Abstract
Objectives
To investigate the relationship between image quality and patient size at 100 kilovoltage (kV) compared to 120 kV ECG-gated Triple-Rule-Out CT angiography (TRO-CTA).
Methods
We retrospectively included 73 patients (age 64 ± 14 years) who underwent retrospective ECG-gated chest CTA. 40 patients were scanned with 100 kV while 33 patients with 120 kV. Body mass index (BMI), patients’ chest circumference (PC) and thoracic surface area (TSA) were recorded. Quantitative image quality was assessed as vascular attenuation in the ascending aorta (AA), pulmonary trunk (PA) and left coronary artery (LCA) and the signal-to-noise ratio (SNR) in the AA.
Results
There was no significant difference in BMI (26.0 ± 4.6 vs. 28.0 ± 6.7 kg/m2), PC (103 ± 7 vs. 104 ± 10 cm2) and TSA (92 ± 15 vs. 91 ± 19 cm2) between 100 kV and 120 kV group. Mean vascular attenuation was significantly higher in the 100 kV compared to the 120 kV group (AA 438 vs. 354 HU, PA 460 vs. 349 HU, LCA 370 vs. 299 HU all p < 0.001). SNR was not significantly different, even after adjusting for patient size. Radiation dose was significantly lower in the 100 kV group (10.7 ± 4.1 vs. 20.7 ± 10.7 mSv; p < 0.001).
Conclusions
100 kV TRO-CTA is feasible in normal-to-overweight patients while maintaining image quality and achieving substantial dose reduction.
Key Points
• 100 kV protocols result in a significantly lower radiation dose.
• Mean vascular attenuation is significantly higher using 100 kV.
• SNR and CNR are not significantly different between 100 kV and 120 kV.
• 100 kV CTA is feasible regardless of patient size while maintaining image quality.
Keywords: Multidetector computed tomography, Radiation dosage, Image enhancement, Body mass index, Chest pain
Introduction
ECG-gated computed tomography angiography (CTA) of the chest has been shown to be a reliable and comprehensive non-invasive imaging test to exclude acute coronary syndrome, aortic dissection and pulmonary embolism in patients presenting to the emergency department with acute chest pain (i.e., Triple Rule Out (TRO)-CTA) [1–9].
However, the potential harm of radiation exposure is of concern, as it has been postulated to be associated with a non-negligible lifetime attributable risk of cancer [10]. This risk varies noticeably and is substantially greater for females, younger patients, and for combined cardiac and aortic scans [10]. Previous studies demonstrated that lowering the kilovoltage (kV) setting from standard 120 kV to 100 kV allows a significant reduction of radiation exposure independent from the CT scanner hardware [11–14]. Radiation dose reduction through kV adjustment, however, may adversely affect image quality due to the inverse relationship between radiation exposure and image noise [15]. Empiric selection criteria for application of reduced peak tube potential include markers of body size (e.g., body mass index [BMI]); however, body size selection criteria are likely crude given interpatient differences in weight distribution and body composition [15]. Patients’ chest area and BMI have been found to frequently be discordant, which can lead to over radiating patients when using BMI to select tube potential [16]. However, there remains hesitation among clinicians to lower kV out of fear for not achieving adequate image quality for diagnosis. The hypothesis of this study was that 100 kV CTA is feasible in normal-to-overweight patients while maintaining appropriate diagnostic image quality. Accordingly, we investigated the relationship between image quality and patient size in patients undergoing 100 kV vs. 120 kV ECG-gated TRO-CTA.
Materials and methods
Patient population
Seventy-three consecutive patients (mean age 64 ± 13, range 25–83 years) were retrospectively enrolled. All patients underwent TRO-CTA for acute chest pain evaluation referred by our chest pain unit between October 2009 and January 2011. The 100 kV scanning was performed at the discretion of the attending radiologist. In our institution, TRO-CTA is used to assess patients with acute chest pain for pulmonary embolism, acute aortic syndrome, acute coronary syndrome and other thoracic pathologic conditions with a single examination.
