Abstract
Key points
Despite an attenuated fluctuation in ovarian hormone concentrations in well‐trained women, one in two of such women believe their menstrual cycle negatively impacts training and performance.
Forthcoming large international events will expose female athletes to hot environments, and studies evaluating aerobic exercise performance in such environments across the menstrual cycle are sparse, with mixed findings.
We have identified that autonomic heat loss responses at rest and during fixed‐intensity exercise in well‐trained women are not affected by menstrual cycle phase, but differ between dry and humid heat.
Furthermore, exercise performance is not different across the menstrual cycle, yet is lower in humid heat, in conjunction with reduced evaporative cooling.
Menstrual cycle phase does not appear to affect exercise performance in the heat in well‐trained women, but humidity impairs performance, probably due to reduced evaporative power.
Abstract
We studied thermoregulatory responses of ten well‐trained [, 57 (7) ml min−1 kg−1] eumenorrheic women exercising in dry and humid heat, across their menstrual cycle. They completed four trials, each of resting and cycling at fixed intensities (125 and 150 W), to assess autonomic regulation, then self‐paced intensity (30 min work trial), to assess behavioural regulation. Trials were in early‐follicular (EF) and mid‐luteal (ML) phases in dry (DRY) and humid (HUM) heat matched for wet bulb globe temperature (WBGT, 27°C). During rest and fixed‐intensity exercise, rectal temperature was ∼0.2°C higher in ML than EF (P < 0.01) independent of environment (P = 0.66). Mean skin temperature did not differ between menstrual phases (P ≥ 0.13) but was higher in DRY than HUM (P < 0.01). Local sweat rate and/or forearm blood flow differed as a function of menstrual phase and environment (interaction: P ≤ 0.01). Exercise performance did not differ between phases [EF: 257 (37), ML: 255 (43) kJ, P = 0.62], but was 7 (9)% higher in DRY than HUM [263 (39), 248 (40) kJ; P < 0.01] in conjunction with equivalent autonomic regulation and thermal strain but higher evaporative cooling [16 (6) W m2; P < 0.01]. In well‐trained women exercising in the heat: (1) menstrual phase did not affect performance, (2) humidity impaired performance due to reduced evaporative cooling despite matched WBGT and (3) behavioural responses nullified thermodynamic and autonomic differences associated with menstrual phase and dry vs. humid heat.
Keywords: exercise, thermoregulation, women
Key points
Despite an attenuated fluctuation in ovarian hormone concentrations in well‐trained women, one in two of such women believe their menstrual cycle negatively impacts training and performance.
Forthcoming large international events will expose female athletes to hot environments, and studies evaluating aerobic exercise performance in such environments across the menstrual cycle are sparse, with mixed findings.
We have identified that autonomic heat loss responses at rest and during fixed‐intensity exercise in well‐trained women are not affected by menstrual cycle phase, but differ between dry and humid heat.
Furthermore, exercise performance is not different across the menstrual cycle, yet is lower in humid heat, in conjunction with reduced evaporative cooling.
Menstrual cycle phase does not appear to affect exercise performance in the heat in well‐trained women, but humidity impairs performance, probably due to reduced evaporative power.
Abbreviations
- BP
blood pressure
- BSA
body surface area
- C
rate of heat transfer from convection
- Cres
rate of respiratory conductive heat transfer
- E
rate of evaporative heat loss
- EF
early follicular
- Emax
maximal evaporative capacity of the environment
- Ereq
required evaporative cooling for heat balance
- Eres
rate of respiratory evaporative heat transfer
- FBF
forearm blood flow
- FVR
forearm vascular resistance
- hc
convective heat transfer coefficient
- HR
heart rate
- HSI
heat strain index
- IAAF
International Association of Athletics Federations
- LR
Lewis Relation
- LSR
local sweat rate
- M
rate of metabolic heat production
- MAP
mean arterial pressure
- ML
mid‐luteal
- PA
ambient vapour pressure
- PETCO2
partial pressure of end‐tidal CO2
- PSk
saturated vapour pressure at the skin
cardiac output
- R
rate of heat transfer from radiation
- RER
respiratory exchange ratio
- RPE
rating of perceived exertion
- S
heat storage
- SV
stroke volume
- TA
ambient temperature
- b
mean body temperature
- Tcore
core body temperature
- TD
thermal discomfort
- Trec
rectal temperature
- TS
thermal sensation
- sk
mean skin temperature
- v
air velocity
rate of expired volume
rate of oxygen uptake
maximal rate of oxygen uptake
rate of carbon dioxide elimination
- W
rate of energy loss from external work generated
- WBGT
wet‐bulb globe temperature
- WBSR
whole‐body sweat rate
Introduction
In eumenorrheic women the approximate monthly rhythm of the reproductive cycle is divided into follicular and luteal phases based on the function of the uterus and ovary, and corresponding fluctuations in hormonal concentrations (Stephenson & Kolka, 1993). Progestogens and oestrogens, the steroidal ovarian hormones, influence several non‐reproductive organs and systems including thermoregulation (Charkoudian & Stachenfeld, 2014). Oestrogens generally promote heat dissipation and lower body temperature whereas progestogens have the opposite effect (Charkoudian & Stachenfeld, 2014). Core body temperature (T core) is regulated approximately 0.3 – 0.5°C higher during the luteal phase (Harvey & Crockett, 1932; Stephenson & Kolka, 1993). The notion of a shift in thermoregulatory set‐point is supported by an elevated T core at rest and during passive and active heat stress, and by an increased T core threshold for thermoregulatory effector responses such as sweating and cutaneous vasodilatation (Stachenfeld et al. 2000; Kuwahara et al. 2005a,b). This shift in set‐point and threshold results in higher T core during the luteal phase particularly when women exercise with environmental heat stress (Avellini et al. 1980; Carpenter & Nunneley, 1988; Kolka & Stephenson, 1997; Tenaglia et al. 1999; Janse de Jonge et al. 2012), leading several authors to suggest that women should avoid competition or face a disadvantage when performing exercise with environmental heat stress during their luteal phase (Stephenson & Kolka, 1993; Janse de Jonge, 2003; Charkoudian & Joyner, 2004; Janse de Jonge et al. 2012). Yet there is a need for norms and recommendations specific to these differing exercise responses (Charkoudian & Joyner, 2004), especially as there remains an under‐representation of women in sport and exercise research (Costello et al. 2014) and that > 40% of women believe that their menstrual cycle has a negative impact on training and performance (Bruinvels et al. 2016).
Only five published investigations appear to have tested the notion of a reduced exercise heat‐stress tolerance or performance during the luteal phase of the menstrual cycle. Two earlier investigations, which employed a fixed‐intensity (constant power) approach, identified that exercise heat stress tolerance was reduced by ∼11 and ∼16% during the mid‐luteal compared to early follicular phase (Avellini et al. 1980; Tenaglia et al. 1999), whereas Kolka & Stephenson (1997) found no difference between early and late follicular or mid‐luteal phases. More recently, Sunderland & Nevill (2003) demonstrated that high‐intensity intermittent running performance in the heat remained unaltered between the mid‐follicular and mid‐luteal phases, whereas Janse de Jonge et al. (2012) reported a ∼6% reduction in endurance time in the mid‐luteal phase during an incremental test to exhaustion in the heat.
