Abstract
Key points
Exercise training effectively improves vascular and skeletal muscle function; however, these effects of training may be blunted in postmenopausal women as a result of the loss of oestrogens.
Accordingly, the capacity to deliver oxygen to the active muscles may also be impaired in postmenopausal women.
In both premenopausal and recent postmenopausal women, exercise training was shown to improve leg vascular and skeletal muscle mitochondrial function. Interestingly, these effects were more pronounced in postmenopausal women.
Skeletal muscle oxygen supply and utilization were similar in the two groups of women.
These findings suggest that the early postmenopausal phase is associated with an enhanced capacity of the leg vasculature and skeletal muscle mitochondria to adapt to exercise training and that the ability to deliver oxygen to match the demand of the active muscles is preserved in the early phase following the menopausal transition.
Abstract
Exercise training leads to favourable adaptations within skeletal muscle; however, this effect of exercise training may be blunted in postmenopausal women as a result of the loss of oestrogens. Furthermore, postmenopausal women may have an impaired vascular response to acute exercise. We examined the haemodynamic response to acute exercise in matched pre‐ and postmenopausal women before and after 12 weeks of aerobic high intensity exercise training. Twenty premenopausal and 16 early postmenopausal (mean ± SEM: 3.1 ± 0.5 years after final menstrual period) women only separated by 4 years of age (mean ± SEM: 50 ± 0 years vs. 54 ± 1 years) were included. Before training, leg blood flow, O2 delivery, O2 uptake and lactate release during knee‐extensor exercise were similar in pre‐ and postmenopausal women. Exercise training reduced (P < 0.05) leg blood flow, O2 delivery, O2 uptake, lactate release, blood pressure and heart rate during the same absolute workloads in postmenopausal women. These effects were not detected in premenopausal women. Quadriceps muscle protein contents of mitochondrial complex II, III and IV; endothelial nitric oxide synthase (eNOS); cyclooxygenase (COX)‐1; COX‐2; and oestrogen‐related receptor α (ERRα) were increased (P < 0.05) with training in postmenopausal women, whereas only the levels of mitochondrial complex V, eNOS and COX‐2 were increased (P < 0.05) in premenopausal women. These findings demonstrate that vascular and skeletal muscle mitochondrial adaptations to aerobic high intensity exercise training are more pronounced in recent post‐ compared to premenopausal women, possibly as an effect of enhanced ERRα signalling. Also, the hyperaemic response to acute exercise appears to be preserved in the early postmenopausal phase.
Keywords: blood flow, menopause, oestrogen
Key points
Exercise training effectively improves vascular and skeletal muscle function; however, these effects of training may be blunted in postmenopausal women as a result of the loss of oestrogens.
Accordingly, the capacity to deliver oxygen to the active muscles may also be impaired in postmenopausal women.
In both premenopausal and recent postmenopausal women, exercise training was shown to improve leg vascular and skeletal muscle mitochondrial function. Interestingly, these effects were more pronounced in postmenopausal women.
Skeletal muscle oxygen supply and utilization were similar in the two groups of women.
These findings suggest that the early postmenopausal phase is associated with an enhanced capacity of the leg vasculature and skeletal muscle mitochondria to adapt to exercise training and that the ability to deliver oxygen to match the demand of the active muscles is preserved in the early phase following the menopausal transition.
Abbreviations
- COX‐1
cyclooxygenase‐1
- eNOS
endothelial nitric oxide synthase
- ER
oestrogen receptor
- ERE
oestrogen response element
- ERRα
oestrogen‐related receptor α
- FABF
femoral arterial blood flow
- FAP
femoral arterial pressure
- FVP
femoral venous pressure
- LVC
leg vascular conductance
- MAP
mean arterial blood pressure
- PGC‐1α
peroxisome proliferator‐activated receptor γ coactivator 1α
- PGI2
prostacyclin
- TXA2
thromboxane A2
Introduction
Exercise training leads to favourable adaptations within skeletal muscle that include improved matching of O2 delivery and O2 demand and increased mitochondrial volume and quality (Blomqvist & Saltin, 1983; Boushel et al. 2014; Hellsten & Nyberg, 2015). Because oestrogens modulate a myriad of molecular pathways related to vascular and skeletal muscle function (Phillips et al. 1993; Enns & Tiidus, 2010; Menazza & Murphy, 2016), one consequence of oestrogen loss at menopause could be an impaired ability of the active skeletal muscle to attain these adaptations. Accordingly, adaptations in endothelial function in response to low‐intensity exercise training have been reported to be reduced in late postmenopausal women compared to age‐matched men (Pierce et al. 2011; Moreau et al. 2013). This impairment appears mainly to be the result of oestrogen loss because oestradiol supplementation concomitant with exercise training can counteract this detrimental consequence of the menopausal transition (Moreau et al. 2013). Furthermore, oestrogen treatment has been shown to augment training‐induced adaptations in skeletal muscle structure and function in recent postmenopausal women (Taaffe et al. 2005).
These beneficial effects of oestrogens are probably mediated via oestrogen receptors (ERs) that are present in both endothelial and skeletal muscle cells and that regulate a number of downstream genes and molecular targets (Enns & Tiidus, 2010; Menazza & Murphy, 2016). The transcriptional activation of ERs by oestrogens is initiated when the receptor binds to specific DNA sequences termed oestrogen response elements (EREs). Interestingly, in addition to oestrogens, muscle contraction can enhance ERE activity in skeletal muscle myotubes (Wiik et al. 2009). Because this effect of contractile activity has been found to be independent of functional ERs (Wiik et al. 2009), the activation of ERE by contraction may be the result of stimulation of oestrogen‐related receptors (ERRs). Activation of ERR by contractile activity is also supported by findings of increased skeletal muscle ERRα mRNA levels following acute exercise in humans (Cartoni et al. 2005). It is thus plausible that repeated exercise‐induced activation of ERE via activation of ERRs can partly compensate for the loss of oestrogens in postmenopausal women. Moreover, it is possible that intense aerobic training is more efficient in inducing vascular adaptations (Nyberg et al. 2016a,b) and skeletal muscle performance (Seidelin et al. 2017) than low to moderate intensity training (Pierce et al. 2011; Moreau et al. 2013).
In women, acceleration of age‐associated declines in vascular and skeletal muscle structure and function are evident after the menopausal transition (Tunstall‐Pedoe et al. 1994; Taddei et al. 1996; Mendelsohn & Karas, 2005; Enns & Tiidus, 2010; Nyberg et al. 2016a). The decline in vascular function has been shown to be accompanied by impaired blood flow to skeletal muscle during exercise (Proctor et al. 2003; Parker et al. 2008). Although the mechanisms underlying the reduced exercise hyperaemia in postmenopausal women remain undisclosed, an impaired vascular response may be attributed to the loss of oestrogens because these strongly influence the regulation of vascular and skeletal muscle function (Phillips et al. 1993; Enns & Tiidus, 2010; Menazza & Murphy, 2016). However, the role of oestrogens vs. age for the hyperaemic response to exercise has not been possible to discern as a result of a large age‐difference (∼40 years) between the pre‐ and postmenopausal women in prior studies (Proctor et al. 2003; Parker et al. 2008).
In the present study, we tested the hypothesis that, compared to premenopausal women, recent postmenopausal women would display an impaired vascular response to submaximal exercise engaging the knee‐extensor muscles. In addition, we hypothesized that aerobic high‐intensity training would improve the vascular and metabolic response to knee‐extensor exercise to a similar extent in recent postmenopausal women and premenopausal women. Accordingly, we measured haemodynamic variables during submaximal knee‐extensor exercise before and after 12 weeks of high‐intensity exercise training. To minimize the effects of age‐related changes, we investigated pre‐ and recent postmenopausal women with a minimal (∼4 years) difference in age.