CT technique
All imaging exams were conducted on a 64-channel dual-source CT scanner (Somatom Definition, Siemens Healthcare, Forchheim, Germany). Retrospectively ECG-gated spiral CTA of the heart was acquired from the level of the carina. Then, 120 ml of iodine contrast (Imeron 400, Bracco Imaging S.p.A., Milan, Italy) was injected using a power injector with an injection rate of 4 ml/s and followed by a saline chaser of 40 ml. Patients were instructed to hold their breath at a mid-inspiratory position. The cranio-caudal acquisition was started using automatic bolus triggering at 2 sec intervals in the ascending aorta (trigger level 100 HU) with a delay of 5 sec. The following additional scan parameters were applied for the TRO-CTA: detector collimation 2 × 32 × 0.6 mm, slice acquisition 2 × 64 × 0.6 mm using z-flying focal spot, gantry rotation time 330 ms, tube current time product 320 mAs, and the pitch automatically adapted to the patient’s heart rate using a reference value of 0.28. Automatic tube current modulation (CARE Dose 4D) and automatic ECG-pulsing for radiation dose reduction were applied. For data reconstruction, axial images of the thoracic scan range were reconstructed with 1.0 mm slice thickness and 0.8 mm increment using a B30f soft tissue kernel. In addition, the heart was reconstructed with a slice thickness of 0.6 mm and 0.5 mm increment using a B26f soft tissue kernel in the optimal diastolic and systolic phase as determined by the CT software. Dose length product (DLP) and volume CT dose index (CTDIvol) were collected. The effective dose was calculated using a conversion coefficient of 0.017 mSv mGy-1cm-1 [17].
Image analysis
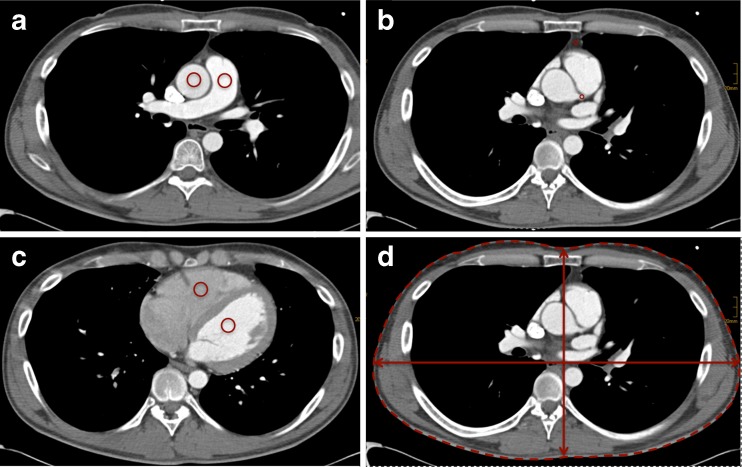
The quantitative measurements were performed on the axial images with 1 mm slice thickness by one experienced reviewer (radiologist with 5 years of experience in cardiothoracic imaging). A circular region of interest (ROI) was placed in the following anatomical regions: main pulmonary artery, ascending aorta, left ventricle, right ventricle, left main coronary artery, and epicardial fat. Calcifications and plaques within the ROIs were avoided. Mean attenuation values were recorded in Hounsfield Units (HU). The signal-to-noise ratio (SNR) was defined as the ratio of the mean attenuation in the ascending aorta to the standard deviation of this ROI. Contrast-to-noise ratio (CNR) was defined as the ratio of the mean attenuation in the ascending aorta minus mean attenuation of epicardial fat to the standard deviation of the attenuation value in the ascending aorta. Patient size was measured as BMI, patients’ chest circumference (PC) at the level of the origin of the left main coronary and thoracic surface area (TSA), defined as the product of dorso-ventral and latero-lateral diameters of the chest at the same level as above (Fig. 1).
Fig. 1.
