduce the quality of patient-provider engagement. We hypothesized a model in which chronic pain, depression, and substance use would be associated with poorer patient-provider engagement, which would be positively associated with adherence, with the latter associated positively with viral suppression. We analyzed data from the BEACON study, which included surveys from 383 PLHIV who were primarily African American, on ART, and had histories of drug use. Due to 6 missing cases on the chronic pain variable, we used data from 377 respondents in a structural equation model. Chronic pain and depressive symptoms were significantly associated with poorer patient-provider engagement, while substance use was associated with better engagement. Patient-provider engagement in turn was associated with better ART adherence, which was associated with higher viral suppression. Results suggest the role of chronic pain in poor patient-physician engagement in this population, which has potential implications for quality of HIV patient care and health outcomes. Findings suggest the need for attention to patient-provider engagement in PLHIV.
Keywords: patient-provider engagement, chronic pain, substance use, depressive symptoms, HIV/AIDS
Introduction
Chronic pain among persons living with HIV (PLHIV) is a common occurrence, especially among urban, disadvantaged individuals with a history of substance use (1,2). In the PLHIV population, pain is highly associated with reductions in physical functioning, retention in care, and overall quality of life (3). While there is a dearth of studies on pain assessment and management among African American PLHIV who inject drugs, research suggests that these individuals may experience disparities in pain management compared to other racial and ethnic groups (4-6).
PLHIV who use drugs are more likely than other groups to have their pain underestimated by medical providers, and to have less pain medication prescribed, which results in poor patient-provider engagement and unmanaged chronic pain (7-10). Patient-provider engagement includes providers thoroughly explaining treatment options to patients, fostering more of a partnership with the patient, and showing respect for patients and their decisions. For people who use drugs, medical providers have been less likely to prescribe effective pain medications due to their drug use history and the medications' potential for abuse (11). Indeed, pain management can be a complex issue, especially among PLHIV with a drug use history that may also heighten their sensitivity to pain (12,13).
However, positive patient-provider engagement may help PLHIV alleviate chronic pain. For example, Lorig and colleagues found that PLHIV who experience pain, but have good rapport with their health care provider fare better in terms of reducing their pain (14). Consistent and respectful patient-provider relationships, which can enhance patient-provider engagement in care, can increase the patient's trust in the provider, mutual agreement on treatment modalities, and patient adherence to treatment regimens. These results are associated with improved pain and other symptom management and quality of life (15). In contrast, patients who experience poorer quality patient-provider engagement are less likely to disclose all of their health problems, which can impede effective treatment (16). Indeed, better patient-provider engagement has been associated with medical care retention, adherence to antiretroviral therapy (ART), and reduced viral loads (17).
Studies investigating relationships between substance use and patient-provider engagement have yielded mixed results. For example, studies have found patients who use substances are less likely to have a good relationship with their medical provider, including physicians, nurse practitioners, and physician assistants(17, 18), while another study indicated better communication between PLHIV patients and their medical providers, which also included primary HIV physicians, nurse practitioners, and physician assistants(19).
In addition, depression also affects patient-provider engagement. Patient depressive symptoms can skew patient perceptions of their providers and their interactions, affecting more negative and less satisfactory perceptions compared to perceptions of similar interactions by non-depressed individuals(20). Depression may also impact patient-provider engagement because depressed patients may lack the energy or motivation to comply with the doctor's recommendations for treatment. This lack of compliance could strain the patient-provider relationship, which could lead to more negative attributions of the relationship by both patient and provider.
The objective of this study was to examine associations between chronic pain, depression, current substance use, with patient-provider engagement among a sample of primarily urban African American PLHIV with a drug use history. We hypothesized that patients with more chronic pain and depressive symptoms, and who use substances would report worse patient-provider engagement. We also hypothesized that patient-provider engagement would be associated with adherence, which would in turn would be associated with undetectable viral load.
