Abstract
Aurora kinase A plays an essential role in mitosis including chromosome separation and cytokinesis. Aberrant expression and activity of Aurora kinase A is associated with numerous malignancies including colorectal cancer followed by poor prognosis. The aim of this study is to determine the inhibitory effects of LDD970, an indirubin derivative, on Aurora kinase A in HT29 colorectal cancer cells. In vitro kinase assay revealed that, LDD970 inhibited levels of activated Aurora kinase A (IC50=0.37 mM). The inhibitory effects of LDD970 on Aurora kinase A, autophosphorylation and phosphorylation of histone H3 (Ser10), were confirmed by immunoblot analysis. Moreover, LDD970 inhibited migration of HT29 cells and upregulated apoptosis-related protein cleaved PARP. In cell viability assay, LDD970 was observed to suppress HT29 cell growth (GI50=4.22 µM). Although further studies are required, results of the present study suggest that LDD970 provide a valuable insight into small molecule indirubin derivative for therapeutic potential in human colorectal cancer.
Keywords: Aurora kinase A, Indirubin derivative, Colorectal cancer
INTRODUCTION
Aurora kinase A belongs to a family of serine/threonine protein kinases that are implicated in important processes during mitosis including centrosome and microtubule activities. Aurora A is involved in controlling several steps of mitosis such as centrosome maturation, bipolar spindle formation (1). Aurora A is phosphorylated on threonine 288 by binding to several protein partners such as targeting protein for Xklp2, p21-activated kinase 1, transforming acidic coiled-coil containing protein 3, and its activity appears to be a peak at G2 phase of the cell cycle (2,3). Overexpression and amplification of Aurora A has been reported to be detected in a number of solid tumors including colorectal tumors, and aberrant expression of Aurora A induces genomic instability by triggering mitotic checkpoints and apoptosis (4). Moreover, Aurora A overexpression leads to increased degradation of p53, resulting in oncogenic transformation of cells, and contributes to anti-cancer drugs resistance of cells (4,5). Furthermore, overexpression and amplification of Aurora A is correlated with poor prognosis in patients with colorectal cancers (6). Thus, Aurora A is an attractive target for anticancer therapy due to its essential role in mitotic progression.
It is known that phosphorylation of histone H3 on serine 10 (Ser 10) is required for the proper execution of mitosis (7,8) and for the initiation of mammalian chromosome condensation (9). In addition, phosphorylation of histone H3 during the G2/M transition is tightly associated with the expression of Aurora A (10).
Several studies have reported that indirubin derivatives inhibit the proliferation of a variety of cells (11,12,13). In the present study, we describe an indirubin derivative LDD970, as a small molecule inhibitor, of Aurora kinase A activity in HT29 colorectal cancer cells.
MATERIALS AND METHODS
Cell culture
HT29 human colorectal cells were purchased from Korean Cell Line Bank (Seoul, Korea) and were cultured in DMEM (Sigma Co., St. Louis, MO, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37℃ in an incubator with an atmosphere of 5% CO2.
In vitro kinase assay
Inhibitory activity of LDD970 against Aurora A kinases was measured by performing HTRF assays. The enzyme was mixed with serially diluted compounds and peptide substrates in a kinase reaction buffer (250 mM HEPES (pH 7.0), 0.1 mM sodium orthovanadate, 5 mM MgCl2, 1 mM DTT, 0.01% bovine serum albumin, 0.02% NaN3). After the addition of detection reagents, TR-FRET signal was measured using Victor multilabel reader (Perkin Elmer, Waltham, MA, USA). IC50 was calculated using nonlinear regression with Prism version 5.01 (GraphPad, La Jolla, CA, USA).
Cell viability assay
Cell viability was measured by performing a tetrazolium-based assay with EZ-Cytox Cell Viability Assay Kit (DaeilLab, Korea). Briefly, HT29 cells were seeded in 96-well plates at a density of 1,000 cells/well and were incubated at 37℃ for 24 h. The cells were treated with serially diluted LDD970 for 72 h. Next, 15 µL EZ-Cytox reagent was added to each well, and the plates were incubated at 37℃ for 4 h. Absorbance was measured using Victor multi-label reader, and IC50 was calculated using nonlinear regression using Prism version 5.01.
