Abstract
Mechanisms underlying fatigue development and limitations for performance during intense exercise have been intensively studied during the past couple of decades. Fatigue development may involve several interacting factors and depends on type of exercise undertaken and training level of the individual. Intense exercise (½–6 min) causes major ionic perturbations (Ca2+, Cl−, H+, K+, lactate− and Na+) that may reduce sarcolemmal excitability, Ca2+ release and force production of skeletal muscle. Maintenance of ion homeostasis is thus essential to sustain force production and power output during intense exercise. Regular speed endurance training (SET), i.e. exercise performed at intensities above that corresponding to maximum oxygen consumption (), enhances intense exercise performance. However, most of the studies that have provided mechanistic insight into the beneficial effects of SET have been conducted in untrained and recreationally active individuals, making extrapolation towards athletes’ performance difficult. Nevertheless, recent studies indicate that only a few weeks of SET enhances intense exercise performance in highly trained individuals. In these studies, the enhanced performance was not associated with changes in and muscle oxidative capacity, but rather with adaptations in muscle ion handling, including lowered interstitial concentrations of K+ during and in recovery from intense exercise, improved lactate−–H+ transport and H+ regulation, and enhanced Ca2+ release function. The purpose of this Topical Review is to provide an overview of the effect of SET and to discuss potential mechanisms underlying enhancements in performance induced by SET in already well‐trained individuals with special emphasis on ion handling in skeletal muscle.
Keywords: endurance, fatigue resilience, high intensity, KATP, KIR, MCT, NHE, NKCC
Abbreviations
- ClC‐1
Cl− channel isoform 1
- FXYD1
phospholemman
- KIR2.1
strong inward rectifying K+ channel
- KATP
ATP‐sensitive K+ channel
- KIR6.2
inward rectifying channel K+ subunit 6.2
- MCT1
monocarboxylate cotransporter isoform 1
- MCT4
monocarboxylate cotransporter isoform 4
- NHE1
Na+–H+ exchanger isoform 1
- NKCC1
Na+–K+–2Cl− exchanger isoform 1
- ROS
reactive oxygen species
- RyR1
ryanodine receptor isoform 1
- SERCAI
sarcoplasmic reticulum Ca2+ ATPase isoform 1
- SERCAII
sarcoplasmic reticulum Ca2+ ATPase isoform 2
- SET
speed endurance training
- SR
sarcoplasmic reticulum
maximum oxygen consumption
Introduction
Training is fundamental to improve exercise performance in the athlete. Optimization of training strategies for performance‐enhancements is thus an important area of research within exercise physiology and sports medicine (Faria et al. 2005; Laursen, 2010; Bishop et al. 2011). In recent years, much research has focused on speed endurance training (SET), with brief intervals (≤ 60 s) performed at intensities above that corresponding to maximum oxygen consumption () (Ross & Leveritt, 2001; Iaia & Bangsbo, 2010; Gibala & Jones, 2013; Bangsbo, 2015). As presented in Table 1, SET is an efficient way to enhance intense exercise performance (½–6 min) in already well‐trained individuals and athletes, even with marked reductions in weekly training volume. It should be emphasised that in several of the studies presented in Table 1, training was also intensified by introducing or extending aerobic high‐intensity training, which is characterized by interval training with intensities close to those eliciting .
Table 1.
Adaptations to SET in performance during intense exercise (½–6 min) and skeletal muscle of trained individuals
| Study | Subjects (no., M/F, exercise type) |
|
Intervention (duration, sets/reps, work/rest, frequency, Δ% volume) | Performance measure | Effect (Δ%) | Muscle adaptation | Effect (Δ%) | |
|---|---|---|---|---|---|---|---|---|
| Houston & Thomson, 1977 | 5 M runners | 59 | 6 wks of 3×60 s/120 s + 5×6 s/24 s + 2×90 s/180 s (4/wk) + leg press (15 RM) |
|
|
ATP content | +15 | |
| Nevill et al. 1989 | 4 M/4F runners | 55 | 8 wks of 2×30 s/10 min + 6–10×6 s/54 s + 2–5×2 min/5 min (3–4/wk) | 30 s all out | +6 |
|
|
|
| Shepley et al. 1992 | 9 M runners | 67 |
|
Intense exercise (∼5 min) | +22 | CS | +18 | |
| Dawson et al. 1998 | 9 M trained | 57 | 6 wks of 3–5×4‐8×10 s/40–60 s (3/wk) |
|
|
|
|
|
| Ørtenblad et al. 2000 | 9 M trained | 61 | 5 wks of 20×10 s/50 s (3/wk) | Repeated sprint | +12 |
|
|
|
| Bickham et al. 2006 | 7 M runners | 58 | 6 wks of 4 × 14–30 × 40–100 m (3/wk) | Intense exercise (∼2½ min) | +11 |
|
|
|
| Bangsbo et al. 2009 | 12 M runners | 63 |
|
|
|
|
|
|
| Iaia et al. 2008, 2009 | 8/9 M runners | 56 |
|
|
|
|
|
|
| Thomassen et al. 2010; Christensen et al. 2011 | 7 M football | 55 |
|
|
|
|
|
|
| Gunnarsson et al. 2012 | 7 M football | 60 |
|
Intermittent running | +11 |
|
|
|
| Gunnarsson et al. 2013; Thomassen et al. 2016 | 8 M cyclists | 59 |
|
|
|
|
|
|
| Puype et al. 2013 | 9 M trained | 53 | 6 wks of 4–9×30 s/270 s (3/wk) | – |
|
|
||
| Skovgaard et al. 2014 | 12 M runners | 59 |
|
|
|
|
|
|
| Nyberg et al. 2016 | 16 M football | – | 9 wks of 2–3×8–10×5 s/10 s (1½/wk) | Intermittent running | +12 |
|
|
|
| Vorup et al. 2016 | 9 M runners | 60 |
|
|
|
|
|
Relative effect size for performance and muscle changes from each study are only presented for statistical significant measures (P ≤ 0.05) or when trending towards significance (P = 0.05–0.10) in parentheses. Abbreviations: CaMKII, Ca2+–calmodulin‐dependent protein kinase II; COX4, cytochrome c oxidase subunit 4; CS, citrate synthase, HAD, hydroxyacyl‐CoA dehydrogenase, KIR2.1, K+ inward rectifier channel 2.1; KIR6.2, K+ inward rectifier channel 6.2; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; NHE1, Na+–H+ exchanger isoform 1; NKCC1, Na+–K+–2Cl− channel isoform 1; OXPHOS, oxidative phosphorylation complex I–V enzymes; PFK, phosphofructokinase; PLN, phospholamban; RyR1, ryanodine receptor 1, SERCA, sarcoplasmic reticulum Ca2+ ATPase; , maximum oxygen consumption (ml kg−1 min−1).
The mechanisms underlying performance enhancements induced by a period of SET are not completely clear. Although several advanced human physiological experiments have been undertaken to investigate the effects of SET, a limitation of the research within this area is that most studies included untrained or recreationally active individuals (Iaia & Bangsbo, 2010; Gibala & Jones, 2013; Weston et al. 2014; Bangsbo, 2015), which makes translation towards athletes’ performance difficult. In untrained and recreationally active individuals, SET augments by 7–15% after only few weeks of training despite a low volume of training (Burgomaster et al. 2008; Jacobs et al. 2013). This observation is not surprising, since the heart rate of the individuals during a SET session with 6–10 × 30 s sprints, separated by 90 s of recovery, may reach mean and peaks of around 85 and 95% of maximum heart rate, respectively (Mohr et al. 2007). Furthermore, faster kinetics, increased capillarization, and augmented mitochondrial function of skeletal muscle have been observed in untrained and recreationally active individuals following a period of SET (Jensen et al. 2004; Gibala et al. 2006; Jacobs et al. 2013; Christensen et al. 2016). In contrast, SET does not increase , capillarization or oxidative capacity of skeletal muscle in already trained individuals (Bangsbo et al. 2009; Iaia et al. 2009; Christensen et al. 2015). Actually, SET has been shown to enhance exercise performance in spite of a concurrent reduction in expression of oxidative enzymes and capillarization of skeletal muscle in well‐trained individuals (Dawson et al. 1998; Nyberg et al. 2016). On the other hand, performance enhancements in trained individuals elicited by a period of SET are associated with several adaptations related to ion handling in skeletal muscle. SET may augment K+ handling (Bangsbo et al. 2009; Thomassen et al. 2010, 2016), lactate−–H+ transport capacity (Bickham et al. 2006; Gunnarsson et al. 2012, 2013; Puype et al. 2013), H+ regulation (Nevill et al. 1989; Gunnarsson et al. 2013; Skovgaard et al. 2014), and Ca2+ handling function (Ørtenblad et al. 2000) of trained individuals. The importance of these adaptations for enhancements in performance during intense exercise (½–6 min) following a period of SET in already trained individuals will be discussed in the present Topical Review.
Development of fatigue during intense exercise
Skeletal muscle fatigue can be defined as a disruption of the force production needed to meet the demand for a given exercise intensity (McKenna et al. 2008). The mechanisms underlying muscle fatigue development are highly debated and complex (Allen et al. 2008; Cairns & Lindinger, 2008; McKenna et al. 2008; MacIntosh & Shahi, 2011). Thus, fatigue development may involve several interacting factors and also depends on exercise modality and inter‐individual differences (Knicker et al. 2011). Intense exercise (½–6 min) causes major metabolic and ionic perturbations (Ca2+, Cl−, H+, K+, lactate−, Mg2+, Na+ and Pi) that impair excitation–contraction coupling of skeletal muscle, decrease mechanical efficiency and lead to force decline (i.e. skeletal muscle fatigue) (Cairns & Lindinger, 2008; Cairns et al. 2015; Grassi et al. 2015). Furthermore, accumulation of metabolites and ions in muscle interstitium stimulates sensory feedback from group III/IV muscle afferents to the central nervous system, thereby inducing the sensation of muscle ache associated with intense exercise (Pollak et al. 2014) and providing a regulatory input for motor command centres (Amann et al. 2011). Maintenance of ion homeostasis is thus essential to sustain force production and power output during intense exercise.
