Abstract
Key points
In human skeletal muscles, the current view is that the capacity for mitochondrial energy production, and thus endurance capacity, is set by the mitochondria volume.
However, increasing the mitochondrial inner membrane surface comprises an alternative mechanism for increasing the energy production capacity.
In the present study, we show that mitochondrial inner membranes in leg muscles of endurance‐trained athletes have an increased ratio of surface per mitochondrial volume.
We show a positive correlation between this ratio and whole body oxygen uptake and muscle fibre mitochondrial content.
The results obtained in the present study help us to understand modulation of mitochondrial function, as well as how mitochondria can increase their oxidative capacity with increased demand.
Abstract
Mitochondrial energy production involves the movement of protons down a large electrochemical gradient via ATP synthase located on the folded inner membrane, known as cristae. In mammalian skeletal muscle, the density of cristae in mitochondria is assumed to be constant. However, recent experimental studies have shown that respiration per mitochondria varies. Modelling studies have hypothesized that this variation in respiration per mitochondria depends on plasticity in cristae density, although current evidence for such a mechanism is lacking. In the present study, we confirm this hypothesis by showing that, in human skeletal muscle, and in contrast to the current view, the mitochondrial cristae density is not constant but, instead, exhibits plasticity with long‐term endurance training. Furthermore, we show that frequently recruited mitochondria‐enriched fibres have significantly increased cristae density and that, at the whole‐body level, muscle mitochondrial cristae density is a better predictor of maximal oxygen uptake rate than muscle mitochondrial volume. Our findings establish an elevating mitochondrial cristae density as a regulatory mechanism for increasing metabolic power in human skeletal muscle. We propose that this mechanism allows evasion of the trade‐off between cell occupancy by mitochondria and other cellular constituents, as well as improved metabolic capacity and fuel catabolism during prolonged elevated energy requirements.
Keywords: electron microscopy, mitochondria, muscle metabolism, oxygen uptake, skeletal muscle
Key points
In human skeletal muscles, the current view is that the capacity for mitochondrial energy production, and thus endurance capacity, is set by the mitochondria volume.
However, increasing the mitochondrial inner membrane surface comprises an alternative mechanism for increasing the energy production capacity.
In the present study, we show that mitochondrial inner membranes in leg muscles of endurance‐trained athletes have an increased ratio of surface per mitochondrial volume.
We show a positive correlation between this ratio and whole body oxygen uptake and muscle fibre mitochondrial content.
The results obtained in the present study help us to understand modulation of mitochondrial function, as well as how mitochondria can increase their oxidative capacity with increased demand.
Abbreviations
- CI
confidence interval
maximal oxygen uptake rate
Introduction
Subsequent to the seminal work by Palladin and colleagues on training‐induced biochemical adaptations in skeletal muscle (Palladin, 1945), followed by that of Holloszy and colleagues on respiration by isolated mitochondria and content of mitochondrial proteins (Holloszy et al. 1967), it has been acknowledged that endurance training enhances skeletal muscle oxygen uptake capacity and induces adaptations at the mitochondrial level (Hoppeler, 1986; Boushel et al. 2014a). The mitochondrion is structurally defined by two membranes: the outer membrane and the inner membrane. ATP production in mitochondria is linked to a movement of protons down an electrochemical gradient through ATP synthase located at the inner membrane (Rich, 2003). The shape and topology of the inner membrane can be visualized in detail by electron microscopy, which reveals a pattern of folds on the inner membrane known as cristae (Mannella, 2006). Cristae morphology depends on the mitochondrial contact site complex (John et al. 2005; Harner et al. 2014) and mathematical modelling suggests that the cristae optimize ATP production per mitochondria by minimizing the distance between adenine nucleotide translocation and transformation sites (Demongeot et al. 2007).
In mammalian skeletal muscle, the density of cristae in mitochondria is assumed to be constant at a level of ∼30 μm2 μm−3, implying that the only mechanism for increasing energy production by mitochondria is an increase in the mitochondrial volumetric content of the muscle cell (Hoppeler & Lindstedt, 1985; Hoppeler, 1986; Lindstedt et al. 1988; Weibel et al. 1991; Weibel & Hoppeler, 2005; Larsen et al. 2012) at the cost of contractile filaments and sarcoplasmic reticulum. However, this assumption is based on only a few studies with small sample sizes that investigated the variability and plasticity of mitochondrial cristae density in mammals (Hoppeler et al. 1973; Hoppeler et al. 1981; Hoppeler et al. 1987; Larsen et al. 2012). In cross‐sectional studies, the mitochondrial cristae density in well‐trained ( 71 ml min−1 kg−1; n = 5), moderately trained ( 61 ml min−1 kg−1; n = 9) and untrained ( 44 ml min−1 kg−1; n = 3) individuals did not differ (Hoppeler et al. 1973), nor was there any correlation with among 13 individuals with values ranging from 30 to 72 ml min−1 kg−1 (although only one subject had a value > 60 ml min−1 kg−1) (Larsen et al. 2012). Furthermore, short‐term training studies do not result in any demonstrable changes in cristae density or mitochondrial composition. For example, chronic stimulation of cat muscles for 28 days did not alter the cristae density (Schwerzmann et al. 1989), nor did 10 weeks of endurance training change the biochemical composition of mitochondria in rats (Davies et al. 1981). By contrast, recent studies show that endurance‐trained athletes have a higher respiration rate per mitochondria (Jacobs & Lundby, 2013) and that endurance training results in higher respiration rates without changes in mitochondrial content (Pesta et al. 2011; Boushel et al. 2014b). However, an understanding of the mechanism responsible for this is lacking.