Example image of measurements (a) ROI in ascending aorta and pulmonary artery. b Left main coronary artery and epicardial fat. c left and right ventricle. d anthropomorphic measures at level of the origin of the left main coronary artery
Statistical analysis
Continuous variables were listed as mean plus standard deviation (SD). Student’s t-test was used to determine statistical significant differences in mean values. Linear regression was applied to generate adjusted p-values. Correlations were calculated using Pearson correlation coefficient or Spearman’s rank. Discrete variables were evaluated using the Fisher’s exact test. A p-value smaller than 0.05 was used to indicate statistical significance. Analyses were performed using SPSS (IBM Corp, Version 23.0. Armonk, NY, USA).
Results
Baseline characteristics
There was no significant difference in BMI between the 100 kV and 120 kV group (26.0 ± 4.6 vs. 28.0 ± 6.7 kg/m2; p = 0.138), PC (103 ± 7 vs. 104 ± 10 cm2; p = 0.737) or TSA (92.4 ± 15.2 vs. 90.9 ± 19.2 cm2; p = 0.815). Both groups showed similar age (65 ± 12 vs. 63 ± 16 years; p = 0.521) and similar proportion of males (27 vs. 20 males, p = 0.540). Detailed characteristics of the study population are listed in Table 1. The correlation between BMI and TSA/PC was moderate while the correlation between TSA and PC was high (Fig. 2).
Table 1.
Patient characteristics and radiation dose
| 100 kV (n = 40) | 120 kV (n = 33) | p-value | |
|---|---|---|---|
| BMI (kg/m2) | 26.0 ± 4.6 | 28.0 ± 6.7 | 0.138 |
| <25 | 19 | 14 | |
| 25.1 – 30 | 17 | 11 | |
| >30.1 | 4 | 8 | |
| CTDI (mGy) | 19.2 ± 6.9 | 41.3 ± 21.5 | <0.001 |
| mAs | 162.4 ± 55.6 | 192.9 ± 70.8 | 0.044 |
| DLP (mGy cm) | 630 ± 240 | 1215 ± 626 | <0.001 |
Fig. 2.
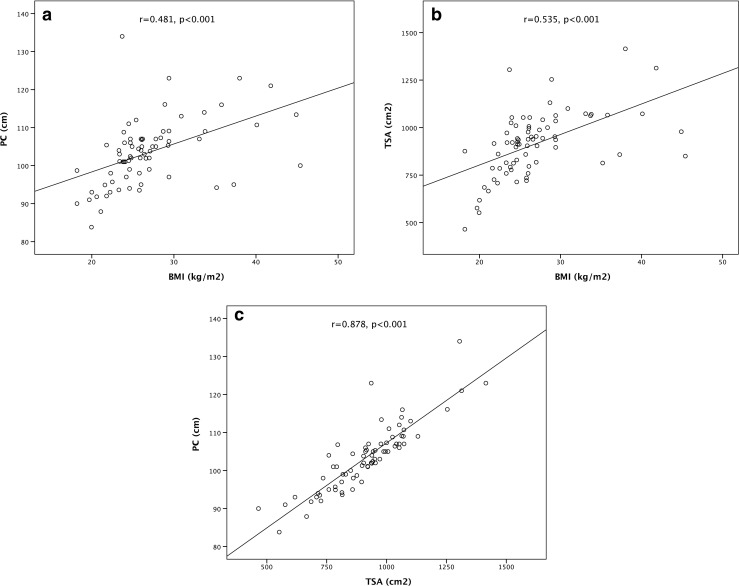
Correlation plot between the various anthropomorphic measures. a PC and BMI, b TSA and BMI, and c PC and TSA
Image quality
A radiologist (5 years of experience) graded all studies as of diagnostic quality. The average attenuation of contrast medium in the low-dose group was significantly higher at all vascular levels (all p < 0.05; Table 2; Fig. 3). There was no significant difference in the mean attenuation values of epicardial fat between the groups (p = 0.40). Image noise in the main pulmonary artery and left main coronary artery was higher in the low-voltage group (p < 0.05). Image noise in the right ventricle and epicardial fat showed no significant difference as presented in Table 2.