From a thermoregulatory standpoint, the limited and conflicting results above cannot be directly applied to a well‐trained, competitive woman for several reasons. First, training status markedly alters effector responses, with trained women demonstrating enhanced sweating and cutaneous vasodilatation compared to untrained women, and aerobic training per se improving these effector responses (Roberts et al. 1977; Drinkwater, 1984; Kuwahara et al. 2005a,b). Trained and competitive female endurance athletes typically display a maximal aerobic uptake () of > 3 l min−1 or > 55 ml kg−1 min−1 (Drinkwater, 1984), so it can be reasoned that in only two (Avellini et al. 1980; Sunderland & Nevill, 2003) of the above‐mentioned investigations would participants fit these criteria. Second and relatedly, trained women have reduced reproductive hormone concentrations and fluctuation between menstrual phases and associated smaller difference in the bi‐phasic T core (Dale et al. 1979; Bullen et al. 1984; Kuwahara et al. 2005a,b). Indeed, Kuwahara et al. (2005a,b) have observed less phase‐related differences in effector and T core responses for trained than untrained women. Third, all previous investigations have used a bout of fixed‐intensity (constant power), sub‐maximal exercise to exhaustion as their mode of investigation. These protocols have poor face‐validity, but more importantly they deprive us of our most effective, powerful and nearly limitless (Benzinger, 1969; Parsons, 2014) form of thermoregulation: behaviour. During (face‐valid) exercise heat stress when able to self‐pace (variable intensity), we have demonstrated that men ‘behave’ by reducing exercise intensity, and therefore metabolic heat production, which modifies heat exchange and allows for an improved compensability of the thermal environment principally via a reduction in required evaporation, that ultimately results in a reduced thermoregulatory strain (Schlader et al. 2011a,b). There is, however, evidence from passive heat stress models indicating that such thermoregulatory behaviour is altered by the menstrual cycle, with reports that the threshold for an affective/behavioural response is shifted during the luteal phase (Cabanac et al. 1971; Scarperi & Bleichert, 1983; Shoemaker & Refinetti, 1996); this remains untested during exercise.
Forthcoming (at the time of writing) large international events (2016 Summer Olympics in Rio de Janeiro, 2018 Commonwealth Games on the Gold Coast, 2018 Asian Games in Jakarta, 2019 IAAF World Championships in Doha) will expose athletes to high levels of environmental heat stress and the number of women participating at this elite level is ever increasing, with the latest editions of the above‐named international events reporting 39–44% of competitors as women. However, these environments differ in their ambient thermal profile, from warm‐humid to dry‐hot, with the latter usually permitting greater (full) evaporation of sweat whilst the former does not and thus high rates of evaporative cooling are not possible. Previous investigations have determined that when exposed to approximate environmental heat but humid versus dry in nature, women sweat less but more efficiently upon exercising when exposed to humid heat as demonstrated by similar T core responses (Morimoto et al. 1967; Frye & Kamon, 1983) although no effect of the menstrual phase was apparent (Shapiro et al. 1980). However, the same limitations as discussed above apply to these studies (training status and fixed‐intensity exercise) and these potential differences in thermoregulatory control across menstrual phase may interact with differences in the thermal environment (i.e. dry vs. evaporative heat transfer), which would warrant examining in highly trained women (i.e. by virtue of higher heat loss requirement). Furthermore, to our knowledge no published investigation has compared how women perform when exposed to equivalent dry and humid heat during exercise.
From the above, it can be concluded that research into how well‐trained women respond to environmental heat stress across the menstrual cycle is markedly sparse. Given these limited and conflicted findings, a hypothesis‐driven experiment was not possible. Instead, we sought to characterise and compare the behavioural and autonomic thermoregulatory responses of well‐trained, eumenorrheic women to exercise when exposed to equivalent dry and humid heat stress during the early follicular and mid‐luteal phase of their menstrual cycle.
Methods
Ethical approval
The study was approved by the Massey University Human Ethics Committee: Southern A (14/99), and performed in accordance with the latest revision of the Declaration of Helsinki, with each participant providing informed, written consent.
Participants
Thirteen eumenorrhoeic, aerobically well‐trained and competitive women cyclists and triathletes volunteered for this study. The participants’ mean (SD) characteristics were: age, 34 (9) years; height, 1.65 (0.05) m; mass, 64 (6) kg; body surface area (BSA), 1.70 (0.09) m2; , 58 (9) ml min−1 kg−1; per cent body fat, 24 (5)%; and peak aerobic power, 270 (35) W.
Experimental overview
All trials were during autumn to spring in Palmerston North, when temperatures rarely exceed 22°C. No participant had spent time in warmer climates or training environments within the month preceding testing. All participants attended the laboratory on six occasions: (1) preliminary submaximal and maximal tests, (2) experimental familiarization and (3–6) experimental trials. The four experimental trials were a full crossover of menstrual phase (early follicular and mid‐luteal) and environment [dry and humid, at matched wet bulb globe temperature (WBGT)]. All trials were counterbalanced except that the same order of dry or humid environment was retained for each menstrual phase within participants. Experimental trials were conducted at the same time of day (± 1 h), and following > 24 h of dietary and exercise control. Each trial consisted of 12 min fixed‐intensity cycling followed immediately by a 30 min self‐paced cycling performance trial. All exercise was on an electronically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) with participant‐specific set up for the seat, handle bars and pedals.
Preliminary testing and familiarisation
Submaximal and maximal capacity tests were undertaken in the follicular phase to minimise potential physiological effects of the menstrual cycle on performance. Following body mass and height measurement, preliminary testing was conducted in a temperate laboratory environment (18–22 °C) with a fan‐generated airflow of 19 km h−1 facing participants. The submaximal test consisted of four consecutive 6 min power outputs, 100, 125, 150 and 175 W, at comfortable but constant cadence. Oxygen consumption was measured during the last 2 min of each stage. Following 10 min rest, a maximal capacity test was undertaken to measure . Work rate began at 100 W and consisted of increments at 25 W min−1 until volitional fatigue. The linear relation between power output and was subsequently used to calculate workload for experimental trials, as 75% (Jeukendrup et al. 1996).
At least 24 h following preliminary testing, the familiarisation trial was undertaken to ensure participants were accustomed to the experimental procedures and to minimise learning effects. These trials replicated entirely the experimental trials outlined below.
Dietary and exercise control
The day of and prior to any experimental trial was marked by abstinence from alcohol, exercise and only habitual caffeine use (as abstinence would in itself confound from withdrawal effects). Additionally, participants were provided with a standardised dinner [two Watties Snack Meals, Heinz Watties, Hastings, New Zealand: 1363 (247) kJ providing 53 (6) g carbohydrate, 12 (4) g protein and 8 (0.3) g fat] the night preceding the trial and were asked to consume the same light meal (consisting of toast or cereal) between 2 and 4 h prior to visiting the laboratory for the trial. This dietary and exercise control minimised variation in pre‐trial metabolic state. Fluid was encouraged and a euhydrated state was further ensured by instructing the participants to drink 500 ml of water 2 h prior to each trial.
Menstrual cycle phase and type of heat stress
Participants were tested during the early follicular (EF) and mid‐luteal (ML) phases, to maximise differences in oestrogen and progesterone concentrations and permit comparison with results of previous studies (Avellini et al. 1980; Kolka & Stephenson, 1997; Tenaglia et al. 1999; Sunderland & Nevill, 2003; Janse de Jonge et al. 2012). Testing occurred on days 3 (1) and 6 (1) (EF), and 18 (2) and 21 (3) (ML) following start of menses, with 12 (2) days separating the second EF and first ML trials. Hatcher et al. (1988) suggested that a progesterone level of > 9.5 nmol l−1 is good evidence that ovulation has occurred. Using this criterion, three participants were excluded from all data analysis whilst another had lower [progesterone] in her first ML trial (4.1 nmol l−1). This participant's results were examined and found to be consistent in magnitude and direction with those of other participants. Furthermore, all statistical analyses were completed with and without this participant and omitting her had no impact on the magnitude or direction of statistical results other than reducing observed power. Therefore, we included her data in the final analyses for n = 10.
In accordance with previous studies investigating the influence of humid (HUM) versus dry (DRY) environmental heat in women (Morimoto et al. 1967; Shapiro et al. 1980; Frye & Kamon, 1983) heat stress was indexed using WBGT because, while limited, it is the most widely used empirical index (Brotherhood, 2008; Budd, 2008). Our decision‐making was guided by what typical or possible extreme conditions athletes would encounter at the 2016 Summer Olympics and 2018 Commonwealth Games (humid) compared to the 2019 IAAF World Championships (dry), so a WBGT equivalent to 27°C was chosen to elicit our HUM [29 (1)°C, 81 (3)% relative humidity] and DRY [34 (0.2)°C, 41 (3)% relative humidity] environments. Absolute humidity in these two environments was 3.4 (0.1) and 2.2 (0.3) kPa, respectively. Within each menstrual phase, exposure to DRY and HUM environments was separated by 3 (1) days.