In skeletal muscle fibres, volumetric constraints dictate a trade‐off between the capacity for mechanical power (work time−1), as produced by contractile filaments and the sarcoplasmic reticulum, and oxidative metabolic power (O2 consumption time−1), as produced by mitochondria. In mammals, the trade‐off is highly predictable in terms of the relative volumetric composition of the fibre for these three main structures (Schaeffer & Lindstedt, 2013). Thus, in muscles with high demands of mechanical and oxidative metabolic power, as in endurance‐trained muscles, qualitative changes in these structures could be important adaptations.
We hypothesize that the mitochondrial cristae density is plastic as a result of endurance‐training and constitutes a mechanism for evading the trade‐off between cell occupancy by mitochondria and other cellular constituents.
To test this hypothesis, we compiled and analysed a large database of transmission electron microscopy images, including those of sedentary, recreationally active and elite endurance‐trained human individuals. Additionally, we tested our hypothesis using a comparative approach and in a short‐term training study.
Methods
Ethical approval
The human muscle biopsy material included in the present study originates from previous studies (Table 1) with ethical approval. The projects were approved by specific boards. Sedentary individuals: The Local Ethics Committee of Funen and Vejle County, Denmark; Recreationally active individuals: The local Ethics Committee in Copenhagen, Denmark (KF01‐322606); Soccer players: The Regional Ethical Review Board in Copenhagen, Denmark; and Cross‐country skiers: the Regional Ethical Review Board in Umeå, Sweden (no. 07–076 M). Before providing their written informed consent to participate, all of the subjects were fully informed about the project, the risks involved and the discomfort associated with the experiment, and were made aware that they could withdraw from the project at any time. The experiments conformed with the standards set by the Declaration of Helsinki. All procedures using zebra finches were carried out in accordance with the requirements of the Danish Animal Experiments Inspectorate (Copenhagen, Denmark).
Table 1.
Subjects characteristics (mean ± SD)
| n | Age (years) | Weight (kg) | BMI (kg m−2) | (ml O2 kg−1 min−1) | Reference | |
|---|---|---|---|---|---|---|
| Sedentary obese type 2 diabetics | 12 | 53 ± 5* | 108 ± 7* | 33 ± 4* | 27 ± 3* | Nielsen et al. (2010a) |
| Sedentary obese | 12 | 53 ± 6* | 110 ± 13* | 33 ± 3* | 28 ± 6* | Nielsen et al. (2010a) |
| Recreationally active | 5 | 23 ± 2 | 75 ± 8 | 22 ± 2 | NA | Nielsen et al. (2010b) |
| Soccer players | 5 | 27 ± 3 | 79 ± 7 | 25 ± 1 | 53 ± 4 | Nielsen et al. (2012) |
| Cross‐country skiers | 10 | 22 ± 1 | 80 ± 9 | 24 ± 2 | 68 ± 5 | Ørtenblad et al. (2011) |
BMI, body mass index; NA, not available. All subjects are males. *Significantly different from the other groups.
Subjects and animals
We obtained a needle biopsy of musculus vastus lateralis on one to four different occasions from several groups of human individuals (Table 1). In addition, we used the superfast vocal muscle (left musculus tracheobronchialis dorsalis) from three male zebra finches dissected immediately after death (Elemans et al. 2008).
Maximal oxygen uptake
Individual's maximal oxygen uptake rate () was determined by indirect calorimetry employing an ergo‐spirometry system (mixing chamber online system, Oxycon Pro; Jaeger, Hoechberg, Germany; or AMIS 2001 model C; Innovision A/S, Odense, Denmark). The gas analysers were calibrated with a high‐precision two‐component gas mixture of 16.0% O2 and 4.0% CO2 and the calibration of the flowmeter was performed at low, medium and high flow rates with a 3 litre air syringe (Hans Rudolph, Kansas City, MO, USA). The sedentary obese type 2 diabetics, the sedentary obese and the soccer players performed their test using a stationary bicycle (Monark model 824E; Monark, Vansbro, Sweden) and the cross‐country skiers used roller skis on a motor‐driven treadmill (RL 3000; Rodby Innivation AB, Vänge, Sweden).