Methods
Participants
Baseline data, collected between 2008 and 2012, were analyzed from the BEACON study, which examined social environments and HIV medical adherence among the study population in Baltimore, Maryland(21). Recruitment was through clinic- and street-based advertising. Inclusion criteria for participants were: (a) age of 18 years or older; (b) documented HIV sero-positive status; (c) current or former injection drug use; (d) current use of ART regimen defined as use in the prior 30 days; and (e) willingness to recruit at least one supportive partner to the study. Participants completed informed consent and received $35 for their participation in the survey. They also consented to a blood test to determine their viral load. The questionnaire was approximately 1.5 hours and included questions on microsocial environmental influences on health behavior using computer-assisted personal interview (CAPI), in addition to more sensitive topics of ART adherence and illicit substance use using audio computer-assisted self-interview (ACASI). The BEACON study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Measures
Outcomes
Undetectable plasma viral load was measured by the Roche Cobas Amplicor. Values were dichotomized to undetectable (<50 copies per mL) = 1 versus detectable (>50 copies per mL) = 0 (22). Adherence was calculated based on self-reported number of pills missed in the past 7 days with 1 = 100% adherence vs. 0 = less than 100% adherence.
Patient-provider engagement was conceptualized as a latent variable that was measured with 7 items (e.g., “My doctor listens to me,” “My doctor answers my questions,” and “My doctor involves me in decisions”) rated on an ordinal scale from 0 = Never to 3 = Always (18).
Independent Variables
Pain was measured by the question, “How often have you been bothered by pain in the past six months?” with response options never, sometimes, often, and always, which were dichotomized into 1=always/often vs. 0=sometimes/never (23). Substance use, which was dichotomized as 1 = current substance use vs. 0 = no current substance use, was assessed by items measuring self-reported use of heroin, prescription opioids, cocaine, crack, stimulants, or barbituates in the previous six months; or binge drinking at least once per week in the previous 30 days(24). Depressive symptoms were measured by the Center for Epidemiologic Studies Depression Scale (CESD-20), and dichotomized using the established cut point of 16 to indicate probable depression (25).
Analyses
Frequencies and means were run on the study variables of interest and demographic characteristics in SPSS v. 2226 and the patient-provider engagement factor was fit in Mplus 7.027. Next, bivariate associations were examined. Subsequently, we used a structural equation model with a chain of three outcomes: patient-provider engagement (first level), adherence (second level) and viral load (third level). The first level outcome was specified to be associated with the independent variables. The higher level outcomes were regressed on lower level outcomes as well as the independent variables. Paths that were statistically non-significant were removed from the model.
Results
In this sample (N=377), the mean age was 48 years (standard deviation = 6 years) and 38.5% were female (Table 1). While the sample was primarily African American (85.9%), other racial groups were included as well, such as White (6.5%), Asian/Asian American (.3%), Native American (2.1%) and multiracial (5.2%). The majority of respondents earned less than $1,000 per month (80.3%). Approximately one-third (32.4%) of respondents reported having pain often or always in the previous 6 months. More than half (63.7%) had used at least one illicit drug in the past six months or binged on alcohol at least weekly in the past 30 days. Two-fifths (40.6%) of respondents had probable depression.
Table 1.
Demographic characteristics of sample (BEACON study; N=377).
| Variable | N (%) or Mean (SDa) |
|---|---|
|
|
|
| Sex (female) | 145 (38.5) |
| Race (African American) | 324 (85.9) |
| Education | |
| 8th grade or less | 31 (9.5) |
| Some high school, no diploma | 124 (38.0) |
| High school diploma/GED | 104 (31.9) |
| Some college or technical training | 54 (16.6) |
| College degree, like B.A. or B.S. | 9 (2.8) |
| Any graduate training | 4 (1.2) |
| Income (per month) | |
| No Income | 3 (.9) |
| < $250 | 20 (6.2) |
| $250-$499 | 31 (9.6) |
| $500-$999 | 187 (57.7) |
| $1,000-$1,499 | 56 (17.3) |
| $1,500-$1,999 | 13 (4.0) |
| $2,000 or more | 14 (4.3) |
| Depression (16+ on CES-D) | 153 (40.6) |
| Current substance use | 240 (63.7) |
| Adherence (100%) | 319 (84.8) |
| Undetectable viral load | 247 (70.8) |
| Pain (often/always) | 122 (32.4) |
| Age (Mean(Standard Deviation)) | 48 (6)b |
SD = Standard Deviation.
Range (minimum and maximum):.28-63.