Methods
Subjects
Twenty premenopausal women with a mean age of 50 years (range 46–55 years) and 16 postmenopausal women with a mean age of 54 years (range 46–57 years) participated in the present study (Table 1). The women were recruited from the Copenhagen area through newspaper advertisement. Eligibility was assessed upon first contact by phone or mail and, second, by evaluation of an online questionnaire and, finally, at a health examination (for details, see below). Recruitment was conducted over four rounds from August 2013 until August 2015 and an almost equal number of pre‐ and postmenopausal women was recruited in each round to prevent seasonal and investigator‐dependent variation. The subjects included in the present study are a part of a larger cohort (Mandrup et al. 2016). All subjects were informed of any risks and discomforts associated with the experiments before giving their written, informed consent to participate in the study.
Table 1.
Subject characteristics
| Premenopausal | Postmenopausal | |||
|---|---|---|---|---|
| Before training | After training | Before training | After training | |
| Variable | (n = 20) | (n = 14) | (n = 16) | (n = 16) |
| Age (years) | 50 ± 0 | – | 54 ± 1# | – |
| Time from last menstrual cycle (years) | – | – | 3.1 ± 0.5 | |
| Height (m) | 1.69 ± 0.01 | – | 1.67 ± 0.01# | – |
| Weight (kg) | 69.2 ± 1.4 | 68.0 ± 1.4* | 64.4 ± 1.8# | 63.1 ± 1.5#, * |
| Body mass index (kg m–2) | 24.4 ± 0.5 | 23.9 ± 0.5* | 23.1 ± 0.5 | 22.7 ± 0.4* |
| Lean body mass (kg) | 43.4 ± 0.9 | 44.2 ± 0.8* | 41.6 ± 1.0 | 42.1 ± 1.0* |
| Fat percentage (%) | 38.5 ± 1.0 | 36.1 ± 1.1* | 36.6 ± 1.1 | 34.9 ± 1.0* |
| Waist circumference (cm) | 83 ± 1 | 80 ± 1* | 80 ± 2 | 76 ± 1* |
| Lean leg mass (kg) | 14.3 ± 0.4 | 14.4 ± 0.4 | 13.1 ± 0.4# | 13.4 ± 0.4* |
| MAP at rest (mmHg) | 90 ± 2 | 89 ± 3 | 95 ± 3# | 90 ± 3* |
| Heart rate at rest (beats min–1) | 67 ± 2 | 65 ± 2 | 62 ± 2# | 58 ± 1#, * |
| (mL min–1) | 2106 ± 58 | 2304 ± 61* | 2033 ± 64 | 2133 ± 65* |
| (mL min−1 kg−1) | 30.6 ± 0.8 | 34.1 ± 1.1* | 31.9 ± 1.3 | 34.1 ± 1.3* |
| Peak (RER) | 1.28 ± 0.02 | 1.27 ± 0.04 | 1.29 ± 0.02 | 1.26 ± 0.03 |
| Peak workload (W) | 199 ± 6 | 224 ± 6* | 194 ± 4 | 209 ± 5* |
| Peak heart rate (beats min–1) | 178 ± 2 | 175 ± 2* | 174 ± 2 | 171 ± 2* |
| Hematocrit (%) | 37.0 ± 0.7 | 36.4 ± 0.7 | 36.9 ± 0.6 | 36.2 ± 0.7 |
| Oestradiol (nmol L−1) | 0.49 ± 0.10 | 0.52 ± 0.09 | 0.09 ± 0.01# | 0.09 ± 0.01# |
| Progesterone (nmol L−1) | 6.4 ± 2.7 | – | 0.9 ± 0.1# | – |
| FSH (IU L−1) | 12.0 ± 2.8 | – | 91.5 ± 6.1# | – |
| LH (IU L−1) | 17.0 ± 2.9 | – | 34.7 ± 5.0# | – |
| Testosterone (nmol L−1) | 0.77 ± 0.08 | – | 0.64 ± 0.07 | – |
| Fasting glucose (mg dL−1) | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.1 |
| Total cholesterol (mmol L−1) | 4.9 ± 0.2 | 4.8 ± 0.1* | 5.5 ± 0.2# | 5.4 ± 0.1*, # |
| HDL‐cholesterol (mmol L−1) | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1# | 2.0 ± 0.1# |
| LDL‐cholesterol (mmol L−1) | 2.9 ± 0.2 | 2.8 ± 0.1* | 3.2 ± 0.2# | 3.1 ± 0.2*, # |
| Triglycerides (mmol L−1) | 0.83 ± 0.07 | 0.83 ± 0.07 | 0.87 ± 0.07 | 0.81 ± 0.06 |
Values are the mean ± SE. RER, respiratory exchange ratio; FSH: follicle‐stimulating hormone; LH, luteinizing hormone; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Mean arterial pressure (MAP) was measured in the femoral artery. *Significant change (P < 0.05) with training; #significantly different (P < 0.05) from premenopausal at that time point.
Premenopausal women were all experiencing regular menstrual cycles and were not taking oral contraceptives, whereas postmenopausal women had not experienced a menstrual cycle during the previous 12 months but were less than 5 years past their final menstrual period (mean ± SE, 3.1 ± 0.5 years). Menopausal status was verified by measurements of hypothalamic and reproductive hormones (Table 1) and none of the postmenopausal women were in hormone replacement therapy. There was no difference between the two groups with respect to previous exposure to reproductive hormones and parity. All subjects were habitually inactive (less than 2 h of moderate intensity exercise per week), although many used a bicycle for daily transportation. All women had normal resting ECG, were non‐smokers, and none of the subjects in either group had been diagnosed with hypertension, cardiovascular disease, renal dysfunction, insulin resistance, diabetes or hypercholesterolaemia. To ensure that all women were normotensive at inclusion, mean arterial blood pressure (MAP) was measured seven consecutive times by an automatic upper arm blood pressure monitor (M7; Omron, Vernon Hills, IL, USA) after at least 15 min of rest in the supine position (premenopausal: 112 ± 3/72 ± 2 mmHg; postmenopausal: 114 ± 4/73 ± 2 mmHg).
The data presented are part of a larger study on the effects of exercise training on women in the menopausal transition funded via the University of Copenhagen Excellence Programme for Interdisciplinary Research and the data presented in Tables 1 and 2 have been reported previously (Mandrup et al. 2016; Nyberg et al. 2016a). The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities Region H (H‐1‐2012‐150) and conducted in accordance with the guidelines of the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (NCT02135575).
Table 2.
Training
| Total training time (% of maximum heart rate) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Training sessions | Average time per session (min:s) | < 61% | 61–70% | 71–80% | 81–85% | 86–90% | 91–95% | 96–100% |
| Premenopausal (n = 20) | 36.3 ± 1.6 | 53:10 ± 00:31 | 2.3 ± 2.0 | 7.9 ± 4.1 | 25.5 ± 6.7 | 22.4 ± 4.7 | 26.9 ± 7.9 | 13.4 ± 7.4 | 1.5 ± 0.4 |
| Postmenopausal (n = 16) | 34.9 ± 1.3 | 51:27 ± 01:11 | 1.8 ± 0.5 | 7.6 ± 1.2 | 21.7 ± 2.2 | 22.1 ± 1.3 | 30.1 ± 3.1 | 15.2 ± 2.2 | 1.4 ± 0.4 |
Values are means ± SE.
Experimental design
The experimental days were performed before and after a 12‐week training period where the subjects participated in supervised exercise training. Experimental testing in premenopausal women was performed in the mid‐follicular phase of their ovarian cycle.