Skeletal muscle has several systems that regulate ion homeostasis of which some are presented in Fig. 1. The figure also provides information about the effect of a period of SET on adaptations in ion transporters, exchangers, and channels as well as in metabolic enzymes of trained individuals. The importance of these adaptations for performance during intense exercise will be discussed in the following sections.
Figure 1. Overview of metabolic and ion handling systems in skeletal muscle of potential importance for performance during intense exercise, and their adaptations to a period of speed endurance training (SET).
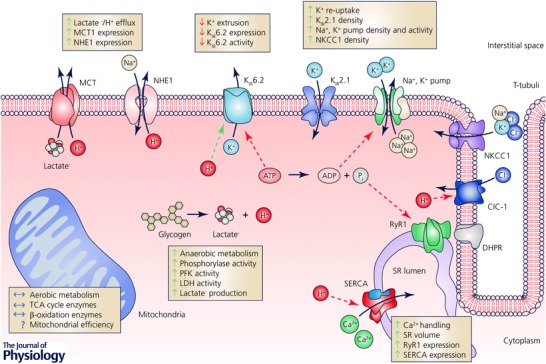
Blue (continuous) arrows, direction of flux. Green (dashed) arrows, potentiation of a given protein/enzyme. Red (dashed) arrows, inhibition of a given protein/enzyme. Green (continuous) arrows, augmented by a period with SET. Red (continuous) arrows, lowered by a period with SET. Question marks (?), the effect of a period with SET is unknown. ClC‐1, Cl− channel isoform 1; DHPR, dihydropyridine receptor; KIR2.1, K+ inward rectifier channel 2.1; KIR6.2, K+ inward rectifier channel 6.2; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; NHE1, Na+–H+ exchanger isoform 1; NKCC1, Na+–K+–2Cl− exchanger isoform 1; PFK, phosphofructokinase; RyR1, ryanodine receptor 1, SERCA, sarcoplasmic reticulum Ca2+ ATPase; SR, sarcoplasmic reticulum; TCA, tricarboxylic acid cycle.
Effect of speed endurance training on muscle K+ homeostasis
Intense exercise causes major perturbations in the rundown of transmembrane ion gradients that may reduce sarcolemmal excitability and impair force production of skeletal muscle (Cairns & Lindinger, 2008; McKenna et al. 2008). Specifically, contraction‐induced accumulation of extracellular K+ depolarizes sarcolemma, which causes inexcitability and muscle force decline in isolated muscle preparations (Clausen, 2013, 2015; Cairns et al. 2015). During intense exercise in humans, the extracellular concentrations of K+ rise from 4 mm to critical levels (> 10 mm), which, based on studies in muscle preparations, contribute to development of muscle fatigue (Cairns et al. 1995, 1997; Cairns & Lindinger, 2008; McKenna et al. 2008). Accordingly, concentrations of K+ have been shown to exceed 12 mm in muscle interstitial fluid during intense one‐legged knee‐extensor exercise and cycling when measured with the microdialysis technique (Juel et al. 2000; Nielsen et al. 2003, 2004a; Nordsborg et al. 2003; Gunnarsson et al. 2013). Interstitial concentrations of K+ are likely to be much higher than that observed because of the low time resolution, geometric placement of probes (e.g. outside the transverse‐tubular system), and probe‐to‐probe variation associated with the microdialysis technique (Green et al. 2000; Juel et al. 2000; Nielsen et al. 2004a). In support of this hypothesis, extracellular concentrations of K+ as high as 50–80 mm have been reported in rat extensor digitorum longus muscle following repeated stimulations, which were associated with pronounced muscle force decline (Clausen, 2008, 2011, 2013). K+ handling is therefore of importance for maintenance of sarcolemmal excitability during muscle contractions and any system that counteracts accumulation of extracellular K+ could potentially delay fatigue development and enhance performance during intense exercise.
The Na+–K+ pump counteracts rundown of transmembrane gradients of Na+ and K+, and is essential for muscle functioning during sustained contractions (Clausen, 2015). Regulation of Na+–K+ pump activity is multifactorial, with activity being stimulated by muscle contractions, intracellular Na+ concentrations, and hormones, such as adrenaline and insulin (Clausen & Flatman, 1977; Clausen & Kohn, 1977; Flatman & Clausen, 1979; Clausen, 2003). Even a few days of training are capable of increasing the abundance of muscle Na+–K+ pumps by ∼15% in recreationally active individuals (Green et al. 1993), and trained aged individuals have been shown to have a higher Na+–K+ pump abundance than untrained elderly subjects (Klitgaard & Clausen, 1989). Intensified training has been shown to increase the abundance of muscle Na+–K+ pumps by ∼10–70% (McKenna et al. 1993; Nielsen et al. 2004a; Bangsbo et al. 2009) when determined by both the [3H]‐ouabain‐binding technique (Madsen et al. 1994; Evertsen et al. 1997) and Western blotting (Iaia et al. 2008; Bangsbo et al. 2009; Thomassen et al. 2010; Vorup et al. 2016). Even in already well‐trained individuals, a period of SET effectively increases expression of the catalytic α subunits and structural β subunits of the Na+–K+ pump. For instance, in trained runners, SET, with a concomitant reduction in training volume, has been shown to elevate expression of Na+–K+ pump subunits α1 (29%), α2 (15–68%) and β1 (10–15%) (Iaia et al. 2008; Bangsbo et al. 2009). The increased abundance of muscle Na+–K+ pumps induced by SET is associated with a lower rate of K+ accumulation in arm venous blood during intense exercise and improved performance (Iaia et al. 2008; Bangsbo et al. 2009).
The question is, however, whether adaptations in muscle Na+–K+ pumps are essential for the improved performance observed following SET in trained individuals, since other studies did not find any change in Na+–K+ pump subunit abundances despite improved K+ handling (Gunnarsson et al. 2013) and performance during intense exercise (Gunnarsson et al. 2012, 2013; Skovgaard et al. 2014). In trained cyclists, Gunnarsson et al. (2013) observed that 7 weeks of intensified training consisting of SET and aerobic high‐intensity training, with a 70% reduction in training volume, increased performance by 18% during intense exercise (∼4 min) and reduced femoral venous plasma K+ concentrations by 0.7–0.8 mm in recovery from exercise, without alterations in expression of muscle Na+–K+ pump subunits. Thus, the lower femoral venous plasma K+ concentration in recovery from intense exercise following the intervention period may be related to other factors than changes in muscle Na+–K+ pump content. In support of this proposition, maximal in vitro Na+–K+ pump activity of muscle homogenate has been shown to be augmented with no contaminant change in abundance of Na+–K+ pump subunits in endurance athletes after 3 weeks of aerobic high intensity training (Aughey et al. 2007).
One such factor that regulates Na+–K+ pump activity and is induced by SET is phospholemman (FXYD1), which interacts with the α and β subunits of the Na+–K+ pump (Crambert et al. 2002; Thomassen et al. 2010, 2016). Phosphorylation of FXYD1 increases affinity for Na+ (lowers K m) of the Na+–K+ pump and augments maximal ATPase activity () (Crambert et al. 2002; Bibert et al. 2008; Rasmussen et al. 2008; Walas & Juel, 2012). In the aforementioned study in trained cyclists (Gunnarsson et al. 2013), expression of FXYD1 increased by 30% with the training period (Thomassen et al. 2016). In addition, non‐specific FXYD1 phosphorylation was higher following the training at rest and during repeated intense exercise, mainly because of increased phosphorylation at serine68 (Thomassen et al. 2016), which regulates K m of the Na+–K+ pump (Han et al. 2006; Juel et al. 2013). Similarly, 2 weeks of SET was shown to elevate phosphorylation of FXYD1 by 27% in elite football players, which correlated with enhancements in repeated sprint performance (10 × 20 m, 15 s active recovery) (Thomassen et al. 2010). Thus, SET‐induced increases in expression and phosphorylation of FXYD1 may accentuate Na+–K+ pump activity and explain the lower femoral venous concentrations of K+ in recovery from intense exercise observed by Gunnarsson et al. (2013).
Aside from Na+–K+ pumps, skeletal muscle expresses Na+–K+–2Cl− exchangers (NKCC1), strong inward rectifying K+ channels (KIR2.1), and ATP‐sensitive K+ (KATP) channels, all of which are involved in K+ homeostasis of skeletal muscle and may be affected by SET (Spruce et al. 1985; Barrett‐Jolley et al. 1999; Nielsen et al. 2003; Kristensen & Juel, 2010). However, only a few studies have investigated the effect of SET on muscle expression of NKCC1, KIR2.1 and KATP channels in well‐trained individuals. In trained runners, 4 weeks of SET was shown to increase expression of NKCC1 by 14% (Iaia et al. 2008). A higher expression of NKCC1 induced by SET may benefit ion homeostasis during intense exercise in two ways. Firstly, NKCC1 regulates myocellular volume via influx of K+ and Na+ with Cl− and water (Gosmanov et al. 2003, 2004; Cairns et al. 2015), thereby counteracting the osmotic changes associated with ionic perturbations during exercise. Secondly, NKCC1‐mediated influx of Na+ stimulates Na+–K+ pump activity and thereby further re‐uptake of K+ (Clausen & Nielsen, 2007). In addition, 7 weeks of SET have been shown to increase expression of KIR2.1 by 18% (P = 0.06) and to reduce expression of the KATP channel subunit KIR6.2 by 14% in trained cyclists (Gunnarsson et al. 2013). A higher expression of KIR2.1 may augment K+ re‐uptake when the electrochemical gradient for K+ is reduced because of interstitial K+ accumulation (Wallinga et al. 1999; Kristensen et al. 2006; Kristensen & Juel, 2010), whereas a lower density of KATP channel KIR6.2 potentially reduces K+ extrusion to the interstitium during intense exercise, since critically low myocellular ATP levels cause opening of KIR6.2 (Davies, 1990; Davies et al. 1992, Xu et al. 2001). The concurrent increase in expression of KIR2.1 and reduction in expression of KIR6.2 may therefore also explain the lower femoral venous plasma K+ observed in recovery from intense exercise and the improved performance observed by Gunnarsson et al. (2013). Nevertheless, the role of NKCC1, KIR2.1 and KATP channels in K+ handling and performance during intense exercise is not completely clear. Studies using muscle preparations indicate the importance of these channels, since pharmacological manipulation of NKCC1, KIR2.1 and KATP channels affects fatigue development (Gong et al. 2003; Kristensen et al. 2006; Selvin & Renaud, 2015). And although muscle fatigue processes in non‐functioning KIR6.2 mice models are uncertain (Boudreault et al. 2010; Bruton, 2010), KATP channels are highly expressed in the transverse‐tubular system (Nielsen et al. 2003) and have been shown to serve a myoprotective role against high cytosolic Ca2+ fluctuations and fibre damage during intense muscle activity (Selvin & Renaud, 2015).