Transmission electron microscopy
Muscle specimens were fixed with a 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.3) for 24 h and subsequently rinsed four times in 0.1 m sodium cacodylate buffer. Following rinsing, fibres were post‐fixed with 1% osmium tetroxide (OsO4) and 1.5% potassium ferrocyanide (K4Fe(CN)6) in 0.1 m sodium cacodylate buffer for 90 min at 4 °C. After post‐fixation, the fibres were rinsed twice in 0.1 m sodium cacodylate buffer at 4 °C, dehydrated through a graded series of alcohol at 4–20 °C, infiltrated with graded mixtures of propylene oxide and Epon at 20 °C, and embedded in 100% Epon at 30 °C. Ultra‐thin (60 nm) sections were cut (using a Ultracut UCT ultramicrotome; Leica Microsystems, Wetzlar, Germany) in three depths (separated by 150 μm) and contrasted with uranyl acetate and lead citrate. Sections were examined and photographed in a pre‐calibrated EM 208 transmission electron microscope (Philips, Eindoven, The Netherlands) and a Megaview III FW camera (Olympus Soft Imaging Solutions, Münster, Germany). All the longitudinally oriented fibres (n = 4–14) were photographed at 40 000× magnification in a randomized systematic order, including 12 images from the subsarcolemmal region and 12 images from the myofibrillar region.
Stereological method
Two blinded investigators examined, in a randomized order, 977 fibres from 49 human individuals, and estimated the mitochondria cristae surface density per mitochondria volume using standard stereological methods. Only mitochondria profiles (Fig. 1 A) of acceptable quality defined as clear visibility and no or few missing spots of the inner membrane were included, resulting in a total of 3925 mitochondrial profiles being analysed. In 32 subjects, more than 19 mitochondria profiles were analysed, and the variation between these profiles indicates that at least eight mitochondria profiles are needed to obtain a satisfactorily high precision of the cristae density (Fig. 1 B). Thereafter, only subjects with a minimum of eight mitochondrial profiles analysed mitochondrial profiles) were included in the present study.
Figure 1. A minimum of eight mitochondrial profiles per subject was required to obtain a satisfactorily high precision of the mitochondrial cristae density estimate.
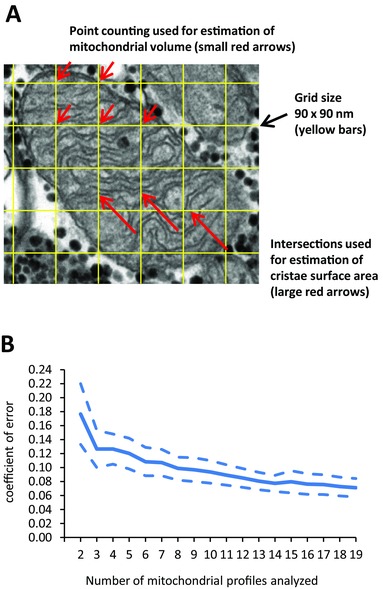
A, transmission electron microscopy image of one representative mitochondria profile used to estimate cristae density. A grid size of 90 × 90 nm was used to estimate mitochondrial volume by point counting (points hitting the mitochondrion) and cristae surface area by intersections of cristae membrane with the yellow bars. B, mean (continuous line) and 95% CI (dashed lines) of coefficient of error after analysis of eight profiles was below 10% (n = 32 subjects).
The mitochondrial cristae surface area per mitochondrial volume (mitochondrial cristae density) (S V) was estimated by the formula: S V = (4/π)B A, where B A is the boundary length density estimated by counting intersections on test lines (I L) multiplied by π/2. The mitochondrial volume density (V v) was estimated by the formula: V V = A A, where A A is the mitochondrial area fraction estimated by point counting. Grid sizes of 60 and 270 nm were used to estimate mitochondria volume and cristae surface area, respectively. The general principle of estimating S v and V V by stereological methods can be found elsewhere (Weibel, 1980). The images were analysed by two blinded investigators, who contributed equally to all the different groups of subjects. An assessment of inter‐investigator reproducibility indicated a coefficient of variation (CV) of 6.3% in the estimate of cristae density based on eight mitochondrial profiles and a bias of 13%. The raw data was adjusted for this bias. The median number of mitochondrial profiles analysed per subject was 27 (interquartile range 15–51).
Fibre typing
Fibre type differences were evaluated by classifying fibres as type 1 or 2, based on a combination of intermyofibrillar mitochondrial volume and Z‐line width (Sjöström et al. 1982). Intermyofibrillar mitochondrial volume fraction was plotted against Z‐line width for all the fibres (n = 6–12) obtained from each biopsy. The fibres with the highest intermyofibrillar mitochondrial volume fraction and thickest Z‐line width were classified as type 1 fibres and vice versa for type 2 fibres. To identify the two main fibre types, all intermediate fibres were discarded and only distinct type 1 and 2 fibres were included, respectively (n = 2–3 fibres of each type per biopsy). Because of the criterion of a minimum of eight mitochondrial profiles per estimate, the sample size decreased when we discriminated between fibre types. We therefore grouped the sedentary and recreationally active individuals as ‘non‐athletes’ and the soccer players and cross‐country skiers as ‘athletes’.
Statistical analysis
Statistical analyses were performed using Stata, version 13.1 (StataCorp LP, College Station, TX, USA). In Table 1 and Fig. 2, differences between groups were tested by the non‐parametric Kruskal–Wallis rank test. In Fig. 2, associations between variables were evaluated using Pearson's correlation coefficient. In Fig. 3, interactions and main effects were tested by a mixed effect model with training and group as fixed effects and subject as random effect. In Fig. 4 A, interactions and main effects were tested by a mixed effect model with fibre type and group as fixed effects and subject as random effect. Regarding the mixed effect models, the assumption of normal distribution was tested by the Shapiro–Wilk W test and the assumption of heteroscedasticity was tested by the Cook–Weisberg test. Values are presented as the mean ± SD, unless stated otherwise. Significance level was set at α = 0.05.