Exercise training
Subjects performed exercise on a cycling ergometer for 1 h three times per week of which two of these occasions were supervised by instructors from the research group and one was supervised by instructors from the local gym. A heart rate monitor was worn during all training sessions (TEAM2 Wearlink+; Polar, Kempele, Finland). Exercise intensity during the training sessions is presented in Table 2.
Before and after the training intervention, peak O2 uptake () was determined (Oxycon Pro; Intramedic, Gentofte, Denmark) using of an incremental exercise test on a cycling ergometer. Briefly, after a standardized 10 min warm‐up, the test was initiated at 50 W and the workload was increased by 25 W every 1 min until volitional fatigue.
Experimental day 1
Subjects refrained from caffeine, alcohol, and exercise for 24 h before arriving at the laboratory after eating breakfast. After local anaesthesia (lidocaine, 20 mg ml−1; Astra Zeneca, Copenhagen, Denmark), catheters (20 G; Arrow International, Reading, PA, USA) were placed in the femoral artery and vein of the experimental leg and in the femoral artery of the non‐experimental leg. After ∼60 min of rest, subjects performed 5 min of one‐leg knee‐extensor exercise (Andersen & Saltin, 1985) at 10 W followed by 5 min at 20 W in an upright seated position. Subjects were instructed to maintain a kicking frequency of 60 rpm and a passive return of the leg to minimize involvement of the hamstring muscles. The workloads were chosen to reflect light/moderate (10 W) and moderate/high‐ intensity (20 W) and to ensure that all women could perform the exercise for 5 min at each workload. Blood flow, blood pressures and arterial and venous blood samples (∼2 ml) were obtained after 4.5 min at each workload.
Experimental day 2
Subjects refrained from caffeine, alcohol and exercise for 24 h before arriving at the laboratory in a fasted state. A blood sample from the antecubital vein and a biopsy from the musculus vastus lateralis (obtained through an incision made in the skin under local anaesthesia; lidocaine, 20 mg mL−1; Astra Zeneca) were then collected. This experimental day was separated by at least 48 h from experimental day 1.
Measurement and analysis
Body composition (fasted state) was assessed by dual X‐ray absorptiometry scanning (Lunar iDXA; GE Healthcare, Little Chalfont, UK) by the same investigator blinded for menopausal status. Whole blood samples obtained from the antecubital vein (experimental day 2) were analysed at the clinical biochemical unit at Rigshospitalet (University Hospital of Copenhagen) via an automatic analyser using a turbidimetric immunoassay (Modular/COBAS P‐Module; Roche Diagnostics, Basel, Switzerland); C‐reactive protein, oestradiol, progesterone and testosterone were analysed via a competitive electrochemiluminescence immunoassay (Modular/COBAS E‐Module); and follicle‐stimulating hormone and luteinizing hormone were analysed via a sandwich electrochemiluminescence immunoassay (Modular/COBAS E‐Module). Plasma high‐density lipoprotein‐cholesterol, low‐density lipoprotein‐cholesterol, total cholesterol and triglyceride were measured using enzymatic absorption photometri (Modular/COBAS C‐Module). Glucose was determined by amperometric metabolite electrode measurement (ABL 837; Radiometer, Copenhagen, Denmark).
Femoral arterial blood flow (FABF) was measured with ultrasound Doppler (Vivid E9; GE Healthcare, Brøndby, Denmark) equipped with a linear probe operating at an imaging frequency of 8.0 MHz and a Doppler frequency of 3.1 MHz as described previously (Nyberg et al. 2014a). Intra‐arterial and intra‐venous pressures were monitored with transducers (Pressure Monitoring Set; Edwards Lifesciences, Irvine, CA, USA) positioned at the level of the tip of the catheter. Blood gases, haemoglobin, pH and lactate were measured using an ABL800 FLEX analyser (Radiometer). Leg vascular conductance (LVC) was calculated as FABF/[mean femoral arterial pressure (FAP) – mean femoral venous pressure (FVP)]. Leg O2 uptake was calculated as the arterio‐venous O2 difference multiplied by FABF; leg O2 delivery as FABF multiplied by arterial O2 content; and leg lactate release as the venous–arterial lactate difference multiplied by FABF.
In the muscle samples, protein expression was determined by western blotting. Freeze‐dried muscle tissue samples obtained at rest were homogenized in a fresh batch of buffer [10% glycerol, 20 mm sodium‐pyrophosphate, 150 mm NaCl, 50 mm Hepes, 1% Nonidet P‐40 (Shell Chemicals, Hague, The Netherlands), 20 mm β‐glycerophosphate, 10 mm NaF, 2 mm phenylmethane sulphonyl fluoride, 1 mm each EDTA and EGTA, 10 μg ml−1 each aprotinin and leupeptin and 3 mm benzamidine] two times for 30 s (Qiagen Tissuelyser II; Retsch, Haan, Germany). After rotation end over end for 1 h, the samples were centrifuged for 30 min at 17 500 g at 4°C and the lysate was collected as the supernatant. Protein concentrations were determined in the lysates (assayed in triplicate) using BSA standards (Pierce Reagents, Rockford, IL, USA). The lysates were diluted to appropriate protein concentrations (2 μg μl−1) in a ×6 sample buffer (0.5 m Tris‐base, dithiothreitol, SDS, glycerol and bromphenol blue) and an equal amount of total protein was loaded for each sample in different wells on precast gels (Bio‐Rad Laboratories, Hercules, CA, USA). Equal amounts of total protein were loaded for each sample in accordance with the antibody optimization: ERRα (5 μg), total OXPHOS (1 μg), endothelial nitric oxide synthase (eNOS) (6 μg), cyclooxygenase (COX)‐1 (5 μg), COX‐2 (8 μg), prostacyclin (PGI2) synthase (8 μg) and thromboxane A2 (TXA2) synthase (4 μg). Samples from each group were distributed evenly across the gel and the sample from before training was placed adjacent to the sample after training for each subject. In addition, a human standard sample was also loaded to control for differences over the gel/membrane. After gel electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane, which was incubated with primary antibody overnight and then washed for 5 min in Tris‐buffered saline with Tween‐20 before incubation with secondary antibody for 1 h.
Antibodies used were: ERRα (dilution 1:1000; 2% non‐fat milk; Abcam, Cambridge, UK), total OXPHOS (dilution 1:1500; 5% non‐fat milk; Abcam), eNOS (dilution 1:500; 2% non‐fat milk; BD Biosciences, Franklin Lakes, NJ, USA), COX‐1 (dilution 1:600; 3% BSA; Abcam), COX‐2 (dilution 1:500; 2% non‐fat milk; Abcam), PGI2 synthase (dilution 1:200; 2% non‐fat milk; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and TXA2 synthase (dilution 1:500; 2% non‐fat milk; Abcam). The secondary HRP‐conjugated antibody (Dako, Glostrup, Denmark) was diluted 1:5000 in 2/5% non‐fat milk or 3% BSA, depending on the primary antibody. The membrane staining was visualized by incubation with a chemiluminescent HRP substrate (Millipore, Billerica, MA, USA) and the image was digitalized on a ChemiDoc MP system (Bio‐Rad). All proteins were expressed in arbitrary units.
Statistical analysis
A linear mixed‐model approach was used to investigate differences within and between groups. Fixed factors were ‘group’ (premenopausal, postmenopausal), ‘time’ (before training, after training) and ‘intensity’ (10 W, 20 W). Subjects were specified as a repeated factor and identifier of random variation. Homogeneity of covariance and normal distribution were confirmed through residual and Q–Q plots. Pairwise differences were identified using Tukey's honestly significant difference post hoc procedure. Pearson correlation analysis was used to determine relations of interest. As a result of technical difficulties, haemodynamic data obtained after training included 14 of the 20 premenopausal women (also clarified in each figure legend). The number of subjects was selected on the basis of detecting differences in blood flow to the exercising leg between the two groups before training and within each group with training. Statistical analyses were performed with R, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) through the interface R studio (R Foundation for Statistical Computing). Data are reported as the mean ± SE. P < 0.05 was considered statistically significant.
