Abstract
Disruptions in early life care, including neglect, extreme poverty, and trauma, influence neural development and increase the risk for and severity of pathology. Significant sex disparities have been identified for affective pathology, with females having an increased risk of developing anxiety and depressive disorder. However, the effects of early life stress (ELS) on cognitive development have not been as well characterized, especially in reference to sex specific impacts of ELS on cognitive abilities over development. In mice, fragmented maternal care resulting from maternal bedding restriction, was used to induce ELS. The development of spatial abilities were tracked using a novel object placement (NOP) task at several different ages across early development (P21, P28, P38, P50, and P75). Male mice exposed to ELS showed significant impairments in the NOP task compared with control reared mice at all ages tested. In female mice, ELS led to impaired NOP performance immediately following weaning (P21) and during peri-adolescence (P38), but these effects did not persist into early adulthood. Prior work has implicated impaired hippocampus neurogenesis as a possible mediator of negative outcomes in ELS males. In the hippocampus of behaviorally naïve animals there was a significant decrease in expression of Ki-67 (proliferative marker) and doublecortin (DCX-immature cell marker) as mice aged, and a more rapid developmental decline in these markers in ELS reared mice. However, the effect of ELS dissipated by P28 and no main effect of sex were observed. Together these results indicate that ELS impacts the development of spatial abilities in both male and female mice and that these effects are more profound and lasting in males.
Highlights
-
•
ELS leads to sex differences in spatial memory abilities in mice.
-
•
Female mice show impaired performance that resolve prior to adolescence.
-
•
Male mice show persistent impairments across early life.
-
•
Effects are restricted to spatial abilities and not other task dimensions.
-
•
Effects are not related to markers of proliferation and differentiation in hippocampus.
1. Introduction
Early in life, parental interactions can serve as a buffer against the negative consequences of stress on physiology and learning (Stanton and Levine, 1990, Kirschbaum et al., 1995, Heinrichs et al., 2003, Shionoya et al., 2007, Taylor et al., 2008, Gee et al., 2014, van Rooij et al., 2016). However, if rearing conditions are suboptimal, parental stress or disruptions in the quality or reliability of care can be rapidly transmitted to the offspring and serve as a primary source of stress, driving changes in development and neurobehavioral outcomes (Levine, 1967, Rosenfeld et al., 1991, Suchecki et al., 1993, Liu et al., 1997, Avishai-Eliner et al., 2001, Rice et al., 2008, Raineki et al., 2010, Roth et al., 2013, Molet et al., 2014, Bath et al., 2016, Heun-Johnson and Levitt, 2016). Significant disruptions in the quality of early life care impact neural structure and functional plasticity of the brain (Teicher et al., 2006, Chen et al., 2008, Chen et al., 2013) and have been identified as potential catalysts for negative health outcomes, including disturbance in cognitive development. For example, in humans, institutionalized rearing or abusive early environments have been associated with the development of significant impairments in general cognitive functioning with specific deficits identified in memory recall (Bremner and Narayan, 1998) and short term memory (Bremner et al., 2000). These same experiences are associated with regional effects on brain development, with significant reductions in hippocampal volume and cortical thinning in frontal regions associated with memory function, attention, and spatial abilities (Bremner et al., 1997).
Significant sex disparities have been identified for stress-associated pathology, with females being twice as likely as males to develop PTSD, depression, and anxiety disorders (Weissman et al., 1996, Breslau et al., 1997a, Breslau et al., 1997b, Felitti et al., 1998, Gater et al., 1998, Burt and Stein, 2002, Kuehner, 2003, Keita, 2007, Breslau, 2009, De Munck et al., 2009, Hankin, 2009, Olino et al., 2010, Pratchett et al., 2010). However, whether similar sex disparities exist for stress-associated cognitive disturbance have not been as well characterized. Recent studies in animal models have provided mixed results, but have identified sex, developmental status, and timing of the stressor, as potential variables contributing to risk for cognitive outcomes. For example, some studies have reported significant impairment following various forms of ELS on spatial learning in female rats (Marco et al., 2013; Wang et al., 2016), while others found a male bias in impairment in both mice and rats (Barha et al., 2007; Mueller and Bale, 2007; Salomon et al., 2011; Schulz et al., 2011; Wang et al., 2011; Naninck et al., 2015). Other studies from both mice and rats failed to identify sex differences in performance (Benoit et al., 2015; Nazeri et al., 2015), while further studies in rats found ELS to be associated with improved cognitive performance (Barha et al., 2007; Zuena et al., 2008; Uysal et al., 2012; Barbie-Shoshani et al., 2016). Thus, considerable confusion exists with regard to the effects of ELS on cognitive functioning, possibly due to the varied forms of stress, timing of stress implementation, and age at testing. It should be noted that the majority of studies focused on assessing outcomes at one or two time points in development, typically late adolescence or adulthood, or failed to take into account possible developmental changes in performance on cognitive measures.