Immunoblot analysis
Expression levels and phosphorylation of Aurora A and histone H3 were evaluated by performing immunoblotting analysis as described previously (14). Antibodies against Aurora A (p-Aurora A) and PARP were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-histone H3 antibody and anti-phosphorylated histone H3 (anti-p-H3; Ser10) antibody were obtained from Abcam (Cambridge, MA, USA) and Millipore (Billerica, MA, USA), respectively. The monoclonal antibody against β-actin (Sigma-Aldrich, St. Louis, MO, USA) was used for a loading control.
Wound healing assay
HT29 cells were plated on 6-well plates and were cultured until they reached 90% confluence. A 10 µL tip was used to create a 2 mm wound in the cell monolayer. Detached cells were removed by washing with DMEM. Cells in the plate were treated with LDD970 and were allowed to migrate for 24 and 48 h. Images of live cells were obtained using a phase-contrast microscope (Carl Zeiss, Germany).
RESULTS
Effect of LDD970 on Aurora A kinase activity
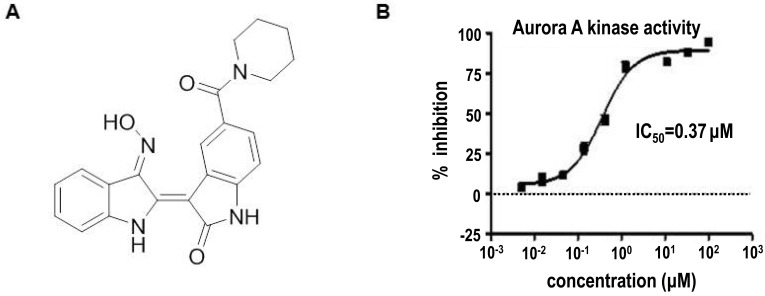
LDD970 (Fig. 1A) was tested against Aurora A kinase activity using purified recombinant Aurora A protein. Aurora A inhibition was measured by performing the HTRF assay as described in Materials and Methods. LDD970 exhibited potent inhibitory activity with an IC50 of 0.37 µM. Inhibitory activities of LDD970 against other kinases are listed in Table I. LDD970 did not affected the in vitro kinase activities of c-Met, ALK, and JAK2 (IC50>10 µM). Moreover, LDD970 was likely to show selectivity toward Aurora A among all the kinases tested despite the use of small number of kinases.
Figure 1. Inhibition of Aurora kinase A by LDD970. (A) Chemical structure of LDD970 compound. (B) Effect of LDD970 on the in vitro kinase activity of Aurora A. This assay was performed on purified recombinant Aurora A enzyme using HTRF method.
Table I. Inhibitory activity against select kinases.
| Kinase | IC50 (µM) |
|---|---|
| c-Met | >10 µM |
| ALK | >10 µM |
| JAK2 | >10 µM |
The inhibitory activities of LDD970 were evaluated against various purified recombinant kinases using HTRF method.
Inhibition of Aurora A autophosphorylation by LDD970 in HT29 cells
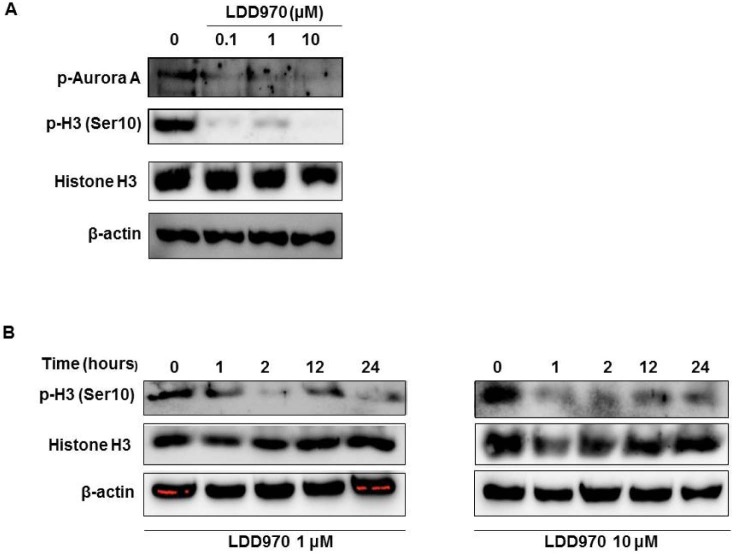
Upon activation, Aurora kinase A undergoes autophosphorylation at threonine 288 (3). Histone H3 is a substrate of Aurora A, and is phosphorylated by Aurora A at Ser10 (7). To determine the inhibitory effects of LDD970 on Aurora A in HT29 cells, we determined p-Aurora A and p-H3 (Ser10) levels in HT29 cells by performing immunoblotting analysis. Our results showed that LDD970 treatment decreased the levels of p-Aurora A and p-H3; however, no change was observed in the total expression level of histone H3 (Fig. 2A). Further, to confirm that LDD970 inhibited Aurora A kinase activity, we performed a time-course experiment to measure p-H3 levels in HT29 cells. Treatment of cells with LDD970 decreased p-H3 levels in a time-dependent manner. Even the inhibitory effect of LDD970 on histone H3 phosphorylation was found to occur before 1 h after treatment of 1 µM and 10 µM of LDD970 (Fig. 2B).