Table 2.
Mean attenuation and noise
| 100 kV (n = 40) | 120 kV (n = 33) | p-value | p-value adjusted for BMI | p-value adjusted for PC | p-value adjusted for TSA | |
|---|---|---|---|---|---|---|
| Attenuation | ||||||
| Pulmonary artery | 459.8 ± 107.2 | 348.6 ± 85.3 | <0.001 | <0.001 | <0.001 | <0.001 |
| Ascending Aorta | 435.7 ± 102.4 | 353.6 ± 66.9 | <0.001 | <0.001 | <0.001 | <0.001 |
| Left ventricle | 407.9 ± 97.6 | 326.2 ± 68.9 | <0.001 | <0.001 | <0.001 | <0.001 |
| Right ventricle | 405.5 ± 135.3 | 335.3 ± 118.9 | 0.024 | 0.040 | 0.029 | 0.021 |
| Left coronary artery | 369.5 ± 117.1 | 299.4 ± 101.3 | 0.009 | 0.015 | 0.011 | 0.009 |
| Epicardial fat | 99.8 ± 25.5 | 93.9 ± 25.6 | 0.335 | 0.404 | 0.367 | 0.336 |
| Noise | ||||||
| Pulmonary artery | 30.3 ± 10.9 | 22.3 ± 8.5 | 0.001 | <0.001 | <0.001 | 0.001 |
| Ascending Aorta | 27.4 ± 9.2 | 22.3 ± 9.1 | 0.057 | 0.061 | 0.049 | 0.060 |
| Left ventricle | 36.6 ± 15.7 | 31.9 ± 22.9 | 0.302 | 0.188 | 0.212 | 0.269 |
| Right ventricle | 46.3 ± 21.7 | 40.8 ± 24.7 | 0.315 | 0.231 | 0.258 | 0.303 |
| Left main coronary artery | 67.9 ± 28.5 | 48.3 ± 21.9 | 0.002 | 0.001 | 0.002 | 0.002 |
| Epicardial fat | 33.9 ± 14.3 | 32.0 ± 20.0 | 0.649 | 0.657 | 0.594 | 0.645 |
| CNR aorta | 12.6 ± 4.7 | 13.8 ± 8.9 | 0.463 | 0.419 | 0.391 | 0.453 |
| SNR aorta | 16.9 ± 6.9 | 17.7 ± 6.9 | 0.623 | 0.460 | 0.529 | 0.666 |
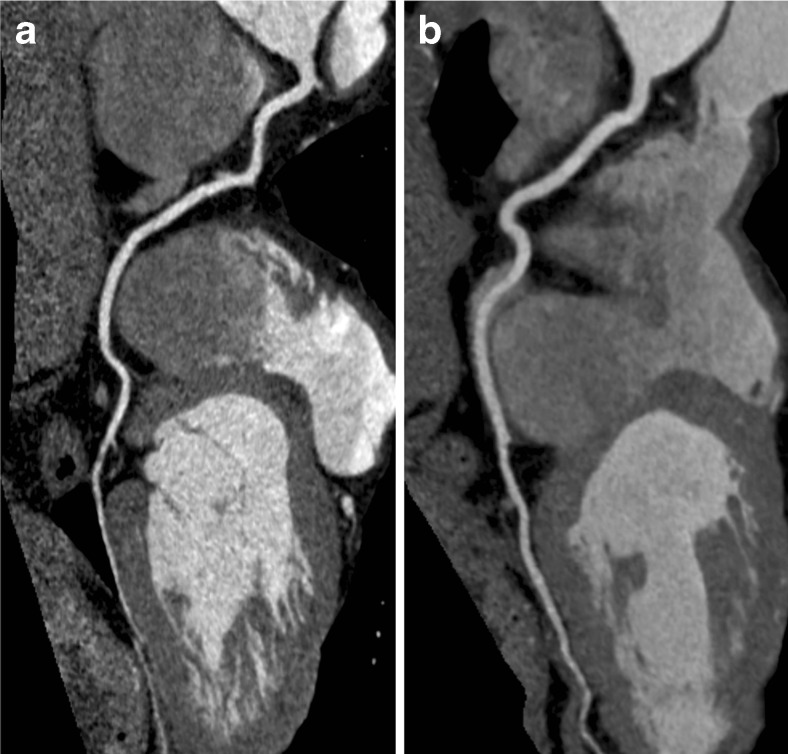
Fig. 3.