Here, the effect of ELS, in the form of maternal bedding restriction from P4-P11 (Rice et al., 2008, Bath et al., 2016), was tested on the development of spatial learning in male and female mice across early development. To do this, ELS and control reared mice were tested on a novel object placement (NOP) task, at postnatal days 21, 28, 38, 50, and/or 75. This allowed for assessment of time points that approximate childhood (P21), the pre-adolescent period (P28), the peri-adolescent period (P38), early adulthood (P50), and adulthood (P75) in mice. To minimize potential practice effects and diminish the contribution of any single litter on the overall results, a large number of litters were sampled (26), and no mouse was tested at more than 2 developmental time points. Significant sex disparities in risk for cognitive outcomes following ELS were observed. Males showed early emergence and persistent impairments in performance on the NOP task, while females showed an earlier but transient impairment in NOP performance. The current data suggest that stress may have sex and developmental selective effects on the emergence of cognitive disturbance, and such factors may be critical in understanding the contribution of stress and sex to developmental pathology.
2. Methods
2.1. Subjects
Breeding stock of male and female C57Bl/6N mice were acquired from Charles River labs and all mice used for the current studies were derived from litters that had been bred in house. Animals were maintained under normal housing conditions on a 12h:12h light cycle with ad libitum access to food and water. Pups were weaned and sex segregated at 21 days of age. All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee and consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Fragmented maternal care
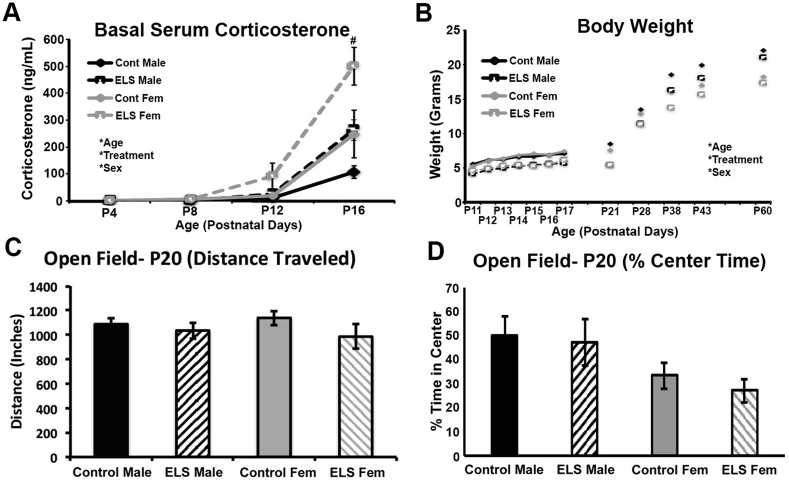
Four days following the birth of a litter, the dam and pups in the fragmented maternal care condition were transferred from their standard home cage, to a home cage with a wire mesh floor and a 2 × 4 cm cotton nestlet as their only source of bedding (as described in Bath et al., 2016). Mice continued to have ad libitum access to food and water. Dam and litters remained in these modified housing conditions for seven days, and were then returned to standard housing, containing cob bedding and a 4 × 4 cm nestlet. Control mice were left undisturbed throughout these procedures. Litters were composed of both male and female pups, and litters ranged in size from 5 to 8 pups per litter. Pups were not culled and natural variation in sex distributions were allowed. Pups were derived from 26 different litters with a range of sex distributions. Previous work in both mice and rats have shown that the bedding restriction manipulation leads to a fragmentation in maternal care and elevations in stress hormones in the dam immediately following the stressor (Avishai-Eliner et al., 2001, Rice et al., 2008, Bath et al., 2016, Heun-Johnson and Levitt, 2016, Molet et al., 2016). In mice and rats, ELS housing leads to an increase in the number of departures by the dam from the nest, but no change in the duration or total time spent licking and grooming or arched back nursing (Heun-Johnson and Levitt, 2016, Molet et al., 2016). Here, detailed assessment of maternal behavior was not carried out. Instead, successful replication of core features of this paradigm were used to verify the efficacy of the manipulation, including diminished weight gain of pups, an effect that was observed in both male and female mice (Fig. 1).
Fig. 1.
ELS effects on hormonal, somatic, and motor development. Data was collected for control and ELS reared male and female mice across development. A) Mean AM basal serum corticosterone levels were higher in female mice compared with males and ELS led to increased basal corticosterone in both sexes. B) mean weight of females were lower than males, with all animals gaining weight across the study period. On average ELS reared mice were 17% smaller than control and ELS mice. C) No effect of sex or treatment was found for total distance traveled in the open field at P20. D) No effect of sex or treatment was found for % center time in the open field. Main effects of age, treatment and sex are identified with (*). Post-hoc ANOVA at a given age are identified with (#). ***p < 0.001, #p < 0.05. Data are presented as mean (+/− SEM).
2.3. Open field task
At 20 days of age, prior to beginning the novel object placement task, all mice received an initial exposure to the open field testing apparatus (the same apparatus used for NOP testing). Open field testing occurred between the hours of 9AM and 12PM, under approximately 15–20Lux of light and lasted for a total of 7 min. During this time, mice were video recorded and their activity was tracked with the aid of digital tracking software (Noldus Ethovision XT 8.5). To determine if ELS or sex significantly impacted locomotion within the testing apparatus, total distance traveled was quantified. Percent time in the center of the open field was used to test if ELS altered anxiety-like behavior.
