Abstract
Specificity is a core principle of exercise training to promote the desired adaptations for maximising athletic performance. The principle of specificity of adaptation is underpinned by the volume, intensity, frequency and mode of contractile activity and is most evident when contrasting the divergent phenotypes that result after undertaking either prolonged endurance or resistance training. The molecular profiles that generate the adaptive response to different exercise modes have undergone intense scientific scrutiny. Given divergent exercise induces similar signalling and gene expression profiles in skeletal muscle of untrained or recreationally active individuals, what is currently unclear is how the specificity of the molecular response is modified by prior training history. The time course of adaptation and when ‘phenotype specificity’ occurs has important implications for exercise prescription. This context is essential when attempting to concomitantly develop resistance to fatigue (through endurance‐based exercise) and increased muscle mass (through resistance‐based exercise), typically termed ‘concurrent training’. Chronic training studies provide robust evidence that endurance exercise can attenuate muscle hypertrophy and strength but the mechanistic underpinning of this ‘interference’ effect with concurrent training is unknown. Moreover, despite the potential for several key regulators of muscle metabolism to explain an incompatibility in adaptation between endurance and resistance exercise, it now seems likely that multiple integrated, rather than isolated, effectors or processes generate the interference effect. Here we review studies of the molecular responses in skeletal muscle and evidence for the interference effect with concurrent training within the context of the specificity of training adaptation.
Keywords: endurance exercise, exercise adaptation, resistance exercise, skeletal muscle
Abbreviations
- ACTN3
α‐actinin‐3
- AMPK
AMP‐activated protein kinase
- 4E‐BP
eukaryotic initiation factor 4E‐binding protein
- MAPK
p38 mitogen‐activated protein kinase
- mTOR
mechanistic target of rapamycin
- PGC‐1α
peroxisome proliferator activated receptor γ coactivator‐1α
- PKB
protein kinase B
- S6K
70 kDa ribosomal protein S6 kinase
maximum oxygen uptake
Introduction
Athletic events are broadly classified as either ‘endurance‐based’ or ‘strength–power‐based’, with the one‐dimensional demands underlying these divergent performance capacities imposing an uncomplicated stimulus for adaptation. Training to increase endurance capacity can be achieved through prolonged (>60 min), continuous or repeated intermittent bouts of submaximal contractions that, when performed for several months or years, elicit a variety of metabolic and morphological adaptations including mitochondrial biogenesis, fast‐to‐slow fibre‐type transformation and shifts in substrate metabolism that favour fat‐ over carbohydrate‐based fuels (Holloszy & Coyle, 1984; Hawley, 2002). In contrast, training to increase strength and/or power requires short duration (<60 s) maximal contractile activity and heavy resistance loading to stimulate the synthesis of myofibrillar proteins and muscle hypertrophy (Damas et al. 2015). Such training, even when performed over several months or years, elicits little or no change to the oxidative profile of the trained muscles, nor major shifts in the patterns of fuel utilisation (MacDougall et al. 1982).
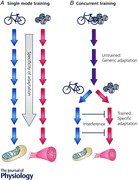
There are, however, numerous athletic disciplines where a combination of both muscular endurance and strength/power are required for successful performance. Under such circumstances endurance and resistance training are undertaken concomitantly as part of a periodised training programme (i.e. ‘concurrent training’). In the context of this review, concurrent training is used generically to describe a single training session during which an individual performs both endurance‐ and resistance‐based exercise, and/or when an athlete incorporates both types of training on different occasions as part of a periodised training programme. During concurrent training, muscle is repeatedly subjected to divergent contractile stimuli and the specificity of the adaptive response is altered to such an extent that gains in hypertrophy, strength and power are typically attenuated compared to when resistance training is undertaken in isolation (Wilson et al. 2012). The simultaneous development of muscular endurance and strength/power arguably represents the highest complexity in exercise prescription, while from a molecular perspective it is an intriguing challenge to dissect the mechanistic bases for the interference effect on adaptive responses with contrasting contractile stimuli.
Concurrent exercise training: evidence for an ‘interference’ effect
Thirty‐five years ago Dr Robert Hickson demonstrated impaired strength development in previously untrained males who incorporated both strength and endurance workouts into a 10 week training programme (Hickson, 1980). Hickson termed the impaired strength gains with concurrent strength and endurance training the ‘interference effect’. Since that seminal work, the results from the majority of studies confirm that gains in muscle hypertrophy and strength are compromised when strength‐ and endurance‐based training are undertaken concurrently (Dudley & Djamil, 1985; Hunter et al. 1987; Hennessy & Watson, 1994; Kraemer et al. 1995; Bell et al. 2000; Putman et al. 2004; Chtara et al. 2008; Rønnestad et al. 2012; Jones et al. 2013). Even where the interference effect has been unclear (McCarthy et al. 1995, 2002; Balabinis et al. 2003; Häkkinen et al. 2003; Hendrickson et al. 2010; Lundberg et al. 2013), this can typically be attributed to the low volume and frequency of training undertaken, a limited intervention period and/or the training status of individuals under investigation. Indeed, a meta‐analysis of concurrent strength and endurance training by Wilson and colleagues clearly demonstrates the negative effect of endurance exercise on muscle hypertrophy, strength and power that occurs in a frequency‐ and duration‐dependent manner (Wilson et al. 2012). The mode of exercise may also have an impact on the magnitude of the interference effect with running likely to have a greater negative effect on strength development than cycling (Wilson et al. 2012), possibly due to the eccentric component of running and concomitant muscle damage. Of note is training‐induced gains in aerobic capacity are not compromised by concurrent strength and endurance training.
In contrast to the impaired strength development when endurance training is undertaken simultaneously with resistance training, there is potential for combined strength and endurance training to amplify endurance performance (Rønnestad & Mujika, 2014). Hickson and co‐workers (Hickson et al. 1988) combined heavy‐resistance workouts (3 sessions per week for 10 weeks) with the normal endurance training regimens of trained runners and cyclists who were already at a steady‐state level of endurance performance. Despite no changes in maximum oxygen uptake () there was a clear benefit of adding strength training to endurance training for both short‐term (4–8 min) running and cycling performance and long‐term (∼80 min) endurance cycling capacity.
The data from concurrent training studies demonstrating attenuated muscle hypertrophy/strength compared to when resistance training is undertaken alone has lead researchers to question the underlying mechanism(s) responsible for this phenomenon. In this regard, quantifying changes in performance capacity and/or muscle morphology has failed to provide insight as to ‘how’ and ‘when’ these divergent exercise modes become discordant with respect to exercise‐induced adaptations (Leveritt et al. 1999). Knowledge of the time course of the molecular and performance changes occurring with concurrent strength and endurance training in various populations (athletes, the elderly) could provide valuable information for practitioners when designing exercise programmes that require the simultaneous development of muscular strength and endurance (Leveritt et al. 1999; Coffey & Hawley, 2007; Fyfe et al. 2014). Clearly, the biology underlying concurrent training responses may confer advantages beyond the athletic arena and has ‘real world’ impact.