Figure 2. Mitochondrial cristae density is elevated in endurance‐trained athletes.
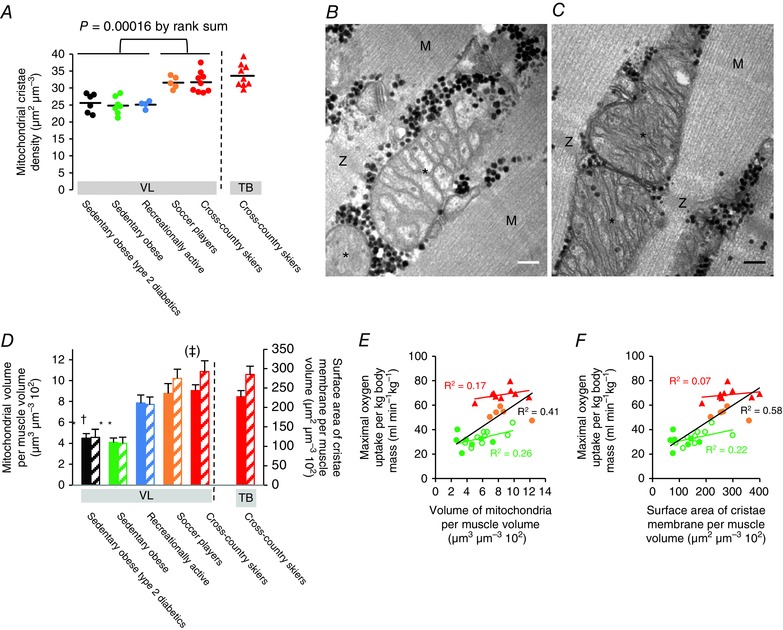
A, cross‐country skiers and soccer players have a higher mitochondrial cristae density than recreationally active and sedentary obese individuals (sedentary obese type 2 diabetics, n = 6; sedentary obese, n = 7; recreationally active, n = 4; soccer players, n = 5; and cross‐country skiers, n = 9). Horizontal lines represent the mean of all individuals in each population. VL, musculus vastus lateralis. TB, musculus triceps brachii. B and C, transmission electron micrographs representing mitochondrial profiles with a low and high cristae density from an obese sedentary man (B) and a cross‐country skier (C), respectively. Asterisks indicate the mitochondrial profile. M, M‐band of the adjacent sarcomeres. Z, Z‐disk of the adjacent sarcomeres. Black dots are glycogen particles. Scale bar = 100 nm. D, endurance‐trained athletes have a higher mean mitochondrial volume per fibre volume (filled bars, left axis) and mean surface area of cristae membrane per volume of fibre (hatched bars, right axis) than sedentary obese individuals. *Different from the other groups (P < 0.05); †Different from soccer players and cross‐country skiers (P < 0.05); (‡)Different from recreationally active (P = 0.07). Values are the mean and horizontal bars represent the SD. E and F, surface area of cristae membrane per fibre volume is a better predictor of maximal oxygen uptake than mitochondrial volume per fibre volume. Colour coding as in (A). Open green symbols represent post 10 weeks of aerobic training (n = 9). Lines represent best fit (E, r 2 = 0.17, P = 0.28; r 2 = 0.26, P = 0.04; r 2 = 0.41, P < 0.001; F, r 2 = 0.07, P = 0.49; r 2 = 0.22, P = 0.07; r 2 = 0.58, P < 0.001).
Figure 3. Short‐term training does not affect mitochondrial cristae density.
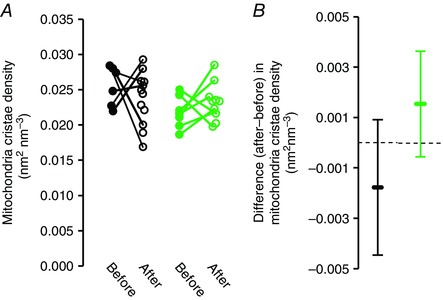
A, 10 weeks of aerobic training did not significantly increase mitochondrial cristae density in sedentary obese individuals with type 2 diabetes (black circles, P = 0.28) or in sedentary obese individuals without type 2 diabetes (green circles, P = 0.25). Furthermore, there was no significant interaction between training and group of sedentary obese individuals (P = 0.11). B, 95% CI of the differences between before and after training indicates that the training was not sufficient to increase cristae density by more than 15% and that detection of a mean 9% higher cristae density after training (as found in the sedentary obese individuals) would require a sample size of 31 individuals. Bars represent 95% CI.
Figure 4. Type 1 fibres have a higher mitochondrial cristae density than type 2 fibres in endurance‐trained athletes.