2.4. Novel object placement task
One day prior to testing, mice were again habituated to the empty open field for 7 min to acclimate them to both handling as well as the testing environment. Testing occurred between the hours of 9AM and 12PM, under approximately 15–20Lux of light. A total of 26 different litters of mice were used. At each age, groups included mice from a minimum of at least 4 different litters. Testing consisted of an exploration phase (T1, exploration trial) and a recognition phase (T2, recognition trial). In the exploration trial, mice were placed in the open field with two identical objects (Supplemental Fig. 1) for 5-min. Investigation of the objects was timed using automated tracking software (Noldus Ethovision XT 8.5), with investigation defined as the subject's nose being directed at and within 1 inch of the object. After the T1 exploration phase, the subject was removed from the open field for 25 min. ITI duration was chosen based on prior work in rats, which found that 21 day old rats could complete the task with short < 1hr, but not long (24hr) intervals (Jablonski et al., 2013, Westbrook et al., 2014). During the ITI, one object was moved to a new location and the arena and object were cleaned with ethanol and dried. The mouse was then returned to the arena for an additional 5-min trial and the time spent exploring the objects in the new (novel) and old (familiar) locations after the delay (T2, recognition trial) were assessed with the aid of automated tracking. All locations for the objects were counterbalanced among groups. Because mice generally show a novelty preference, (e.g. more time spent exploring the object in the novel rather than familiar location) a bias toward investigation of the object in novel location was used to assess the subjects memory of objects in familiar relative to novel locations (Aggleton et al., 1997, Luine et al., 2003).
2.5. Realtime quantitative polymerase chain reaction (RT-qPCR)
For each developmental time point, the hippocampus (whole hippocampus from one hemisphere-side randomly selected) was collected from behaviorally naïve animals from at least 2 different litters to eliminate the possibility of cohort effects on measures of gene expression (n = 5 animals per group/per age). Brains were dissected on ice, hippocampus was isolated followed by homogenization in RNAzol (Molecular Research Center, Cincinnati, OH) and stored at −80C until processing. RNA isolation was in accordance with the manufacturers protocol. First strand cDNA synthesis was in accordance with New England Biolabs MmULV protocols (NEB, Ipswitch, MA). Predesigned and pre-validated Taqman assays from Applied Biosystems (Life Technologies, Norwalk, CT) for Ki-67 (assay# Mm01278616_m1) and DCX (assay# Mm00438400_m1) were used and run in multiplex with housekeeping gene (18S cat# 4319413E). For each plate and assay, gene expression was calculated based upon a standard curve included on each plate. A CFX384 RT-qPCR system (Biorad, Hercules, CA) and associated software was used for all gene expression profiling.
2.6. Statistics
Effects of sex, age, and treatment were calculated for gene expression, locomotor activity, object visits, and object exploration time during the T1 exploration and T2 recognition phase of the task using ANOVA. For T2 recognition testing, a single sample t-test (tested against 50- defined as chance) was used to test for successful performance on the task. Correlation analysis was used to test for relationships between T1 exploration (object visits and object investigation) and T2 recognition performance. For all tests alpha was set at p < 0.05.
3. Results
3.1. ELS effects on somatic and stress measures
Between P11 and P60, a significant effect of age was observed on weight (F(11,780) = 1719.732, p < 0.001; eta2 = 0.960), with all groups showing a significant increase in weight over development. A significant main effect of sex was also observed (F(1,780) = 73.120, p < 0.001; eta2 = 0.086), with females being smaller than males. In addition, a significant main effect of treatment was found for weight (F(1,780) = 350.234, p < 0.000; eta2 = 0.310; Fig. 1A), with ELS animals weighing on average 17% less than control reared mice. The effect of ELS on weight gain was similar in both male and female mice, with no sex x age × treatment interaction (F(11,780) = 0.467, p = 0.924; eta2 = 0.007).
For corticosterone, basal serum levels significantly increased with age (F(1,50) = 71.797, p < 0.001; eta2 = 0.812), and a main effect of treatment was found (F(1,50) = 25.128, p < 0.001; eta2 = 0.334; Fig. 1B) with ELS leading to a significant elevation in basal corticosterone levels in both male and female mice. In addition, there was a significant main effect of sex (sex: F(1,50) = 18.837, p < 0.001; eta2 = 0.274) with corticosterone levels in females being significantly higher than those observed in males for both control and ELS reared conditions (Fig. 1B). Follow-up analyses at each independent age show a significant effect of treatment that emerged at P16 (ANOVA, F(1,12) = 14.780, p = 0.002; eta2 = 0.552), but did not reach significance at P12 (ANOVA, F(1,14) = 2.515, p = 0.135; eta2 = 0.152).