Are strength and endurance training molecularly incompatible?
The molecular bases of skeletal muscle adaptations to exercise (i.e. increased mitochondrial mass, altered substrate metabolism, angiogenesis, or myofibre hypertrophy) involve increased expression and/or activity of key proteins, mediated by an array of signalling events, pre‐ and post‐transcriptional processes, regulation of translation and protein expression, and modulation of protein (enzyme) activities and/or intracellular localisation (Hawley et al. 2014; Egan et al. 2016). Multiple stimuli are associated with endurance‐ and resistance‐based exercise, various signalling kinases that respond to these divergent stimuli, and numerous downstream pathways and targets of these kinases (Coffey & Hawley, 2007; Egan & Zierath, 2013; Egan et al. 2016). In addition, there is ‘cross‐talk’ between these various elements that combine to produce the integrated response to an exercise challenge and ultimately result in functional improvements and alterations in phenotype (Hawley et al. 2015; Egan et al. 2016). The various signalling pathways involved in endurance‐ and resistance/strength‐based adaptation are numerous and have been reviewed in detail elsewhere (Coffey & Hawley, 2007; Egan & Zierath, 2013; Hawley et al. 2014). Briefly, endurance training adaptation requires the stimulation of several transcription factors (including nuclear respiratory factor‐1 and ‐2 (NRF‐1, NRF‐2)) that bind to their promoters and activate the transcription of genes that encode mitochondrial respiratory chain proteins. Not all promoters of genes transcribing mitochondrial proteins have NRF binding sites, so other transcription factors are involved in contractile‐modulated mitochondrial biogenesis, including the oestrogen‐receptor‐related receptors (ERRs) and the peroxisome proliferator‐activated receptor coactivators (PPARs), which regulate expression of the mitochondrial fatty acid oxidative enzymes. The AMP‐activated protein kinase (AMPK) and p38 mitogen‐activated protein kinase (MAPK) are two other important signalling cascades that converge upon the regulation of peroxisome proliferator activated receptor γ coactivator‐1α (PGC‐1α) and consequently the regulation of mitochondrial biogenesis (Fig. 1). Resistance training adaptation is less defined than endurance training with regard to specific signalling pathways or critical nodes that are necessary to generate hypertrophy. The mechanistic target of rapamycin (mTOR) complex 1 has been characterised as a focal point for hypertrophy with an important role in contraction‐induced increases in muscle protein synthesis. The most well‐defined effectors of mTOR signalling are proteins implicated in translational control: 70 kDa ribosomal protein S6 kinase (S6K) and eukaryotic initiation factor 4E‐binding protein (4E‐BP) (Philp et al. 2011) (Fig. 2).
Figure 1. Putative mediators of exercise‐induced regulation of peroxisome proliferator activated receptor γ coactivator‐1α (PGC‐1α).
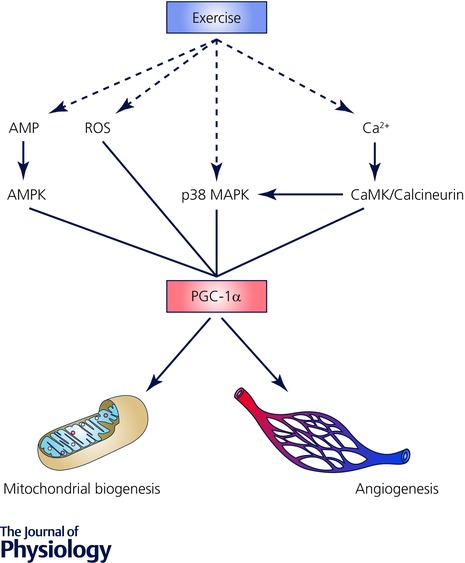
Dashed lines show the primary sensors of contractile activity, continuous lines show secondary mediators of signalling pathways proposed to upregulate PGC‐1α expression. The adenosine monophosphate kinase (AMPK) is an energy sensing protein, reactive oxygen species (ROS) are a metabolic by‐product of oxidative metabolism, which in concert with the p38 mitogen activated protein kinase (MAPK) all respond to the metabolic stress generated within skeletal muscle by exercise. Modulation of cellular calcium concentration with contraction upregulates calmodulin kinase (CaMK) and/or calcineurin proteins, which also act as a metabolic signal for adaptation responses. Together, these intermediaries coordinate complex signalling pathways regulating PGC‐1α expression and subsequent mitochondrial biogenesis and angiogenesis in skeletal muscle (Lira et al. 2010; Olesen et al. 2010).
Figure 2. Proposed mechanical activation of signals leading to increased translation and ribosome biogenesis in skeletal muscle.
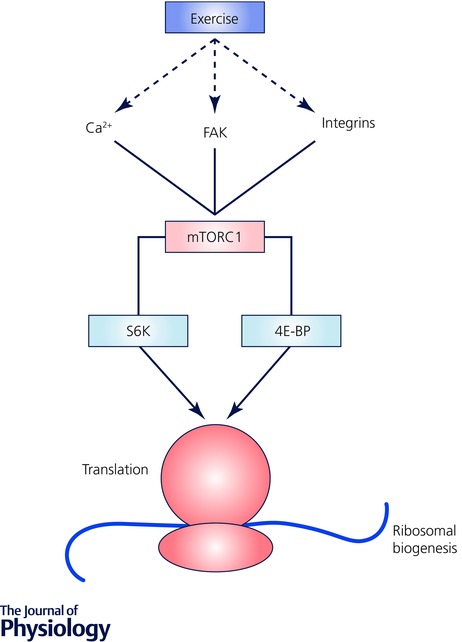
Dashed lines show the primary sensors of contractile activity, continuous lines show secondary mediators of signalling pathways proposed to ultimately upregulate protein synthesis. Calcium, focal adhesion kinases (FAK) and integrin linked kinases have each been proposed as mechanosensors that initiate the signal that generates greater subsequent activity of the mechanistic target of rapamycin complex 1 (mTORC1). The downstream effectors of mTORC1 are the 70 kDa ribosomal S6 protein kinase (S6K) and eukaryotic initiation factor 4E binding protein (4E‐BP) that have been shown to have key roles in promoting translation initiation and ribosome biogenesis (Philp et al. 2011).