As outlined above, SET elicits several adaptations related to K+ handling of skeletal muscle in already trained individuals. SET may augment abundance and regulation of Na+–K+ pumps, increase expression of NKCC1 and KIR2.1, and reduce expression of KIR6.2. These adaptations are associated with lower concentrations of interstitial and femoral venous K+ during exercise and a more rapid decline in recovery from exercise (Nielsen et al. 2004a; Gunnarson et al. 2013). Given that the concentrations of interstitial and femoral venous K+ reach same levels at exhaustion after compared to before a period of SET, the adaptations related to K+ handling induced by SET may, at least in part, explain the improved performance during intense exercise in already trained individuals.
Effect of speed endurance training on accumulation of lactate− and H+
A major contributor to muscular energy production during intense exercise (½–6 min) is glycolysis, leading to production of lactate− and accumulation of H+ (Hultman & Sahlin, 1980; Cheetham et al. 1986; Nevill et al. 1989; Gaitanos et al. 1993; Juel et al. 2004). Estimates of energy production derived from glycolysis range from 60–70% during 30 s of maximal cycling (Bogdanis et al. 1995; Kalsen et al. 2016) to 30–40% during 2–5 min of intense knee extensor exercise (Bangsbo et al. 1990, 1992). Thus, muscle lactate− may accumulate from ∼1 to > 25 mmol (kg ww)−1 and pH may decline from ∼7.2 to < 6.6 during intense exhaustive exercise lasting a few minutes (Juel et al. 1990; Bangsbo et al. 1993; Hostrup et al. 2014). Skeletal muscle has several transport systems that facilitate efflux of lactate− and H+ from myocellular compartments to the interstitium (Juel, 1998a; Juel & Halestrap, 1999; Pilegaard et al. 1999b). The main muscle transporters of lactate− and H+ during exercise are the lactate−–H+ monocarboxylate cotransporters (MCT) of which the predominant isoforms 1 (MCT1) and 4 (MCT4) account for 70–80% of the lactate− and H+ efflux (Mason & Thomas, 1988; Juel, 1997; Pilegaard et al. 1999b; Thomas et al. 2012).
While several studies have investigated the effect of SET on muscle lactate− and H+ transport capacity and regulation in humans (Thomas et al. 2012), only few have been conducted in trained individuals. Unlike the marked improvements SET exerts in lactate−–H+ transport capacity of skeletal muscle in untrained and recreationally active individuals (Pilegaard et al. 1999a; Juel et al. 2004; Burgomaster et al. 2007), the adaptive response to SET in trained individuals is moderate and sometimes not evident. A few weeks of SET with reduced training volume have been shown to increase expression of MCT1 by ∼10% in elite football players and by up to ∼70% in trained men (Bickham et al. 2006; Thomassen et al. 2010; Gunnarsson et al. 2012; Puype et al. 2013), whereas no changes were observed in trained runners and cyclists (Bangsbo et al. 2009; Gunnarsson et al. 2013; Vorup et al. 2016). Unlike MCT1, expression of MCT4 does not seem to change with a period of SET in trained individuals (Bickham et al. 2006; Thomassen et al. 2010; Gunnarsson et al. 2013; Puybe et al. 2013; Skovgaard et al. 2014). However, it may be that alterations in MCT4 only occur in sarcolemma, since Dubouchaud et al. (2000) observed that endurance training increased expression of MCT4 in sarcolemmal fractions, but not in whole‐muscle homogenate.
Apart from MCT, skeletal muscle has several systems that regulate myocellular H+, including Na+–H+ exchangers (NHE1), carbonic anhydrase enzymes, HCO3 − transporters, and H+ buffers (Sahlin & Henriksson, 1984; Geers & Gros, 2000; Messonnier et al. 2007). While data on the effect of SET on carbonic anhydrase enzymes and HCO3 − transporters are scant, studies have shown that SET increases content of NHE1 (Iaia et al. 2008; Skovgaard et al. 2014) and H+ buffering capacity of skeletal muscle (Parkhouse & McKenzie, 1984; Bell & Wenger, 1988; Gibala et al. 2006; Baguet et al. 2011). NHE1 was shown to increase by 30–35% during a period of intensified training with reduced volume when SET was performed alone or in combination with resistance training in trained runners (Iaia et al. 2008; Skovgaard et al. 2014). Given that a higher expression of NHE1 may increase Na+ uptake and H+ efflux, it could be speculated that such adaptation may counteract contraction‐induced accumulation of interstitial K+ and sarcolemmal depolarization during intense exercise, since intracellular Na+ stimulates Na+–K+ pump activity (Nielsen et al. 2004b) and H+ accumulation causes opening of KATP channels and hence extrusion of K+ (Davies, 1990; Davies et al. 1992, Xu et al. 2001; Street et al. 2005). While most studies found no change in in vitro muscle H+ buffering capacity of muscle homogenate in trained individuals during a period of SET (Nevill et al. 1989; Iaia et al. 2008), in vivo muscle buffering capacity has been shown to be increased in some studies (Nevill et al. 1989; Gunnarsson et al. 2013). Thus, Nevill et al. (1989) observed no change in buffering capacity of muscle homogenate in vitro, whereas the estimated in vivo muscle buffering capacity from H+ and lactate− during 30 s of maximal cycling was 44% higher after 8 weeks of SET in male and female runners. In line with this, Gunnarsson et al. (2013) observed that in vivo muscle buffering capacity estimated during intense exercise (∼4 min) increased by ∼18% with 8 weeks of combined SET and aerobic high‐intensity training in trained cyclists. The difference between the in vitro and in vivo measurements observed may be due to a relative greater increase in the capacity of the trained muscle to release H+ than lactate (Juel et al. 1998a,b), e.g. due to an increased expression of NHE1 (Gunnarsson et al. 2013), which per se will increase the estimated in vivo buffer capacity but not the in vitro equivalent.
The implications of adaptations in muscle lactate−–H+ transport and H+ regulation for performance during intense exercise following a period of SET in trained individuals are not completely clear. While SET increases rate of H+ release during intense exercise and lactate−–H+ clearance in the first few minutes of recovery from exercise, lactate− and H+ formation greatly exceed muscle transport capacity during intense exercise (Bangsbo et al. 1990, 1993; Juel et al. 1990, 2004). To what extent accumulation of lactate− and H+ contribute to fatigue development is also controversial (Korzeniewski & Zoladz, 2002; Pedersen et al. 2004; Bangsbo & Juel, 2006; Fitts, 2006; Lamb & Stephenson, 2006; Lindinger, 2007). Studies in muscle preparations have challenged the importance of lactate− and H+ as muscle fatigue inducers (de Paoli et al. 2007), since low pH has little detrimental effect on fatigue development at physiological relevant temperatures, and lactate− has been shown to restore the depressing effect of raised extracellular K+ on sarcolemmal excitability because of its inhibitory effect on Cl− channels (ClC‐1) (Nielsen et al. 2001; Pedersen et al. 2004; Hansen et al. 2005, 2005; de Paoli et al. 2010). In simulation models, Korzeniewski & Zoladz (2002) also demonstrated that H+ accumulation may counteract disturbances in energy system integrity, especially oxidative phosphorylation in working muscles, in that glycolytic production of H+ compensates the cytosolic alkalization caused by H+ consumption of creatine kinase at the onset of exercise. Nevertheless, although these studies in muscle preparations in vitro and in simulation models imply that lactate− and H+ may even act protectively against fatigue (Allen & Westerblad, 2004), studies in humans indicate that accumulation of lactate− and H+ may be detrimental for performance during intense exercise. For instance, accumulation of lactate− induced by intense arm ergometer exercise was associated with greater accumulation of myocellular H+ (pH: 6.65 vs. 6.82), reduced lactate− release (19 vs. 50 mmol), and a ∼30% reduction in performance of the quadriceps during subsequent intense knee‐extensor exercise (Bangsbo et al. 1996; Nordsborg et al. 2003). Notably, preceding arm ergometer exercise also caused a higher rate of extracellular K+ accumulation during the subsequent knee‐extensor exercise (Bangsbo et al. 1996; Nordsborg et al. 2003). The latter observation may be related to the greater myocellular accumulation H+, thus causing opening of KATP channels (Davies, 1990; Davies et al. 1992, Xu et al. 2001; Street et al. 2005). In addition, lactate−–H+ transport and buffering capacity of skeletal muscle are good predictors for intense exercise performance (Nevill et al. 1989; Pilegaard et al. 1994; Bishop et al. 2003, 2004; Thomas et al. 2005; Messonnier et al. 2007), and athletes have higher lactate−–H+ transport and buffering capacity of skeletal muscle than trained and untrained individuals (McKenzie et al. 1982; Sahlin & Henriksson, 1984; Pilegaard et al. 1994; Edge et al. 2006). Lastly, β‐alanine and sodium bicarbonate supplementation enhance intense exercise performance in trained individuals (Carr et al. 2011; Hobson et al. 2012). These effects are respectively related to increased muscle content of the H+ buffer carnosine (Hill et al. 2007) and systemic HCO3− concentrations (Sostaric et al. 2006). Augmented muscle carnosine content may also increase Ca2+ sensitivity and affect cross‐bridge formation because of carnosine's potential role as a diffusible Ca2+–H+ exchanger (Dutka et al. 2012; Swietach et al. 2013), whereas increased systemic HCO3− concentrations have been shown to reduce the rate of accumulation of extracellular H+ and K+ (Street et al. 2005; Sostaric et al. 2006). Collectively, these observations underpin the importance of improved muscle lactate−–H+ transport and H+ regulation induced by a period of SET for performance during intense exercise in already trained individuals.