3.2. Open field locomotor testing (P20)
In previous work, ELS has been shown to impair early motor development (Bath et al., 2016). Differences in locomotor activity can influence performance on the NOP task by altering the number of visits and amount of time investigating objects in the environment. To test for effects of sex (male and female) and treatment (control and ELS) on general locomotor activity prior to study enrollment, aged P20 mice were tested in the open field for 7 min. No main effect of treatment was found for total distance traveled (ANOVA; F(1,85) = 2.247, p = 0.138; eta2 = 0.026). There was also no effect of sex on distance traveled (ANOVA; F(1,85) = 0.000, p = 0.993; eta2 0.000) with male and female control and ELS mice traveling similar overall distances (Fig. 1C). To determine if ELS alters the expression of anxiety-like behavior at this young age, the percent time that mice spent in the center of the open field was assessed. There was no main effect of treatment (F(1,47) = 0.198, p = 0.658), sex (F(1,47) = 3.448, p = 0.070), or treatment × sex interaction (F(1,47) = 0.032, p = 0.858) for this measure, indicating that ELS does not alter locomotor activity or anxiety-like behavior at this age (Fig. 1D).
3.3. Novel object placement performance (T2 recognition)
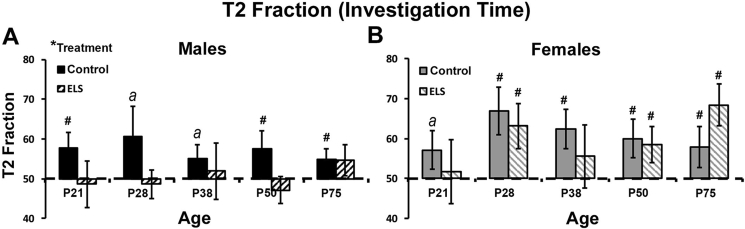
Prior work in mice has shown sex differences in NOP performance when using a 24 h ITI (Frick and Gresack, 2003). Here, a 25-min ITI was used and no difference was found between control reared male and female mice (F(1,110) = 1.065, p = 0.304, eta2 = 0.010), indicating that both male and female mice can perform successfully on this task. However, for mice reared under ELS conditions, a significant main effect of sex was found (F(1,127) = 6.581, p = 0.011, eta2 = 0.049) with males performing significantly worse than females. In follow-up analyses, the effect of treatment was assessed within sex, and showed that ELS led to impaired NOP performance in male (F(1,135) = 4.663, p = 0.033; eta2 = 0.033), but not female mice (F(1,107) = 0.148, p = 0.701; eta2 = 0.001).
To test for successful performance on the NOP task at each age tested, a single sample t-test was used to test group performance against chance levels of investigation (50–e.g. equal investigation of the object in the novel relative to the familiar location). Successful performance was defined as a T2 fraction significantly greater than 50. Data from control and ELS male mice is presented in Fig. 2A and data from control and ELS female mice is presented in Fig. 2B.
Fig. 2.
ELS has developmental and sex selective effects on NOP performance. T2 fraction for mice during the test phase of the NOP task. T2 fraction was calculated as the ratio (novel object investigation/(novel + familiar object investigation)*100. There was no difference in performance between control male and female mice. (A) For male mice, a significant main effect fo treatment was found, with ELS mice failing to perform above chance at all ages tested. (B) For female mice, ELS led to impaired performance at P21 and P38 but not other developmental time points. *denote significant main effect (ANOVA). #denotes significant single sample t-test, comparing T2 fraction to chance (50). adenotes an effect approaching but failing to reach significance (p < 0.10). Data represent the group mean (+/-SEM).
3.3.1. Male NOP performance
At P21, control reared male mice showed successful discrimination (t(10) = 2.827, p = 0.009). However, ELS male mice failed to differentiate between the object in the novel relative to familiar locations (t(16) = 0.224, p = 0.413), performing at levels indistinguishable from chance. At P28, performance of control reared male mice approached but did not reach significance (t(8) = 1.397, p = 0.100), while male mice reared under ELS conditions again failed to show successful discrimination (t(7) = 0.368, p = 0.362). At P38, control reared male mice again approached but did not reach significance with regard to successful performance (t(13) = 1.470, p = 0.083). However, at this same developmental time point ELS reared males failed to show significant discrimination (t(11) = 0.265, p = 0.398). At P50, control reared male mice were again successful at performing the task (t(13) = 2.085, p = 0.029), while ELS males again failed to show successful discrimination (t(23) = 0.827, p = 0.209). Finally, at P75, control reared male mice showed successful performance (t(7) = 1.941, p = 0.047), while ELS male mice continued to fail to discriminate between the objects in the novel relative to familiar location (t(21) = 1.186, p = 0.125).
3.3.2. Female NOP performance
For female mice, at P21, control reared female performance approached, but did not reach significance (t(11) = 1.509, p = 0.080). At this age, ELS female mice failed to differentiate between the object in the novel relative to familiar locations, performing at levels indistinguishable from chance (t(8) = 0.218, p = 0.417). At P28, Both control reared females (t(12) = 2.848, p = 0.008) and ELS reared females (t(8) = 2.326, p = 0.024) successfully performed the task. At P38, control reared female mice could successfully discriminate between the novel and familiar object location (t(16) = 2.496, p = 0.012), however, ELS reared females failed to show significant discrimination at this time (t(7) = 0.706, p = 0.252). At P50, both control reared female mice (t(15) = 2.083, p = 0.028) as well as ELS female mice (t(15) = 1.923, p = 0.037) performed successfully on the task. Finally, at P75, again, both control reared female mice (t(4) = 1.560, p = 0.097) as well as ELS female mice (t(11) = 3.482, p = 0.003) were successful in discriminating between the object in the novel relative to familiar location.