We (Coffey et al. 2009,b) and others (Jones et al. 2016) have examined the acute effect of temporal proximity and exercise order of resistance and endurance/high‐intensity exercise bouts on translational signalling and transcription of select genes implicated in exercise‐induced adaptation in recreationally trained humans. While we have observed some differences in the magnitude of effect in kinase phosphorylation of regulators of translation such as S6K and ribosomal protein S6 (rpS6), and mRNA content of PGC‐1α and myogenic regulatory factors, the overall responses in the ‘metabolic’ and ‘myogenic’ pathways were often similar, regardless of exercise mode, and any differences moderate (Coffey et al. 2009,b). Jones and colleagues (Jones et al. 2016) were also unable to clearly differentiate between acute signalling responses in human skeletal muscle with alternate concurrent exercise order compared with resistance exercise alone. The impact of exercise order notwithstanding, other studies have compared molecular responses of combined endurance and resistance exercise versus single‐mode exercise and show similar or enhanced signalling and gene responses with concurrent exercise in moderately trained or recreationally active individuals (Apro et al. 2013, 2015; Lundberg et al. 2014; Kazior et al. 2016). An important consideration when comparing the outcomes of studies of single mode versus concurrent exercise bouts is that more often than not, the contractile stimulus to the working muscles (i.e. the total work performed) is vastly different. In an attempt to mitigate the influence of total work on acute molecular responses, Donges and co‐workers (Donges et al. 2013) studied sedentary, middle‐aged men who performed separate single resistance and aerobic exercise bouts, and a concurrent exercise bout comprising 50% of the total work undertaken during each of the resistance and aerobic exercise bouts. They reported no meaningful change in S6K and AMPK phosphorylation between the different exercise modes, comparable increases in skeletal muscle myofibrillar protein synthesis with concurrent compared with resistance exercise, and similar rates of mitochondrial protein synthesis between concurrent and aerobic exercise.
The inability to match total work as well as the type of stimulus and/or exercise mode make comparisons between the results of studies of concurrent training difficult. Indeed, differences in experimental design and dependent variable selection that often limit the knowledge gained from studies using only performance‐based outcomes (Leveritt et al. 1999) also confounds information gained from investigations that have measured molecular responses to divergent exercise modes. However, perhaps the primary factors determining the molecular profiles generated by concurrent exercise are the training status of subjects and inter‐individual responses. We previously hypothesised that training history would impact the concurrent training response due to an underlying incompatibility in the training‐induced phenotype when undertaking an opposing exercise stimulus (Coffey et al. 2006). To investigate this we studied highly trained athletes with a prolonged history of either endurance or strength training (but not concurrent training) who performed both an acute bout of exercise in their specialised discipline and then ‘crossed over’ and undertook a bout of unfamiliar exercise (Coffey et al. 2006). Muscle biopsies were taken at rest, immediately, and 3 h post exercise. AMPK phosphorylation increased after cycling in strength‐trained but not endurance‐trained subjects. Conversely, AMPK and S6K phosphorylation was elevated after resistance exercise in endurance‐ but not strength‐trained subjects. These early signalling responses to divergent exercise stimuli in skeletal muscle from well‐trained humans clearly demonstrate that prior training history alters the exercise‐specific signalling responses involved in single mode adaptations to training and that a degree of ‘response plasticity’ is conserved at opposite ends of the endurance–hypertrophic adaptation continuum.
Compared to trained athletes, untrained individuals have a greater capacity to activate the molecular machinery in muscle in response to contractile activity because any overload stimulus induces large perturbations to cellular homeostasis regardless of the mode of exercise (Benziane et al. 2008; Perry et al. 2010; Nader et al. 2014). Given the generic ‘molecular footprint’ generated in untrained individuals in response to different exercise modes, it seems reasonable to conclude that training history has a large bearing on any molecular signature induced by concurrent training (Fig. 3). In addition, individual variation in both the molecular and functional responses to exercise is likely to impact on the precision to detect molecular incompatibility with concurrent training. The characterisation of individual responses is not a new phenomenon, but the results of studies of individuals undertaking resistance and endurance training in isolation suggest that ‘low responders’ to each exercise stimulus may be as high as 25% (Timmons et al. 2005; Phillips et al. 2013; Gurd et al. 2016). Intuitively, it might be expected that the number of ‘low responders’ would double (or at least increase) after concurrent compared to single mode training, but the little data currently available suggest that individuals are not systematically low or high responders when exposed to different training stimuli (Karavirta et al. 2011). Nonetheless, the variation of human responses to divergent stimuli adds complexity to the molecular bases of the interference phenomenon.
Figure 3. Hypothetical time course for skeletal muscle and functional adaptation from the untrained to a trained state.
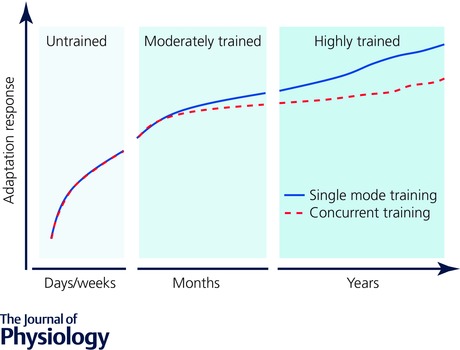
In the first days/weeks when an individual commences training, the initial skeletal muscle adaptation responses are similar between single mode and concurrent training adaptation as are any functional performance measures. During this early phase the mechanosensors and subsequent mechanotransduction of the adaptive signal fail, at least in part, to differentiate between the endurance‐ and resistance‐like stimuli often undertaken at low–moderate intensity and volume. As training progresses (i.e. months to years) repeated bouts of divergent exercise begin to generate a specificity of training adaptation that initiates transformation of the skeletal muscle phenotype. The change in phenotype coincides with the need for greater training loads to disrupt homeostasis and promote further adaptation which results in impaired responses to concurrent training compared with single mode training. The impaired adaptive response with concurrent versus single mode training is exacerbated with increasing training history.
The specificity of training adaptation in skeletal muscle
Differences in the skeletal muscle phenotype and the associated performance capacities of highly trained endurance‐ compared to resistance‐trained individuals are clear (Coffey et al. 2006). These phenotypes result from adaptation in response to the cumulative overload generated by individual bouts of exercise repeated over days, months and years of training. Detailed characterisation of the time course of adaptation over such extended periods has proved difficult to study. Instead, we are left with the results of investigations that have determined acute responses or outcomes to short‐term training interventions (weeks to months) to underpin our current understanding of the adaptation process. The work of Perry and colleagues (Perry et al. 2010) provides the first time course of molecular sequelae in human skeletal muscle that accompany repetitive training stimuli. These workers examined the time course of responses of mitochondrial biogenesis and fusion/fission proteins, along with selected transcriptional and mitochondrial mRNAs and proteins in human skeletal muscle during a ∼2 week intervention period in which individuals performed seven bouts of high‐intensity training. They demonstrated that the repeated, transient increases in mRNA induced after each exercise session were necessary to elicit the sustained increases observed in the content of transcription and metabolic proteins. Of note was that even during such a short‐term intervention, the mRNA responses to exercise were attenuated as the muscle adapted to the exercise challenge, even in the face of an increasing training intensity. Increases in mitochondrial proteins occurred within 5 days and following three sessions of high‐intensity interval training, while at the end of the 2 weeks, had also increased by 12% (Perry et al. 2010). Similar characterisation of the chronic time course of adaptation to resistance training is currently lacking. Nevertheless, it is interesting to consider the extent to which the molecular profiles generated in skeletal muscle may differ following acute bouts of concurrent exercise.