Figure 2. Effects of LDD970 on phosphorylation of Aurora A and histone H3 in HT29 cells. Immunoblot analysis of phosphorylated Aurora A (p-Aurora A), phosphorylated histone H3 (p-H3) on Ser10 on HT29 cells. Cells were treated with LDD970 for 2 h with indicated concentration (A) or indicated times with 1 µM and 10 µM concentration (B). Lysates were analyzed using antibodies against p-Aurora A, p-H3 on Ser10. The β-actin antibody was used as a loading control.
Effects of LDD970 on HT29 cell growth and apoptosis
Cytotoxicity of LDD970 was measured to evaluate HT29 colorectal cancer cells growth. Cells were treated with LDD970 for 72 h, and cell viability was measured. We found that LDD970 treatment suppressed the growth of HT29 cells with IC50 of 4.22 µM (Fig. 3A).
Figure 3. Effects of LDD970 on cell growth and apoptosis. (A) Determination of cytotoxicity activity of LDD970 on HT29 cells. Cells were treated with LDD970 for 72 h. Percentage of cell growth was calculated using 0.5% DMSO treatment as a negative control. (B) Immunoblotting analysis of PARP and cleaved PARP after 48 h of treatment of LDD970.
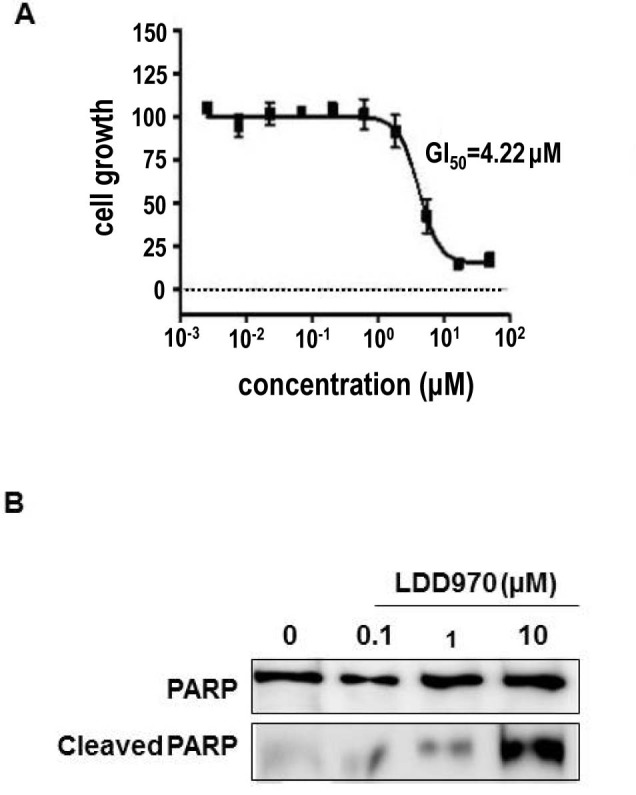
Inhibition of Aurora A activity induced mitotic spindle defect resulting in cell death through apoptosis (2). LDD970-mediated induction of apoptosis was confirmed by evaluating the levels of PARP cleavage. We observed a dose-dependent increase in PARP cleavage (Fig. 3B), indicating that LDD970 induced the apoptosis of HT29 cells.