3.4. Effects of test re-test on NOP performance
A subset of mice (96) were tested at two separate developmental time points. Comparing performance between first and second test, no effects of test repetition on performance were found (Paired t-test- T(95) = −1.669, p = 0.098). Mean performance on test 1 was 53.9 (SEM = 2.1) while mean performance on test 2 was 58.2 (SEM = 1.8). Performance across age was also assessed in control and ELS reared animals to determine if age significantly contributed to changes in performance over development. No significant main effect of age was found for either control (F(4,110) = 0.438, p = 0.748, eta2 = 0.017) or ELS reared mice (F(4,127) = 1.345, p = 0.257, eta2 = 0.041), indicating no differences in performance across the ages tested.
3.5. Additional metrics of NOP performance
As mentioned above, deficits in performance can be due to failures in a number of different phases of this task beyond impaired memory or spatial abilities. In some instances, failures can emerge as a consequence of diminished initial investigation of the objects during habituation, a failure to approach the objects during the recognition phase, and/or increased or decreased locomotion which may impair overall investigation time and number or duration of object visits. Data was collected on all of these metrics to test if any of these variables might explain impairments in performance observed in ELS male and female mice, as well as test for potential effects of age, sex, and treatment on these measures.
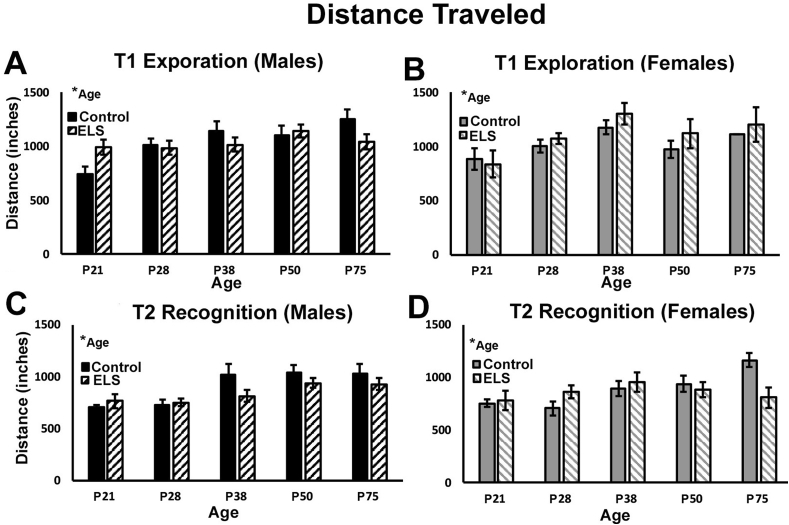
3.6. Distance traveled during T1 exploration and T2 recognition testing
Group differences in total distance traveled during the T1 (exploration) as well as T2 (recognition) phases of the NOP task were collected using automated tracking software (Noldus Ethovision), to investigate the effects of age, sex, and treatment on locomotor activity. During the exploration phase of the task, no effects of sex (ANOVA; F(1,162) = 0.280, p = 0.598; eta2 = 0.002) or of treatment (ANOVA; F(1,162) = 0.380, p = 0.539; eta2 = 0.002) were found for total distance traveled (Fig. 3A and B). In addition, there was no sex*treatment interaction (ANOVA; F(1,162) = 0.858, p = 0.356; eta2 = 0.005). However, a significant effect of age was found for distance traveled (ANOVA; F(4,162) = 6.873, p < 0.001; eta2 = 0.145; Fig. 3A and B) with mice showing a general trend toward greater distance traveled with increasing age. However, there were no interactions between age and any other variables. During the T2 recognition phase of testing, no effect of sex (ANOVA; F(1,162) = 0.016, p = 0.901; eta2 = 0.001), treatment (ANOVA; F(1,162) = 1.813, p = 0.179; eta2 = 0.007), or sex*treatment interaction (ANOVA; F(1,162) = 0.192, p = 0.661; eta2 = 0.001; Fig. 3C and D) were observed. For the T2 recognition phase, a significant effect of age was observed (ANOVA; F(4,162) = 8.124, p < 0.001; eta2 = 0.118; Fig. 3C and D), with greater distance traveled in older mice, but no interaction between age and any other variable.
Fig. 3.
ELS does not alter motor activity in the NOP task. We observed a significant effect of age, but no effects of sex or treatment on locomotor activity during T1 exploration phase for either (A) male or (B) female mice. During the T2 recognition phase, we also observed a significant effect of age, but no effects of sex or treatment on locomotor activity for (C) male or (D) female mice. *alpha was set at (p < 0.05). Data represent the group mean (+/-SEM).