Effect of speed endurance training on sarcoplasmic reticulum Ca2+ handling and myofibrillar Ca2+ sensitivity
Skeletal muscle force production and relaxation are regulated by myoplasmic concentrations of Ca2+ and myofibrillar Ca2+ sensitivity. Force production is controlled by Ca2+ release via opening of sarcoplasmic reticulum (SR) ryanodine receptors (RyR1) and relaxation by subsequent re‐sequestration of Ca2+ from the myoplasm into the SR, which predominantly is controlled by the Ca2+ uptake rate of SR Ca2+‐ATPase isoforms SERCAI and SERCAII, but also by the dissociation off‐kinetics of troponin C, and the Ca2+ loading rate of Ca2+‐binding proteins (Westerblad & Allen, 1996a,b; Nogueira et al. 2013). Based on studies in muscle preparations, impairment of Ca2+ release in particular appears to contribute to muscle fatigue development (Allen et al. 2008, 2011; Nogueira et al. 2013). However, while the importance of Ca2+ handling for fatigue resistance is well described in muscle preparations, surprisingly few studies have investigated the effect of training on SR Ca2+ handling function and myofibrillar Ca2+ sensitivity in humans, and only two studies have, to our knowledge, been undertaken with SET in trained individuals (Ørtenblad et al. 2000; Thomassen et al. 2016).
Ørtenblad et al. (2000) observed that 5 weeks of SET augmented maximal AgNO3 stimulated Ca2+ release in SR vesicles of muscle homogenate by 9%, which was associated with a 12% better mean power output during a repeated sprint test (10 × 8 s, 32 s recovery) in highly trained men. The adaptations in Ca2+ release function were presumably related to a training‐induced increase in total SR‐volume, since expression of RyR1, SERCAI and SERCAII was increased by SET, whereas [H3]‐ryanodine‐specific binding remained unchanged (Ørtenblad et al. 2000). In spite of the increased expression of SERCAI and II, however, the authors observed no changes in SR vesicle Ca2+ uptake kinetics or Ca2+‐ATPase activity of muscle homogenate with the intervention. Because these measures were performed in in vitro preparations, extrapolation to in vivo Ca2+ release and uptake function during exercise is speculative. However, reduction in the in vitro SR Ca2+ release function of muscle homogenate has been shown to predict a decline in muscle torque after intense knee‐extensor exercise of untrained individuals (Hill et al. 2001). In addition, 7 weeks of SET, combined with aerobic high‐intensity training, was shown to increase total expression and level of phosphorylation of the SERCAII‐regulatory protein phospholamban and Ca2+–calmodulin‐dependent protein kinase II (CaMKII) in cyclists (Thomassen et al. 2016). A higher expression and phosphorylation of phospholamban and CaMKII may increase the rate of SR Ca2+ release and re‐uptake because of the reduced affinity for Ca2+ of SERCAII and increased opening probability of RyR1 (Suko et al. 1993; Rose et al. 2006; Kemi et al. 2007). Such effects might potentially improve SR Ca2+ handling during intense exercise. For myofibrillar Ca2+ sensitivity, currently no data are available regarding the effect of SET in trained individuals. In untrained individuals, Lynch et al. (1994) observed that sprint training lowered sensitivity for Sr2+, while sensitivity for Ca2+ remained unaltered at normal pH but was lower at pH 6.6, which reflects the pH during intense exercise.
The limited number of studies that have investigated the effects of SET on Ca2+ handling makes interpretation of its implications difficult. Cross‐sectional data have shown that endurance‐trained individuals have lower in vitro Ca2+ handling function than resistance‐trained and untrained individuals (Li et al. 2002). In line with this, aerobic training has been shown to reduce expression of SERCA and to lower in vitro Ca2+ release and uptake function of skeletal muscle (Majerczak et al. 2008; Green et al. 2011; Zoladz et al. 2013). Although these observations may imply that Ca2+ handling function is not the main determinant factor for endurance performance, studies have shown that Ca2+ uptake function may predict some of the variance during intense exercise (Harmer et al. 2014). Furthermore, 6 weeks of knee‐extensor training was shown to increase rate of Ca2+ release and reduce Ca2+ leak from the SR, which were associated with enhanced peak power in untrained men (Munkvik et al. 2010). Nonetheless, while potential enhancing effects of SET in SR Ca2+ release function (Ørtenblad et al. 2000) as well as in expression and phosphorylation of phospholamban (Thomassen et al. 2016) may augment Ca2+ handling during intense exercise in trained individuals, more studies are needed on this issue.
Influence of speed endurance training on energy metabolism and reactive oxygen species: interaction with ion handling
Myocellular energy metabolism is important for excitation–contraction coupling during intense exercise. Na+–K+ pumps and SERCA account for ∼40% of energy expenditure of skeletal muscle during repeated contractions (Clausen, 2003; Walsh et al. 2006; Barclay et al. 2007) and any local limitation in energy availability and/or accumulation of metabolites impairs ion handling and accelerates fatigue development (Matar et al. 2000; Gong et al. 2003; Nogueira et al. 2013; Ørtenblad et al. 2013; Place et al. 2015; Selvin & Renaud, 2015). Low levels of ATP with a concomitant rise in free Mg2+, H+, ADP, IMP and Pi activates KATP channels (Allard et al. 1995; Barrett‐Jolley & Davies, 1997; Pedersen et al. 2009), causes Ca2+–Pi precipitation inside the SR (Allen et al. 2011), impairs Ca2+ handling (Li et al. 2002; Allen et al. 2008; Nogueira et al. 2013), and reduces Na+–K+ pump activity (Juel et al. 2014). Furthermore, anaerobic energy production through glycolysis leads to lactate− production and the accompanying H+ accumulation changes SERCA activity and KATP channel sensitivity for ATP (Davies, 1990, 1992, Westerblad & Allen, 1993, Xu et al. 2001; Flagg et al. 2010; Nogueira et al. 2013). Anaerobic enzymes lactate dehydrogenase and creatine kinase may also interact with KATP channels (Crawford et al. 2002a,b), and Na+–K+ pump activity and SR Ca2+ handling are regulated by glycogenolysis and glycolysis (James et al. 1999; Okamoto et al. 2001; Dutka & Lamb, 2007; Nogueira et al. 2013; Ørtenblad et al. 2013). As such, a higher capacity for glycolytic ATP provision following a period of SET may potentially improve regulation of ion homeostasis during intense exercise.
SET has been shown to accentuate glycolytic enzymes (Dawson et al. 1998; Iaia et al. 2008; Puype et al. 2013: Vorup et al. 2016), increase glycogen content, and augment capacity for glycolytic energy production of skeletal muscle in trained individuals (Nevill et al. 1989; Shepley et al. 1992). Furthermore, given that CaMKII regulates the rate of glycogenolysis and glycolysis (Rose et al. 2006), a higher expression and phosphorylation of CaMKII induced by SET may accelerate the rate of glycolytic ATP provision during intense exercise (Gunnarsson et al. 2013; Thomassen et al. 2016). Notably, Iaia et al. (2008) observed that glycogen and phosphocreatine utilization during a second bout of intense exercise to exhaustion (∼1½ min) were lower after a period of SET, despite of higher accumulation of blood lactate− and longer time to exhaustion. This observation suggests that muscle oxidation and/or mechanical efficiency was improved by SET. Such adaptations may enhance metabolic stability and delay the impairments in contraction–excitation contraction coupling of skeletal muscle during intense exercise (MacIntosh & Shahi, 2011; Grassi et al. 2015). Furthermore, any potential glycogen‐sparing effect of a period of SET might potentially counteract exercise‐induced reductions in SR Ca2+ handling, since critically low glycogen levels in proximity to the SR have been shown to be associated with impaired Ca2+ handling function in highly trained individuals (Ørtenblad et al. 2011; Hostrup et al. 2014; Nielsen et al. 2014).
Intense exercise leads to formation of reactive oxygen species (ROS) and glutathione in skeletal muscle that reacts with thiol groups (glutathionylation) of various myocellular proteins of importance for ion handling (Juel et al. 2015; Cheng et al. 2016). Among these proteins, the Na+–K+ pump is subjected to glutathionylation in human skeletal muscle during intense exercise, which has been shown to attenuate maximal in vitro Na+–K+ pump activity by ∼25% in highly trained individuals (Hostrup et al. 2014; Juel et al. 2015); a phenomenon known as Na+–K+ pump inactivation (McKenna et al. 2008). Furthermore, ROS‐production reduces SR Ca2+ release function and myofibrillar Ca2+ sensitivity (Place et al. 2015; Cheng et al. 2016). Interestingly, in recreationally active individuals, muscle Ca2+ release channel RyR1 is subjected to fragmentation following one session of SET, whereas endurance athletes have no fragmentation (Place et al. 2015). This observation indicates that training augments muscle oxidant defence and protects the integrity of proteins related to ion handling during intense exercise. In support of these observations, a few weeks of SET has been shown to increase activity of antioxidant enzymes glutathione peroxidase and glutathione reductase in recreationally active individuals (Hellsten et al. 1996; Bogdanis et al. 2013). In addition, Green et al. (1998) observed that 7 and 11 weeks of high‐intensity resistance training attenuated reductions in Ca2+‐ATPase activity induced by intense cycling. While it could be speculated that SET would have similar adaptive effects in already trained individuals, future studies are warranted to elucidate whether SET augments muscle oxidant defence and counteracts glutathionylation of the Na+–K+ pump and fragmentation of RyR1 in the trained population.