The work of Atherton and colleagues (Atherton et al. 2005) provided an elegant example of the specificity of the adaptation response to divergent contractile overload, albeit in rodent skeletal muscle, which is highly homogeneous with respect to fibre type and metabolic potential. High frequency intermittent (60 × 3 s, 100 Hz) or low frequency continuous (3 h, 10 Hz) electrical stimulation was used to mimic resistance‐ or endurance‐like overload. The results demonstrated that ‘resistance‐like’ overload exclusively promoted an ‘anabolic signature’ but little activation of the metabolic pathways involved in upregulating mitochondrial biogenesis and oxidative metabolism. In contrast, the endurance‐like stimuli resulted in a coordinated upregulation of metabolic pathways with putative roles in mitochondrial biogenesis, but little or no activation of the anabolic pathways. To explain the specificity of adaptive responses to the divergent contractile overload, the authors hypothesised the existence of an ‘AMPK–protein kinase B (PKB) switch’. However, since that study, there have been few data from human exercise studies to support the hypothesis of a simple ‘AMPK–PKB switch’ to explain specificity of training adaptation.
Following the work of Atherton and co‐workers (Atherton et al. 2005), and in an attempt to explain the molecular underpinning of the specificity of training, AMPK and mTOR became focal points for many exercise studies. There are a number of instances showing that acute bouts of resistance and endurance exercise generate similar post‐exercise signalling responses in skeletal muscle where endurance exercise upregulates mTOR‐mediated signalling (Mascher, 2007; Wilkinson et al. 2008; Benziane et al. 2008; Camera et al. 2010) and resistance‐based exercise increases AMPK phosphorylation (Dreyer et al. 2006; Koopman et al. 2006; Wilkinson et al. 2008). Furthermore, elevated AMPK activity does not supress mTOR mediated signalling when endurance exercise is undertaken prior to resistance exercise (Apro et al. 2015). It would be easy to dismiss the AMPK–PKB ‘switch’ hypothesis to explain the molecular bases of training specificity and simply conclude that there is an inability to extrapolate the findings of animal research in humans. On the other hand, it could be argued that the electrical stimulation model of contractile overload employed by Atherton and colleagues (Atherton et al. 2005) and the subsequent molecular response observed in rodent skeletal muscle are in fact more representative of adaptation responses to the extreme regimens of highly trained athletes than untrained individuals undertaking low/moderate exercise loads.
The transcriptional changes in skeletal muscle induced by resistance and endurance exercise also provide evidence of similar molecular responses. Yang and co‐workers (Yang et al. 2005) and Louis and colleagues (Louis et al. 2007) compared the acute time course of mRNA content of metabolic, myogenic and proteolytic mRNA targets after bouts of divergent exercise and show gene profiles that are remarkably comparable for endurance running and resistance exercise. However, most studies aimed at determining the adaptation response of single exercise modes do not typically quantify candidate gene expression of targets associated with the ‘alternate’ type of exercise: such lack of measures limit our current understanding of the specificity of training. It is also becoming evident that individual gene responses may not mirror chronic functional changes in the muscle (i.e. increased protein abundance) and the study of the phosphoproteome (Hoffman et al. 2015), transcriptome and functional gene clusters offer a more powerful analytical approach to discovering the unexplored complexity of acute exercise signalling. While several studies have determined the transcriptome response to resistance (Raue et al. 2012; Phillips et al. 2013; Thalacker‐Mercer et al. 2013; Nader et al. 2014) and endurance training (Timmons et al. 2005, 2010) in untrained individuals over prolonged (6–20 week) periods, a direct comparison between the transcriptome profiles of resistance‐ and endurance‐training‐induced gene expression has not been undertaken.
Despite the advancing knowledge of molecular changes with specific exercise modes, fundamental questions remain unanswered. Principal among them within the context of concurrent training is what is the time course for adaptation to a ‘trained’ state and if/when does specificity in adaptive responses occur in skeletal muscle (Fig. 3)? If modest gains in muscle size can be achieved after endurance training in untrained individuals (Konopka & Harber, 2014) (Fig. 4 A) but not trained cyclists (Rønnestad et al. 2010) (Fig. 4 B), and oxidative potential can be enhanced by resistance training in the muscle of untrained individuals (Tang et al. 2006) (Fig. 4 A) but decreases in powerlifters/bodybuilders (MacDougall et al. 1982) (Fig. 4 B), this has important implications for understanding molecular responses and training induced phenotype. It seems reasonable to suggest that when previously sedentary or recreationally active individuals commence any concurrent training programme, the response to the two exercise modes is additive and promotes a generic adaptation in the absence of a true specificity of training effect. Accordingly, we propose that the molecular bases for the ‘interference effect’ may be indistinguishable in such individuals, particularly if the total work in the single modes of training is not matched. Given the practical difficulties associated with accessing muscle biopsies from highly trained athletes, and differences in the modes and complexity of periodised training for different sports, unravelling the molecular bases for the interference effect will likely remain a scientific conundrum for some time.
Figure 4. Adaptation to training in skeletal muscle of untrained compared with trained individuals.
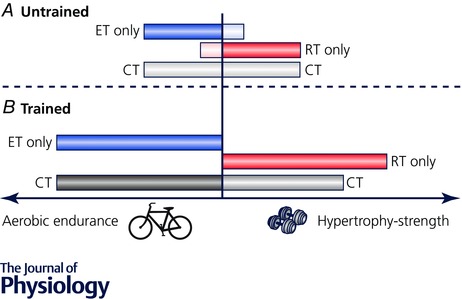
Schematic diagram showing, in A, the potential for endurance training (ET; light blue bar) to induce modest hypertrophy and resistance training (RT; pink bar) to promote oxidative capacity in the untrained state. The capacity for the different exercise modes to promote adaptive responses associated with the ‘opposing’ exercise also contributes to a lack of meaningful interference during concurrent training (CT; light gray) with short‐term training in untrained or recreationally active individuals. B, specificity of adaptation with prolonged, intense training in well‐trained athletes shows no significant ‘cross‐over’ effects between exercise modes. Resistance training does not impair continued development of oxidative metabolism and endurance capacity but endurance training compromises gains in hypertrophy and strength with concurrent training (black/gray).
Potential molecular candidates to explain the interference effect
The complexity of sensing and transducing a contractile stimulus and its subsequent conversion to a stable molecular response dictates almost countless potential sites of regulation of exercise‐induced adaptation. The AMPK signalling cascade has been considered a major pathway through which endurance training‐induced responses may impair muscle hypertrophy and strength with concurrent training. Studies in cell culture and animal models provide compelling evidence for the cross‐talk between AMPK and mTOR signalling pathways, and support the notion that activation of AMPK‐related pathways suppresses translation through decreased S6K and 4E‐BP phosphorylation (Bolster et al. 2002; Thomson et al. 2008; Lantier et al. 2010; Egawa et al. 2014). However, there is little evidence to support a direct AMPK‐induced impairment to rates of myofibrillar protein synthesis and resistance training‐induced muscle hypertrophy in humans. This may be, in part, related to the combination of limited muscle sampling time points and the temporal patterns of activation of the various signalling machinery. Complicating the issue is that the AMPKα1 isoform has recently been associated with promoting satellite cell activation and muscle regeneration (Fu et al. 2015), which contrasts the conventional role of AMPK as a ‘metabolic sensor’ in skeletal muscle. AMPK phosphorylation and activation has also been demonstrated following acute bouts of resistance exercise indicating that contraction‐induced AMPK activation is not solely restricted to an endurance‐like training stimulus (Dreyer et al. 2006; Koopman et al. 2006). For now it appears safe to conclude that exercise‐induced increases in AMPK‐signalling are not the sole moderators underlying the molecular bases of the interference effect observed with concurrent training.