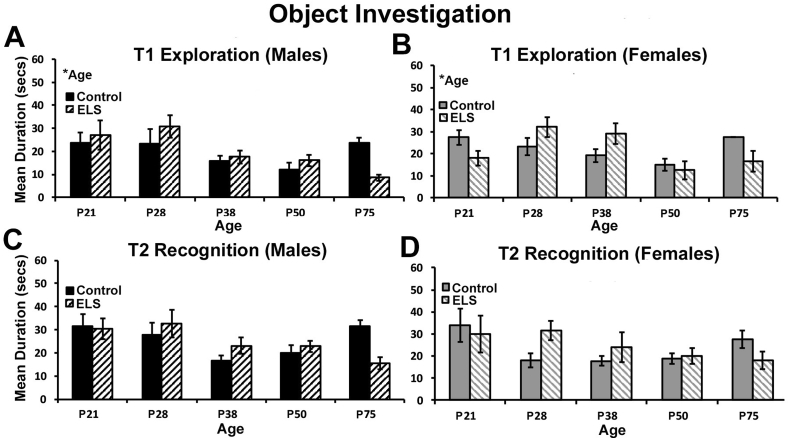
3.7. Object investigation during T1 exploration and T2 recognition
ELS may impact the overall duration or frequency of object investigation during the exploration or recognition phase of testing, an indicator of object interest or willingness to explore objects. Thus, total time spent engaged in object exploration for each phase of testing was assessed. For the exploration phase, similar overall levels of investigation were observed for male and female control and ELS reared mice. For total time spent investigating objects, no main effect of sex (ANOVA; F(1,162) = 0.577, p = 0.449; eta2 = 0.004), treatment (ANOVA; F(1,162) = 0.001, p = 0.978; eta2 = 0.001), or sex * treatment interaction (ANOVA; F(1,162) = 0.003, p = 0.956; eta2 = 0.001; Fig. 4A and B) were found. However, a main effect of age was found (ANOVA; F(4,162) = 5.450, p < 0.001; eta2 = 0.119, Fig. 4A and B), with a trend toward lower levels of investigation at later ages in development. Near identical effects were observed for the number of object visits during the exploration phase of testing (data not shown). For the number of object visits, no main effect of sex (ANOVA; F(1,162) = 0.193, p = 0.661; eta2 = 0.001), treatment (ANOVA; F(1,162) = 1.003, p = 0.318; eta2 = 0.006), or sex*treatment interaction (ANOVA: F(1,162) = 0.035, p = 0.852; eta2 = 0.001) were found. For number of object visits during T1 exploration, a significant effect of age was found (ANOVA; F(4,162) = 2.969, p = 0.021; eta2 = 0.068) but no interaction between age and any other variables.
Fig. 4.
ELS does not alter object exploration during the NOP task. For object exploration during the T1 exploration phase of testing we observed a significant effect of age for (A) male and (B) female mice, but no effects of sex or treatment or interactions. During the T2 recognition phase of testing, we observed a main effect of sex, but no effect of treatment, age, or interactions for (C) male and (D) female mice. *alpha was set at (p < 0.05). Data represent the group mean (+/-SEM).
For the T2 recognition phase of the task, a main effect of sex was found (ANOVA; F(1,242) = 6.714, p = 0.010; eta2 = 0.027) with males showing slightly higher overall levels of investigation, but no main effect of treatment (ANOVA; F(1,242) = 2.899, p = 0.090; eta2 = 0.012) as well as no treatment*sex interaction (ANOVA; F(1,242) = 1.238, p = 0.267; eta2 = 0.005; Fig. 4C and D). For time spent investigating objects during the recognition phase of the task, there was no main effect of age (ANOVA; F(4,242) = 0.729, p = 0.573; eta2 = 0.012, Fig. 4C and D), or interaction between age and any other variable. For the number of object visits during the recognition phase, a significant main effect of treatment was found (ANOVA; F(1,242) = 4.060, p = 0.045; eta2 = 0.016, data not shown), with ELS animals tending to visits objects more frequently. A marginal main effect of sex was also found for this measure (ANOVA; F(1,242) = 3.455, p = 0.064; eta2 = 0.014, data not shown), but no treatment*sex interaction (ANOVA; F(1,242) = 0.486, p = 0.486; eta2 = 0.002, data not shown). Finally, there was no significant main effect of age on object visits (ANOVA; F(4,242) = 0.219, p = 0.928; eta2 = 0.004, data not shown) or interaction between age and any other variable.