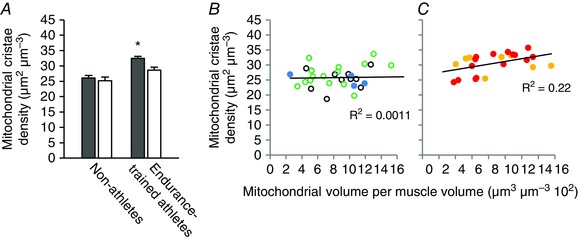
A, mitochondria cristae density in type 1 fibres (grey bars) and type 2 fibres (white bars) of non‐athletes (n = 20 and 10 subjects for type 1 and 2 fibres, respectively) and endurance‐trained athletes (n = 12 and 11 subjects for type 1 and 2 fibres, respectively). Values are the mean and horizontal bars represent SD. There was a significant interaction between fibre types and group of individuals (P = 0.024). *Significantly different from type 2 fibres (P < 0.001). B and C, mitochondrial cristae density plotted against mitochondrial volume fraction of non‐athletes (b) and endurance‐trained athletes (C). Colour coding: open black circles, sedentary obese type 2 diabetic patients post 10 weeks of training; open green circles, sedentary obese post 10 weeks of training; blue circles, recreationally active; yellow circles, soccer players; red circles, cross‐country skiers. Lines represent linear fit (B, r 2 = 0.0011, P = 0.86; C, r 2 = 0.22, P = 0.024).
Results
We found that the endurance‐trained athletes (cross‐country skiers and soccer players) have, on average, a 23% (17:29, 95% confidence interval; CI) higher mean mitochondrial cristae density (31.7 ± 3.1 and 31.6 ± 1.8 μm2 μm−3, respectively) in the leg muscle musculus vastus lateralis compared to both recreationally active men of the same age (25.1 ± 1.1 μm2 μm−3) and sedentary obese men with or without type 2 diabetes (25.6 ± 2.8 and 24.8 ± 2.6 μm2 μm−3, respectively) (P = 0.00016) (Fig. 2 A–C).
In cross‐country skiers, we also found a high mitochondrial cristae density in the arm muscle musculus triceps brachii (33.6 ± 3.3 μm2 μm−3) (Fig. 2 A), which is highly recruited during training and competition (Holmberg et al. 2005; Ørtenblad et al. 2011). Interestingly, the range of observations, from the lowest value of 21.2 μm2 μm−3 (found in a sedentary obese individual) to the highest value of 39.3 μm2 μm−3 (found in the musculus triceps brachii of a cross‐country skier), reveals high variability between human individuals.
In addition to the higher mitochondrial cristae density, the endurance‐trained athletes have 110% higher (P < 0.05) muscle mitochondrial volume density compared to the sedentary individuals (Fig. 2 D), which together gives a 150% higher (P < 0.05) surface area of mitochondrial cristae membrane per muscle volume in the endurance‐trained athletes compared to the sedentary individuals (Fig. 2 D). An intriguing finding is that the cross‐country skiers have only a non‐significant 15% higher (P = 0.28) mitochondrial volume density compared to the recreationally active men, although their higher mitochondrial cristae density gives them a higher (41%, P = 0.07) surface area of mitochondrial cristae membrane per muscle volume (Fig. 2 D). Interestingly, the surface area of mitochondrial cristae membrane per muscle volume is a better predictor of a subject's maximal oxygen uptake than mitochondrial volume per muscle volume (Fig. 2 E and F).
The recreationally active subjects, despite having a substantially higher mitochondrial volume fraction, do not have a higher cristae density than the sedentary obese men with type 2 diabetes (P = 0.48) or without type 2 diabetes (P = 0.43) (Fig. 2 D). Furthermore, short‐term (10 weeks) endurance training did not change the mitochondrial cristae density in either sedentary obese men with type 2 diabetes (P = 0.28) or those without type 2 diabetes (P = 0.25) (Fig. 3 A and B). In a comparative approach, we also demonstrated that, in muscles with a very high mitochondrial volume, the cristae density can be increased. In a superfast muscle with a high oxidative capacity (musculus tracheobronchialis dorsalis) found in the vocal organ of the zebra finch (Taenopygia guttata) (Elemans et al. 2008), the mitochondrial cristae density is 36.7 μm2 μm−3 (n = 3, range: 35.5 – 38.2 μm2 μm−3).
A discrimination between fibre types (see Methods) revealed that, in athletes, the type 1 fibres have, on average, a 14% higher cristae density compared to the type 2 fibres (P < 0.001), whereas no difference is observed between fibre types in the non‐athletes (P = 0.68) (Fig. 4 A). In line with these findings, the mitochondrial cristae density of the different fibre types shows a significant positive correlation with mitochondrial volume fraction (Fig. 4 B and C), although only in the endurance‐trained athletes.
Discussion
The oxidative capacity of the muscle is primarily determined by the mitochondrial oxidative capacity, which, in turn, is defined by the oxygen delivery, the oxidative enzymatic machinery and the electron transport chain localized at the cristae of the mitochondria. In the present study, we show that endurance‐trained athletes with an above normal oxidative capacity, in addition to a largely expanded mitochondrial network, have an enlarged cristae surface area within the mitochondria.