Results
Subject characteristics
Before the training period, there was no difference in between pre‐ and postmenopausal women (Table 1). Exercise training increased (P < 0.05) by 9.9 ± 2.4% and 5.4 ± 2.1% in the pre‐ and postmenopausal women. Before training, MAP at rest was higher (P < 0.05) in postmenopausal women compared to premenopausal women, although this difference in MAP was not detected after training as a result of a training‐induced lowering (P < 0.05) of MAP in the postmenopausal group. Heart rate at rest was lower (P < 0.05) in postmenopausal women before and after training. Exercise training lowered heart rate at rest in postmenopausal women, whereas there was a non‐significant reduction in premenopausal women (P = 0.053).
Vascular and metabolic responses to knee‐extensor exercise
FABF and leg O2 delivery
During knee‐extensor exercise, FABF and leg O2 delivery were similar in the pre‐ and postmenopausal women before training (Fig. 1 A and B). In the postmenopausal group, exercise training reduced (P < 0.05) FABF (−10.8 ± 3.6% and −6.4 ± 2.8 %; 10 and 20 W) and O2 delivery (−12.6 ± 3.9% and −13.8 ± 3.5%) and FABF and O2 delivery were lower (P < 0.05) in postmenopausal compared to premenopausal women after training.
Figure 1. Leg haemodynamics during exercise.

Femoral arterial blood flow (A), leg O2 delivery (B), leg venous arterial‐lactate difference (C), leg arterio‐venous O2 difference (D), leg lactate release (E) and leg O2 uptake (F) in pre‐ (n = 14–20) and postmenopausal (n = 16) women during knee‐extensor exercise performed at 10 and 20 W before and after training. *Significantly different (P < 0.05) from before training; #significantly different (P < 0.05) from premenopausal at that time point.
Leg arterio‐venous O2 difference and O2 uptake
Leg arterio‐venous O2 difference was similar in both groups of women and was not changed with training (Fig. 1 D). Leg O2 uptake was lower (−9.2 ± 3.4% and −10.3 ± 3.7 %; 10 and 20 W; P < 0.05) after training in postmenopausal women and leg O2 uptake was lower (P < 0.05) in postmenopausal compared to premenopausal women after training (Fig. 1 F).
Difference between leg O2 delivery and O2 uptake
Before training, the difference between leg O2 delivery and leg O2 uptake was not different between the pre‐ and postmenopausal women (Fig. 2 A). The difference between leg O2 delivery and leg O2 uptake was lower (P < 0.05) after training in both the pre‐ (−6.4 ± 9.3% and −4.6 ± 5.8 %; 10 and 20 W) and postmenopausal group (−18.3 ± 5.3% and −19.0 ± 5.2 %). The difference between leg O2 delivery and leg O2 uptake was reduced to a larger extent (P < 0.05) at 20 W in the postmenopausal compared to the premenopausal group (−17 ± 12 vs. −46 ± 11 mL O2 min−1, pre‐ and postmenopausal). There was a linear relationship (r 2 = 0.97, P < 0.05) between leg O2 uptake and leg O2 delivery irrespective of training status (Fig. 2 B).
Figure 2. Matching of O2 delivery and O2 utilization during exercise.
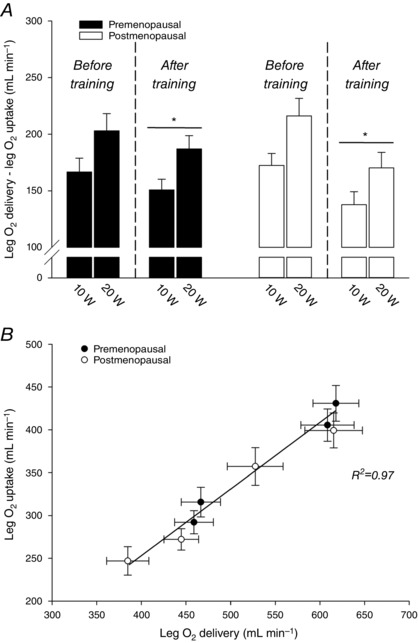
Difference between leg O2 delivery and leg O2 uptake (A) and the association between leg O2 delivery and leg O2 uptake (B) in pre‐ (n = 14–20) and postmenopausal (n = 16) women during knee‐extensor exercise performed at 10 and 20 W before and after training. *Significantly different (P < 0.05) from before training.
Leg venous–arterial lactate difference and lactate release
Before training, leg venous–arterial lactate difference and lactate release were not different between the two groups (Fig. 1 C). Exercise training reduced (P < 0.05) leg venous–arterial lactate difference in pre‐ (−0.2 ± 0.1 and −0.3 ± 0.1 mmol L−1, 10 and 20 W) and postmenopausal (−0.3 ± 0.1 and −0.2 ± 0.1 mmol L−1) women, whereas lactate release was only reduced (P < 0.05) in postmenopausal women (−0.8 ± 0.2 and −0.9 ± 0.4 mmol min−1) (Fig. 1 C and E).
Mean femoral arterial blood pressure
FAP was higher (P < 0.05) in postmenopausal women compared to premenopausal women before but not after training because training reduced (P < 0.05) FAP in the postmenopausal group (−12.0 ± 2.9 and −10.5 ± 2.9 mmHg; 10 and 20 W) (Fig. 3 A). LVC was not different between groups before training (Fig. 3 B). Although changes in LVC with training did not reach statistical difference in any of the groups, LVC was lower (P < 0.05) in postmenopausal women compared to premenopausal women after training. Before training, a negative correlation was detected between LVC during 10 W and follicle‐stimulating hormone when accounting for all women (r2 = 0.16, P = 0.024).
Figure 3. Blood pressure and vascular conductance during exercise.
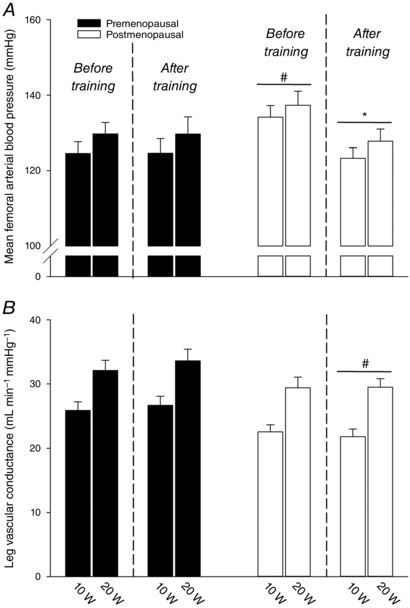
Mean femoral arterial blood pressure (A) and leg vascular conductance (B) in pre‐ (n = 14–20) and postmenopausal (n = 16) women during knee‐extensor exercise performed at 10 and 20 W before and after training. *Significantly different (P < 0.05) from before training; #significantly different (P < 0.05) from premenopausal at that time point.
Blood pO2, O2 content, pH, O2 saturation, haemoglobin, heart rate and FVP are presented in Table 3.
Table 3.