Experimental procedure
These four sessions were conducted in the same environmental chamber with the 19 km h−1 airflow mentioned above; however, the fan was turned off for each ∼2 min data collection period (of each 6 min stage/interval) to minimise interference of airflow on measurement. On arrival to the laboratory participants voided to produce a urine sample to confirm a urine‐specific gravity < 1.010 and hence euhydration, nude weight was recorded and they then self‐inserted a rectal thermistor. A blood sample was obtained from the antecubital vein, following which participants entered the environmental chamber wearing only cycling shorts and top, shoes and socks. Participants rested seated on the ergometer for 20 min during which they were instrumented and baseline measurements were recorded. Participants then completed 6 min cycling at each of 125 and 150 W, to allow sufficient warm‐up and fixed‐intensity responses to be recorded. Physiological measurements taken during the final 2 min of each intensity included expired gas, heart rate (HR), blood pressure (BP), forearm blood flow (FBF), cardiac output () and perceptual responses, whilst rectal (T rec) and skin ( sk) temperatures as well as local sweat rate (LSR) were measured continuously. Immediately on completion of the 150 W bout, the ergometer was set to linear mode based on the formula of Jeukendrup et al. (1996), where participants were instructed to perform as much work as possible over 30 min. During this 30 min self‐paced period, work completed (kJ), HR, expired gas and perceptual responses were recorded every 6 min, whilst T rec, sk and LSR were measured continuously. Total work completed was used as our performance criterion, whereas the time profile of power output was used as our behavioural criterion. Immediately following the 30 min self‐paced exercise, FBF was measured and following towel‐drying participants’ nude weight was recorded to allow estimation of whole‐body sweat rate (WBSR). Tap water at 20°C and in aliquots of 3 ml kg−1 bodyweight was provided to drink ad libitum either at 15 min intervals or when requested throughout each trial to minimise dehydration.
Measurements
Anthropometric
Participant height and mass were measured using a stadiometer (Seca, Hamburg, Germany; accurate to 0.1 cm) and scale (Jadever, Taipei, Taiwan; accurate to 0.01 kg), from which surface area was estimated (Dubois & Dubois, 1916). Body composition was measured using multi‐frequency bioelectrical impedance analysis (InBody 230, Seoul, Korea) using a standard procedure (Kyle et al. 2004).
Respiratory
Expired respiratory gases were collected and analysed for and carbon dioxide elimination (), ventilation () and respiratory exchange ratio (RER) using an online, breath‐by‐breath system (VacuMed Vista Turbofit, Ventura, CA, USA) using a 30 s average. The system was calibrated before each trial using a zero and β‐standard gas concentrations, and volume (VacuMed 3l Calibration Syringe).
Cardiovascular
HR was recorded from detection of R–R intervals (Polar Vantage XL, Polar Electro, Kempele, Finland) whilst BP was measured using a stethoscope and a sphygmomanometer over the right brachial artery, in duplicate and by the same experienced operator. Mean arterial pressure (MAP) was calculated as diastolic blood pressure + 1/3 pulse pressure. FBF was measured using venous occlusion plethysmography (Whitney, 1953) with a mercury‐in‐silastic strain‐gauge on the widest part of the forearm, supported at heart level. The voltage output was acquired (PowerLab, ADInstruments, Dunedin, New Zealand) and displayed (Labchart Pro, ADInstruments) in real time, as well as for offline analysis. The venous occlusion pressure was 50 mmHg. Forearm vascular resistance (FVR) was calculated as FBF/MAP. was measured using CO2 rebreathing (Defares, 1958), as described previously in our laboratory (Schlader et al. 2010). End‐tidal CO2 (P ETCO2) during the rebreathing procedure was measured (O2/CO2 gas analyser, ADInstruments), with data acquisition and display as mentioned above (AD Instruments). Differences between P ETCO2 and venous and arterial were corrected according to Paterson & Cunningham (1976) and Jones et al. (1979). The CO2 content difference was calculated according to McHardy (1967). Stroke volume (SV) was calculated from the Fick equation.
Body temperatures
T core was indexed from T rec, measured using a calibrated rectal thermistor (Covidien Mon‐a‐Therm, USA; accurate to 0.1°C) inserted 10 cm beyond the anal sphincter. sk was measured at four sites using calibrated skin thermistors (Grant Instrument Ltd, Cambridge, UK; accurate to 0.2°C) fastened on calf, thigh, chest and forearm using surgical tape (3M Healthcare, USA). Area‐weighted mean sk was calculated according to the equation of Ramanathan (1964). Core and skin temperatures were recorded using TracerDAQ software (Measurement Computing Corporation, Norton, MA, USA). To account for the relative influence of T core and sk on the activation of heat loss responses (Hertzman et al. 1952) mean body temperature ( b) was calculated as: 0.8 × T rec + 0.2 × sk (Stolwijk & Hardy, 1966).
Sweat rates
LSR was measured using a ventilated capsule (Graichen et al. 1982). The capsule (3.5 cm2) was attached to the neck dorsally and ventilated with dry air at 0.4 litres min−1. The effluent gas was sensed for humidity (Honeywell Ltd, New Zealand) and temperature (National Semiconductor, Santa Clara, CA, USA). The neck was used because all limbs were used for other measures and it was not exposed directly to the fan. WBSR was estimated from body mass loss, corrected for fluid consumed.
Thermodynamics
Heat stress compensability was estimated using the heat strain index (HSI), with > 1.0 indicating uncompensable heat stress (Cheung et al. 2000). HSI was calculated as the ratio of the required evaporative cooling for heat balance (E req; in W m2) and the maximal evaporative capacity of the environment (E max; in W m2) (Belding & Hatch, 1955). E req was calculated as , where: M is the rate of metabolic heat production (W m2), calculated as follows (Kenney, 1998): . W is the rate of energy lost as external work (W m2). C + R is the rate of heat transfer from convection (C; W m2) and radiation (R; W m2), calculated as the sum of: (Kerslake, 1972) and (Kenney, 1998) where: h c is the convective heat transfer coefficient (W m2 °C) (Kerslake, 1972) and T A is the ambient temperature (°C). C res + E res is the rate of respiratory conductive (C res) and evaporative (E res) heat transfer, and was calculated as follows (Kenney, 1998): , where P A is ambient vapour pressure (kPa). E max was calculated as , where LR is the Lewis Relation (16.5°C kPa) and P Sk is the saturated vapour pressure at the skin (in kPa). Additionally, the rate of evaporative heat loss (E; in W m2) was estimated according to the following equation (Kerslake, 1972): where v is air velocity (0.5 m s−1). The rate of body heat storage (S, in W m2) at every recording interval was calculated as follows: .
Perceptual
Borg's rating of perceived exertion (RPE) was measured using the 15‐grade scale, from 6 to 20 (Borg, 1970), whilst thermal sensation (TS) and discomfort (TD) were measured using seven and four point scales, as described by Gagge et al. (1967).
Hormonal
Blood was collected by venipuncture into a vacutainer (Becton‐Dickinson, Oxford, UK) containing clot activator. Following inversion and clotting, the whole blood was centrifuged at 4°C and 805 g for 12 min and aliquots of serum were transferred into Eppendorf tubes (Genuine Axygen Quality, USA) and stored at −80 °C until further analysis. Serum samples were analysed using enzyme‐linked immune assays for 17β‐oestradiol (Demeditec Diagnostics, Kiel, Germany) and progesterone (IBL International, Hamburg, Germany) with a sensitivity of 22.7 pmol l−1 and 0.14 nmol l−1, respectively, and an intra‐assay variation of 4 and 6%, respectively.