Inhibition of HT29 cell migration by LDD970
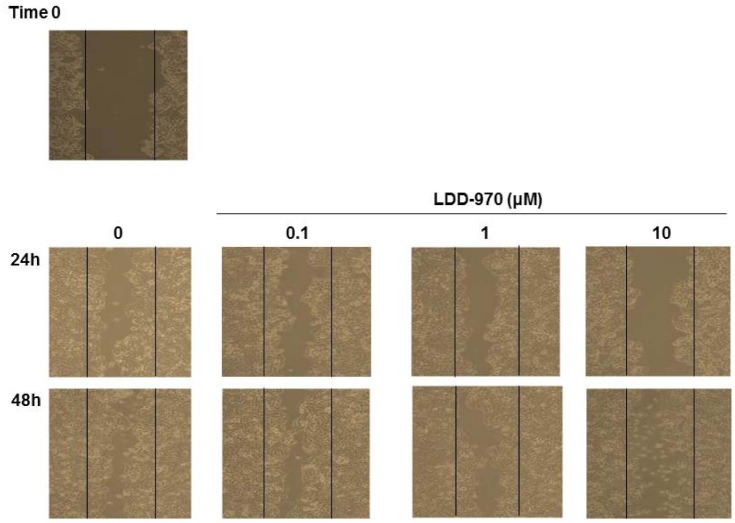
Cell migration was assessed by performing the wound healing assay, followed by LDD970 treatment. After wound induction, cells were washed and were treated with the indicated concentrations of LDD970. LDD970 treatment suppressed the migration of HT29 cells in a concentration-dependent manner as compared to untreated cells (Fig. 4).
Figure 4. Inhibition of cell migration by LDD970. After making the wound, cells were washed and treated with the indicated concentrations of LDD970. Cells were photographed at the time scratch (Time 0, upper panel). Movement of cells into the wound was observed after 24 h (middle panel) and 48 h (lower panel) using a microscope. Cell migration was assessed by recovery of the scratch.
DISCUSSION
The microtubules play an important role during mitosis (15), providing an ideal drug target for cancer therapy. It has been demonstrated that antimitotic drugs such as taxanes and vinca alkaloids have specific interaction with microtubules. They inhibit the microtubule dynamics by disturbing the spindle assembly checkpoint (16). Although these drugs are currently the best approach for treatment of variety of tumors, there are still issues on severe side effects. Moreover, Antimitotic agents exert cytotoxic effects on non-tumorigenic cells and induce drug resistance (16). Development of anti-cancer agents that disrupt mitosis and enhance therapeutic potency with fewer side effects is the main focus of new Antimitotic drug research. Aurora A is a mitotic specific kinase that has been identified as a promising target for anti-cancer therapy.
In this study, we reported that LDD970, an indirubin derivative, possessed inhibitory activity against Aurora kinase A, resulting in decreased proliferative capacity and viability, and inhibition of migration as well as induction of apoptosis in HT29 human colorectal cancer cells. This result clearly showed that LDD970 displayed a potent inhibitory activity against Aurora kinase A. Inhibition of Aurora kinase A activity using Aurora A recombinant enzyme was attested by the evaluation of autophosphorylation of histone H3 on serine 10. The phosphorylation of histone H3 (Ser10) plays an important role in chromosome condensation and is controlled by Aurora kinase A during G2/M transition (10). Following inhibition of histone H3 phosphorylation, chromosome condensation is prevented and entry to mitosis is knocked down. The levels of histone H3 phosphorylation were measured to determine whether LDD970 had the effect on the cells. Our data revealed that the levels of phosphorylated histone H3 were inhibited with LDD970 treatment in HT29 cells. Moreover, exposure to LDD970 was found to inhibit phosphorylation of histone H3 in both short-term and long-term treated periods. These results were consistent with previous reports that treatment of Aurora A inhibitors in human cancer cells overexpressing Aurora A resulted in loss of phosphorylated histone H3 (17). This indicates that LDD970 is an Aurora A kinase inhibitor since phosphorylation of histone H3 is a direct downstream target of Aurora kinase A. Further, using the wound healing assay, we demonstrated that cells treated with LDD970 for 24 h and 48 h showed a reduced capacity to undergo migration. In addition, when LDD970 was applied to HT29 cells, PARP cleavage was increased (18), suggesting that LDD970 induced apoptosis. These results are in agreement with previous findings that treatment with Aurora kinase A inhibitors not only block cell proliferation and migration, but also induce apoptotic cell death (17,19).