Blood and haemodynamic variables during knee‐extensor exercise
| Premenopausal | Postmenopausal | |||||||
|---|---|---|---|---|---|---|---|---|
| Before training | After training | Before training | After training | |||||
| (n = 20) | (n = 14) | (n = 16) | (n = 16) | |||||
| Blood variable | 10 W | 20 W | 10 W | 20 W | 10 W | 20 W | 10 W | 20 W |
| (mmHg) | ||||||||
| Arterial | 99 ± 2 | 102 ± 2 | 102 ± 2 | 106 ± 2 | 102 ± 2 | 108 ± 3 | 103 ± 2 | 105 ± 2 |
| Venous | 24 ± 1 | 24 ± 1 | 23 ± 1* | 23 ± 1* | 25 ± 1 | 25 ± 1 | 25 ± 1* | 23 ± 1* |
| Haemoglobin (mmol L−1) | ||||||||
| Arterial | 8.0 ± 0.1 | 8.1 ± 0.1 | 7.8 ± 0.2 | 7.9 ± 0.2 | 8.1 ± 0.2 | 8.4 ± 0.2 | 7.7 ± 0.2* | 7.7 ± 0.2* |
| Venous | 7.9 ± 0.1 | 8.1 ± 0.1 | 7.8 ± 0.2 | 7.9 ± 0.2 | 8.0 ± 0.1 | 8.1 ± 0.2 | 7.7 ± 0.2* | 7.7 ± 0.2* |
| O2 saturation (%) | ||||||||
| Arterial | 97.8 ± 0.1 | 98.0 ± 0.2 | 97.9 ± 0.2* | 98.6 ± 0.5* | 98.0 ± 0.2 | 98.1 ± 0.2 | 97.8 ± 0.1 | 98.0 ± 0.1 |
| Venous | 35.8 ± 1.4 | 32.5 ± 1.5 | 31.9 ± 1.5* | 29.9 ± 1.5* | 38.7 ± 1.7 | 35.6 ± 1.7 | 35.8 ± 2.0* | 31.6 ± 1.4* |
| O2 content (mL L−1) | ||||||||
| Arterial | 172 ± 3 | 175 ± 3 | 168 ± 4 | 171 ± 4 | 174 ± 3 | 181 ± 3 | 166 ± 3* | 165 ± 3* |
| Venous | 62 ± 2 | 58 ± 3 | 55 ± 3* | 52 ± 3* | 67 ± 3 | 63 ± 3 | 59 ± 3* | 53 ± 2* |
| pH | ||||||||
| Arterial | 7.405 ± 0.003 | 7.410 ± 0.004 | 7.413 ± 0.005 | 7.417 ± 0.008 | 7.403 ± 0.005 | 7.393 ± 0.009 | 7.407 ± 0.004* | 7.412 ± 0.004* |
| Venous | 7.319 ± 0.006 | 7.295 ± 0.008 | 7.329 ± 0.008* | 7.314 ± 0.01* | 7.310 ± 0.007 | 7.280 ± 0.011 | 7.332 ± 0.005* | 7.318 ± 0.004* |
| Lactate (mmol L−1) | ||||||||
| Arterial | 1.2 ± 0.1 | 1.9 ± 0.2 | 1.1 ± 0.2 | 1.6 ± 0.3 | 1.2 ± 0.1 | 2.3 ± 0.3 | 0.8 ± 0.1* | 1.3 ± 0.2* |
| Venous | 1.7 ± 0.2 | 2.8 ± 0.3 | 1.5 ± 0.2 | 2.3 ± 0.4 | 1.8 ± 0.2 | 2.9 ± 0.3 | 1.2 ± 0.1* | 1.8 ± 0.3* |
| Heart rate (beats min−1) | ||||||||
| 92 ± 2 | 104 ± 3 | 89 ± 3 | 101 ± 5 | 86 ± 3 | 102 ± 3 | 74 ± 2#, * | 86 ± 4#, * | |
| FVP (mL min−1 mmHg−1) | ||||||||
| 19.7 ± 0.8 | 20.4 ± 1.1 | 19.1 ± 0.8 | 19.8 ± 0.9 | 19.6 ± 1.4 | 20.0 ± 1.6 | 18.5 ± 1.2 | 20.1 ± 1.5 | |
Data are the mean ± SE. #Significantly different (P < 0.05) from premenopausal (main effect). *Significant change (P < 0.05) with training (main effect).
Skeletal muscle protein content
In muscle homogenate, the content of ERRα was similar in the two groups before training (Fig. 4). In the postmenopausal group but not in the premenopausal group, the level of ERRα increased (∼62%, P < 0.05) with training so that the protein content of ERRα was higher (P < 0.05) in the post‐ compared to premenopausal group after training.
Figure 4. Protein content of ERRα in vastus lateralis muscle.
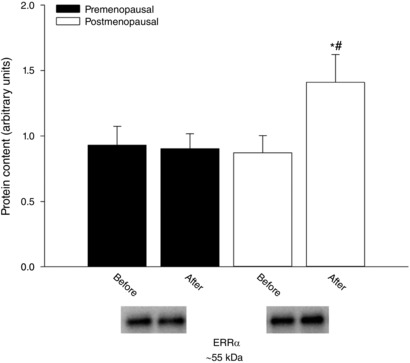
Protein content of ERRα in vastus lateralis muscle before and after training in pre‐ (n = 15) and postmenopausal (n = 13) women. *Significantly different (P < 0.05) from before training; #significantly different (P < 0.05) from premenopausal at that time point.
The protein level of complex I–V (OXPHOS) was similar in the two groups before training (Fig. 5). In premenopausal women, the protein content of complex V was higher (∼28%, P < 0.05) after training, whereas the content of complex II (∼50%), III (∼33%) and IV (∼33%) was higher (P < 0.05) in the postmenopausal group after training. The level of complex II and III was higher (P < 0.05) in the post‐ compared to premenopausal women after training. A negative correlation (r 2 = 0.35, P = 0.047) was detected between the training‐induced change in complex IV and lactate release during 10 W in postmenopausal women
Figure 5. Protein content of mitochondrial complex I–V in vastus lateralis muscle.
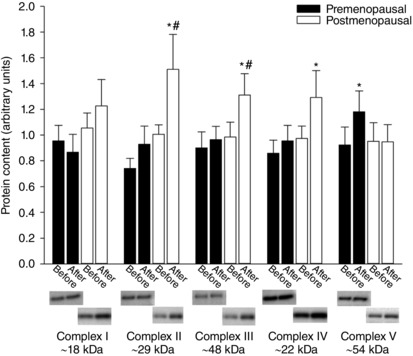
Protein content of mitochondrial complex I–V in vastus lateralis muscle before and after training in pre‐ (n = 14–17) and postmenopausal (n = 12–14) women. *Significantly different (P < 0.05) from before training; #significantly different (P < 0.05) from premenopausal at that time point.
Before training, the protein content of eNOS, COX‐1, COX‐2, PGI2 synthase and TXA2 synthase was similar in the two groups of women (Fig. 6). In the premenopausal women, the level of eNOS (∼29%) and COX‐2 (∼60%) was higher (P < 0.05) after training. In the postmenopausal women, the level of eNOS (∼49%), COX‐1 (∼55%) and COX‐2 (∼105%) was increased (P < 0.05) with training. These effects of training resulted in a higher (P < 0.05) level of eNOS and COX‐1, although there were similar levels of COX‐2 in the post‐ compared to premenopausal women after training. The protein content of PGI2 synthase and TXA2 synthase was not changed as a result of training.
Figure 6. Protein content of eNOS, COX‐1, COX‐2, PGI2 synthase and TXA2 synthase in vastus lateralis muscle.
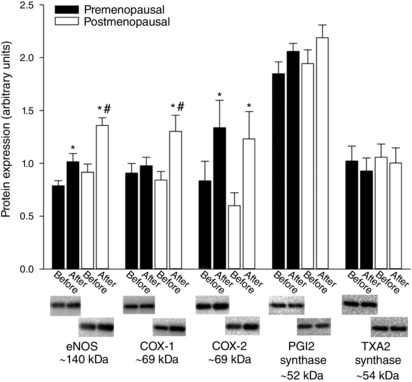
Protein content of eNOS, COX‐1, COX‐2, PGI2 synthase and TXA2 synthase in vastus lateralis muscle before and after training in pre‐ (n = 16–17) and postmenopausal (n = 13–14) women. *Significantly different (P < 0.05) from before training; #significantly different (P < 0.05) from premenopausal at that time point.