The right coronary artery in patients of similar BMI scanned with Triple-Rule-Out CT angiography at 100 kV (a) and 120 kV (b)
The difference in the calculated SNR and CNR between the two groups was not statistically significant (p = 0.46 and p = 0.62), which was maintained after adjusting for body size measures.
Radiation dose
Mean CTDIvol in the 100 kV group was 19.2 ± 6.9 mGy and was significantly lower compared to the 120 kV group (41.3 ± 21.5 mGy, p < 0.001). Mean DLP in the 100 kV group was 630 ± 240 mGy cm and was significantly lower in comparison with the 120 kV group (1215 ± 626 mGy cm, p < 0.001). The mean estimated effective radiation dose for the 100 kV group was 10.7 ± 4.1 mSv and 20.7 ± 10.7 mSv (p < 0.001), corresponding to a 55 % reduction in radiation exposure by lowering tube voltage to 100 kV. Radiation dose results are summarized in Table 1. A moderate correlation was found between BMI and effective radiation dose for both the 100 kV group and the 120 kV group (r = 0.326, p = 0.043 and r = 0.357, p = 0.041, respectively).
Discussion
Our results show that 100 kV TRO-CTA of the chest can be performed with appropriate diagnostic image quality and substantial dose reduction in our patient population, which included predominantly normal-to-overweight individuals. Even after adjusting for measures of patient size, no significant differences were found in image quality between 100 kV and 120 kV. Reducing tube voltage to 100 kV results in an approximately 55 % lower effective radiation dose compared to a 120 kV TRO-CTA protocol performed while maintaining diagnostic image quality. Similar reductions were reported by Feuchtner et al. [12], where a radiation dose reduction of 47 % was achieved using 100 kV.
The mean attenuation values of the pulmonary artery, the ascending aorta, the left ventricle, the right ventricle and the left main coronary artery were significantly higher at 100 kV, which is in line with published data on low kV imaging [18, 19].
This observation can be explained by the photoelectric effect, which results in increased photon attenuation as tube voltages approach the k-edge of a given element; in case of iodine-based contrast materials, vascular attenuation is expected to increase with decreasing tube voltages to approximately 33 kV (the approximate k-edge of iodine) [20, 21]. The photoelectric effect is the dominant mechanism of photon attenuation in materials containing high-atomic number elements (e.g., iodinated contrast material). In contrast, Compton scatter is the dominant interaction in tissues lacking high-atomic number elements (e.g., soft tissue and fat). Accordingly, no significant difference in mean attenuation of the (fatty) tissue adjacent to the left coronary artery was observed [22]. A disadvantage of lower tube voltage (and the major reason for diagnostic tube voltage ranges used in current clinical imaging) is the concomitant increase in image noise [20]. While the noise level for the 100 kV CTA protocol was found to be higher in the current study, as expected, all imaging studies were considered of diagnostic quality, which was supported by non-significant differences in SNR and CNR between both protocols. In a prior study on 100 kV imaging by Krissak et al. [22] the feasibility of TRO-CTA with 100 kV was demonstrated in non-obese patients. In this current study we extend the use of 100 kV imaging to normal-to-overweight patients, which was maintained even after adjusting for measures of patient size.