Statistical analysis
All statistical analyses were performed with SPSS software for windows (IBM SPSS Statistics 20, NY, USA). Descriptive values were obtained and reported as means and standard deviation (SD) unless stated otherwise. Levene's test was used to ensure data did not differ substantially from a normal distribution. Data were analysed by three‐way (menstrual phase × heat stress × time) ANOVA for repeated measures. Resting and fixed‐intensity exercise data were analysed separately from self‐paced exercise data. Sphericity was assessed and where the assumption of sphericity could not be assumed, adjustments to the degrees of freedom were made (ε > 0.75 = Huynh‐Feldt; ε < 0.75 = Greenhouse‐Geisser). Where main or interaction effects occurred, post hoc pairwise analyses were performed using a paired samples t‐test (Bonferroni correction where relevant), with statistical significance set at P ≤ 0.05. To examine how menstrual phase and type of heat stress affected the thermal control of the effector responses (LSR and FBF), the visually determined linear portion of each response against b was analysed using simple linear regression [y = y 0+a * x] and compared using two‐way (menstrual phase × heat stress) ANOVA. The onset threshold was defined as the y‐intercept (y 0) of the regression line with values at baseline, while the thermosensitivity was defined as the slope (a) of the regression line. Given its putative role in the shift in thermoregulatory set‐point and threshold for effector responses between menstrual phases, we sought to further determine whether [progesterone] was associated with or predicted the key variables of exercise performance and resting T rec, FBF and LSR. First, we described the form and strength of bivariate association using Pearson's correlation coefficient for both the absolute values and change (Δ) between menstrual phases. Next we used hierarchical multiple regression to estimate the effect of [progesterone] on these key variables while controlling for potential confounding from [oestrogen].
Results
Ovarian hormone concentrations
Progesterone [EF: 1.7 (1.2) vs ML: 53.5 (51.8) nmol l−1] and 17β‐oestradiol [EF: 185 (166) vs ML: 364 (259) pmol l−1] concentrations were significantly higher in the luteal phase (both P < 0.001) but not different for each environment (P = 0.43 and P = 0.24, respectively).
Exercise performance and behaviour
Work capacity was similar between menstrual phases [EF: 257 (37) vs ML: 255 (43) kJ, P = 0.62] but was 7 (9)% higher in DRY than in HUM [263 (39) vs 248 (40) kJ; P = 0.001] (Fig. 1). Accordingly, mean power output was unaffected by menstrual phase (P = 0.87) but 5 (8)% higher in DRY than in HUM [146 (22) vs 139 (22) W; P < 0.01]. When viewing behaviour as the self‐paced exercise profile, behaviour differed between environments as a function of menstrual phase (environment × phase × time: P = 0.03). Specifically, participants reduced workload more rapidly in HUM, which was more pronounced in EF than in ML.
Figure 1. Mean (SD) power output (n = 10) and individual and mean (SD) work capacity (n = 10) during exercise in dry (DRY) and humid (HUM) heat during the early follicular (EF) and mid‐luteal (ML) phase.
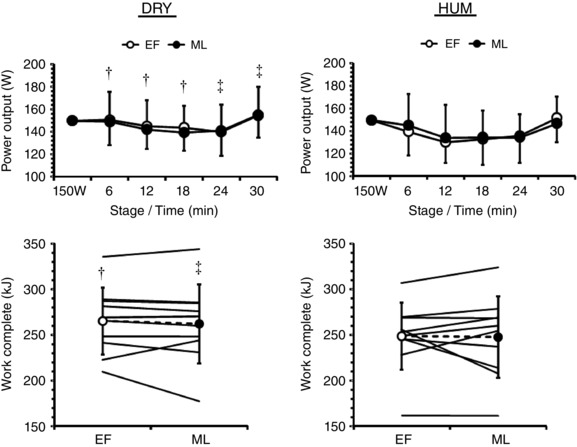
†Significant difference between corresponding EF‐HUM value, ‡significant difference between corresponding ML‐HUM value.
Thermoregulatory measures
Body temperatures
T rec when resting was 0.21 (0.14)°C higher in ML than in EF (P < 0.01) and remained higher by 0.16 (0.10)°C during fixed‐intensity exercise (P < 0.01) (Fig. 2). The rise in T rec throughout this exercise (P < 0.01) was not dependent on menstrual phase or environment (interaction: P = 0.66). During self‐paced exercise T rec differed between environments as a function of menstrual phase (environment × phase × time: P = 0.02). Specifically, the between‐phase differences seen at rest [0.13 (0.14)°C] and fixed‐intensity exercise [0.45 (0.24)°C] during DRY were not evident during self‐paced exercise [−0.04 (0.21)°C], whilst the resting [0.29 (0.29)°C] and fixed‐intensity [0.25 (0.14)°C] differences persisted throughout self‐paced exercise [0.23 (0.28)°C] during HUM. The rise in T rec was smaller during ML than EF [1.23 (0.27) vs. 1.40 (0.18)°C, P = 0.05] but similar between environments [DRY: 1.34 (0.26) vs. HUM: 1.28 (0.24)°C, P = 0.55].
Figure 2. Mean (SD) rectal temperature (T rec, n = 10) and weighted mean skin temperature ( sk, n = 10) during exercise in dry (DRY) and humid (HUM) heat during the early follicular (EF) and mid‐luteal (ML) phase.
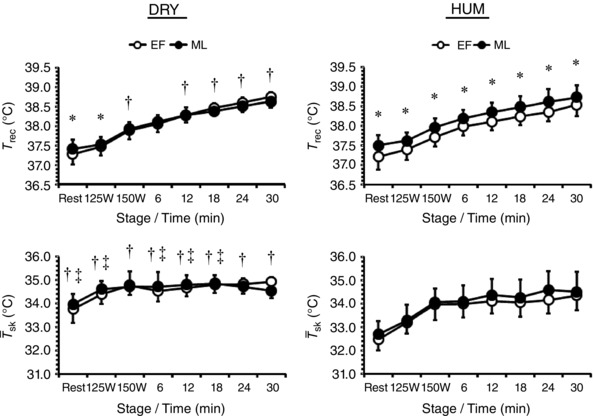
*Significant difference between EF‐ML within environment, †significant difference between corresponding EF‐HUM value, ‡significant difference between corresponding ML‐HUM value.
Resting sk was similar between menstrual phases (P = 0.13) but was 1.3 (0.5)°C higher during DRY than HUM (P < 0.01) (Fig. 2). During fixed‐intensity exercise sk was similar between menstrual phases (P = 0.26) but differed between environments as a function of work‐rate (environment × work‐rate: P = 0.01) such that the difference between environments was halved to 0.7 (0.4)°C. During self‐paced exercise sk differed between environments as a function of menstrual phase (environment × phase × time: P = 0.04). Specifically, end‐exercise sk values were attained in the following order: EF‐DRY > ML‐DRY > ML‐HUM > EF‐HUM.
Cardiovascular and thermoeffectors
Resting , SV and MAP were similar between menstrual phases and environments (all P > 0.38) whereas resting FVR differed between environments as a function of menstrual phase (environment × phase: P = 0.02) being higher in ML‐DRY than in EF‐DRY and ML‐HUM (Table 1 and Fig. 3). During fixed‐intensity exercise and SV were similar between menstrual phases (both P > 0.74) and environments (both P > 0.54) but increased above resting values before plateauing (both P < 0.01). During fixed‐intensity exercise MAP differed between menstrual phases as a function of work‐rate (phase × time: P = 0.03) whilst FVR differed between environments as a function of menstrual phase (environment × phase: P = 0.01) such that ML‐DRY > EF‐DRY > ML‐HUM > EF‐HUM.
Table 1.