Aurora A has received much attention because of its important role during mitosis and association with cancer progression. Drugs that inhibit Aurora kinase A have been discovered and currently pursued in stages of preclinical and clinical development (20). However, further in-depth studies require increasing the efficacy and reducing side effects. This LDD970 study provides useful pharmacological information in the field of Aurora A research.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A2A01053928).
Abbreviations
- HTRF
homogeneous time-resolved fluorescence
- PARP
Poly (ADP-ribose) polymerase
- TR-FRET
time resolved-fluorescence resonance energy transfer
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Giet R, Prigent C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999;112:3591–3601. doi: 10.1242/jcs.112.21.3591. [DOI] [PubMed] [Google Scholar]
- 2.Mehra R, Serebriiskii IG, Burtness B, Astsaturov I, Golemis EA. Aurora kinases in head and neck cancer. Lancet Oncol. 2013;14:e425–e435. doi: 10.1016/S1470-2045(13)70128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodson CA, Bayliss R. Activation of Aurora-A kinase by protein partner binding and phosphorylation are independent and synergistic. J Biol Chem. 2012;287:1150–1157. doi: 10.1074/jbc.M111.312090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XX, Liu R, Jin SQ, Fan FY, Zhan QM. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res. 2006;16:356–366. doi: 10.1038/sj.cr.7310046. [DOI] [PubMed] [Google Scholar]
- 5.Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004;10:2065–2071. doi: 10.1158/1078-0432.ccr-1057-03. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003;116:3677–3685. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- 8.Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene. 2001;20:3021–3027. doi: 10.1038/sj.onc.1204326. [DOI] [PubMed] [Google Scholar]
- 9.Van HA, Goodrich DW, Allis CD, Brinkley BR, Mancini MA. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J Cell Sci. 1998;111:3497–3506. doi: 10.1242/jcs.111.23.3497. [DOI] [PubMed] [Google Scholar]
- 10.Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, Niederberger E, Tang W, Eisenbrand G, Meijer L. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 12.Moon MJ, Lee SK, Lee JW, Song WK, Kim SW, Kim JI, Cho C, Choi SJ, Kim YC. Synthesis and structure-activity relationships of novel indirubin derivatives as potent anti-proliferative agents with CDK2 inhibitory activities. Bioorg Med Chem. 2006;14:237–246. doi: 10.1016/j.bmc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Gaboriaud-Kolar N, Vougogiannopoulou K, Skaltsounis AL. Indirubin derivatives: a patent review (2010-present) Expert Opin Ther Pat. 2015;25:583–593. doi: 10.1517/13543776.2015.1019865. [DOI] [PubMed] [Google Scholar]
- 14.Chung HJ, Kamli MR, Lee HJ, Ha JD, Cho SY, Lee J, Kong JY, Han SY. Discovery of quinolinone derivatives as potent FLT3 inhibitors. Biochem Biophys Res Commun. 2014;445:561–565. doi: 10.1016/j.bbrc.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Goshima G, Vale RD. The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J Cell Biol. 2003;162:1003–1016. doi: 10.1083/jcb.200303022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vuuren RJ, Visagie MH, Theron AE, Joubert AM. Antimitotic drugs in the treatment of cancer. Cancer Chemother Pharmacol. 2015;76:1101–1112. doi: 10.1007/s00280-015-2903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, Galvin KM, Hoar KM, Huck JJ, LeRoy PJ, Ray ET, Sells TB, Stringer B, Stroud SG, Vos TJ, Weatherhead GS, Wysong DR, Zhang M, Bolen JB, Claiborne CF. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amours D, Sallmann FR, Dixit VM, Poirier GG. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci. 2001;114:3771–3778. doi: 10.1242/jcs.114.20.3771. [DOI] [PubMed] [Google Scholar]
- 19.Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, Ikeda H, Bianchi G, Hu Y, Cirstea D, Santo L, Tai YT, Nahar S, Zheng M, Bandi M, Carrasco RD, Raje N, Munshi N, Richardson P, Anderson KC. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115:5202–5213. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollareddy M, Zheleva D, Dzubak P, Brahmkshatriya PS, Lepsik M, Hajduch M. Aurora kinase inhibitors: progress towards the clinic. Invest New Drugs. 2012;30:2411–2432. doi: 10.1007/s10637-012-9798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]