Discussion
There were two primary findings of the present study. (i) A period of aerobic high‐intensity exercise training induced leg vascular and skeletal muscle mitochondrial adaptations in both pre‐ and postmenopausal women; however, these effects of training were most pronounced in postmenopausal women. (ii) Before initiation of training, pre‐ and postmenopausal women displayed a similar vascular and metabolic response to acute exercise engaging the knee‐extensor muscles.
Role of menopausal status in the adaptive response to exercise training
Salient responses to aerobic exercise training include augmented maximal skeletal muscle blood flow and mitochondrial oxidative capacity. Moreover, exercise training leads to a more precise matching of O2 delivery and oxidative metabolism during submaximal exercise through optimization of microvascular blood flow distribution in the muscle (Saltin et al. 1976; Kalliokoski et al. 2001; Hellsten & Nyberg, 2015). In the present study, the difference between O2 delivery and O2 uptake was reduced in the pre‐ and postmenopausal women, indicating that training improved the matching of blood flow and oxidative metabolism in both groups. This effect of training was evident in both groups of women and, interestingly, the effect was more pronounced in the postmenopausal group during the highest workload. Along these lines, lactate release from the exercising leg was reduced after training only in postmenopausal women, indicating that, in this group, the contribution of energy from aerobic sources was enhanced. This effect of training is in agreement with the marked increase in mitochondrial content in postmenopausal women because a lower lactate production following exercise training has been shown to be a result of increased mitochondrial volume (Holloszy, 1967; Boushel et al. 2014). One mechanism underlying the more prominent response to exercise training in postmenopausal women could be an enhanced signalling through the ERRα pathway. ERRα has been shown to be an effector of peroxisome proliferator‐activated receptor γ coactivator 1α (PGC‐1α) and a regulator of genes involved in oxidative phosphorylation and mitochondrial biogenesis (Schreiber et al. 2004). In agreement with this proposed mechanism of action, the skeletal muscle protein content of ERRα was found to increase in postmenopausal women only. Because oestrogens readily interact with ERRα (Vanacker et al. 1999), this pathway might not be further stimulated by muscle contraction in the premenopausal state. In this setting, a loss of oestrogens may lead to an enhanced contraction‐induced signalling through the ERRα pathway. This altered signalling in response to contraction may require sufficiently high exercise intensity because intense exercise (Nyberg et al. 2016a,b) but not low‐ to moderate‐intensity exercise (Pierce et al. 2011; Moreau et al. 2013) is associated with improved vascular function in postmenopausal women. However, it is also plausible that the proposed contraction‐activated ERRα pathway is no longer as readily stimulated many years after menopause because the latter studies used late postmenopausal women (Pierce et al. 2011; Moreau et al. 2013. The putative role of the PGC‐1α ‐ ERRα signalling pathway in mitochondrial adaptations to exercise training in postmenopausal women deserves further investigation.
An improved matching of microvascular blood flow and oxidative metabolism in contracting skeletal muscle could be explained, at least in part, by the enhanced function of the NO and prostanoid systems because these systems have been shown to be essential for exercise hyperaemia (Radegran & Saltin, 1999; Mortensen et al. 2007) and to be improved with exercise training (Nyberg et al. 2012). In agreement, skeletal muscle eNOS and COX‐2 content was increased in both groups, indicating that both the capacity and the function of these systems were improved. Also, the more pronounced increase in content of eNOS with training in postmenopausal women is in agreement with an enhanced ERRα signalling in this group of women because ERRα is involved in the regulation of eNOS mRNA and protein expression (Sumi & Ignarro, 2003).
Despite substantial peripheral training adaptations in postmenopausal women, including increased skeletal muscle mitochondrial content, no increase in leg arterio‐venous O2 difference was observed. Exercise training has been shown to increase O2 extraction and reduce blood flow during submaximal exercise (Kiens et al. 1993; Kalliokoski et al. 2001; Hellsten & Nyberg, 2015), although this is not a consistent finding (Beere et al. 1999; Mortensen et al. 2012; Gifford et al. 2016). The reason for the lack of change is not clear, although it may be that more enhanced structural and functional adaptations than those observed in the present study are needed for a greater O2 extraction during submaximal exercise.
Skeletal muscle blood flow and O2 delivery are closely matched to the O2 demand of the contracting muscles during one‐leg knee extensor exercise and submaximal cycling (Andersen & Saltin, 1985; Gonzalez‐Alonso et al., 2002, 2008; Mortensen et al. 2008). Given the unaltered leg arterio‐venous O2 difference, the lower blood flow in postmenopausal women was likely a consequence of reduced O2 demand of the exercising leg. It is well established that exercise training can reduce the metabolic cost of locomotion as indicated by a lower steady‐state O2 consumption at a given submaximal intensity following a period of training (Bransford & Howley, 1977; Proctor et al. 2001; Nyberg et al. 2016b). Such an effect of training was also observed in the present study because leg O2 uptake during exercise was lower in postmenopausal women after training. Although more efficient mitochondria (i.e. an improved P/O ratio) is an attractive candidate for the lower O2 uptake, the finding of a similar P/O ratio in sedentary and well trained subjects (Mogensen et al. 2006) suggests that other mechanisms may be more important. Both in vitro (Barclay, 1996 ) and in vivo studies (Krustrup et al. 2008) have provided evidence showing that type I fibres are more economical than type II fibres and enhanced PGC‐1α signalling has also been shown to induce fibre type switching from fast, glycolytic fibres towards slow oxidative fibres (Lin et al. 2002). Hence, an enhanced PGC‐1α signalling via ERRα in postmenopausal women may have induced not only an increased mitochondrial capacity, but also a fibre type switch towards more oxidative fibres that reduced the metabolic cost of contraction. In addition, the reduced oxidative metabolism may also reflect an optimized recruitment of accessory and/or active muscle fibres (higher dependence on type I fibres), which would reduce the overall O2 demand (Krustrup et al. 2008), although the mechanisms by which menopausal status affects such adaptations are unclear.
The classical view of is that maximal rates of O2 utilization in skeletal muscle are limited under most circumstances by the ability of the heart to deliver O2 to the mitochondria (Saltin & Strange, 1992). This is probably true in physically active subjects (Levine, 2008; Hellsten & Nyberg, 2015); however, evidence for being limited by the capacity of the mitochondria to utilize O2 in sedentary subjects has been reported (Jacobs et al. 2013; Gifford et al. 2016). Hence, the rate‐limiting step in the O2 cascade may also be shifted in postmenopausal women because the action of skeletal muscle ERα has been demonstrated to be critical for mitochondrial function (Ribas et al. 2016). Accordingly, although the difference in the training‐induced change in and peak workload between the two groups did not reach statistical significance, the numerically greater change in the premenopausal group is intriguing. Because this observation was paralleled by enhanced peripheral adaptations in postmenopausal women (although the functional adaptations were detected during knee‐extensor exercise where muscle blood flow is not expected to be limited by cardiac output), it may be speculated that the increase in in this group was mediated by improved mitochondrial capacity, whereas the increase in premenopausal women was mediated by a greater peak cardiac output.