Mean arterial pressure (MAP, n = 10), cardiac output (, n = 8), forearm vascular resistance (FVR, n = 10) and stroke volume (SV, n = 8) at rest and during fixed‐intensity exercise in dry (DRY) and humid (HUM) heat during the early follicular (EF) and mid‐luteal (ML) phase
| DRY | HUM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EF | ML | EF | ML | |||||||||
| Rest | 125 W | 150W | Rest | 125 W | 150W | Rest | 125 W | 150W | Rest | 125 W | 150W | |
| MAP (mmHg) | 83 (6) | 93 (8)* | 100 (7) | 85 (5) | 95 (5)* | 100 (4) | 85 (5) | 94 (6)* | 102 (6) | 84 (6) | 94 (9)* | 99 (9) |
| (litres min–1) | 7 (3) | 22 (4)* | 21 (4) | 7 (3) | 20 (4)* | 22 (3) | 7 (2) | 21 (5)* | 21 (5) | 7 (2) | 20 (5)* | 23 (4) |
| FVR (ml dl min mmHg–1) | 5.8 (1.3) | 4.4 (1.2)* | 3.8 (1.2)* | 7.6 (2.3)† | 5.8 (1.4)*, † | 4.4 (0.8)* | 5.2 (1.1)† | 3.5 (0.4)*, † | 3.2 (0.5)* | 5.4 (1.3)† | 3.9 (0.7)*, † | 3.3 (0.6)*, † |
| SV (ml) | 98 (32) | 160 (25)* | 136 (36) | 103 (37) | 146 (30)* | 144 (22) | 97 (36) | 154 (28)* | 135 (25) | 94 (30) | 145 (35)* | 143 (20) |
Values are mean (SD).
*Significant difference to preceding time‐point.
†Significant difference to corresponding EF‐DRY time‐point. ‡Significant difference to corresponding ML‐DRY time‐point.
Figure 3. Mean (SD) local sweat rate (LSR, n = 9) and forearm blood flow (FBF, n = 10) against time and mean body temperature ( b) during exercise in dry (DRY) and humid (HUM) heat during the early follicular (EF) and mid‐luteal (ML) phase.
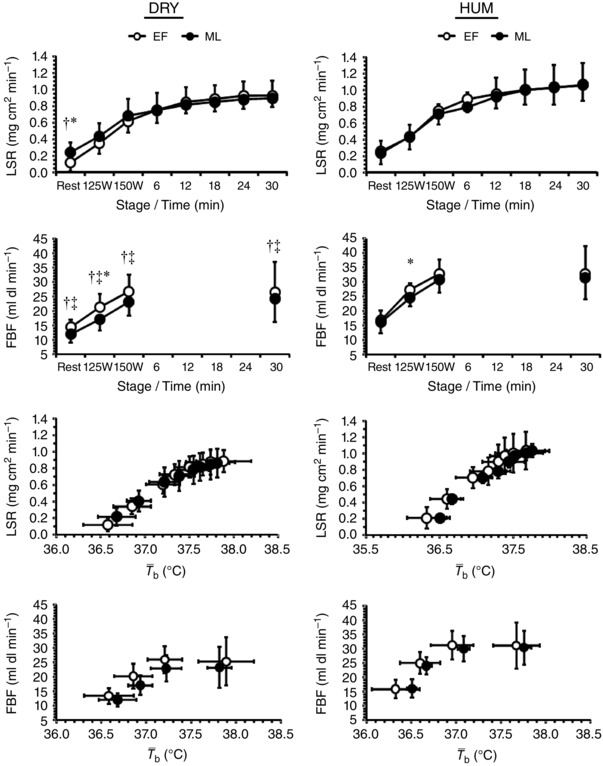
*Significant difference between EF‐ML within environment, †significant difference between corresponding EF‐HUM value, ‡significant difference between corresponding ML‐HUM value.
Resting LSR differed between environments as a function of menstrual phase (environment × phase: P = 0.01) such that EF‐DRY was lower than ML‐DRY and EF‐HUM, whilst resting FBF was similar between menstrual phases (P = 0.15) but was lower in DRY than HUM (P < 0.01; Fig. 3). During fixed‐intensity exercise LSR was similar between menstrual phases and environments (both P > 0.14) but increased with work‐rate (P < 0.01) whilst FBF differed between environments as a function of menstrual phase (environment × menstrual phase × work‐rate: P = 0.01). During self‐paced exercise LSR was similar between menstrual phases and environments (both P > 0.11) but increased over time (P < 0.01). Neither onset threshold nor thermosensitivity of the effector responses were affected by menstrual phase or environment (all P > 0.26). Water consumption was similar between menstrual phases and environments [806 (422) ml; both P > 0.14] whilst WBSR was similar between menstrual phases and environments [937 (262) g h−1; both P > 0.42], resulting in a 1.6 (0.5)% loss of body mass that was similar between menstrual phases and environments (both P > 0.43).
Thermodynamics
Resting M was similar between menstrual phases and environments (both P > 0.79) whereas resting E was similar between menstrual phases (P = 0.41) but was 22 (10) W m2 higher in DRY than HUM (P < 0.01), with negligible S (Fig. 4). Resting E max was similar between menstrual phases (P = 0.48) but was 39 (16) W m2 higher in DRY than HUM (P < 0.01) whereas E req was similar between menstrual phases and environments (both P > 0.48), which meant that the HSI was similar between menstrual phases and environments (both P > 0.44). During fixed‐intensity exercise M and E were similar between menstrual phases (both P > 0.25) but differed between environments as a function of work‐rate (environment × time: both P < 0.03). As a result, S was similar between menstrual phases (P = 0.58) but was 35 (18) W m2 lower in DRY than HUM (P < 0.01). During fixed‐intensity exercise E max, E req and consequently HSI were similar between menstrual phases (all P > 0.29) but differed between environments as a function of work‐rate (environment × work‐rate: all P < 0.04). During self‐paced exercise M, E and S were similar between menstrual phases (all P > 0.67) with only E being 16 (6) W m2 higher in DRY than HUM (P < 0.01). During self‐paced exercise E max, E req and consequently HSI were similar between menstrual phases (all P > 0.39) with only E max being 27 (10) W m2 higher in DRY than HUM (P < 0.01).
Figure 4. Mean (SD) rate of metabolic heat production (M, n = 9), rate of evaporative heat loss (E, n = 9), rate of heat storage (S, n = 9), maximal evaporative capacity of the environment (E max, n = 9), required evaporative cooling for heat balance (E req, n = 9) and heat strain index (HSI, n = 9) during exercise in dry (DRY) and humid (HUM) heat during the early follicular (EF) and mid‐luteal (ML) phase.

*Significant difference between EF‐ML within environment, †significant difference between corresponding EF‐HUM value, ‡significant difference between corresponding ML‐HUM value.
Perceptual measures
Participants felt similar TS at rest between menstrual phases (P = 0.31) but felt warmer during DRY than HUM [5.1 (0.9) vs. 4.2 (1.2); P = 0.01] (Fig. 5), whilst resting TD was similar between menstrual phases and environments (both P > 0.09). During fixed‐intensity exercise TS was similar between menstrual phases (P = 0.08) but differed between environments as a function of work‐rate (environment × work‐rate: P = 0.01). By contrast, TD during fixed‐intensity exercise was similar between menstrual phases (P = 0.54) but participants felt more thermally uncomfortable during DRY than HUM [2.0 (0.4) vs. 1.8 (0.4); P = 0.03]. During fixed‐intensity exercise RPE was similar between menstrual phases and environments (both P > 0.32) but increased with work‐rate (P < 0.01). During self‐paced exercise TS, TD and RPE were similar between menstrual phases and environments (all P > 0.09) but increased progressively with time (P < 0.01).
Figure 5. Mean (SD) thermal sensation (n = 10), thermal discomfort (n = 10) and rating of perceived exertion (n = 10) during exercise in dry (DRY) and humid (HUM) heat during the early follicular (EF) and mid‐luteal (ML) phase.