Ratings for the patient-provider engagement items were high, with means ranging from 2.52 to 2.84 on the 0 – 3 rating scale (Table 2). An exploratory factor analysis with the seven items measuring patient-provider engagement indicated an underlying single dimension with good model fit (Comparative Fit Index [CFI] = .99, Tucker-Lewis Index [TLI] = .97, Root Mean Square Error of Approximation [RMSEA] < .08, 90% Confidence Interval = (.06, .11)) (28). Factor loadings ranged from .79 to .88.
Table 2.
Factor loadings for patient-provider engagement indicator variables.
| Mean (SD) | Factor Loadings | |
|---|---|---|
|
|
|
|
| Listens to me | 2.78 (.59) | .88 |
| Answers my questions | 2.84 (.46) | .88 |
| Involves me in decisions | 2.70 (.70) | .83 |
| Respects my choices | 2.62 (.73) | .84 |
| Respects me | 2.83 (.52) | .88 |
| Supports my decisions | 2.53 (.77) | .80 |
| Provides information I need | 2.79 (.54) | .79 |
Comparative Fit Index (CFI) = .98; Tucker-Lewis Index (TLI) = .97; Root Mean Square Error of Approximation < .08 [.06, .11].
Bivariate associations between the patient-provider engagement factor and study variables revealed significant negative associations between the outcome and experiencing pain often or always (β = -.23, p < .001) and between the outcome and having depression (β = -.22, p < .001).
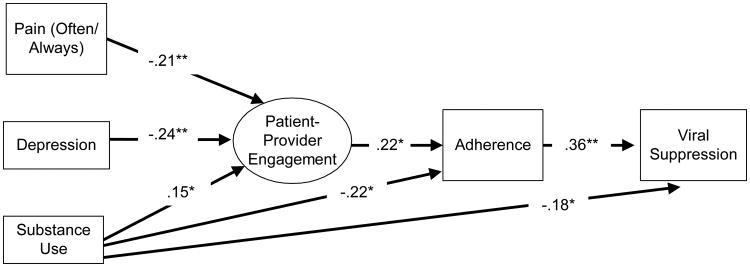
The structural equation model indicated that pain (Standardized Coefficient = -.21, p < .01), depression (Standardized Coefficient = -.24, p < .01), and substance use (Standardized Coefficient = .15, p < .05) were significantly associated with patient-provider engagement (Figure 1). Also, substance use was associated with adherence (Standardized Coefficient = -.22, p < .05) and viral load (Standardized Coefficient = -.18, p < .05). Paths between depression and adherence, and between depression and viral load were non-significant and consequently trimmed. Paths between pain and adherence, and between pain and viral load were also trimmed. However, patient-provider engagement was associated with adherence (Standardized Coefficient = .22, p < .05), which was in turn associated with undetectable viral load (Standardized Coefficient = .36, p < .01). The model achieved good fit (Comparative Fit Index = .99, Tucker-Lewis Index = .99, RMSEA < .03, 90% Confidence Interval = (.00, .05)) (28).
Figure 1.
Structural equation modeling analysis showing standardized path coefficients of associations between pain, depression, and current substance use associated with patient-provider engagement, which in turn is associated with adherence and viral load (Beacon study; N=377).
Comparative Fit Index (CFI) = .99; Tucker-Lewis Index (TLI) = .99, Root Mean Square Error of Approximation (RMSEA) < .03 [.00, .05].
Bold paths are significant at p < .05 or below.
*p < .05; ** p < .01.
Discussion
The purpose of the present study was to assess the effects of chronic pain, depression, and current substance use on patient-provider engagement among a cohort of African American PLHIV with a history of substance use. Our results suggest the vast majority of current or former drug-using, primarily African American PLHIV reported good patient-provider engagement, characterized by positive, supportive relationships with their primary care provider. Specifically, ratings of better engagement were based on patients' reports of having medical providers who listen to them and provide them with needed information, as well as providers who share decision-making with them and respect their decisions.
Relationships characterized by open engagement and patients' perceptions of physician respect were associated with PLHIV having less chronic pain, not being depressed, and currently using substances. Our findings were contradictory to previous research indicating that African Americans, especially among those with histories of drug use, tend to experience disparities in pain management (4-6). Compared to participants in previous studies, it is likely that the drug-using respondents in our study differed in patient-provider engagement because they were regular patients at an HIV clinic and the majority were adherent to highly-active antiretroviral therapy (HAART) and achieved viral suppression. Also, our results should be considered in light of HIV patients' pain adversely affecting their HIV health outcomes through its effects on their relationship with their health care provider.