PGC‐1α is an exercise‐responsive transcriptional co‐activator that has been described as a master regulator of oxidative metabolism and mitochondrial biogenesis, promoting many of the adaptations to endurance training in skeletal muscle (Hood, 2009). A single bout of endurance exercise induces a rapid and sustained increase in PGC‐1α gene and protein in skeletal muscle (Mathai et al. 2008), whereas muscle‐specific overexpression of PGC‐1α results in a large increase in functional mitochondria (Lin et al. 2002). The specific proteins generated through expression of different PGC‐1α isoforms display differential regulation and tissue distribution and, most importantly, exert specific biological functions (Martinez‐Redondo et al. 2015). PGC‐1α4, a transcript from the PGC‐1α gene, is abundantly expressed in skeletal muscle and appears to play a role in the adaptive response to exercise, particularly in the setting of resistance training (Ruas et al. 2012; White et al. 2014). This protein does not appear to regulate the same set of oxidative genes induced by PGC‐1α but, rather, activates the expression of insulin‐like growth factor 1 (IGF‐1) while concomitantly suppressing myostatin (an inhibitor of muscle cell differentiation and growth) pathways. Accordingly, the PGC‐1α protein and PGC‐1α4 isoform could play a role in modulating the training response‐adaptation after concurrent exercise. In this regard, Ruas and co‐workers (Ruas et al. 2012) have shown that after training consisting of either endurance exercise, resistance exercise, or a combination of both endurance and resistance exercise, increases in PGC‐1α4 are confined to resistance‐only and combined exercise training programmes, with no changes in this transcript after endurance‐only training (Ruas et al. 2012). Few human studies have been undertaken to examine the specificity of activation of the various PGC‐1α isoforms. However, in contrast to the early results from Ruas et al. (2012), others have failed to show a specificity of adaptation response in PGC‐1α isoforms with acute bouts of endurance compared to resistance exercise or indeed differences in expression with resistance versus concurrent exercise bouts albeit in untrained individuals (Ydfors et al. 2013; Lundberg et al. 2014). Of note, Nader et al. (Nader et al. 2014) showed PGC‐1α4 expression was not induced in the muscle of untrained subjects in response to an acute bout of resistance exercise undertaken at the beginning of a 12 week training programme, but was selectively upregulated by the same exercise session at the end of the intervention period. Taken collectively, these data provide further support that training status is a major regulator of the molecular response to exercise, and suggest that exercise‐induced PGC‐1α isoform specificity may provide clues to mechanisms underlying the molecular bases of the interference effect with concurrent training.
Satellite cells are niche stem cells located in skeletal muscle with the capacity to promote adaptation through the contribution of new myonuclei within existing muscle fibres and/or myocytes that can fuse and form new myofibres (Dumont et al. 2015). Accordingly, the role of muscle satellite cells has generally been associated with the hypertrophy response through regeneration and repair of myofibres, and subsequent muscle growth. Babcock and co‐workers (Babcock et al. 2012) have undertaken the only study to date to determine whether aerobic exercise might attenuate satellite cell responses important to hypertrophy within the concurrent training context. They found that a conventional resistance exercise bout transiently increases muscle fibre satellite cell density (∼38%) after 4 days recovery, but the addition of a 90 min cycling bout (∼60% maximal workload (W max)) undertaken immediately after resistance exercise completely suppressed this response. They proposed that the altered satellite cell response when aerobic exercise is undertaken following resistance exercise contributes to the interference effect with concurrent exercise (Babcock et al. 2012). It should be noted that resistance and aerobic exercise independently have the potential to induce satellite cell responses for skeletal muscle remodelling (Joanisse et al. 2013; Bellamy et al. 2014). Joanisse and colleagues (2013) have shown that aerobic interval training (6 weeks, 3 sessions per week) expands the muscle satellite cell pool and increases satellite cell activity without subsequent hypertrophy, indicating exercise‐induced satellite cell activation in skeletal muscle is not limited to resistance exercise. It may be that the satellite cell activity generating non‐hypertrophic adaptation with endurance‐based exercise compromises the resistance exercise‐induced satellite cell response for hypertrophy. The different exercise modes also appear to generate a fibre type specific satellite cell response which may also increase the complexity in delineating its role in the specificity of adaptation and any incompatibility with concurrent training (Babcock et al. 2012; Joanisse et al. 2013; Bellamy et al. 2014). Consequently, more work is needed to elucidate the satellite cell contribution to aerobic and resistance training adaptation, and the potential to modify the training response with concurrent training.
Advances in our understanding of how individual genotype may determine athletic potential may also influence the capacity to adapt to concurrent training. The α‐actinin‐3 (ACTN3) gene has emerged as a candidate that may influence exercise performance capacity. Early studies of elite endurance‐ and strength/power‐trained athletes provided data indicating that ACTN3 gene deficiency, which prevents its subsequent protein expression in type II muscle fibres, may be unfavourable for the development of muscular power but could promote an endurance‐like phenotype (Eynon et al. 2013, 2014). Eynon and co‐workers (Eynon et al. 2014) compared the ACTN3 genotype of team sport athletes with endurance and power athletes. They showed the ACTN3 genotype (577RR) associated with muscular power was under‐represented in team sport compared with power athletes, but that the distributions of the polymorphism that may promote endurance capacity (R577X) were similar between team sport and endurance athletes. These findings are supported by Massidda and colleagues (Massidda et al. 2015) who also showed that the ACTN3 genotype was not different between endurance and elite team sport athletes. To better understand the potential influence of genotype in determining exercise training adaptation, invasive studies with large subject cohorts that quantify the magnitude of the adaptive response associated with specific genotype following prolonged periods of single mode and concurrent training would be required. Nonetheless, as concurrent training is a prerequisite for performance in many team sports, the potential for using genetic screening to identify an individual's capability to adapt to divergent exercise modes is intriguing. The results of studies using candidate gene analysis have generated largely inconclusive findings and characterising genetic variants that may be responsible for specific phenotypes will continue to be a difficult proposition (Wang et al. 2013).
Although major breakthroughs in the knowledge of how exercise activates numerous cellular, molecular and biochemical pathways have been observed during the past decades, evidence linking such effects to specific performance outcomes has proved elusive and a challenge for future research. In the final analyses, when considering the multiplicity, complexity and redundancy of the many signalling cascades involved in endurance‐ and resistance exercise‐induced responses, it seems unlikely that a few select proteins could mediate such events and explain the interference effect. Indeed, we have previously suggested that changes at the transcriptional level may be more likely to elucidate the mechanistic underpinning of exercise‐specific adaptive profiles (Camera et al. 2010). While the various ‘omics’ technologies and the application of computational and systems biology approaches to problems in exercise biology should facilitate future progress, it is likely that multiple integrated, rather than isolated, effectors or processes are required to generate the interference effect.