Despite growing evidence of non-inferior image quality using low kV protocols in nonobese patients [11, 22], data regarding the impact of body habitus, i.e., chest dimensions, PC, and TSA has been lacking. This is of particular concern, as a recent anthropomorphic phantom study found that patients’ chest area and BMI classes were frequently discordant, which could potentially lead to over-radiating patients when using BMI to select the tube voltage [16]. BMI, while an imperfect but convenient proxy for human body fat, does not reliably represent the body shape, especially in patients with central obesity; this can become particularly troublesome in TRO-CTA, where the scan field is limited to the chest. Conversely, a large-chested woman probably requires more radiation than a small-chested man with a similar BMI [23]. Thus, the findings of our study extend prior data, establishing radiation dose reduction and maintaining image quality even after adjusting for patient size.
Our study is limited by its retrospective nature. Second, we only had a small percentage of subjects with a BMI above 30, which may hamper the generalizability of the results to morbidly obese individuals. Finally, in this study we did not evaluate the effect of iterative image reconstruction techniques, which would reduce image noise [24, 25].
In conclusion, 100 kV TRO-CTA can be performed in normal-to-overweight patients, while maintaining image quality and achieving substantial dose reductions.
Acknowledgments
The scientific guarantor of this publication is Richard A.P. Takx. The authors of this manuscript declare relationships with the following companies: The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, cross sectional study, performed at one institution.
Abbreviations
- BMI
body mass index
- CTA
computed tomography angiography
- CTDIvol
volume CT dose index
- DLP
dose length product
- kV
kilovoltage
- PC
patients’ chest circumference
- TRO
triple rule out
- TSA
thoracic surface area
References
- 1.Halpern EJ. Triple-rule-out CT angiography for evaluation of acute chest pain and possible acute coronary syndrome. Radiology. 2009;252:332–345. doi: 10.1148/radiol.2522082335. [DOI] [PubMed] [Google Scholar]
- 2.Frauenfelder T, Appenzeller P, Karlo C, et al. Triple rule-out CT in the emergency department: protocols and spectrum of imaging findings. Eur Radiol. 2009;19:789–799. doi: 10.1007/s00330-008-1231-3. [DOI] [PubMed] [Google Scholar]
- 3.Christiaens L, Duchat F, Boudiaf M, et al. Impact of 64-slice coronary CT on the management of patients presenting with acute chest pain: results of a prospective two-centre study. Eur Radiol. 2012;22:1050–1058. doi: 10.1007/s00330-011-2354-5. [DOI] [PubMed] [Google Scholar]
- 4.Schertler T, Scheffel H, Frauenfelder T, et al. Dual-source computed tomography in patients with acute chest pain: feasibility and image quality. Eur Radiol. 2007;17:3179–3188. doi: 10.1007/s00330-007-0724-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang LJ, Lu GM, Meinel FG, McQuiston AD, Ravenel JG, Schoepf UJ. Computed tomography of acute pulmonary embolism: state-of-the-art. Eur Radiol. 2015;25:2547–2557. doi: 10.1007/s00330-015-3679-2. [DOI] [PubMed] [Google Scholar]
- 6.Durmus T, Rogalla P, Lembcke A, Muhler MR, Hamm B, Hein PA. Low-dose triple-rule-out using 320-row-detector volume MDCT--less contrast medium and lower radiation exposure. Eur Radiol. 2011;21:1416–1423. doi: 10.1007/s00330-011-2088-4. [DOI] [PubMed] [Google Scholar]
- 7.Rist C, Johnson TR, Becker CR, Reiser MF, Nikolaou K. New applications for noninvasive cardiac imaging: dual-source computed tomography. Eur Radiol. 2007;17:F16–F25. doi: 10.1007/s10406-007-0224-7. [DOI] [PubMed] [Google Scholar]
- 8.Wnorowski AM, Halpern EJ. Diagnostic yield of triple-rule-Out CT in an emergency setting. AJR Am J Roentgenol. 2016;207:295–301. doi: 10.2214/AJR.15.15717. [DOI] [PubMed] [Google Scholar]