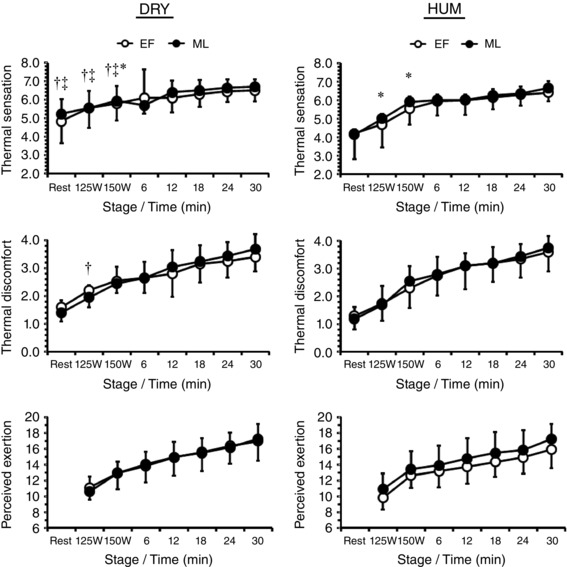
*Significant difference between EF‐ML within environment, †significant difference between corresponding EF‐HUM value, ‡significant difference between corresponding ML‐HUM value.
Correlation and regression analyses
The [progesterone] correlated with [oestrogen] in absolute terms (r = 0.69, P < 0.01) and in the extent of rise from EF to ML (r = 0.47, P = 0.04). The [progesterone] moderately predicted resting T rec (r 2 = 0.14, β = 0.37, P = 0.02), while the rise in [progesterone] moderately to strongly predicted the rise in resting LSR (r 2 = 0.31, β = −0.55, P = 0.02), with no more than 4% of remaining variability in T rec or LSR being accounted for by the rise in oestrogen.
Discussion
Research into how well‐trained women respond to either dry or humid heat stress across the menstrual cycle remains sparse despite it being known that women sweat more efficiently than men, and that ovarian hormones exert multiple physiological effects including on sweating function. Therefore, this investigation characterised and compared the behavioural and autonomic thermoregulatory responses of well‐trained, eumenorrheic women to exercise when exposed to equivalent dry and humid heat stress during the early follicular and mid‐luteal phases of their menstrual cycle. The novel results are that: (1) self‐paced exercise performance (i.e. total work) was not affected by menstrual phase but was impaired by a humid environment, (2) whilst the autonomic (thermoeffector onset thresholds and thermosensitivities) thermoregulatory responses were similar between menstrual phases and environments, the behavioural response (i.e. exercise pacing) differed between environments as a function of menstrual phase, and (3) the ovarian hormone concentrations and fluctuations between menstrual phases were not attenuated in these well‐trained women relative to values previously reported in less‐trained women. These results indicate that under the conditions of this investigation, trained women behaviourally thermoregulate to minimise autonomic differences but at the expense of their exercise performance under humid heat stress.
Performance was unaffected by menstrual phase in either environment. Our performance data (Fig. 1) support some earlier findings that menstrual phase does not affect heat‐stress tolerance (Kolka & Stephenson, 1997; Sunderland & Nevill, 2003), but are in contrast to other reports in which tolerance/performance was reduced by ∼6–16% in ML compared to EF (Avellini et al. 1980; Tenaglia et al. 1999; Janse de Jonge et al. 2012). Importantly, however, these previous investigations did not allow participants to pace themselves and were performed to exhaustion, thereby limiting their ecological validity for performance requirements.
Thermoregulatory differences across menstrual phases and ambient conditions were nullified by thermoregulatory behaviour. We have previously demonstrated in men that reductions in work‐rate in the heat are a form of behavioural thermoregulation that serve to improve heat exchange (Schlader et al. 2011b) that cannot be achieved when the work‐rate is constant (Schlader et al. 2011a). The current results extend these observations to women. This is perhaps best illustrated by the impact of changing from one exercise modality to the other (constant vs. variable intensity). For instance, at rest or during fixed‐intensity exercise (125 or 150 W) the effector (LSR, FBF, FVR; Fig. 3 and Table 1) and thermodynamic (M, S, E req; Fig. 4) responses display differences between menstrual phase and/or environments, but these differences disappear when allowed to self‐pace. At its most pronounced this exercise behaviour constituted a reduction in power output ∼31 W or ∼12% of peak aerobic power (Fig. 1). Such a sustained (6 min) reduction in work‐rate resulted in a ∼238 W m2 lower M that required ∼219 W m2 less evaporative cooling for heat balance. It has been shown previously that total heat loss, predominantly from E, during exercise in compensable environments, such as those in this study (Fig. 4, HSI), is dependent on E req, and M largely determines E req (e.g. Gagnon et al. 2013). Thus, it follows that in our participants the behavioural adjustments during exercise minimised autonomic differences (LSR, WBSR). The behavioural adjustments displayed by our participants in the current study (Fig. 1) display ‘classic’ pacing in the heat, whereby physiological strain is constrained and allows a ‘reserve’ for an end‐spurt that reduces the likelihood of exhaustion and heat illness (Schlader et al. 2011 c). That this behaviour/pacing is probably mediated, at least in part, by perceptual cues appears likely and is supported by there being no differences between all three scales used in the current study (Fig. 5), as discussed elsewhere (see Flouris & Schlader, 2015).
Thermoregulatory behaviour differed between environments as a function of menstrual phase. Another novel result is that these women reduced workload more rapidly and performed worse in HUM compared to DRY, despite matched WBGTs. This is probably due to low(er) rates of evaporative cooling (Fig. 4) driven by the reduced vapour pressure gradient between skin and environment, which is consistent with previous observations (Morimoto et al. 1967; Shapiro et al. 1980; Frye & Kamon, 1983). However, it should be noted that whilst this performance decrement in humid versus dry heat has been demonstrated previously in men (e.g. Sen Gupta et al. 1984) it was unknown whether this would be replicated in women due to their greater sweating efficiency in this environment (Morimoto et al. 1967; Shapiro et al. 1980; Frye & Kamon, 1983). Furthermore, our observation that the initiation of thermal behaviour in HUM occurred earlier in EF than in ML (Fig. 1) supports previous investigations that used passive‐heating behavioural models to determine that the threshold for affective/behavioural response is menstrual phase‐dependent (Cabanac et al. 1971; Scarperi & Bleichert, 1983; Shoemaker & Refinetti, 1996). Nevertheless, it should be highlighted that this behaviour during HUM was not sufficient to completely diminish the differences observed for T rec (from rest) in spite of no differences for S.
Phase‐related differences in Tcore but not ovarian [hormone] were not attenuated compared to less trained women. Our finding of an elevated T core at rest and during fixed‐intensity exercise during ML compared to EF is consistent with those of others (Avellini et al. 1980; Carpenter & Nunneley, 1988; Kolka & Stephenson, 1997; Tenaglia et al. 1999; Janse de Jonge et al. 2012). Our results also support previous observations that trained women have a smaller difference in the bi‐phasic T core and no phase‐related difference for the T core threshold for sweating or cutaneous vasodilatation (Kuwahara et al. 2005a,b). Yet, our participants did not have the reduced ovarian hormone concentrations or between‐phase fluctuations that were evident in previous studies (Dale et al. 1979; Bullen et al. 1984; Kuwahara et al. 2005a,b). However, when examining our participant data further by separating into greater (n = 5, 59–70 ml min−1 kg−1) and lesser (n = 5, 48–56 ml min−1 kg−1) trained, a clear difference in the absolute and relative (Δ) hormone concentrations appeared such that a posteriori analysis confirmed correlated with [oestrogen] (r = 0.79, P < 0.01) but not [progesterone] (r = −0.14, P = 0.55).
Considerations
The design of comparing the responses of women between EF and ML used within this study has the specific advantage of being applicable to competitive women experiencing their natural, endogenous hormonal changes. Furthermore, our rationale was based on (1) maximising the differences in [oestrogen] and [progesterone] occurring naturally/endogenously, (2) permitting comparison with and therefore expansion beyond previous results, and (3) the previous research indicating that women self‐report training and performance to be impacted negatively by their menstrual cycle. However, whilst this approach ‘captures’ the phases of lowest hormone exposure and peak [progesterone] it does not include for comparison the late‐follicular (pre‐ovulatory) phase, when [oestrogen] peaks and during which it has been demonstrated that resting T core and the threshold for thermoregulatory effector responses are shifted to a lower T core (Stephenson & Kolka, 1999). However, the same authors did not observe any exercise performance change between this late‐follicular, EF and ML phases (Kolka & Stephenson, 1997). It must also be noted that women are in EF and ML for ≤50% of their reproductive lives, and that hormone exposure does not determine effect, i.e. receptor activity (Stachenfeld & Taylor, 2014). Moreover, the ovarian and other reproductive (luteinising and follicle stimulating) hormones exert independent effects, and in combination their effects on the body's systems are complex and multi‐faceted, and extend beyond thermoregulation. Thus, other experimental designs are required (e.g. hormonal contraception or suppression) to allow causal inferences to be made (Stachenfeld & Taylor, 2014).