Conclusion and perspectives
As presented in this review, only a few weeks of SET leads to significant performance enhancements during intense exercise in already trained individuals. Given that a period of SET has no apparent effects on , haemoglobin mass, plasma volume, muscle mass, capillarization or oxidative capacity in trained individuals, the enhancements in performance during intense exercise induced by SET may be related to muscle adaptations in ion handling, including improved regulation of K+ homeostasis, enhanced lactate−–H+ transport, and in some instances augmented H+ buffering capacity and SR Ca2+ handling. The implications of these adaptations with relevance for performance during intense exercise may involve:
Lower rate of accumulation of interstitial K+ of exercising muscles due to increased Na+–K+ pump activity and repression of KATP channels.
Reduced extrusion of K+ from KATP channels because of augmented lactate−–H+ transport and H+ regulation.
Augmented Ca2+ handling.
Higher capacity for glycolytic ATP provision.
Because of the marked perturbations of multiple ions (Cairns & Lindinger, 2008; McKenna et al. 2008) and their interactions with energy production and ROS formation during muscle activity (MacIntosh & Shahi, 2011; Place et al. 2015; Cheng et al. 2016), fatigue processes during intense exercise in trained individuals are complex, and further studies are needed to elucidate the exact mechanisms underlying the enhancing effects of SET on performance. For instance, recent observations in muscle preparations indicate that extracellular Ca2+ interacts with lowered transsarcolemmal K+ gradients during muscle contractions, where a reduction in extracellular Ca2+ lowers the intracellular activity of K+ and contributes to fatigue development (Cairns et al. 2015). In this perspective, studies are warranted that investigate the importance of muscle Ca2+–H+ and Na+–Ca2+ exchangers, as well as ClC‐1 channels for exercise performance in humans and their potential adaptability to training. For example, ClC‐1 channels are of interest because of their role in regulating Cl− conductance and sarcolemmal excitability during muscle activity (Dutka et al. 2008; de Paoli et al. 2010, 2013). Furthermore, improvements related to ion handling, lactate− transport, and H+ regulation after a period of SET could possibly affect regulation of myocellular and plasma osmolarity during exercise, and the implication of such changes for performance should be studied (Lindinger et al. 1995, 2013). Despite the difficulty in recruiting athletes for advanced physiological training interventions, more studies are needed in the athlete population. Lastly, it is relevant to probe the long‐term effects of SET, since the intervention period of most studies that have provided an emphasis for this training modality only lasted a few weeks.
Additional information
Competing interests
None declared.
Biographies
Morten Hostrup, Ph.D., is Assistant Professor in the Department of Nutrition, Exercise and Sports (NEXS), University of Copenhagen and the Department of Respiratory Research at Bispebjerg Hospital in Copenhagen, Denmark. He studies fatigue processes and limitations in exercise performance of humans using various interventions, including training, nutrition and pharmacology.

Jens Bangsbo, Ph.D., is Professor and Deputy Head at NEXS, University of Copenhagen, Denmark, and leader of the Integrative Physiology Group at NEXS. His research focuses on optimizing exercise performance, including how development of muscle fatigue is affected by high‐intensity training.
References
- Allard B, Fournet G, Rougier O, Descans B & Vivaudou M (1995). Dose‐dependent activation and block by bisG10, a K+ channel blocker, of mouse and frog skeletal muscle KATP channels. FEBS Lett 375, 215–219. [DOI] [PubMed] [Google Scholar]
- Allen D & Westerblad H (2004). Physiology. Lactic acid – the latest performance‐enhancing drug. Science 305, 1112–1113. [DOI] [PubMed] [Google Scholar]
- Allen DG, Clugston E, Petersen Y, Röder IV, Chapman B & Rudolf R (2011). Interactions between intracellular calcium and phosphate in intact mouse muscle during fatigue. J Appl Physiol 111, 358–366. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD & Westerblad H (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88, 287–332. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2011). Implications of group III and IV muscle afferents for high‐intensity endurance exercise performance in humans. J Physiol 589, 5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aughey RJ, Murphy KT, Clark SA, Garnham AP, Snow RJ, Cameron‐Smith D, Hawley JA & McKenna MJ (2007). Muscle Na+‐K+‐ATPase activity and isoform adaptations to intense interval exercise and training in well‐trained athletes. J Appl Physiol 103, 39–47. [DOI] [PubMed] [Google Scholar]
- Baguet A, Everaert I, De Naeyer H, Reyngoudt H, Stegen S, Beeckman S, Achten E, Vanhee L, Volkaert A, Petrovic M, Taes Y & Derave W (2011). Effects of sprint training combined with vegetarian or mixed diet on muscle carnosine content and buffering capacity. Eur J Appl Physiol 111, 2571–2580. [DOI] [PubMed] [Google Scholar]
- Bangsbo J & Juel C (2006). Counterpoint: lactic acid accumulation is a disadvantage during muscle activity. J Appl Physiol 100, 1412–1413. [DOI] [PubMed] [Google Scholar]
- Bangsbo J (2015). Performance in sports – With specific emphasis on the effect of intensified training. Scand J Med Sci Sports 25, 88–99. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Gollnick PD, Graham TE, Juel C, Kiens B, Mizuno M & Saltin B (1990). Anaerobic energy production and O2 deficit‐debt relationship during exhaustive exercise in humans. J Physiol 422, 539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Graham T, Johansen L, Strange S, Christensen C & Saltin B (1992). Elevated muscle acidity and energy production during exhaustive exercise in humans. Am J Physiol 263, 891–899. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Gunnarsson TP, Wendell J, Nybo L & Thomassen M (2009). Reduced volume and increased training intensity elevate muscle Na+‐K+ pump α2‐subunit expression as well as short‐ and long‐term work capacity in humans. J Appl Physiol 107, 1771–1780. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Johansen L, Graham T & Saltin B (1993). Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol 462, 115–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B & Richter EA (1996). Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol 495, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay CJ, Woledge RC & Curtin NA (2007). Energy turnover for Ca2+ cycling in skeletal muscle. J Muscle Res Cell Motil 28, 259–274. [DOI] [PubMed] [Google Scholar]
- Barrett‐Jolley R & Davies NW (1997). Kinetic analysis of the inhibitory effect of glibenclamide on KATP channels of mammalian skeletal muscle. J Membr Biol 155, 257–262. [DOI] [PubMed] [Google Scholar]
- Barrett‐Jolley R, Dart C & Standen NB (1999). Direct block of native and cloned (Kir2.1) inward rectifier K+ channels by chloroethylclonidine. Br J Pharmacol 128, 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GJ & Wenger HA (1988). The effect of one‐legged sprint training on intramuscular pH and nonbicarbonate buffering capacity. Eur J Appl Physiol Occup Physiol 58, 158–164. [DOI] [PubMed] [Google Scholar]
- Bibert S, Roy S, Schaer D, Horisberger JD & Geering K (2008). Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K‐ATPase isozymes. J Biol Chem 283, 476–486. [DOI] [PubMed] [Google Scholar]
- Bickham DC, Bentley DJ, Le Rossignol PF & Cameron‐Smith D (2006). The effects of short‐term sprint training on MCT expression in moderately endurance‐trained runners. Eur J Appl Physiol 96, 636–643. [DOI] [PubMed] [Google Scholar]
- Bishop D, Edge J & Goodman C (2004). Muscle buffer capacity and aerobic fitness are associated with repeated‐sprint ability in women. Eur J Appl Physiol 92, 540–547. [DOI] [PubMed] [Google Scholar]
- Bishop D, Girard O & Mendez‐Villanueva A (2011). Repeated‐sprint ability – part II: recommendations for training. Sports Med 41, 741–756. [DOI] [PubMed] [Google Scholar]
- Bishop D, Lawrence S & Spencer M (2003). Predictors of repeated‐sprint ability in elite female hockey players. J Sci Med Sport 6, 199–209. [DOI] [PubMed] [Google Scholar]
- Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK & Nevill AM (1995). Recovery of power output and muscle metabolites following 30 s of maximal sprint cycling in man. J Physiol 482, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanis GC, Stavrinou P, Fatouros IG, Philippou A, Chatzinikolaou A, Draganidis D, Ermidis G & Maridaki M (2013). Short‐term high‐intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem Toxicol 61, 171–177. [DOI] [PubMed] [Google Scholar]
- Boudreault L, Cifelli C, Bourassa F, Scott K & Renaud JM (2010). Fatigue preconditioning increases fatigue resistance in mouse flexor digitorum brevis muscles with non‐functioning KATP channels. J Physiol 588, 4549–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J (2010). What does the membrane KATP channel really do in skeletal muscle? J Physiol 588, 4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgomaster KA, Cermak NM, Phillips SM, Benton CR, Bonen A & Gibala MJ (2007). Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol 292, R1970–R1976. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL & Gibala MJ (2008). Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP & Lindinger MI (2008). Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol 586, 4039–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA & Clausen T (1995). Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+‐K+ pump. Pflugers Arch 430, 909–915. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Hing WA, Slack JR, Mills RG & Loiselle DS (1997). Different effects of raised [K+]o on membrane potential and contraction in mouse fast‐ and slow‐twitch muscle. Am J Physiol 273, 598–611. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Leader JP, Loiselle DS, Higgins A, Lin W & Renaud JM (2015). Extracellular Ca2+‐induced force restoration in K+‐depressed skeletal muscle of the mouse involves an elevation of [K+]i: implications for fatigue. J Appl Physiol 118, 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Hopkins WG & Gore CJ (2011). Effects of acute alkalosis and acidosis on performance: a meta‐analysis. Sports Med 41, 801–814. [DOI] [PubMed] [Google Scholar]