Prior researchers have found that medical providers may have little empathy for patients' pain and limited knowledge regarding prescribing medications to help PLHIV manage their pain effectively (29-31). This may explain why PLHIV in our study with chronic pain rated their physicians lower on patient-provider engagement. Therefore, it is possible that PLHIV with medical providers who are more empathetic and have greater knowledge of pain treatment may receive higher engagement ratings by their patients who may feel they have a better patient-centered relationship with their provider. Indeed, better engagement is linked with a more patient-centered relationship, which could affect the quality of assessment and treatment options.
Our results suggest the importance of patient-provider relationships in the management of chronic pain. The provider can be a valuable source of social support and information that is useful to the PLHIV pain patient in managing pain symptoms. Our results suggest that ensuring a supportive, patient-centered relationship between HIV primary care physicians and their patients could be a useful intervention as part of a comprehensive approach to patient pain management. This result supports a previous study, which found that patient-centered engagement was associated with better pain-related outcomes among patients with chronic pain in primary care settings (32).
In addition, we found that patients who were depressed rated patient-provider engagement lower. This is likely due to depressed individuals having more negative thinking and attributions. Depression may contribute to worse doctor-patient relationships by making patients less responsive and less forthcoming about their ailments. These actions could decrease the likelihood of the physician being able to ascertain a full description of the pain symptoms, which would impede the physician's ability to provide effective help in managing chronic pain. Also, treating chronic pain along with the depressive symptoms could lead to prioritization of problems with some health conditions, such as depression, being considered secondary to the chronic physical condition (20,33). These findings may also be explained by prior research that demonstrates greater depressive symptoms, in conjunction with problem alcohol use, was associated with less counseling on the part of the physician, suggesting worse doctor-patient engagement (19). Therefore, poor patient-provider engagement among people who use drugs likely has implications for provider counseling, especially for addictions counseling in particular.
Moreover current substance use was positively associated with patient-provider engagement. This finding supports previous research which found a similar relationship between illicit drug use and better patient-provider communication (19). Specifically, among PLHIVs, illicit drug use was associated with more negative statements made by primary HIV physicians, nurse practitioners, or physician assistants with their patients, which suggests that patients who make more negative statements are confronting, rather than avoiding, conflict; thereby, having a more open and honest interaction with their medical provider (19). Having more open conversations with their providers likely helps the patient because the medical professional is more informed about barriers to health maintenance, such as drug use, that the patient may be confronting. Therefore, the physician may be better equipped to help drug-using patients achieve their health goals, including maintenance of HAART treatment. However, it is important to note that current drug use still had an independent and negative effect on adherence.
As hypothesized, patient provider engagement was associated with adherence, which was in turn associated with undetectable viral load. These results suggest support for previous findings that patient provider engagement can facilitate better health outcomes for patients, likely by increasing retention in care and adherence to medical regimens, such as HAART therapy for PLHIV (34).
Limitations
This study has several limitations. First, the data were cross-sectional, which prevents the ascertainment of fluctuations over time of factors such as depression and drug use. Second, participants were predominantly African American, insured, and on HAART, which limits the representativeness of the sample and thus generalizability of the findings. Next, several measurement limitations exist, such as lack of distinction with regard to frequency and severity of pain symptoms over time. In addition, it was not ascertained for this study whether or not the HIV primary medical provider was the one to prescribe pain medication, if any was prescribed. Also, we did not assess duration of time spent seeing a particular physician and we did not ascertain the number of visits for illnesses when participants saw doctors other than their HIV primary care physicians.
Conclusions
Our results underscore the important role of the medical provider in helping patients with HIV reduce their incidence for chronic pain and suggest the importance of supportive patient-provider engagement regarding chronic pain reduction. These results suggest that future interventions to reduce chronic pain should target providers and their engagement skills as part of a pain-alleviation intervention. Patient-provider engagement and supportive relationships, which are principles of palliative care that aim to reduce suffering and improve quality of life, may help to promote physical and mental health outcomes (35).