Summary
While the study of concurrent training has received less attention than that of single mode training for endurance or strength/power, existing evidence supports the existence of an interference effect of endurance training on resistance training induced muscle hypertrophy and strength. Importantly, the specificity of the molecular training responses with divergent exercise modes and the time course over which these events occur provides the essential context in which concurrent training adaptation and performance outcomes should be evaluated. Recommendations to individuals to undertake divergent exercise modes on different days to avoid adaptation interference with concurrent training is oversimplistic and not representative of the ‘real world’ scenarios under which athletes train. The demands and management of professional athletes often restricts the ‘optimal’ timing for different exercise modes within a periodised programme. In this review we have limited discussion to the effects of endurance‐ on resistance‐based training adaptation and have not even begun to consider the effects of alternative modes of exercise (e.g. high‐intensity sprint training, plyometrics) that would undoubtedly add complexity to the training adaptation responses and the ensuing interference effect. The molecular profiles generated by endurance and resistance exercise are complex, an effect that is magnified when individuals undertake concurrent training. Are we guilty of overlooking the obvious or simply making the issue more complicated than it is? For example, it is possible that acute residual fatigue (from the previous exercise session) and/or chronic fatigue (due to undertaking a greater total work load to match adaptive responses of single mode training) are generating the interference effect. If so, such training would surely induce marked metabolic consequences and result in a unique ‘molecular footprint’ directly proportional to the energetic demands of training? If this were the case then such a ‘footprint’ should have the potential to inform the ‘how’ and ‘when’ of concurrent training in order to minimise interference and optimise adaptation. A decade ago we reviewed the molecular bases of training adaptation (Coffey & Hawley, 2007) and while the application of molecular techniques to exercise biology during the past decade has provided novel insight into the molecular pathways engaged in both acute and chronic responses to exercise, our progress in understanding how concurrent training creates an ‘interference effect’ at the molecular level has been less than spectacular. Consequently, the challenge to bridge the gap between ‘basic’ and ‘applied’ sciences remains and there should be no shortage of work for the exercise scientist who dares to mix their exercise modes!
Additional information
Competing interests
None declared.
Funding
Work being undertaken in the laboratory of V.G.C. is supported by the Collaborative Research Network for Advancing Exercise and Sports Science (201202) from the Department of Education and Training, Australia. Work being undertaken in the lab of J.A.H. is supported by the Collaborative Research Network for Advancing Exercise and Sports Science (2013000443).
Acknowledgements
The authors thank Dr Donny Camera for his helpful comments in preparation of the manuscript.
Biographies
Vernon Coffey is Associate Professor of Exercise and Sports Science at Bond University. He studies the specificity of training and effect of nutrition on the adaptation response in skeletal muscle, working to bridge the gap between basic sciences and applied exercise science.

John Hawley is Director of the Centre for Exercise and Nutrition in the Mary MacKillop Institute for Health Research at the Australian Catholic University, Melbourne, Australia. The focus of his research is training–nutrient interactions to optimise health and performance.
This review was presented at the symposium “Impact of training modalities on physiological function and adaptation”, which took place at the meeting of The Biomedical Basis of Elite Performance in Nottingham, UK, 6–8 March 2016.
References
- Apro W, Moberg M, Hamilton DL, Ekblom B, van Hall G, Holmberg HC & Blomstrand E (2015). Resistance exercise‐induced S6K1 kinase activity is not inhibited in human skeletal muscle despite prior activation of AMPK by high‐intensity interval cycling. Am J Physiol Endocrinol Metab 308, E470–E481. [DOI] [PubMed] [Google Scholar]
- Apro W, Wang L, Ponten M, Blomstrand E & Sahlin K (2013). Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 305, E22–E32. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ & Wackerhage H (2005). Selective activation of AMPK–PGC‐1α or PKB–TSC2–mTOR signaling can explain specific adaptive responses to endurance or resistance training‐like electrical muscle stimulation. FASEB J 19, 786–788. [DOI] [PubMed] [Google Scholar]
- Babcock L, Escano M, D'Lugos A, Todd K, Murach K & Luden N (2012). Concurrent aerobic exercise interferes with the satellite cell response to acute resistance exercise. Am J Physiol Regul Integr Comp Physiol 302, R1458–R1465. [DOI] [PubMed] [Google Scholar]
- Balabinis CP, Moukas M, Behrakis PK, Psarakis CH & Vassiliou MP (2003). Early phase changes by concurrent endurance and strength training. J Strength Cond Res 17, 393–401. [DOI] [PubMed] [Google Scholar]
- Bell GJ, Syrotuik DG, Martin TP, Burnham R & Quinney HA (2000). Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol 81, 418–427. [DOI] [PubMed] [Google Scholar]
- Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, Baker S & Parise G (2014). The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 9, e109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benziane B, Burton TJ, Scanlan B, Galuska D, Canny BJ, Chibalin AV, Zierath JR & Stepto NK (2008). Divergent cell signaling after short‐term intensified endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 295, E1427–E1438. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR & Jefferson LS (2002). AMP‐activated protein kinase suppresses protein synthesis in rat skeletal muscle through down‐regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277, 23977–23980. [DOI] [PubMed] [Google Scholar]
- Camera DM, Edge J, Short MJ, Hawley JA & Coffey VG (2010). Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42, 1843–1852. [DOI] [PubMed] [Google Scholar]
- Chtara M, Chaouachi A, Levin GT, Chaouachi M, Chamari K, Amri M & Laursen PB (2008). Effect of concurrent endurance and circuit resistance training sequence on muscular strength and power development. J Strength Cond Res 22, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Coffey VG & Hawley JA (2007). The molecular bases of training adaptation. Sports Med 37, 737–763. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Jemiolo B, Edge J, Garnham AP, Trappe SW & Hawley JA (2009. a). Effect of consecutive repeated sprint and resistance exercise bouts on acute adaptive responses in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297, R1441–R1451. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Pilegaard H, Garnham AP, O'Brien BJ & Hawley JA (2009. b). Consecutive bouts of diverse contractile activity alter acute responses in human skeletal muscle. J Appl Physiol (1985) 106, 1187–1197. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR & Hawley JA (2006). Early signaling responses to divergent exercise stimuli in skeletal muscle from well‐trained humans. FASEB J 20, 190–192. [DOI] [PubMed] [Google Scholar]