Many elite athletes often have irregular menstrual cycles or take the oral contraceptive pill (OCP) for reasons of contraception and/or to negate pre‐menstrual symptoms and manipulate the menstrual cycle timing for travel, training and competition (Bennell et al. 1999). Previous investigations on OCP‐users have reported that the phase‐related elevation in T core is maintained during exercise in the heat (Tenaglia et al. 1999; Sunderland & Nevill, 2003). However, this increased resting and exercising T core and the concomitant increase in the T core threshold for sweating differs according to the type of OCP used, i.e. combined synthetic oestrogen + progesterone versus progesterone only (Stachenfeld et al. 2000). Nevertheless, to our knowledge, only two published studies have measured exercise performance with heat stress using matched groups (OCP vs. eumenorrheic) and the same limitations apply of not having used self‐paced protocols or well‐trained women (Tenaglia et al. 1999; Sunderland & Nevill, 2003). Therefore, whether well‐trained women using OCP differ in their thermo‐behavioural and performance responses from matched eumenorrheic or to different thermal stress (dry vs. humid) remains unknown.
One (de)limitation includes the lack of an untrained cohort. They were not included because – at least in a sporting context – performance is less imperative, and hence they may rely on behavioural thermoregulation to a relatively greater extent (i.e. reduce power output) and may introduce other confounding effects by exhibiting different perceptual and behavioural tolerance and motivation (e.g. McLellan, 2001; Tikuisis et al. 2002). One limitation was not including other physiological measures that are impacted by ovarian (and other reproductive) hormones and could contribute to performance/behaviour or homeostasis during exercise in the heat (especially leg blood flow and arterial oxygenation). Our focus was on autonomic and behavioural thermoregulation, and even during more prolonged and severe hyperthermia with marked dehydration, oxygen delivery to the active musculature is preserved despite a reduced blood flow – at least in men (e.g. Gonzalez‐Alonso et al. 1998, 2008). Furthermore, using our P ETCO2 data (not shown) as an indication of alveolar ventilation, arterial oxygenation seems unlikely to have been influenced by menstrual phase or environment, as no significant main or interaction effects were evident. Finally, the current design included a period of fixed‐intensity exercise and a period of variable‐intensity exercise that were unequal in duration, and it is unlikely that a thermoregulatory steady state had been achieved during the fixed‐intensity exercise; therefore, as per our previous observations in men (Schlader et al. 2011a,b), a longer (> 20 min) and duration‐matched protocol would strengthen the current results.
Conclusion
This study demonstrates that when well‐trained women are allowed to behaviourally thermoregulate (self‐pace) during exercise in heat‐stressful environments, thermodynamic and autonomic differences associated with menstrual cycle phase (early follicular vs. mid‐luteal) and the type of heat stress (dry vs. humid) were abolished. However, this is to their performance detriment during humid heat and in this environment the phase‐related (post‐ovulation) increase in T rec persists.
Additional information
Competing interests
No potential conflicts or competing interests are disclosed; no external funding was received.
Author contributions
THL: design of the work; data acquisition, analysis, and interpretation; drafting and critically revising important intellectual content. SRS: design of the work; data acquisition and interpretation; critically revising important intellectual content. BGP: design of the work; data acquisition; critically revising important intellectual content. ZJS: design of the work; data analysis and interpretation; drafting and critically revising important intellectual content. JDC: design of the work; data analysis and interpretation; drafting and critically revising important intellectual content. TM: conception and design of the work; data acquisition, analysis and interpretation; drafting and critically revising important intellectual content. All authors approved the final version of the manuscript. All experimental procedures were performed in the School of Sport and Exercise, Massey University, Palmerston North.
Acknowledgements
We would like to thank the very dedicated group of women who participated in this study.
References
- Avellini B, Kamon E & Krajewski J (1980). Physiological responses of physically fit men and women to acclimation to humid heat. J Appl Physiol 49, 254–261. [DOI] [PubMed] [Google Scholar]
- Belding HS & Hatch TF (1955). Index for evaluating heat stress in terms of resulting physiological strain. Heat Piping Air Cond 27, 129–136. [Google Scholar]
- Bennell K, White S & Crossley K (1999). The oral contraceptive pill: a revolution for sportswomen? Br J Sports Med 33, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger TH (1969). Heat regulation: homeostasis of central temperature in man. Physiol Rev 49, 671–759. [DOI] [PubMed] [Google Scholar]
- Borg G (1970). Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2, 92–98. [PubMed] [Google Scholar]
- Brotherhood JR (2008). Heat stress and strain in exercise and sport. J Sci Med Sport 11, 6–19. [DOI] [PubMed] [Google Scholar]
- Bruinvels G, Burden R, Brown N, Richards T & Pedlar C (2016). The prevalence and impact of heavy menstrual bleeding (menorrhagia) in elite and non‐elite athletes. PLoS ONE 11,e0149881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd GM (2008). Wet‐bulb globe temperature (WBGT)—its history and its limitations. J Sci Med Sport 11, 20–32. [DOI] [PubMed] [Google Scholar]
- Bullen BA, Skrinar GS, Beitins IZ, Carr DB, Reppert S, Dotson C, Fencl M, Gervino E & McArthur J (1984). Endurance training effects on plasma hormonal responsiveness and sex hormone excretion. J Appl Physiol 56, 1453–1463. [DOI] [PubMed] [Google Scholar]
- Carpenter A & Nunneley S (1988). Endogenous hormones subtly alter women's response to heat stress. J Appl Physiol 65, 2313–2317. [DOI] [PubMed] [Google Scholar]
- Charkoudian N & Joyner MJ (2004). Physiologic considerations for exercise performance in women. Clin Chest Med 25, 247–255. [DOI] [PubMed] [Google Scholar]
- Charkoudian N & Stachenfeld NS (2014). Reproductive hormone influences on thermoregulation in women. Compr Physiol 4, 793–804. [DOI] [PubMed] [Google Scholar]
- Cheung SS, McLellan TM & Tenaglia S (2000). The thermophysiology of uncompensable heat stress. Physiological manipulations and individual characteristics. Sports Med 29, 329–359. [DOI] [PubMed] [Google Scholar]
- Costello JT, Bieuzen F & Bleakley CM (2014. Where are all the female participants in Sports and Exercise Medicine research? Eur J Sport Sci 14, 847–851. [DOI] [PubMed] [Google Scholar]
- Cunningham D & Cabanac M (1971). Evidence from behavioural thermoregulatory responses of a shift in setpoint temperature related to the menstrual cycle. J Physiol (Paris) 63, 236–238. [PubMed] [Google Scholar]
- Dale E, Gerlach DH & Wilhite AL (1979). Menstrual dysfunction in distance runners. Obstet Gynecol 54, 47–53. [DOI] [PubMed] [Google Scholar]
- Defares J (1958). Determination of PvCO2 from the exponential CO2 rise during rebreathing. J Appl Physiol 13, 159–164. [DOI] [PubMed] [Google Scholar]
- Drinkwater BL (1984). Women and exercise: physiological aspects. Exerc Sport Sci Rev 12, 21–52. [PubMed] [Google Scholar]
- Dubois D & Dubois EF (1916). A formula to estimate approximate surface area if height and weight be known. Arch Intern Med 17, 863–871. [Google Scholar]