Merlin and colleagues (2015) (35) note the dearth of interventions targeting PLHIV to address chronic pain management. Our research findings suggest the relevance of patient and physician interactions in addressing chronic pain. In sum, our results suggest that having chronic pain and being depressed may strain the patient-provider relationship by affecting the patient's perceptions of engagement quality. Based on previous research, it is likely that these poorer quality relationships affect PLHIV's retention in care, adherence to HAART, and maintenance of low viral loads. Therefore, intervention is warranted to strengthen the patient-provider relationship, especially among patients with chronic pain and depression.
Acknowledgments
Funding: This study was funded by R01 DA019413, R01NR014050 and T-32DA007292 from the National Institutes of Health and Center For AIDS Research (CFAR) grant 1P30AI094189.
Footnotes
Conflicts of interest: Mary M. Mitchell declares that she has no conflict of interest.
Trang Q. Nguyen declares that she has no conflict of interest.
Allysha C. Maragh-Bass declares that she has no conflict of interest.
Sarina R. Isenberg declares that she has no conflict of interest.
Mary Catherine Beach declares that she has no conflict of interest.
Amy R. Knowlton declares that she has no conflict of interest.
Informed consent was obtained from all individual participants included in the study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Merlin JS, Zinski A, Norton WE, et al. A conceptual framework for understanding chronic pain in patients with HIV. Pain Practice. 2014;14(3):207–216. doi: 10.1111/papr.12052. [DOI] [PubMed] [Google Scholar]
- 2.Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. The Journal of Pain. 2011;12(9):1004–1016. doi: 10.1016/j.jpain.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlin JS, Westfall AO, Chamot E, et al. Pain is Independently Associated with Impaired Physical Function in HIV-Infected Patients. Pain Medicine. 2013;14(12):1985–1993. doi: 10.1111/pme.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey JA, Hurley RW, Gold MS. Crossroads of pain and addiction. Pain Medicine. 2010;11(12):1803–1818. doi: 10.1111/j.1526-4637.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 5.Blankenship KM, Smoyer AB, Bray SJ, Mattocks K. Black-white disparities in HIV/AIDS: the role of drug policy and the corrections system. J Health Care Poor Underserved. 2005;16(4 Suppl B):140–156. doi: 10.1353/hpu.2005.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kral AH, Bluthenthal RN, Booth RE, Watters JK. HIV seroprevalence among street-recruited injection drug and crack cocaine users in 16 US municipalities. Am J Public Health. 1998;88(1):108–113. doi: 10.2105/ajph.88.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anziska Y, Helzner EP, Crystal H, et al. The relationship between race and HIV-distal sensory polyneuropathy in a large cohort of US women. J Neurol Sci. 2012;315(1):129–132. doi: 10.1016/j.jns.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45(12):1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malla KY, Manchikanti L. A prospective evaluation of psychotherapeutic and illicit drug use in patients presenting with chronic pain at the time of initial evaluation. Pain physician. 2013;16:E1–E13. is an Interventional. [PubMed] [Google Scholar]
- 10.Stewart DW, Jones GN, Minor KS. Smoking, depression, and gender in low-income African Americans with HIV/AIDS. Behavioral Medicine. 2011;37(3):77–80. doi: 10.1080/08964289.2011.583946. [DOI] [PubMed] [Google Scholar]
- 11.Selwyn PA. Palliative care for patient with human immunodeficiency virus/acquired immune deficiency syndrome. J Palliat Med. 2005;8(6):1248–1268. doi: 10.1089/jpm.2005.8.1248. [DOI] [PubMed] [Google Scholar]
- 12.Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage S, Schofferman J. Pharmacological therapies for drug and alcohol addictions. New York: Dekker; 1995. Pharmacological therapies of pain in drug and alcohol addictions; pp. 373–409. [Google Scholar]
- 14.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Annals of behavioral medicine. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh AT, Atchison JW, Berger JJ, et al. Patient satisfaction with treatment for chronic pain: predictors and relationship to compliance. Clin J Pain. 2005;21(4):302–310. doi: 10.1097/01.ajp.0000113057.92184.90. [DOI] [PubMed] [Google Scholar]