This finding is in contrast to the current consensus that human skeletal muscle mitochondrial cristae density is fixed at a level of ∼30 μm2 μm−3 (Hoppeler & Lindstedt, 1985; Hoppeler, 1986; Lindstedt et al. 1988; Weibel et al. 1991; Weibel & Hoppeler, 2005; Larsen et al. 2012). We show that endurance‐trained athletes have, on average, a mitochondrial cristae density of ∼32 μm2 μm−3 compared to only ∼25 μm2 μm−3 in recreationally active and sedentary individuals. Thus, if the muscle oxidative capacity of endurance‐trained athlete is evaluated only by mitochondrial volume fraction, it may be severely underestimated. Although it was observed in different muscles of one wildebeest (Connochates taurinus) and one dik‐dik (Madoqua kirkii) that mitochondrial cristae density can vary between 20 and 40 μm2 μm−3 (with no systematic difference could be found between muscles with different oxidative capacities (Hoppeler et al. 1981), it has been concluded that it is invariant with respect to species, body mass, aerobic capacity and training (Hoppeler & Lindstedt, 1985; Weibel et al. 1991; Weibel & Hoppeler, 2005). The discrepancy between the present study and previous studies may reside in either an inaccurate estimation of mitochondrial cristae density or the relatively low sample size of the previous studies. Most studies have not reported the number of mitochondria profile analysed per muscle (Hoppeler et al. 1973; Schwerzmann et al. 1989) or have a low sample size (Hoppeler et al. 1981; Larsen et al. 2012).
The variability in cristae density and the finding that the surface area of mitochondrial cristae membrane per muscle volume (Fig. 2 D) is a better predictor of a subject's maximal oxygen uptake than mitochondrial volume per muscle volume (Fig. 2 E and F) suggest that mitochondrial volume per muscle volume is not as accurate a measure of the oxidative capacity of a muscle. This means that precautions should be taken with physiological interpretations of muscle oxidative capacity from measures of muscle mitochondrial volume fractions per se, in particular in muscles from athletes with a high oxidative capacity.
Our finding that recreationally active subjects, despite substantially higher mitochondrial volume fraction (Fig. 2 D), do not have a higher cristae density than sedentary individuals suggests that regular but moderate aerobic activity can be met by increasing mitochondrial content. To test whether this correlation is causal, we investigated the effect of short‐term (10 weeks) endurance training on cristae density in sedentary obese men (with or without type 2 diabetes). Although maximal oxygen uptake rate and muscle mitochondrial volume fraction increased (15% and 40%, respectively) (Nielsen et al. 2010a), cristae density was not different after 10 weeks of endurance training in these previously sedentary individuals (Fig. 3 A and B). This observation is in agreement with the lack of any change in mitochondrial cristae density in cat muscles after 28 days of chronic stimulation (Schwerzmann et al. 1989) or in the biochemical composition of rat mitochondria after 10 weeks of endurance training (Davies et al. 1981). Thus, the higher cristae density found in endurance‐trained athletes cannot be achieved through short‐term training, whereas an increase in mitochondrial volume percentages from 4.8% to 6.8% appears to be adequate to cover the higher metabolic demand (Nielsen et al. 2010a). The training‐induced increase in mitochondrial volume percentage is in accordance with previous studies, as well as the general consensus that such an increase is accompanied by enhanced oxidative capacity (Kiessling et al. 1973; Hoppeler et al. 1985; Howald et al. 1985; Rösler et al. 1985). We therefore suggest that muscles with a low mitochondrial content may benefit more from an increased spatial distribution of mitochondria than an increased quality of existing mitochondria after aerobic training. Although still subject to debate (Hesselink et al. 2016), it has been speculated that altered morphology of mitochondria in patients with type 2 diabetes may be of pathological significance (Kelley et al. 2002), in which case the altered mitochondrial cristae density in our patients may represent a mitochondrial myopathy. Importantly, we observed no difference between this density and those in obese, non‐diabetic and lean, recreationally active individuals (Fig. 2).
Because there is a clear fibre type‐dependent discrepancy in oxidative capacity and mitochondrial volume, we addressed the role of the oxidative capacity of the two main fibre types. This is of importance because the endurance‐trained athletes predominantly recruit oxidative fibres during training and competition (Gollnick et al. 1974). At the ultrastructural level, the slow‐oxidative (type 1) and fast‐glycolytic (type 2) fibres can be identified as fibres with a high mitochondrial content and a thick Z‐disk and a low mitochondrial content and a thin Z‐disk, respectively (Sjöström et al. 1982). Our data revealed that, in the athletes, the type 1 fibres have a higher cristae density compared to the type 2 fibres, whereas no difference was observed between fibre types in the non‐athletes (Fig. 4 A). Interestingly, cross‐sectional studies indicate that higher physical activity levels are associated with a relatively larger increase in mitochondrial respiration compared to citrate synthase activity (Bishop et al. 2014), a marker of mitochondrial content (Larsen et al. 2012). Indeed, the mitochondrial cristae density of the different fibre types shows a significant positive correlation with mitochondrial volume fraction (Fig. 4 B and C), although only in the endurance‐trained athletes. This indicates that qualitative changes in mitochondrial structure and function (i.e. increased cristae density) may be an essential adaptation in the muscles of these athletes with a high proportional mitochondrial volume and oxidative capacity. This implication is supported by the high mitochondrial cristae density in a highly oxidative muscle from the vocal organ of the zebra finch, confirming previous studies that have shown a very high cristae density in specialized muscles with high metabolic power, such as hummingbird flight muscle (Suarez et al. 1991) and tuna oxidative muscle (Moyes et al. 1991).