- Damas F, Phillips S, Vechin FC & Ugrinowitsch C (2015). A review of resistance training‐induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med 45, 801–807. [DOI] [PubMed] [Google Scholar]
- Donges CE, Duffield R, Guelfi KJ, Smith GC, Adams DR & Edge JA (2013). Comparative effects of single‐mode vs. duration‐matched concurrent exercise training on body composition, low‐grade inflammation, and glucose regulation in sedentary, overweight, middle‐aged men. Appl Physiol Nutr Metab 38, 779–788. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E & Rasmussen BB (2006). Resistance exercise increases AMPK activity and reduces 4E‐BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA & Djamil R (1985). Incompatibility of endurance‐ and strength‐training modes of exercise. J Appl Physiol (1985) 59, 1446–1451. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Wang YX & Rudnicki MA (2015). Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142, 1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Hawley JA & Zierath JR (2016). Snapshot: Exercise metabolism. Cell Metab 24, 342–342.e1. [DOI] [PubMed] [Google Scholar]
- Egan B & Zierath JR (2013). Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17, 162–184. [DOI] [PubMed] [Google Scholar]
- Egawa T, Ohno Y, Goto A, Ikuta A, Suzuki M, Ohira T, Yokoyama S, Sugiura T, Ohira Y, Yoshioka T & Goto K (2014). AICAR‐induced activation of AMPK negatively regulates myotube hypertrophy through the HSP72‐mediated pathway in C2C12 skeletal muscle cells. Am J Physiol Endocrinol Metab 306, E344–E354. [DOI] [PubMed] [Google Scholar]
- Eynon N, Banting LK, Ruiz JR, Cieszczyk P, Dyatlov DA, Maciejewska‐Karlowska A, Sawczuk M, Pushkarev VP, Kulikov LM, Pushkarev ED, Femia P, Stepto NK, Bishop DJ & Lucia A (2014). ACTN3 R577X polymorphism and team‐sport performance: a study involving three European cohorts. J Sci Med Sport 17, 102–106. [DOI] [PubMed] [Google Scholar]
- Eynon N, Hanson ED, Lucia A, Houweling PJ, Garton F, North KN & Bishop DJ (2013). Genes for elite power and sprint performance: ACTN3 leads the way. Sports Med 43, 803–817. [DOI] [PubMed] [Google Scholar]
- Fu X, Zhu MJ, Dodson MV & Du M (2015). AMP‐activated protein kinase stimulates Warburg‐like glycolysis and activation of satellite cells during muscle regeneration. J Biol Chem 290, 26445–26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe JJ, Bishop DJ & Stepto NK (2014). Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med 44, 743–762. [DOI] [PubMed] [Google Scholar]
- Gurd BJ, Giles MD, Bonafiglia JT, Raleigh JP, Boyd JC, Ma JK, Zelt JG & Scribbans TD (2016). Incidence of nonresponse and individual patterns of response following sprint interval training. Appl Physiol Nutr Metab 41, 229–234. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Alen M, Kraemer WJ, Gorostiaga E, Izquierdo M, Rusko H, Mikkola J, Häkkinen A, Valkeinen H, Kaarakainen E, Romu S, Erola V, Ahtiainen J & Paavolainen L (2003). Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol 89, 42–52. [DOI] [PubMed] [Google Scholar]
- Hawley JA (2002). Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol 29, 218–222. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ & Zierath JR (2014). Integrative biology of exercise. Cell 159, 738–749. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Maughan RJ & Hargreaves M (2015). Exercise metabolism: Historical perspective. Cell Metab 22, 12–17. [DOI] [PubMed] [Google Scholar]
- Hendrickson NR, Sharp MA, Alemany JA, Walker LA, Harman EA, Spiering BA, Hatfield DL, Yamamoto LM, Maresh CM, Kraemer WJ & Nindl BC (2010). Combined resistance and endurance training improves physical capacity and performance on tactical occupational tasks. Eur J Appl Physiol 109, 1197–1208. [DOI] [PubMed] [Google Scholar]
- Hennessy LC & Watson AWS (1994). The interference effects of training for strength and endurance simultaneously. J Strength Cond Res 8, 12–19. [Google Scholar]
- Hickson RC (1980). Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol 45, 255–263. [DOI] [PubMed] [Google Scholar]
- Hickson RC, Dvorak BA, Gorostiaga EM, Kurowski TT & Foster C (1988). Potential for strength and endurance training to amplify endurance performance. J Appl Physiol (1985) 65, 2285–2290. [DOI] [PubMed] [Google Scholar]
- Hoffman NJ, Parker BL, Chaudhuri R, Fisher‐Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stockli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JF, Richter EA & James DE (2015). Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise‐regulated kinases and AMPK substrates. Cell Metab 22, 922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO & Coyle EF (1984). Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56, 831–838. [DOI] [PubMed] [Google Scholar]
- Hood DA (2009). Mechanisms of exercise‐induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab 34, 465–472. [DOI] [PubMed] [Google Scholar]
- Hunter G, Demment R & Miller D (1987). Development of strength and maximum oxygen uptake during simultaneous training for strength and endurance. J Sports Med Phys Fitness 27, 269–275. [PubMed] [Google Scholar]
- Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky MA, Gibala MJ & Parise G (2013). Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J 27, 4596–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TW, Howatson G, Russell M & French DN (2013). Performance and neuromuscular adaptations following differing ratios of concurrent strength and endurance training. J Strength Cond Res 27, 3342–3351. [DOI] [PubMed] [Google Scholar]
- Jones TW, Walshe IH, Hamilton DL, Howatson G, Russell M, Price OJ, St Clair Gibson A & French DN (2016). Signalling responses following varying sequencing of strength and endurance training in a fed state. Int J Sports Physiol Perform 11, 868–875. [DOI] [PubMed] [Google Scholar]
- Karavirta L, Hakkinen K, Kauhanen A, Arija‐Blazquez A, Sillanpaa E, Rinkinen N & Hakkinen A (2011). Individual responses to combined endurance and strength training in older adults. Med Sci Sports Exerc 43, 484–490. [DOI] [PubMed] [Google Scholar]
- Kazior Z, Willis SJ, Moberg M, Apro W, Calbet JA, Holmberg HC & Blomstrand E (2016). Endurance exercise enhances the effect of strength training on muscle fiber size and protein expression of Akt and mTOR. PLoS One 11, e0149082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR & Harber MP (2014). Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev 42, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R, Zorenc AHG, Gransier RJJ, Cameron‐Smith D & van Loon LJC (2006). Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab 290, E1245–E1252. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Patton JF, Gordon SE, Harman EA, Deschenes MR, Reynolds K, Newton RU, Triplett NT & Dziados JE (1995). Compatibility of high‐intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol (1985) 78, 976–989. [DOI] [PubMed] [Google Scholar]
- Lantier L, Mounier R, Leclerc J, Pende M, Foretz M & Viollet B (2010). Coordinated maintenance of muscle cell size control by AMP‐activated protein kinase. FASEB J 24, 3555–3561. [DOI] [PubMed] [Google Scholar]
- Leveritt MD, Abernethy PJ, Barry BK & Logan PA (1999). Concurrent strength and endurance training: A review. Sports Med 28, 413–427. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel‐Duby R & Spiegelman BM (2002). Transcriptional co‐activator PGC‐1α drives the formation of slow‐twitch muscle fibres. Nature 418, 797–801. [DOI] [PubMed] [Google Scholar]