- 16.Peters S, Rogers A, Salmon P, et al. What do patients choose to tell their doctors? Qualitative analysis of potential barriers to reattributing medically unexplained symptoms. Journal of general internal medicine. 2009;24(4):443–449. doi: 10.1007/s11606-008-0872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beach MC, Keruly J, Moore RD. Is the Quality of the Patient Provider Relationship Associated with Better Adherence and Health Outcomes for Patients with HIV? Journal of general internal medicine. 2006;21(6):661–665. doi: 10.1111/j.1525-1497.2006.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakken S, Holzemer WL, Brown M, et al. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. AIDS Patient Care STDS. 2000;14(4):189–197. doi: 10.1089/108729100317795. [DOI] [PubMed] [Google Scholar]
- 19.Korthuis P Todd, et al. Substance use and the quality of patient-provider communication in HIV clinics. AIDS Behav. 2011;15(4):832–841. doi: 10.1007/s10461-010-9779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenker Y, Stewart A, Na B, Whooley MA. Depressive symptoms and perceived doctor-patient communication in the Heart and Soul study. Journal of general internal medicine. 2009;24(5):550–556. doi: 10.1007/s11606-009-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell MM, Maragh-Bass AC, Nguyen TQ, Isenberg S, Knowlton AR. The role of chronic pain and current substance use in predicting negative social support among disadvantaged persons living with HIV/AIDS. AIDS Care. 2016:1–7. doi: 10.1080/09540121.2016.1168916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arribas JR, Pulido F, Delgado R, et al. Lopinavir/ritonavir as single-drug therapy for maintenance of HIV-1 viral suppression: 48-week results of a randomized, controlled, open-label, proof-of-concept pilot clinical trial (OK Study) JAIDS J Acquired Immune Defic Syndromes. 2005;40(3):280–287. doi: 10.1097/01.qai.0000180077.59159.f4. [DOI] [PubMed] [Google Scholar]
- 23.Holzemer WL, Henry SB, Nokes KM, et al. Validation of the Sign and Symptom Check-List for Persons with HIV Disease (SSC HIV) J Adv Nurs. 1999;30(5):1041–1049. doi: 10.1046/j.1365-2648.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell MM, Knowlton A. Caregiver role overload and network support in a sample of predominantly low-income, African-American caregivers of persons living with HIV/AIDS: a structural equation modeling analysis. AIDS Behav. 2012;16(2):278–287. doi: 10.1007/s10461-011-9886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 26.IBM Corporation. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corporation; 1999. [Google Scholar]
- 27.Muthén L, Muthén B. Mplus user's guide. 7th. Los Angeles: Muthén & Muthé; 1998-2012. [Google Scholar]
- 28.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal. 1999;6(1):1–55. [Google Scholar]
- 29.Hughes HK, Korthuis PT, Saha S, et al. A mixed methods study of patient–provider communication about opioid analgesics. Patient Educ Couns. 2015;98(4):453–461. doi: 10.1016/j.pec.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krashin D, Trescot AM. Opioid therapy in chronic painful disease. Expert Decision Making on Opioid Treatment. 2013 [Google Scholar]
- 31.Lum PJ, Little S, Botsko M, et al. Opioid-prescribing practices and provider confidence recognizing opioid analgesic abuse in HIV primary care settings. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S91–7. doi: 10.1097/QAI.0b013e31820a9a82. [DOI] [PubMed] [Google Scholar]
- 32.Dorflinger L, Kerns RD, Auerbach SM. Providers' roles in enhancing patients' adherence to pain self management. Translational behavioral medicine. 2013;3(1):39–46. doi: 10.1007/s13142-012-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swenson SL, Rose M, Vittinghoff E, Stewart A, Schillinger D. The influence of depressive symptoms on clinician-patient communication among patients with type 2 diabetes. Med Care. 2008;46(3):257–265. doi: 10.1097/MLR.0b013e31816080e9. [DOI] [PubMed] [Google Scholar]
- 34.Barrier PA, Li JT, Jensen NM. Two words to improve physician-patient communication: what else? Mayo Clinic Proceedings. 2003;78(2):211–214. doi: 10.4065/78.2.211. [DOI] [PubMed] [Google Scholar]
- 35.Radbruch L, Downing J. Principles of Palliative Care. Guide to Pain Management in Low-Resource Settings. 2010:47. [Google Scholar]
- 36.Merlin JS, Walcott M, Kerns R, Bair MJ, Burgio KL, Turan JM. Pain Self-Management in HIV-Infected Individuals with Chronic Pain: A Qualitative Study. Pain Medicine. 2015;16(4):706–714. doi: 10.1111/pme.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]