Another observation in the present study was a training‐induced reduction in heart rate and blood pressure during exercise observed only in postmenopausal women. These adaptations are in agreement with previous findings (Saltin et al. 1976; Mortensen et al. 2013) and the lower heart rate observed in the present study probably reflects an increased stroke volume in addition to lower cardiac output as a result of the reduced leg blood flow (Hellsten & Nyberg, 2015). Local factors within the skeletal muscle probably play an important role in this altered haemodynamic response to acute exercise with training. Accordingly, afferent fibres located within skeletal muscles respond to mechanical distortion (group III) and changes in the chemical environment (group IV) (e.g. interstitial pH, ATP and lactate), which contributes to the increase in sympathetic nervous activity and consequently heart rate and blood pressure during exercise (i.e. the exercise pressor reflex) (Mitchell et al. 1983; Fadel, 2015). The observed reduction in lactate release with training probably reflects lower interstitial levels and could partly explain the lower heart rate and blood pressure during exercise observed only in the postmenopausal women after training.
Longitudinal and cross‐sectional data have provided compelling evidence that exercise training leads to an increase in total haemoglobin mass, whereas hematocrit either remains unaltered or is slightly reduced as a result of plasma expansion (Hellsten & Nyberg, 2015). The reason that the Hb concentration was only reduced in the postmenopausal women after the training is unclear, although it may be related to a greater increase in plasma volume or potentially a reduced ability to synthesize erythrocytes in the premenopausal group.
Role of menopausal status in the regulation of exercise hyperaemia
There is accumulating evidence for an impaired blood flow response to exercise engaging the leg muscles in older postmenopausal women (Proctor et al. 2003; Parker et al. 2008). This altered vascular response may be attributable to the loss of sex hormones associated with menopause rather than age per se because exercise hyperaemia has been reported to be reduced in the early phase following the menopausal transition (Moore et al. 2012) and menopausal stage (and not age) appears to predict the vascular response to exercise in women (Parker et al. 2011). Based on these findings, we hypothesized that blood flow and O2 delivery to the exercising leg would be lower in the postmenopausal women before training. However, we were not able to detect any differences in the haemodynamic response between the two groups. This finding of similar exercise hyperaemia is in contrast to observations made in the study by Moore et al. (2012) in which late peri‐ and early postmenopausal women displayed lower blood flow and vascular conductance than early perimenopausal women during knee‐extensor exercise. This discrepancy may be explained by physiological changes associated with the transition from the pre‐ to the early perimenopausal phase because these changes may lead to compensatory vascular mechanisms and consequently enhanced exercise hyperaemia during the menopausal transition (Clarkson et al. 2013; Hodis et al. 2016). The finding that O2 uptake and lactate release were similar in the two groups of women prior to training suggests that the matching of O2 delivery and oxidative metabolism was not affected by the menopausal transition (Proctor & Moore, 2012). This preserved capacity of the circulation to deliver O2 to meet the O2 demand of the contracting muscles despite a marked reduction in vascular function, as assessed by arterial ACh and epoprostenol infusion, in the present group of postmenopausal women (Nyberg et al. 2016a) is probably the result of a greater contribution from redundant vasodilator mechanisms (Hellsten & Nyberg, 2015; Joyner & Casey, 2015). Because the function of the prostanoid system was found to be unaffected by the menopausal transition in the present group of women (Nyberg et al. 2016a), it is plausible that this system compensated for an impaired function of the NO system (Novella et al. 2012) given the reported redundancy between these vasodilator systems (Radegran & Saltin, 1999; Mortensen et al. 2007).
Experimental considerations
Despite strict inclusion criteria aiming to limit the confounding influence of age, it cannot be completely ruled out that the mean age difference of 4 years may have influenced study outcomes. However, the primary reason for the observed differences between the groups was probably the loss of oestrogens. We determined the content of relevant vasoactive enzymes and mitochondrial proteins in skeletal muscle samples in parallel with an assessment of the content of ERRα, aiming to understand whether there was a concomitant adaptation in the different proteins and whether the two groups of women responded differently. However, to what extent the observed changes in proteins had a direct functional consequence cannot be known because functional measures of vasoactive systems and mitochondrial respiration were not assessed. Lastly, the reduced sample size in the premenopausal group after training could also have impacted the study outcomes. However, the calculated power for this group was adequate and exclusion of the premenopausal women without post‐training data did not change the outcome of the statistical analyses.
Conclusions
Aerobic high‐intensity exercise training induced adaptations in leg vascular function and skeletal muscle mitochondrial proteins in pre‐ and recent postmenopausal women, with the greatest effect being attained in the postmenopausal women. This altered sensitivity to exercise training in postmenopausal women was accompanied by a greater upregulation of skeletal muscle ERRα and we propose that contraction‐induced signalling through the ERRα pathway may be more pronounced in the absence of oestrogens. Furthermore, despite reductions in vascular function, the hyperaemic response to acute exercise is preserved in the early postmenopausal phase.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
MN, BS, JB and YH conceived and designed the experiments. MN, JE, CMM, CBA, KMBEH, IFG, NV, RFS and YH collected, analysed and interpreted the data. MN, JE, CMM, CBA, KMBEH, IFG, NV, RFS, BS, JB and YH drafted the article and revised it critically for important intellectual content. All authors have read and approved the final submission. All authors agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The study is part of The Copenhagen Women Study for which financial support was provided by the University of Copenhagen Excellence Programme for Interdisciplinary Research. The study was also supported by The Danish Ministry of Culture: Sports Research.
Acknowledgements
Rasmus Damsgaard, Karina Olsen and Gemma Kroos are gratefully acknowledged for their excellent technical assistance.
References
- Andersen P & Saltin B (1985). Maximal perfusion of skeletal muscle in man. J Physiol 366, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay CJ (1996). Mechanical efficiency and fatigue of fast and slow muscles of the mouse. J Physiol 497, 781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW & Higginbotham MB (1999). Aerobic exercise training can reverse age‐related peripheral circulatory changes in healthy older men. Circulation 100, 1085–1094. [DOI] [PubMed] [Google Scholar]
- Blomqvist CG & Saltin B (1983). Cardiovascular adaptations to physical training. Annu Rev Physiol 45, 169–189. [DOI] [PubMed] [Google Scholar]
- Boushel R, Lundby C, Qvortrup K & Sahlin K (2014). Mitochondrial plasticity with exercise training and extreme environments. Exerc Sport Sci Rev 42, 169–174. [DOI] [PubMed] [Google Scholar]
- Bransford DR & Howley ET (1977). Oxygen cost of running in trained and untrained men and women. Med Sci Sports 9, 41–44. [PubMed] [Google Scholar]
- Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A & Russell AP (2005). Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TB, Melendez GC & Appt SE (2013). Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause 20, 342–353. [DOI] [PubMed] [Google Scholar]
- Enns DL & Tiidus PM (2010). The influence of estrogen on skeletal muscle: sex matters. Sports Med 40, 41–58. [DOI] [PubMed] [Google Scholar]
- Fadel PJ (2015). Reflex control of the circulation during exercise. Scand J Med Sci Sports 25 (Suppl 4), 74–82. [DOI] [PubMed] [Google Scholar]
- Gifford JR, Garten RS, Nelson AD, Trinity JD, Layec G, Witman MA, Weavil JC, Mangum T, Hart C, Etheredge C, Jessop J, Bledsoe A, Morgan DE, Wray DW, Rossman MJ & Richardson RS (2016). Symmorphosis and skeletal muscle VO2 max: in vivo and in vitro measures reveal differing constraints in the exercise‐trained and untrained human. J Physiol 594, 1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA & Dufour SP (2008). Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586, 2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Alonso J, Olsen DB & Saltin B (2002). Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Hellsten Y & Nyberg M (2015). Cardiovascular adaptations to exercise training. Compr Physiol 6, 1–32. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang‐Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH & Azen SP (2016). Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 374, 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO (1967). Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242, 2278–2282. [PubMed] [Google Scholar]