- Cheetham ME, Boobis LH, Brooks S & Williams C (1986). Human muscle metabolism during sprint running. J Appl Physiol 61, 54–60. [DOI] [PubMed] [Google Scholar]
- Cheng AJ, Yamada T, Rassier D, Andersson DC, Westerblad H & Lanner JT (2016). Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J Physiol 594, 5149–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen PM, Gunnarsson TP, Thomassen M, Wilkerson DP, Nielsen JJ, Bangsbo J (2015). Unchanged content of oxidative enzymes in fast‐twitch muscle fibers and kinetics after intensified training in trained cyclists. Physiol Rep 3, pii: e12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen PM, Jacobs RA, Bonne T, Flück D, Bangsbo J & Lundby C (2016). A short period of high‐intensity interval training improves skeletal muscle mitochondrial function and pulmonary oxygen uptake kinetics. J Appl Physiol 120, 1319–1327. [DOI] [PubMed] [Google Scholar]
- Christensen PM, Krustrup P, Gunnarsson TP, Kiilerich K, Nybo L & Bangsbo J (2011). VO2 kinetics and performance in soccer players after intense training and inactivity. Med Sci Sports Exerc 43, 1716–1724. [DOI] [PubMed] [Google Scholar]
- Clausen T & Flatman JA (1977). The effect of catecholamines on Na‐K transport and membrane potential in rat soleus muscle. J Physiol 270, 383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T & Kohn PG (1977). The effect of insulin on the transport of sodium and potassium in rat soleus muscle. J Physiol 265, 19–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T & Nielsen OB (2007). Potassium, Na+,K+‐pumps and fatigue in rat muscle. J Physiol 584, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T (2003). Na+‐K+ pump regulation and skeletal muscle contractility. Physiol Rev 83, 1269–1324. [DOI] [PubMed] [Google Scholar]
- Clausen T (2008). Clearance of extracellular K+ during muscle contraction – roles of membrane transport and diffusion. J Gen Physiol 131, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T (2011). In isolated skeletal muscle, excitation may increase extracellular K+ 10‐fold; how can contractility be maintained? Exp Physiol 96, 356–368. [DOI] [PubMed] [Google Scholar]
- Clausen T (2013). Excitation‐induced exchange of Na+, K+, and Cl− in rat EDL muscle in vitro and in vivo: physiology and pathophysiology. J Gen Physiol 141, 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T (2015). Excitation of skeletal muscle is a self‐limiting process, due to run‐down of Na+,K+ gradients, recoverable by stimulation of the Na+,K+ pumps. Physiol Rep 3, pii: e12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crambert G, Fuzesi M, Garty H, Karlish S & Geering K (2002). Phospholemman (FXYD1) associates with Na,K‐ATPase and regulates its transport properties. Proc Natl Acad Sci USA 99, 11476–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RM, Budas GR, Jovanović S, Ranki HJ, Wilson TJ, Davies AM & Jovanović A (2002a). M‐LDH serves as a sarcolemmal KATP channel subunit essential for cell protection against ischemia. EMBO J 21, 3936–39348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RM, Ranki HJ, Botting CH, Budas GR & Jovanovic A (2002b). Creatine kinase is physically associated with the cardiac ATP‐sensitive K+ channel in vivo . FASEB J 16, 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NW (1990). Modulation of ATP‐sensitive K+ channels in skeletal muscle by intracellular protons. Nature 343, 375–377. [DOI] [PubMed] [Google Scholar]
- Davies NW, Standen NB & Stanfield PR (1992). The effect of intracellular pH on ATP‐dependent potassium channels of frog skeletal muscle. J Physiol 445, 549–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson B, Fitzsimons M, Green S, Goodman C, Carey M & Cole K (1998). Changes in performance, muscle metabolites, enzymes and fibre types after short sprint training. Eur J Appl Physiol Occup Physiol 78, 163–169. [DOI] [PubMed] [Google Scholar]
- de Paoli FV, Broch‐Lips M, Pedersen TH & Nielsen OB (2013). Relationship between membrane Cl− conductance and contractile endurance in isolated rat muscles. J Physiol 591, 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paoli FV, Ørtenblad N, Pedersen TH, Jørgensen R & Nielsen OB (2010). Lactate per se improves the excitability of depolarized rat skeletal muscle by reducing the Cl− conductance. J Physiol 588, 4785–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paoli FV, Overgaard K, Pedersen TH & Nielsen OB (2007). Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+ . J Physiol 581, 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC & Brooks GA (2000). Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 278, E571–E579. [DOI] [PubMed] [Google Scholar]
- Dutka TL & Lamb GD (2007). Na+‐K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. Am J Physiol Cell Physiol 293, C967–C977. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamboley CR, McKenna MJ, Murphy RM & Lamb GD (2012). Effects of carnosine on contractile apparatus Ca²+ sensitivity and sarcoplasmic reticulum Ca²+ release in human skeletal muscle fibers. J Appl Physiol 112, 728–736. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Murphy RM, Stephenson DG & Lamb GD (2008). Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation–contraction coupling and fatigue. J Physiol 586, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge J, Bishop D, Hill‐Haas S, Dawson B & Goodman C (2006). Comparison of muscle buffer capacity and repeated‐sprint ability of untrained, endurance‐trained and team‐sport athletes. Eur J Appl Physiol 96, 225–234. [DOI] [PubMed] [Google Scholar]
- Evertsen F, Medbo JI, Jebens E & Nicolaysen K (1997). Hard training for 5 mo increases Na+‐K+ pump concentration in skeletal muscle of cross‐country skiers. Am J Physiol 272, 1417–1424. [DOI] [PubMed] [Google Scholar]
- Faria EW, Parker DL & Faria IE (2005). The science of cycling: physiology and training – part 1. Sports Med 35, 285–312. [DOI] [PubMed] [Google Scholar]
- Fitts RH (2006). The muscular system: fatigue processes In ACSM's Advanced Exercise Physiology, ed. Tipton CM, pp. 178–197. Lippincott Williams & Wilkins. [Google Scholar]
- Flagg TP, Enkvetchakul D, Koster JC & Nichols CG (2010). Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev 90, 799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman JA & Clausen T (1979). Combined effects of adrenaline and insulin on active electrogenic Na+‐K+ transport in rat soleus muscle. Nature 281, 580–581. [DOI] [PubMed] [Google Scholar]
- Gaitanos GC, Williams C, Boobis LH & Brooks S (1993). Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 75, 712–719. [DOI] [PubMed] [Google Scholar]
- Geers C & Gros G (2000). Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80, 681–715. [DOI] [PubMed] [Google Scholar]
- Gibala MJ & Jones AM (2013). Physiological and performance adaptations to high‐intensity interval training. Nestle Nutr Inst Workshop Ser 76, 51–60. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S & Tarnopolsky MA (2006). Short‐term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Legault D, Miki T, Seino S & Renaud JM (2003). KATP channels depress force by reducing action potential amplitude in mouse EDL and soleus muscle. Am J Physiol Cell Physiol 285, C1464–C1474. [DOI] [PubMed] [Google Scholar]
- Gosmanov AR, Fan Z, Mi X, Schneider EG & Thomason DB (2004). ATP‐sensitive potassium channels mediate hyperosmotic stimulation of NKCC in slow‐twitch muscle. Am J Physiol Cell Physiol 286, C586–C595. [DOI] [PubMed] [Google Scholar]
- Gosmanov AR, Schneider EG & Thomason DB (2003). NKCC activity restores muscle water during hyperosmotic challenge independent of insulin, ERK, and p38 MAPK. Am J Physiol Regul Integr Comp Physiol 284, R655–R665. [DOI] [PubMed] [Google Scholar]
- Grassi B, Rossiter HB & Zoladz JA (2015). Skeletal muscle fatigue and decreased efficiency: two sides of the same coin? Exerc Sport Sci Rev 43, 75–83. [DOI] [PubMed] [Google Scholar]
- Green HJ, Burnett M, Kollias H, Ouyang J, Smith I & Tupling S (2011). Malleability of human skeletal muscle sarcoplasmic reticulum to short‐term training. Appl Physiol Nutr Metab 36, 904–912. [DOI] [PubMed] [Google Scholar]
- Green HJ, Chin ER, Ball‐Burnett M & Ranney D (1993). Increases in human skeletal muscle Na+‐K+‐ATPase concentration with short‐term training. Am J Physiol 264, 1538–1541. [DOI] [PubMed] [Google Scholar]
- Green HJ, Grange F, Chin C, Goreham C & Ranney D (1998). Exercise‐induced decreases in sarcoplasmic reticulum Ca2+‐ATPase activity attenuated by high‐resistance training. Acta Physiol Scand 164, 141–146. [DOI] [PubMed] [Google Scholar]
- Green S, Langberg H, Skovgaard D, Bulow J & Kjaer M (2000). Interstitial and arterial‐venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J Physiol 529, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson TP, Christensen PM, Holse K, Christiansen D & Bangsbo J (2012). Effect of additional speed endurance training on performance and muscle adaptations. Med Sci Sports Exerc 44, 1942–1948. [DOI] [PubMed] [Google Scholar]
- Gunnarsson TP, Christensen PM, Thomassen M, Nielsen LR & Bangsbo J (2013). Effect of intensified training on muscle ion kinetics, fatigue development, and repeated short‐term performance in endurance‐trained cyclists. Am J Physiol Regul Integr Comp Physiol 305, R811–R821. [DOI] [PubMed] [Google Scholar]
- Han F, Bossuyt J, Despa S, Tucker AL & Bers DM (2006). Phospholemman phosphorylation mediates the protein kinase C‐dependent effects on Na+/K+ pump function in cardiac myocytes. Circ Res 99, 1376–1383. [DOI] [PubMed] [Google Scholar]
- Hansen AK, Clausen T & Nielsen OB (2005). Effects of lactic acid and catecholamines on contractility in fast‐twitch muscles exposed to hyperkalemia. Am J Physiol Cell Physiol 289, C104–C112. [DOI] [PubMed] [Google Scholar]
- Harmer AR, Ruell PA, Hunter SK, McKenna MJ, Thom JM, Chisholm DJ & Flack JR (2014). Effects of type 1 diabetes, sprint training and sex on skeletal muscle sarcoplasmic reticulum Ca2+ uptake and Ca2+‐ATPase activity. J Physiol 592, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Apple FS & Sjödin B (1996). Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J Appl Physiol 81, 1484–1487. [DOI] [PubMed] [Google Scholar]
- Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK & Wise JA (2007). Influence of beta‐alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32, 225–233. [DOI] [PubMed] [Google Scholar]
- Hill CA, Thompson MW, Ruell PA, Thom JM & White MJ (2001). Sarcoplasmic reticulum function and muscle contractile character following fatiguing exercise in humans. J Physiol 531, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RM, Saunders B, Ball G, Harris RC & Sale C (2012). Effects of β‐alanine supplementation on exercise performance: a meta‐analysis. Amino Acids 43, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Ørtenblad N, Juel C, Mørch K, Rzeppa S, Karlsson S, Backer V & Bangsbo J (2014). β2‐Adrenergic stimulation enhances Ca2+ release and contractile properties of skeletal muscles, and counteracts exercise‐induced reductions in Na+‐K+‐ATPase in trained men. J Physiol 592, 5445–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston ME & Thomson JA (1977). The response of endurance‐adapted adults to intense anaerobic training. Eur J Appl Physiol Occup Physiol 36, 207–213. [DOI] [PubMed] [Google Scholar]
- Hultman E & Sahlin K (1980). Acid‐base balance during exercise. Exerc Sport Sci Rev 8, 41–128. [PubMed] [Google Scholar]
- Iaia FM & Bangsbo J (2010). Speed endurance training is a powerful stimulus for physiological adaptations and performance improvements of athletes. Scand J Med Sci Sports 20, 11–23. [DOI] [PubMed] [Google Scholar]
- Iaia FM, Hellsten Y, Nielsen JJ, Fernström M, Sahlin K & Bangsbo J (2009). Four weeks of speed endurance training reduces energy expenditure during exercise and maintains muscle oxidative capacity despite a reduction in training volume. J Appl Physiol 106, 73–80. [DOI] [PubMed] [Google Scholar]
- Iaia FM, Thomassen M, Kolding H, Gunnarsson T, Wendell J, Rostgaard T, Nordsborg N, Krustrup P, Nybo L, Hellsten Y & Bangsbo J (2008). Reduced volume but increased training intensity elevates muscle Na+‐K+ pump α1‐subunit and NHE1 expression as well as short‐term work capacity in humans. Am J Physiol Regul Integr Comp Physiol 294, R966–R974. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Flück D, Bonne TC, Bürgi S, Christensen PM, Toigo M & Lundby C (2013). Improvements in exercise performance with high‐intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol 115, 785–793. [DOI] [PubMed] [Google Scholar]
- James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, Friend LA, Shelly DA, Paul RJ & Fischer JE (1999). Stimulation of both aerobic glycolysis and Na+‐K+‐ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol 277, 176–186. [DOI] [PubMed] [Google Scholar]
- Jensen L, Bangsbo J & Hellsten Y (2004). Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol 557, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C & Halestrap AP (1999). Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. J Physiol 517, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C (1997). Lactate–proton cotransport in skeletal muscle. Physiol Rev 77, 321–358. [DOI] [PubMed] [Google Scholar]
- Juel C (1998a). Muscle pH regulation: role of training. Acta Physiol Scand 162, 359–366. [DOI] [PubMed] [Google Scholar]
- Juel C (1998b). Skeletal muscle Na+/H+ exchange in rats: pH dependency and the effect of training. Acta Physiol Scand 164, 135–140. [DOI] [PubMed] [Google Scholar]
- Juel C, Bangsbo J, Graham T & Saltin B (1990). Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol Scand 140, 147–159. [DOI] [PubMed] [Google Scholar]
- Juel C, Hostrup M & Bangsbo J (2015). The effect of exercise and β2‐adrenergic stimulation on glutathionylation and function of the Na,K‐ATPase in human skeletal muscle. Physiol Rep 3, pii: e12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M & Bangsbo J (2004). Effect of high‐intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab 286, E245–E251. [DOI] [PubMed] [Google Scholar]
- Juel C, Nordsborg NB & Bangsbo J (2013). Exercise‐induced increase in maximal in vitro Na‐K‐ATPase activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 304, R1161–R1165. [DOI] [PubMed] [Google Scholar]
- Juel C, Nordsborg NB & Bangsbo J (2014). Purinergic effects on Na,K‐ATPase activity differ in rat and human skeletal muscle. PLoS One 9, e91175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ & Bangsbo J (2000). Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol 278, R400–R406. [DOI] [PubMed] [Google Scholar]
- Kalsen A, Hostrup M, Backer V & Bangsbo J (2016). Effect of formoterol, a long‐acting β2‐adrenergic agonist, on muscle strength and power output, metabolism, and fatigue during maximal sprinting in men. Am J Physiol Regul Integr Comp Physiol 310, R1312–R1321. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Ellingsen O, Ceci M, Grimaldi S, Smith GL, Condorelli G & Wisløff U (2007). Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr‐17 of phospholamban. J Mol Cell Cardiol 43, 3354–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard H & Clausen T (1989). Increased total concentration of Na‐K pumps in vastus lateralis muscle of old trained human subjects. J Appl Physiol 67, 2491–2494. [DOI] [PubMed] [Google Scholar]
- Knicker AJ, Renshaw I, Oldham AR & Cairns SP (2011). Interactive processes link the multiple symptoms of fatigue in sport competition. Sports Med 41, 307–328. [DOI] [PubMed] [Google Scholar]
- Korzeniewski B & Zoladz JA (2002). Influence of rapid changes in cytosolic pH on oxidative phosphorylation in skeletal muscle: theoretical studies. Biochem J 365, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M & Juel C (2010). Potassium‐transporting proteins in skeletal muscle: cellular location and fibre‐type differences. Acta Physiol (Oxf) 198, 105–123. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Hansen T & Juel C (2006). Membrane proteins involved in potassium shifts during muscle activity and fatigue. Am J Physiol Regul Integr Comp Physiol 290, R766–R772. [DOI] [PubMed] [Google Scholar]
- Lamb GD & Stephenson DG (2006). Point: lactic acid accumulation is an advantage during muscle activity. J Appl Physiol 100, 1410–1412. [DOI] [PubMed] [Google Scholar]
- Laursen PB (2010). Training for intense exercise performance: high‐intensity or high‐volume training? Scand J Med Sci Sports 20, 1–10. [DOI] [PubMed] [Google Scholar]
- Li JL, Wang XN, Fraser SF, Carey MF, Wrigley TV & McKenna MJ (2002). Effects of fatigue and training on sarcoplasmic reticulum Ca2+ regulation in human skeletal muscle. J Appl Physiol 92, 912–922. [DOI] [PubMed] [Google Scholar]
- Lindinger MI (2007). Combating muscle fatigue: extracellular lactic acidosis and catecholamines. J Physiol 581, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger MI, Leung MJ & Hawke TJ (2013). Inward flux of lactate⁻ through monocarboxylate transporters contributes to regulatory volume increase in mouse muscle fibres. PLoS One 8, e84451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger MI, McKelvie RS & Heigenhauser GJ (1995). K+ and Lac− distribution in humans during and after high‐intensity exercise: role in muscle fatigue attenuation? J Appl Physiol 78, 765–777. [DOI] [PubMed] [Google Scholar]
- Lynch GS, McKenna MJ & Williams DA (1994). Sprint‐training effects on some contractile properties of single skinned human muscle fibres. Acta Physiol Scand 152, 295–306. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR & Shahi MR (2011). A peripheral governor regulates muscle contraction. Appl Physiol Nutr Metab 36, 1–11. [DOI] [PubMed] [Google Scholar]
- Madsen K, Franch J & Clausen T (1994). Effects of intensified endurance training on the concentration of Na,K‐ATPase and Ca‐ATPase in human skeletal muscle. Acta Physiol Scand 150, 251–258. [DOI] [PubMed] [Google Scholar]
- Majerczak J, Karasinski J & Zoladz JA (2008). Training induced decrease in oxygen cost of cycling is accompanied by down‐regulation of SERCA expression in human vastus lateralis muscle. J Physiol Pharmacol 59, 589–602. [PubMed] [Google Scholar]
- Mason MJ & Thomas RC (1988). A microelectrode study of the mechanisms of l‐lactate entry into and release from frog sartorius muscle. J Physiol 400, 459–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar W, Nosek TM, Wong D & Renaud J (2000). Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during muscle fatigue. Am J Physiol Cell Physiol 278, C404–C416. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Bangsbo J & Renaud JM (2008). Muscle K+, Na+, and Cl disturbances and Na+‐K+ pump inactivation: implications for fatigue. J Appl Physiol 104, 288–295. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Schmidt TA, Hargreaves M, Cameron L, Skinner SL & Kjeldsen K (1993). Sprint training increases human skeletal muscle Na+‐K+‐ATPase concentration and improves K+ regulation. J Appl Physiol 75, 173–180. [DOI] [PubMed] [Google Scholar]
- McKenzie DC, Parkhouse WS & Hearst WE (1982). Anaerobic performance characteristics of elite Canadian 800 meter runners. Can J Appl Sport Sci 7, 158–160. [PubMed] [Google Scholar]
- Messonnier L, Kristensen M, Juel C & Denis C (2007). Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J Appl Physiol 102, 1936–1944. [DOI] [PubMed] [Google Scholar]
- Mohr M, Krustrup P, Nielsen JJ, Nybo L, Rasmussen MK, Juel C & Bangsbo J (2007). Effect of two different intense training regimens on skeletal muscle ion transport proteins and fatigue development. Am J Physiol Regul Integr Comp Physiol 292, R1594–R1602. [DOI] [PubMed] [Google Scholar]
- Munkvik M, Rehn TA, Slettaløkken G, Hasic A, Hallén J, Sjaastad I, Sejersted OM & Lunde PK (2010). Training effects on skeletal muscle calcium handling in human chronic heart failure. Med Sci Sports Exerc 42, 847–855. [DOI] [PubMed] [Google Scholar]
- Nevill ME, Boobis LH, Brooks S & Williams C (1989). Effect of training on muscle metabolism during treadmill sprinting. J Appl Physiol 67, 2376–2382. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Cheng AJ, Ørtenblad N & Westerblad H (2014). Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. J Physiol 592, 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J & Juel C (2003). Localization and function of ATP‐sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 284, R558–R563. [DOI] [PubMed] [Google Scholar]