- 9.Kligerman SJ, White CS. Image quality and feasibility of an ultralow-dose high-pitch helical triple-rule-out computed tomography angiography acquired in the caudocranial direction. J Thorac Imaging. 2014;29:50–59. doi: 10.1097/RTI.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 10.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff B, Hein F, Meyer T, et al. Impact of a reduced tube voltage on CT angiography and radiation dose: results of the PROTECTION I study. JACC Cardiovasc Imaging. 2009;2:940–946. doi: 10.1016/j.jcmg.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Feuchtner GM, Jodocy D, Klauser A, et al. Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: a comparative study. Eur J Radiol. 2010;75:e51–e56. doi: 10.1016/j.ejrad.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Fink C, Krissak R, Henzler T, et al. Radiation dose at coronary CT angiography: second-generation dual-source CT versus single-source 64-MDCT and first-generation dual-source CT. AJR Am J Roentgenol. 2011;196:W550–W557. doi: 10.2214/AJR.10.5153. [DOI] [PubMed] [Google Scholar]
- 14.Leschka S, Stolzmann P, Schmid FT, et al. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol. 2008;18:1809–1817. doi: 10.1007/s00330-008-0966-1. [DOI] [PubMed] [Google Scholar]
- 15.Ghafourian K, Younes D, Simprini LA, Weigold WG, Weissman G, Taylor AJ. Scout view X-ray attenuation versus weight-based selection of reduced peak tube voltage in cardiac CT angiography. JACC Cardiovasc Imaging. 2012;5:589–595. doi: 10.1016/j.jcmg.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Ghoshhajra BB, Engel LC, Major GP, et al. Direct chest area measurement: a potential anthropometric replacement for BMI to inform cardiac CT dose parameters? J Cardiovasc Comput Tomogr. 2011;5:240–246. doi: 10.1016/j.jcct.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Commission E (1999) European guidelines on quality criteria for computed tomography. Brussels, Belgium Report EUR 16262 EN
- 18.Kalva SP, Sahani DV, Hahn PF, Saini S. Using the K-edge to improve contrast conspicuity and to lower radiation dose with a 16-MDCT: a phantom and human study. J Comput Assist Tomogr. 2006;30:391–397. doi: 10.1097/00004728-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Kidoh M, Nakaura T, Nakamura S, et al. Contrast material and radiation dose reduction strategy for triple-rule-out cardiac CT angiography: feasibility study of non-ECG-gated low kVp scan of the whole chest following coronary CT angiography. Acta Radiol. 2014;55:1186–1196. doi: 10.1177/0284185113514886. [DOI] [PubMed] [Google Scholar]
- 20.Huda W, Scalzetti EM, Levin G. Technique factors and image quality as functions of patient weight at abdominal CT. Radiology. 2000;217:430–435. doi: 10.1148/radiology.217.2.r00nv35430. [DOI] [PubMed] [Google Scholar]
- 21.Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF. Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology. 2004;231:169–174. doi: 10.1148/radiol.2311030191. [DOI] [PubMed] [Google Scholar]
- 22.Krissak R, Henzler T, Prechel A, et al. Triple-rule-out dual-source CT angiography of patients with acute chest pain: Dose reduction potential of 100kV scanning. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Li JL, Huang MP, Liang CH, et al. Individualized radiation dose control in 256-slice CT coronary angiography (CTCA) in retrospective ECG-triggered helical scans: Using a measure of body size to adjust tube current selection. Eur J Radiol. 2012 doi: 10.1016/j.ejrad.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Moscariello A, Takx RA, Schoepf UJ, et al. Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique-comparison with traditional filtered back projection. Eur Radiol. 2011;21:2130–2138. doi: 10.1007/s00330-011-2164-9. [DOI] [PubMed] [Google Scholar]
- 25.Lu GM, Luo S, Meinel FG, et al. High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. Eur Radiol. 2014;24:3260–3268. doi: 10.1007/s00330-014-3365-9. [DOI] [PubMed] [Google Scholar]