- Flouris AD & Schlader ZJ (2015). Human behavioural thermoregulation during exercise in the heat. Scand J Med Sci Sports 25 Suppl 1, 52–64 [DOI] [PubMed] [Google Scholar]
- Frye A & Kamon E (1983). Sweating efficiency in acclimated men and women exercising in humid and dry heat. J Appl Physiol 54, 972–977. [DOI] [PubMed] [Google Scholar]
- Gagge AP, Stolwijk J & Hardy J (1967). Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res 1, 1–20. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Jay O & Kenny GP (2013). The evaporative requirement for heat balance determines whole‐body sweat rate during exercise under conditions permitting full evaporation. J Physiol 591, 2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Alonso J, Calbet JA & Nielsen B (1998). Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol 513, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Alonso J, Crandall CG & Johnson JM (2008). The cardiovascular challenge of exercising in the heat. J Physiol 586, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graichen H, Rascati R & Gonzalez R (1982). Automatic dew‐point temperature sensor. J Appl Physiol 52, 1658–1660. [DOI] [PubMed] [Google Scholar]
- Harvey O & Crockett HE (1932). Individual differences in temperature changes of women during the course of the menstrual cycle. Hum Biol 4, 453–468. [Google Scholar]
- Hatcher RA, Guest F & Stewart F (1988). Contraceptive Technology 1988–1989. Irvington Publishers, New York. [Google Scholar]
- Hertzman A, Randall W, Peiss C & Seckendorf R (1952). Regional rates of evaporation from the skin at various environmental temperatures. J Appl Physiol 5, 153–161. [DOI] [PubMed] [Google Scholar]
- Janse de Jonge XA (2003). Effects of the menstrual cycle on exercise performance. Sports Med 33, 833–851. [DOI] [PubMed] [Google Scholar]
- Janse de Jonge XA, Thompson MW, Chuter VH, Silk LN & Thom JM (2012). Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med Sci Sports Exerc 44, 2190–2198. [DOI] [PubMed] [Google Scholar]
- Jeukendrup A, Saris W, Brouns F & Kester AD (1996). A new validated endurance performance test. Med Sci Sports Exerc 28, 266–270. [DOI] [PubMed] [Google Scholar]
- Jones NL, Robertson DG & Kane JW (1979). Difference between end‐tidal and arterial in exercise. J Appl Physiol 47, 954–960. [DOI] [PubMed] [Google Scholar]
- Kenney WL (1998). Heat flux and storage in hot environments. Int J Sports Med 19 Suppl 2, S92–95. [DOI] [PubMed] [Google Scholar]
- Kerslake DM (1972). The Stress of Hot Environments. Cambridge University Press, London. [PubMed] [Google Scholar]
- Kolka MA & Stephenson LA (1997). Interaction of menstrual cycle phase, clothing resistance and exercise on thermoregulation in women. J Therm Biol 22, 137–141. [Google Scholar]
- Kuwahara T, Inoue Y, Abe M, Sato Y & Kondo N (2005a). Effects of menstrual cycle and physical training on heat loss responses during dynamic exercise at moderate intensity in a temperate environment. Am J Physiol Regul Integr Comp Physiol 288, R1347–R1353. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Inoue Y, Taniguchi M, Ogura Y, Ueda H & Kondo N (2005b). Effects of physical training on heat loss responses of young women to passive heating in relation to menstrual cycle. Eur J Appl Physiol 94, 376–385. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent‐Smith L, Melchior JC & Pirlich M (2004). Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 23, 1226–1243. [DOI] [PubMed] [Google Scholar]
- Mchardy GJR (1967). Relationship between differences in pressure and content of carbon dioxide in arterial and venous blood. Clin Sci 32, 299–309. [PubMed] [Google Scholar]
- McLellan TM (2001). The importance of aerobic fitness in determining tolerance to uncompensable heat stress. Comp Biochem Physiol A Mol Integr Physiol 128, 691–700. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Slabochova Z, Naman R & Sargent F (1967). Sex differences in physiological reactions to thermal stress. J Appl Physiol 22, 526–532. [DOI] [PubMed] [Google Scholar]
- Parsons K (2014). Human Thermal Environments: The Effects of Hot, Moderate, and Cold Environments on Human Health, Comfort, and Performance. CRC Press, Boca Raton. [Google Scholar]
- Paterson DH & Cunningham DA (1976). Comparison of methods to calculate cardiac output using the CO2 rebreathing method. Eur J Appl Physiol Occup Physiol 35, 223–230. [DOI] [PubMed] [Google Scholar]
- Ramanathan N (1964). A new weighting system for mean surface temperature of the human body. J Appl Physiol 19, 531–533. [DOI] [PubMed] [Google Scholar]
- Roberts MF, Wenger CB, Stolwijk J & Nadel ER (1977). Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol 43, 133–137. [DOI] [PubMed] [Google Scholar]
- Scarperi M & Bleichert A (1983). Non‐thermal influences on thermoregulatory behaviour. J Therm Biol 8, 179–181. [Google Scholar]
- Schlader ZJ, Mündel T, Barnes MJ & Hodges LD (2010). Peak cardiac power output in healthy, trained men. Clin Physiol Func Imag 30, 480–484. [DOI] [PubMed] [Google Scholar]
- Schlader ZJ, Raman A, Morton RH, Stannard SR & Mündel T (2011a). Exercise modality modulates body temperature regulation during exercise in uncompensable heat stress. Eur J Appl Physiol 111, 757–766. [DOI] [PubMed] [Google Scholar]
- Schlader ZJ, Stannard SR & Mündel T (2011b). Evidence for thermoregulatory behaviour during self‐paced exercise in the heat. J Therm Biol 36, 390–396. [Google Scholar]
- Schlader ZJ, Stannard SR & Mundel T (2011. c) Exercise and heat stress: performance, fatigue and exhaustion—a hot topic. Br J Sports Med 45, 3–5. [DOI] [PubMed] [Google Scholar]
- Sen Gupta J, Swamy YV, Pichan G & Dimri GP (1984). Physiological responses during continuous work in hot dry and hot humid environments in Indians. Int J Biometeor 28, 137–146. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Pandolf KB, Avellini BA, Pimental NA & Goldman RF (1980). Physiological responses of men and women to humid and dry heat. J Appl Physiol 49, 1–8. [DOI] [PubMed] [Google Scholar]
- Shoemaker JA & Refinetti R (1996). Day–night difference in the preferred ambient temperature of human subjects. Physiol Behav 59, 1001–1003. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Silva C & Keefe DL (2000). Estrogen modifies the temperature effects of progesterone. J Appl Physiol 88, 1643–1649. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS & Taylor HS (2014). Challenges and methodology for testing young healthy women in physiological studies. Am J Physiol Endocrinol Metab 306, E849–E853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LA & Kolka MA (1993). Thermoregulation in women. Exerc Sport Sci Rev 21, 231–262. [PubMed] [Google Scholar]
- Stephenson LA & Kolka MA (1999). Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol 86, 22–28. [DOI] [PubMed] [Google Scholar]
- Stolwijk JAJ & Hardy JD (1966). Partitional calorimetric studies of man during exposures to thermal transients. J Appl Physiol 21, 967–977. [DOI] [PubMed] [Google Scholar]
- Sunderland C & Nevill M (2003). Effect of the menstrual cycle on performance of intermittent, high‐intensity shuttle running in a hot environment. Eur J Appl Physiol 88, 345–352. [DOI] [PubMed] [Google Scholar]
- Tenaglia SA, McLellan TM & Klentrou PP (1999). Influence of menstrual cycle and oral contraceptives on tolerance to uncompensable heat stress. Eur J Appl Physiol Occup Physiol 80, 76–83. [DOI] [PubMed] [Google Scholar]
- Tikuisis P, McLellan TM & Selkirk G (2002). Perceptual versus physiological heat strain during exercise‐heat stress. Med Sci Sports Exerc 34, 1454–1461. [DOI] [PubMed] [Google Scholar]
- Whitney R (1953). The measurement of volume changes in human limbs. J Physiol 121, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