3.8. Relationship between T1 exploration and T2 recognition performance
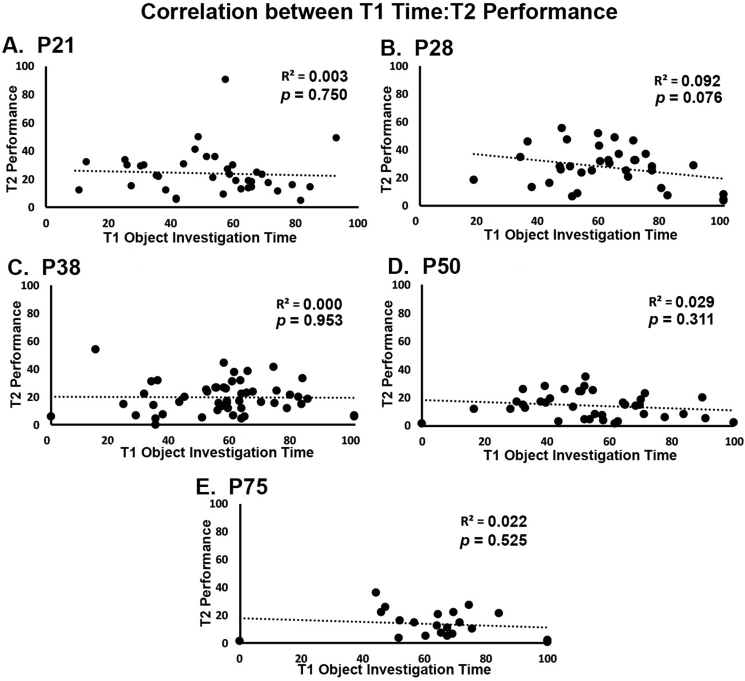
Multiple methodologies exist for carrying out NOP testing, with some groups suggesting that increasing object investigation time or equating object investigation time during the exploration phase can lead to enhanced performance during the recognition phase of the task. Here, a fixed trial duration was used for both the T1 exploration and T2 recognition phases of testing. Correlation analysis was used to investigate whether differences in object investigation time or number of visits to the objects during the T1 exploration phase significantly correlated with performance in the T2 recognition phase. Interestingly, no correlation was found between object visits during the T1 exploration phase and performance during the T2 recognition phase (r(182) = 0.017, p = 0.822) and no correlation between object investigation time during the T1 exploration phase and performance during the T2 recognition phase (r(182) = 0.023, p = 0.760), indicating that increased investigation during the exploration phase did not lead to better performance in the recognition phase of the task. When correlations were carried out at each age (Fig. 5A–E), no significant relationships were found between time investigating the objects during the T1 phase and T2 performance at P21 (r(35) = 0.003, p = 0.750), P28 (r(33) = 0.092, p = 0.076), P38 (r(49) = 0.000, p = 0.953), P50 (r(36) = 0.029, p = 0.311), or P75 (r(19) = 0.022, p = 0.525) (see Fig. 6).
Fig. 5.
Degree of T1 investigation is not correlated with performance during the T2 phase of the NOP task. The relationship between performance during the T2 recognition phase of testing and duration of object exploration during the T1 exploration phase of testing is plotted for each individual animal. Plots at R2 are included for animals at A) P21 B) P28 C) P38 D) P50 and E) P75. No significant relationship between T1 exploration measures and performance during the T2 recognition phase of the task at any age. Based upon these data, greater investigation of the objects during the T1 exploration phase does not promote better performance on during the T2 recognition phase.
Fig. 6.
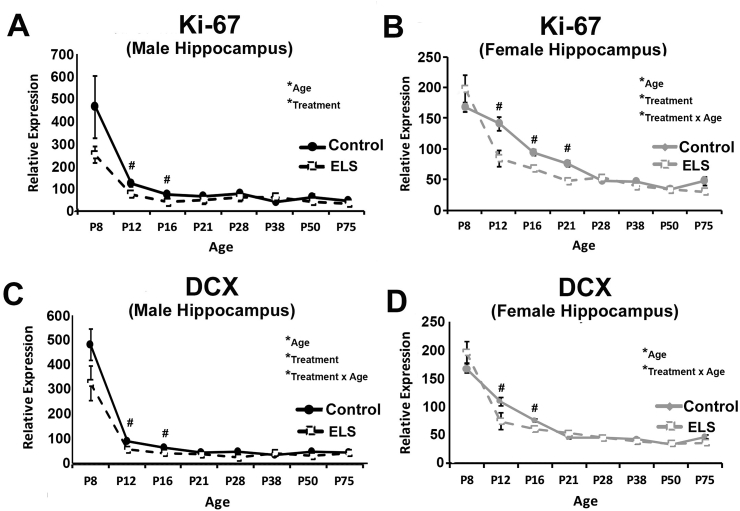
ELS alters the developmental expression of markers of neuronal proliferation and differentiation similarly in male and female hippocampus. Gene expression for Ki-67 in (a) male and (b) female whole hippocampus as well as doublecortin (DCX) in (c) male and (d) female whole hippocampus. Gene expression was assessed by realtime qPCR and calculated relative to 18S. We observed a significant decrease in expression over development, and observed lower levels of expression in ELS reared hippocampus at early developmental ages for both sexes and both markers, but no significant effects of sex or treatment beyond P28. *significant main effects #significant post-hoc (Tukey LSD). Alpha was set at (p < 0.05). Data represent the group mean (+/−SEM).