Our data unequivocally demonstrate that human skeletal muscle mitochondria cristae density varies between populations with different physical activity levels. This is in agreement with endurance‐trained athletes having a higher respiration rate per mitochondria (Pesta et al. 2011; Jacobs and Lundby, 2013; Boushel et al. 2014b) and the fact that aerobic training is known to increase mitochondrial fusion (Iqbal et al. 2013), which increases mitochondrial intrinsic ATP production (Mitra et al. 2009). Elevated cristae density could provide the mechanism for enhancing skeletal muscle endurance via a more optimal selection of fuel stores, delaying exhaustion of endogenous glycogen reserves and, in turn, fatigue during prolonged physical activity (Allen et al. 2008),as well as confer an evolutionary advantage in energy savings during prolonged fasting by some animals (Monternier et al. 2014). Furthermore, this mechanism may be the consequence of a trade‐off between the capacity for mechanical power (work time−1), as produced by contractile filaments and the sarcoplasmic reticulum, and oxidative metabolic power (O2 consumption time−1), as produced by mitochondria, as well as a mechanism for increasing metabolic power without constraining contractile function.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
All authors were involved in the design of this study. JN, KDG and NØ analysed the data and wrote the manuscript. All authors were involved in the revisions and reviewed the final, submitted version of the manuscript. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The present study was supported by grants to NØ from the Lundbeck foundation (424/06) and the Team Denmark elite sports organisation (06‐40215/3), as well as to JN from the Ministry of Culture Committee on Sports Research (TKIF2006‐049).
Acknowledgements
We would like to thank Kirsten Hansen, Karin Trampedach and Benthe Jørgensen for their technical assistance. We thank Professor D George Stephenson for critical reading and commenting of the manuscript. Transmission electron microscopy analysis was carried out at the Department of Pathology and Department of Sports Science and Clinical Biomechanics at the University of Southern Denmark, Odense, Denmark.
Linked articles This article is highlighted by a Perspective by Perry. To read this Perspective, visit https://doi.org/10.1113/JP273549. This article featured in a Journal Club article by Leveille et al. To read this Journal Club article, visit https://doi.org/10.1113/JP274158.
References
- Allen DG, Lamb GD & Westerblad H (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88, 287–332. [DOI] [PubMed] [Google Scholar]
- Bishop DJ, Granata C & Eynon N (2014) Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim Biophys Acta 1840, 1266–1275 [DOI] [PubMed] [Google Scholar]
- Boushel R, Lundby C, Qvortrup K & Sahlin K (2014a). Mitochondrial plasticity with exercise training and extreme environments. Exerc Sport Sci Rev 42, 169–174 [DOI] [PubMed] [Google Scholar]
- Boushel R, Ara I, Gnaiger E, Helge JW, González‐Alonso J, Munck‐Andersen T, Sondergaard H, Damsgaard R, van Hall G, Saltin B & Calbet JA (2014b). Low‐intensity training increases peak arm VO2 by enhancing both convective and diffusive O2 delivery. Acta Physiol 211, 122–134. [DOI] [PubMed] [Google Scholar]
- Davies KJA, Packer L & Brooks GA (1981). Biochemical adaptation of mitochondria, muscle, and whole‐animal respiration to endurance training. Arch Biochem Biophys 209, 539–554 [DOI] [PubMed] [Google Scholar]
- Demongeot J, Glade N, Hansen O & Moreira A (2007). An open issue: the inner mitochondrial membrane (IMM) as a free boundary problem. Biochimie 89, 1049–1057. [DOI] [PubMed] [Google Scholar]
- Elemans CPH, Mead AF, Rome LC & Goller F (2008) Superfast muscles control sound production in songbirds. PLoS ONE 3, e2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K & Saltin B (1974). Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and varying pedalling rates. J Physiol 241, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner M, Korner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F & Neupert W (2014). The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J 30, 4356–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselink MKC, Schrauwen‐Hinderling V & Schrauwen P (2016). Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat Rev Endo 12, 633–645. [DOI] [PubMed] [Google Scholar]
- Holloszy JO (1967). Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242, 2278–2282 [PubMed] [Google Scholar]
- Holmberg H‐C, Lindinger S, Stöggl T, Eitzlmair E & Müller E (2005). Biomechanical analysis of double poling in elite cross‐country skiers. Med Sci Sports Exerc 37, 807–818. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Hudlicka O & Uhlmann E (1987). Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol 385, 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H, Lüthi P, Claassen H, Weibel ER & Howald H (1973). The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well‐trained orienteers. Pflügers Arch 344, 217–232. [DOI] [PubMed] [Google Scholar]
- Hoppeler H (1986). Exercise‐induced ultrastructural changes in skeletal muscle. Int J Sports Med 7, 187–204. [DOI] [PubMed] [Google Scholar]