- Jacobs RA, Fluck D, Bonne TC, Burgi S, Christensen PM, Toigo M & Lundby C (2013). Improvements in exercise performance with high‐intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol (1985 ) 115, 785–793. [DOI] [PubMed] [Google Scholar]
- Joyner MJ & Casey DP (2015). Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95, 549–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J & Nuutila P (2001). Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance‐trained men. Am J Physiol Endocrinol Metab 280, E1015–E1021. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen‐Gustavsson B, Christensen NJ & Saltin B (1993). Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol 469, 459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Secher NH, Relu MU, Hellsten Y, Soderlund K & Bangsbo J (2008). Neuromuscular blockade of slow twitch muscle fibres elevates muscle oxygen uptake and energy turnover during submaximal exercise in humans. J Physiol 586, 6037–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD (2008). VO2max: what do we know, and what do we still need to know? J Physiol 586, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel‐Duby R & Spiegelman BM (2002). Transcriptional co‐activator PGC‐1 alpha drives the formation of slow‐twitch muscle fibres. Nature 418, 797–801. [DOI] [PubMed] [Google Scholar]
- Mandrup CM, Egelund J, Nyberg M, Lundberg Slingsby MH, Andersen C, Logstrup S, Bangsbo J, Suetta C, Stallknecht B & Hellsten Y (2016). Effects of high‐intensity training on cardiovascular risk factors in pre‐ and postmenopausal women. Am J Obstet Gynecol. [DOI] [PubMed] [Google Scholar]
- Menazza S & Murphy E (2016). The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res 118, 994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME & Karas RH (2005). Molecular and cellular basis of cardiovascular gender differences. Science 308, 1583–1587. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP & Iwamoto GA (1983). The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45, 229–242. [DOI] [PubMed] [Google Scholar]
- Mogensen M, Bagger M, Pedersen PK, Fernstrom M & Sahlin K (2006). Cycling efficiency in humans is related to low UCP3 content and to type I fibres but not to mitochondrial efficiency. J Physiol 571, 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Gonzales JU, Tucker SH, Elavsky S & Proctor DN (2012). Exercise‐induced vasodilation is associated with menopause stage in healthy middle‐aged women. Appl Physiol Nutr Metab 37, 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Stauffer BL, Kohrt WM & Seals DR (2013). Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98, 4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Damsgaard R, Dawson EA, Secher NH & Gonzalez‐Alonso J (2008). Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high‐intensity whole‐body exercise in humans. J Physiol 586, 2621–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez‐Alonso J, Damsgaard R, Saltin B & Hellsten Y (2007). Inhibition of nitric oxide and prostaglandins, but not endothelial‐derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581, 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Morkeberg J, Thaning P, Hellsten Y & Saltin B (2012). Two weeks of muscle immobilization impairs functional sympatholysis but increases exercise hyperemia and the vasodilatory responsiveness to infused ATP. Am J Physiol Heart Circ Physiol 302, H2074–H2082. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Svendsen JH, Ersboll M, Hellsten Y, Secher NH & Saltin B (2013). Skeletal muscle signaling and the heart rate and blood pressure response to exercise: insight from heart rate pacing during exercise with a trained and a deconditioned muscle group. Hypertension 61, 1126–1133. [DOI] [PubMed] [Google Scholar]
- Novella S, Dantas AP, Segarra G, Medina P & Hermenegildo C (2012). Vascular aging in women: is estrogen the fountain of youth? Front Physiol 3, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Christensen PM, Mortensen SP, Hellsten Y & Bangsbo J (2014a). Infusion of ATP increases leg oxygen delivery but not oxygen uptake in the initial phase of intense knee‐extensor exercise in humans. Exp Physiol 99, 1399–1408. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J & Hellsten Y (2016a). Early postmenopausal phase is associated with reduced prostacyclin‐induced vasodilation that is reversed by exercise training: The Copenhagen Women Study. Hypertension 68, 1011–1020. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Fiorenza M, Lund A, Christensen M, Romer T, Piil P, Hostrup M, Christensen PM, Holbek S, Ravnholt T, Gunnarsson TP & Bangsbo J (2016b). Adaptations to Speed Endurance Training in Highly Trained Soccer Players. Med Sci Sports Exerc 48, 1355–1364. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Jensen LG, Thaning P, Hellsten Y & Mortensen SP (2012). Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high‐intensity aerobic training. J Physiol 590, 1481–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Seidelin K, Andersen TR, Overby NN, Hellsten Y & Bangsbo J (2014b). Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am J Physiol Regul Integr Comp Physiol 306, R510–R517. [DOI] [PubMed] [Google Scholar]
- Parker B, Capizzi J, Augeri A, Grimaldi A, Proctor D & Thompson P (2011). Sex‐specific effect of aging on submaximal leg exercise hemodynamics in middle‐aged and older adults. Eur J Appl Physiol 111, 1369–1379. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD & Proctor DN (2008). Sex‐specific influence of aging on exercising leg blood flow. J Appl Physiol (1985 ) 104, 655–664. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Rook KM, Siddle NC, Bruce SA & Woledge RC (1993). Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 84, 95–98. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN & Seals DR (2011). Sex‐specific effects of habitual aerobic exercise on brachial artery flow‐mediated dilation in middle‐aged and older adults. Clin Sci (Lond) 120, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU & Leuenberger UA (2003). Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol (1985 ) 95, 1963–1970. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Miller JD, Dietz NM, Minson CT & Joyner MJ (2001). Reduced submaximal leg blood flow after high‐intensity aerobic training. J Appl Physiol 91, 2619–2627. [DOI] [PubMed] [Google Scholar]
- Proctor DN & Moore DJ (2012). Lifelong physical activity and blood flow to active muscles: sufficient supply to meet the demand. J Physiol 590, 5927–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radegran G & Saltin B (1999). Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol 276, H1951–H1960. [DOI] [PubMed] [Google Scholar]
- Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS & Hevener AL (2016). Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 8, 334ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B & Gollnick D (1976). The nature of the training response; peripheral and central adaptations of one‐legged exercise. Acta Physiol Scand 96, 289–305. [DOI] [PubMed] [Google Scholar]
- Saltin B & Strange S (1992). Maximal oxygen uptake: ‘old’ and ‘new’ arguments for a cardiovascular limitation. Med Sci Sports Exerc 24, 30–37. [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ & Kralli A (2004). The estrogen‐related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC‐1alpha)‐induced mitochondrial biogenesis. Proc Natl Acad Sci USA 101, 6472–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidelin K, Nyberg M, Piil P, Jorgensen NR, Hellsten Y & Bangsbo J (2017). Adaptations with intermittent exercise training in post‐ and premenopausal women. Med Sci Sports Exerc 49, 96–105. [DOI] [PubMed] [Google Scholar]
- Sumi D & Ignarro LJ (2003). Estrogen‐related receptor alpha 1 up‐regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci USA 100, 14451–14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J & Suominen H (2005). The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging 25, 297–304. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S & Salvetti A (1996). Menopause is associated with endothelial dysfunction in women. Hypertension 28, 576–582. [DOI] [PubMed] [Google Scholar]
- Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM & Pajak A (1994). Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation 90, 583–612. [DOI] [PubMed] [Google Scholar]
- Vanacker JM, Pettersson K, Gustafsson JA & Laudet V (1999). Transcriptional targets shared by estrogen receptor‐related receptors (ERRs) and estrogen receptor (ER)alpha, but not by ERbeta. EMBO J 18, 4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiik A, Hellsten Y, Berthelson P, Lundholm L, Fischer H & Jansson E (2009). Activation of estrogen response elements is mediated both via estrogen and muscle contractions in rat skeletal muscle myotubes. Am J Physiol Cell Physiol 296, C215–C220. [DOI] [PubMed] [Google Scholar]