- Lira VA, Benton CR, Yan Z & Bonen A (2010). PGC‐1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 299, E145–E161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E, Raue U, Yang Y, Jemiolo B & Trappe S (2007). Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103, 1744–1751. [DOI] [PubMed] [Google Scholar]
- Lundberg TR, Fernandez‐Gonzalo R, Gustafsson T & Tesch PA (2013). Aerobic exercise does not compromise muscle hypertrophy response to short‐term resistance training. J Appl Physiol (1985) 114, 81–89. [DOI] [PubMed] [Google Scholar]
- Lundberg TR, Fernandez‐Gonzalo R, Norrbom J, Fischer H, Tesch PA & Gustafsson T (2014. a). Truncated splice variant PGC‐1α4 is not associated with exercise‐induced human muscle hypertrophy. Acta Physiol (Oxf) 212, 142–151. [DOI] [PubMed] [Google Scholar]
- Lundberg TR, Fernandez‐Gonzalo R & Tesch PA (2014. b). Exercise‐induced AMPK activation does not interfere with muscle hypertrophy in response to resistance training in men. J Appl Physiol (1985) 116, 611–620. [DOI] [PubMed] [Google Scholar]
- McCarthy JP, Agre JC, Graf BK, Pozniak MA & Vailas AC (1995). Compatibility of adaptive responses with combining strength and endurance training. Med Sci Sports Exerc 27, 429–436. [PubMed] [Google Scholar]
- McCarthy JP, Pozniak MA & Agre JC (2002). Neuromuscular adaptations to concurrent strength and endurance training. Med Sci Sports Exerc 34, 511–519. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Sale DG, Elder GC & Sutton JR (1982). Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur J Appl Physiol Occup Physiol 48, 117–126. [DOI] [PubMed] [Google Scholar]
- Martinez‐Redondo V, Pettersson AT & Ruas JL (2015). The hitchhiker's guide to PGC‐1α isoform structure and biological functions. Diabetologia 58, 1969–1977. [DOI] [PubMed] [Google Scholar]
- Mascher H, Andersson H, Nilsson P‐A, Ekblom B, Blomstrand E (2007). Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol (Oxf) 191, 67–75. [DOI] [PubMed] [Google Scholar]
- Massidda M, Bachis V, Corrias L, Piras F, Scorcu M, Culigioni C, Masala D & Calo C (2015). ACTN3 R577X polymorphism is not associated with team sport athletic status in Italians. Sports Medicine‐Open 1 DOI: 10.1186/s40798-015-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai AS, Bonen A, Benton CR, Robinson DL & Graham TE (2008). Rapid exercise‐induced changes in PGC‐1α mRNA and protein in human skeletal muscle. J Appl Physiol (1985) 105, 1098–1105. [DOI] [PubMed] [Google Scholar]
- Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE & Gordon PM (2014). Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (1985) 116, 693–702. [DOI] [PubMed] [Google Scholar]
- Olesen J, Kiilerich K & Pilegaard H (2010). PGC‐1α‐mediated adaptations in skeletal muscle. Pflugers Arch 460, 153–162. [DOI] [PubMed] [Google Scholar]
- Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A & Spriet LL (2010). Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588, 4795–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA & Atherton PJ (2013). Molecular networks of human muscle adaptation to exercise and age. PLoS Genet 9, e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp A, Hamilton DL & Baar K (2011). Signals mediating skeletal muscle remodeling by resistance exercise: PI3‐kinase independent activation of mTORC1. J Appl Physiol (1985) 110, 561–568. [DOI] [PubMed] [Google Scholar]
- Putman C, Xu X, Gillies E, MacLean I & Bell G (2004). Effects of strength, endurance and combined training on myosin heavy chain content and fibre‐type distribution in humans. Eur J Appl Physiol 92, 376–384. [DOI] [PubMed] [Google Scholar]
- Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC & Trappe S (2012). Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 112, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnestad BR, Hansen EA & Raastad T (2010). Effect of heavy strength training on thigh muscle cross‐sectional area, performance determinants, and performance in well‐trained cyclists. Eur J Appl Physiol 108, 965–975. [DOI] [PubMed] [Google Scholar]
- Rønnestad BR, Hansen EA & Raastad T (2012). High volume of endurance training impairs adaptations to 12 weeks of strength training in well‐trained endurance athletes. Eur J Appl Physiol 112, 1457–1466. [DOI] [PubMed] [Google Scholar]
- Rønnestad BR & Mujika I (2014). Optimizing strength training for running and cycling endurance performance: A review. Scand J Med Sci Sports 24, 603–612. [DOI] [PubMed] [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA & Spiegelman BM (2012). A PGC‐1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151, 1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JE, Hartman JW & Phillips SM (2006). Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl Physiol Nutr Metab 31, 495–501. [DOI] [PubMed] [Google Scholar]
- Thalacker‐Mercer A, Stec M, Cui X, Cross J, Windham S & Bamman M (2013). Cluster analysis reveals differential transcript profiles associated with resistance training‐induced human skeletal muscle hypertrophy. Physiol Genomics 45, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DM, Fick CA & Gordon SE (2008). AMPK activation attenuates S6K1, 4E‐BP1, and eEF2 signaling responses to high‐frequency electrically stimulated skeletal muscle contractions. J Appl Physiol (1985) 104, 625–632. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, Rachman J & Sundberg CJ (2005). Modulation of extracellular matrix genes reflects the magnitude of physiological adaptation to aerobic exercise training in humans. BMC Biol 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NB, Nielsen S, Akerstrom T, MacDougald OA, Jansson E, Greenhaff PL, Tarnopolsky MA, van Loon LJ, Pedersen BK, Sundberg CJ, Wahlestedt C, Britton SL & Bouchard C (2010). Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol (1985) 108, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Padmanabhan S, Wolfarth B, Fuku N, Lucia A, Ahmetov II, Cieszczyk P, Collins M, Eynon N, Klissouras V, Williams A & Pitsiladis Y (2013). Genomics of elite sporting performance: what little we know and necessary advances. Adv Genet 84, 123–149. [DOI] [PubMed] [Google Scholar]
- White JP, Wrann CD, Rao RR, Nair SK, Jedrychowski MP, You JS, Martinez‐Redondo V, Gygi SP, Ruas JL, Hornberger TA, Wu Z, Glass DJ, Piao X & Spiegelman BM (2014). G protein‐coupled receptor 56 regulates mechanical overload‐induced muscle hypertrophy. Proc Natl Acad Sci USA 111, 15756–15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA & Rennie MJ (2008). Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586, 3701–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Marin PJ, Rhea MR, Wilson SM, Loenneke JP & Anderson JC (2012). Concurrent training: a meta‐analysis examining interference of aerobic and resistance exercises. J Strength Cond Res 26, 2293–2307. [DOI] [PubMed] [Google Scholar]
- Yang Y, Creer A, Jemiolo B & Trappe S (2005). Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98, 1745–1752. [DOI] [PubMed] [Google Scholar]
- Ydfors M, Fischer H, Mascher H, Blomstrand E, Norrbom J & Gustafsson T (2013). The truncated splice variants, NT‐PGC‐1α and PGC‐1α4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep 1, e00140. [DOI] [PMC free article] [PubMed] [Google Scholar]