- Hoppeler H. Mathieu O, Krauer R, Claassen H, Armstrong RB & Weibel ER (1981). Design of the mammalian respiratory system. VI. Distribution of mitochondria and capillaries in various muscles. Respir Physiol 44, 87–111. [DOI] [PubMed] [Google Scholar]
- Hoppeler H & Lindstedt SL (1985). Malleability of skeletal muscle in overcoming limitations: structural elements. J Exp Biol 115, 355–364. [DOI] [PubMed] [Google Scholar]
- Howald H, Hoppeler H, Claassen H, Mathieu O & Straub R (1985). Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflügers Arch 403, 369–376. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Ostojic O, Singh K, Joseph A‐M & Hood D (2013). Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 48, 963–970. [DOI] [PubMed] [Google Scholar]
- Jacobs RA & Lundby C (2013). Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol 114, 344–350. [DOI] [PubMed] [Google Scholar]
- John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JML, Rangell L, Bennett MJ & Zha J (2005). The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell 16, 1543–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV & Ritov VB (2002). Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950. [DOI] [PubMed] [Google Scholar]
- Kiessling K‐H, Pilström L, Karlsson J & Piehl K (1973). Mitochondrial volume in skeletal muscle from young and old physically untrained and trained healthy men and from alcoholics. Clin Sci 44, 547–554. [DOI] [PubMed] [Google Scholar]
- Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helger JW, Dela F & Hey‐Mogensen M (2012). Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590, 3349–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt SL, Wells DJ, Jones JH, Hoppeler H & Thronson HA Jr (1988). Limitations to aerobic performance in mammals: interaction of structure and demand. Int J Sports Med 9, 210–217. [DOI] [PubMed] [Google Scholar]
- Mannella CA (2006). Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 1763, 542–548. [DOI] [PubMed] [Google Scholar]
- Mitra K, Wunder C, Roysam B, Lin G & Lippincott‐Schwartz JA (2009). A hyperfused mitochondrial state achieved at G1‐S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA 106, 11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monternier P‐A, Marmillot V, Rouanet J‐L & Roussel D (2014). Mitochondrial phenotypic flexibility enhances energy savings during winter fast in king penguin chicks. J Exp Biol 217, 2691–2697. [DOI] [PubMed] [Google Scholar]
- Moyes CD, Mathieu‐Costello OA, Brill RW & Hochachka PW (1991). Mitochondrial metabolism of cardiac and skeletal muscle rom a fast (Katsuwonus pelamis) and a slow (Cyprinus carpio) fish. Can J Zool 70, 1246–1253. [Google Scholar]
- Nielsen J, Krustrup P, Nybo L, Gunnarsson TP, Madsen K, Schrøder HD, Bangsbo J & Ortenblad N (2012). Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high‐level competitive soccer game. Eur J Appl Physiol 112, 3559–3567. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Mogensen M, Vind BF, Sahlin K, Højlund K, Schrøder HD & Ortenblad N (2010a). Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Metab 298, E706–E713. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Suetta C, Hvid LG, Schrøder HD, Aagaard P & Ortenblad N (2010b). Subcellular localization‐dependent decrements in skeletal muscle glycogen and mitochondria content following short‐term disuse in young and old men. Am J Physiol Endocrinol Metab 299, E1053–E1060. [DOI] [PubMed] [Google Scholar]
- Ørtenblad N, Nielsen J, Saltin B & Holmberg H‐C (2011). Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol 589, 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladin AV (1945). The biochemistry of muscle training. Science 102, 576–578 [DOI] [PubMed] [Google Scholar]
- Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M & Gnaiger E (2011). E. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301, R1078–R1087. [DOI] [PubMed] [Google Scholar]
- Rich P (2003). Chemiosmotic coupling: the cost of living. Nature 421, 583. [DOI] [PubMed] [Google Scholar]
- Rösler K, Hoppeler H, Conley KE, Claassen H, Gehr P & Howald H (1985). Transfer effects in endurance exercise. Adaptations in trained and untrained muscles. Eur J Appl Physiol 54, 355–362. [DOI] [PubMed] [Google Scholar]
- Schaeffer PJ, Lindstedt SL (2013). How animals move: comparative lessons on animal locomotion. Compr Physiol 3, 289–314. [DOI] [PubMed] [Google Scholar]
- Schwerzmann K, Hoppeler H, Kayar SR & Weibel ER (1989). Oxidative capacity of muscle and mitochondria: Correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci USA 86, 1583–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström M, Angquist KA, Bylund AC, Fridén J, Gustavsson L & Scherstén T (1982). Morphometric analyses of human muscle fiber types. Muscle Nerve 5, 538–553. [DOI] [PubMed] [Google Scholar]
- Suarez RK, Lighton JRB, Brown GS & Mathieu‐Costello O (1991). Mitochondrial respiration in hummingbird flight muscles. Proc Natl Acad Sci USA 88, 4870–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER & Hoppeler H (2005). Exercise‐induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol 208, 1635–1644. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Taylor CR & Hoppeler H (1991). The concept of symmorphosis: A testable hypothesis of structure–function relationship. Proc Natl Acad Sci USA 88, 10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER (1980). Stereological Methods. Vol. 2: Theoretical Foundations. Academic Press, London. [Google Scholar]