- Nielsen JJ, Mohr M, Klarskov C, Kristensen M, Krustrup P, Juel C & Bangsbo J (2004a). Effects of high‐intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol 554, 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F & Overgaard K (2001). Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol 536, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, Ørtenblad N, Lamb GD & Stephenson DG (2004b). Excitability of the T‐tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+–K+ pump activity. J Physiol 557, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira L, Shiah AA, Gandra PG & Hogan MC (2013). Ca²⁺‐pumping impairment during repetitive fatiguing contractions in single myofibers: role of cross‐bridge cycling. Am J Physiol Regul Integr Comp Physiol 305, R118–R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H & Bangsbo J (2003) Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol 285, R143–R148. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Fiorenza M, Lund A, Christensen M, Rømer T, Piil P, Hostrup M, Christensen PM, Holbek S, Ravnholt T, Gunnarsson TP & Bangsbo J (2016). Adaptations to speed endurance training in highly trained soccer players. Med Sci Sports Exerc 48, 1355–1364. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Wang W, Rounds J, Chambers EA & Jacobs DO (2001). ATP from glycolysis is required for normal sodium homeostasis in resting fast‐twitch rodent skeletal muscle. Am J Physiol Endocrinol Metab 281, E479–E488. [DOI] [PubMed] [Google Scholar]
- Ørtenblad N, Lunde PK, Levin K, Andersen JL & Pedersen PK (2000). Enhanced sarcoplasmic reticulum Ca2+ release following intermittent sprint training. Am J Physiol Regul Integr Comp Physiol 279, R152–R160. [DOI] [PubMed] [Google Scholar]
- Ørtenblad N, Nielsen J, Saltin B & Holmberg HC (2011). Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol 589, 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad N, Westerblad H & Nielsen J (2013). Muscle glycogen stores and fatigue. J Physiol 591, 4405–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse WS & McKenzie DC (1984). Possible contribution of skeletal muscle buffers to enhanced anaerobic performance: a brief review. Med Sci Sports Exerc 16, 328–338. [PubMed] [Google Scholar]
- Pedersen TH, de Paoli F & Nielsen OB (2005). Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol 125, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli FV, Flatman JA & Nielsen OB (2009). Regulation of ClC‐1 and KATP channels in action potential‐firing fast‐twitch muscle fibers. J Gen Physiol 134, 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD & Stephenson DG (2004). Intracellular acidosis enhances the excitability of working muscle. Science 305, 1144–1147. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Bangsbo J, Richter EA & Juel C (1994). Lactate transport studied in sarcolemmal giant vesicles from human muscle biopsies: relation to training status. J Appl Physiol 77, 1858–1862. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP & Bangsbo J (1999a). Effect of high‐intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol 276, 255–261. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Terzis G, Halestrap A & Juel C (1999b). Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am J Physiol 276, 843–848. [DOI] [PubMed] [Google Scholar]
- Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevičius G, Paužas H, Mekideche A, Kayser B, Martinez‐Redondo V, Ruas JL, Bruton J, Truffert A, Lanner JT, Skurvydas A & Westerblad H (2015). Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high‐intensity interval exercise. Proc Natl Acad Sci USA 112, 15492–15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M & Light AR (2014). Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99, 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puype J, Van Proeyen K, Raymackers JM, Deldicque L & Hespel P (2013). Sprint interval training in hypoxia stimulates glycolytic enzyme activity. Med Sci Sports Exerc 45, 2166–2174. [DOI] [PubMed] [Google Scholar]
- Rasmussen MK, Kristensen M & Juel C (2008). Exercise‐induced regulation of phospholemman (FXYD1) in rat skeletal muscle: implications for Na+/K+‐ATPase activity. Acta Physiol (Oxf) 194, 67–79. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Kiens B & Richter EA (2006). Ca2+‐calmodulin‐dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol 574, 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A & Leveritt M (2001). Long‐term metabolic and skeletal muscle adaptations to short‐sprint training: implications for sprint training and tapering. Sports Med 31, 1063–1082. [DOI] [PubMed] [Google Scholar]
- Sahlin K & Henriksson J (1984). Buffer capacity and lactate accumulation in skeletal muscle of trained and untrained men. Acta Physiol Scand 122, 331–339. [DOI] [PubMed] [Google Scholar]
- Selvin D & Renaud JM (2015). Changes in myoplasmic Ca2+ during fatigue differ between FDB fibers, between glibenclamide‐exposed and Kir6.2‐/‐ fibers and are further modulated by verapamil. Physiol Rep 3, pii: e12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepley B, MacDougall JD, Cipriano N, Sutton JR, Tarnopolsky MA & Coates G (1992). Physiological effects of tapering in highly trained athletes. J Appl Physiol 72, 706–711. [DOI] [PubMed] [Google Scholar]
- Skovgaard C, Christensen PM, Larsen S, Andersen TR, Thomassen M & Bangsbo J (2014). Concurrent speed endurance and resistance training improves performance, running economy, and muscle NHE1 in moderately trained runners. J Appl Physiol 117, 1097–1109. [DOI] [PubMed] [Google Scholar]
- Sostaric SM, Skinner SL, Brown MJ, Sangkabutra T, Medved I, Medley T, Selig SE, Fairweather I, Rutar D & McKenna MJ (2006). Alkalosis increases muscle K+ release, but lowers plasma [K+] and delays fatigue during dynamic forearm exercise. J Physiol 570, 185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce AE, Standen NB & Stanfield PR (1985). Voltage‐dependent ATP‐sensitive potassium channels of skeletal muscle membrane. Nature 316, 736–738. [DOI] [PubMed] [Google Scholar]
- Street D, Nielsen JJ, Bangsbo J & Juel C (2005). Metabolic alkalosis reduces exercise‐induced acidosis and potassium accumulation in human skeletal muscle interstitium. J Physiol 566, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suko J, Maurer‐Fogy I, Plank B, Bertel O, Wyskovsky W, Hohenegger M & Hellmann G (1993). Phosphorylation of serine 2843 in ryanodine receptor‐calcium release channel of skeletal muscle by cAMP‐, cGMP‐ and CaM‐dependent protein kinase. Biochim Biophys Acta 1175, 193–206. [DOI] [PubMed] [Google Scholar]
- Swietach P, Youm JB, Saegusa N, Leem CH, Spitzer KW & Vaughan‐Jones RD (2013). Coupled Ca2+/H+ transport by cytoplasmic buffers regulates local Ca2+ and H+ ion signaling. Proc Natl Acad Sci USA 110, 2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Bishop DJ, Lambert K, Mercier J & Brooks GA (2012). Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: current status. Am J Physiol Regul Integr Comp Physiol 302, R1–R14. [DOI] [PubMed] [Google Scholar]
- Thomas C, Perrey S, Lambert K, Hugon G, Mornet D & Mercier J (2005). Monocarboxylate transporters, blood lactate removal after supramaximal exercise, and fatigue indexes in humans. J Appl Physiol 98, 804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M, Christensen PM, Gunnarsson TP, Nybo L & Bangsbo J (2010). Effect of 2‐wk intensified training and inactivity on muscle Na+‐K+ pump expression, phospholemman (FXYD1) phosphorylation, and performance in soccer players. J Appl Physiol 108, 898–905. [DOI] [PubMed] [Google Scholar]
- Thomassen M, Gunnarsson TP, Christensen PM, Pavlovic D, Shattock MJ & Bangsbo J (2016). Intensive training and reduced volume increases muscle FXYD1 expression and phosphorylation at rest and during exercise in athletes. Am J Physiol Regul Integr Comp Physiol 310, R659–R669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorup J, Tybirk J, Gunnarsson TP, Ravnholt T, Dalsgaard S & Bangsbo J (2016). Effect of speed endurance and strength training on performance, running economy and muscular adaptations in endurance‐trained runners. Eur J Appl Physiol 116, 1331–1341. [DOI] [PubMed] [Google Scholar]
- Walas H & Juel C (2012). Purinergic activation of rat skeletal muscle membranes increases Vmax and Na+ affinity of the Na,K‐ATPase and phosphorylates phospholemman and α1 subunits. Pflugers Arch 463, 319–326. [DOI] [PubMed] [Google Scholar]
- Wallinga W, Meijer SL, Alberink MJ, Vliek M, Wienk ED & Ypey DL (1999). Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T‐tubular system. Eur Biophys J 28, 317–329. [DOI] [PubMed] [Google Scholar]
- Walsh B, Howlett RA, Stary CM, Kindig CA & Hogan MC (2006). Measurement of activation energy and oxidative phosphorylation onset kinetics in isolated muscle fibers in the absence of cross‐bridge cycling. Am J Physiol Regul Integr Comp Physiol 290, R1707–R1713. [DOI] [PubMed] [Google Scholar]
- Westerblad H & Allen DG (1993). The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol 466, 611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H & Allen DG (1996a). Mechanisms underlying changes of tetanic [Ca2+]i and force in skeletal muscle. Acta Physiol Scand 156, 407–416. [DOI] [PubMed] [Google Scholar]
- Westerblad H & Allen DG (1996b). Slowing of relaxation and [Ca2+]i during prolonged tetanic stimulation of single fibres from Xenopus skeletal muscle. J Physiol 492, 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston M, Taylor KL, Batterham AM & Hopkins WG (2014). Effects of low‐volume high‐intensity interval training (HIT) on fitness in adults: a meta‐analysis of controlled and non‐controlled trials. Sports Med 44, 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Wu J, Giwa LR, Abdulkadir L, Sharma P & Jiang C (2001). Direct activation of cloned KATP channels by intracellular acidosis. J Biol Chem 276, 12898–12902. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Grassi B, Majerczak J, Szkutnik Z, Korostyński M, Karasiński J, Kilarski W & Korzeniewski B (2013). Exp Physiol 98, 883–898. [DOI] [PubMed] [Google Scholar]