3.9. ELS and markers of cell proliferation and differentiation
Previous work has suggested that ELS effects on hippocampus neurogenesis may contribute to sex differences in performance on the NOP task (Naninck et al., 2015). Here, whole hippocampus was collected from one hemisphere of behavioral naïve male and female control and ELS mice across development. Using RT-qPCR, effects of development, sex, and treatment on markers of cell proliferation (Ki-67) and cellular differentiation (Doublecortin- DCX) were tested. For Ki-67 levels in male mice, a significant main effect of age (F(7,65) = 16.247, p < 0.001; eta2 = 0.636), treatment (F(1,65) = 4.887, p = 0.031; eta2 = 0.070), and marginal treatment × age interaction (F(7,65) = 1.870, p = 0.089; eta2 = 0.168) was found. Specifically, a significant reduction in Ki-67 levels across early development was observed, with reduced Ki-67 expression in ELS hippocampus at P12 (p < 0.011) and P16 (p < 0.022). For DCX in male hippocampus, a significant effect of treatment (F(1,65) = 6.411, p = 0.014; eta2 = 0.090), age (F(7,65) = 54.231, p < 0.001; eta2 = 0.854), and a treatment*age interaction (F(7,65) = 2.313, p < 0.036; eta2 = 0.199) were observed, with decreasing expression over development and lower levels of expression in ELS mice at P8 (p = 0.070), P12 (p < 0.001), and P16 (p = 0.014). For female mice, a nearly identical pattern of results was found. Specifically, for Ki-67, a significant effect of age (F(7,64) = 75.712, p < 0.001; eta2 = 0.892), treatment (F(1,64) = 9.150, p = 0.004; eta2 = 0.125), and treatment*sex interaction (F(7,64) = 5.055, p < 0.001; eta2 = 0.356) were observed, with decreasing expression over development and lower levels of Ki-67 in ELS female hippocampus compared with controls at P12 (p = 0.006), P16 (p < 0.001), and P21 (p = 0.001), but not later time points. For DCX levels in female hippocampus, a significant effect of age (F(7,63) = 93.407, p < 0.001; eta2 = 0.912), no main effect of treatment (F(1,63) = 0.804; p = 0.373; eta2 = 0.013) but a significant treatment*age interaction (F(7,63) = 3.240, p = 0.005; eta2 = 0.265) were found. Similar to males, there was a decrease in expression of DCX over development with lower DCX levels in ELS hippocampus at P12 (p = 0.033) and P16 (p = 0.009). Thus, ELS does impact the expression of markers of neurogenesis over development, however, all significant effects are restricted to early developmental time points (prior to P38).
4. Discussion
Here, the effects of ELS on the development of spatial memory in a mouse model were tested. Specifically, testing was carried out to investigate if sex differences exist in risk for the development of disturbances in spatial memory, and if effects were present, to identify when they emerge, and how long they persisted. ELS, in the form of maternal bedding restriction, led to early and persistent impairments in male performance on the NOP task. In females, ELS was associated with impaired performance during the pre-adolescent period, but these effects resolved prior to young adulthood. The current results suggest that ELS may have sex and developmentally selective effect on cognitive functioning, with effects in males being early emerging and more persistent than those observed in female mice.
In the current study, control reared male mice could perform the NOP task as early as 21 days of age (P21) with females showing a trend toward successful performance at P21 and successful performance by P28. These results are consistent with the work of Stanton and colleagues, who have found in rats that novel object location abilities develop as early as P21 (Jablonski et al., 2013, Westbrook et al., 2014). Thus, the task and chosen time points for measuring NOP function were appropriate and allowed us to assess the impact of ELS on this behavior throughout early development.
ELS led to persistent impairments in spatial performance in male mice and early but transient effects in females. The current study leveraged a modified version of the fragmented maternal care paradigm developed by the lab of Dr. Tallie Baram (Rice et al., 2008, Bath et al., 2016). Using a similar model of ELS, Naninck et al. (2015). observed significant effects of ELS on cognitive outcomes in adult mice on the NOP task: impaired performance in adult male mice and no impairments in adult females. The current results confirm the adult effects observed in that report, and significantly extend that work to demonstrate that female cognitive ability is also affected by ELS. However, the effects of ELS on female cognitive ability appear to be dependent upon the age of testing. Specifically, impaired performance in ELS female mice was observed immediately post weaning (P21) as well as during pre-adolescent stage (P38), but not at other developmental time points. The current data suggest that female function may in fact be impacted by ELS, but that the effects in females either resolve, or that females develop an alternative strategy to promote success on this task by early adulthood. However, the mechanisms underlying the persistent impairments in memory function observed in males, or the transient impairments observed in females, are not fully understood.
The neural mechanisms supporting NOP performance have been investigated across a variety of species including rodents and non-human primates. Based upon that literature, multiple nodes within a broader network have been identified as being that critical for successful performance on this task. Based on lesion studies, the broader circuit supporting NOP function includes the hippocampus (Aggleton et al., 1992, Barker and Warburton, 2011) and more specifically a reliance upon an intact dentate gyrus (DG) and CA3, but not CA1 (Lee et al., 2005). Additional studies have shown that success on the NOP task further relies upon an intact fornix (Aggleton et al., 1991, Chudasama and Muir, 1997, Eacott and Norman, 2004), anterior thalamus (Aggleton et al., 1991), anterior cingulate cortex (Weible et al., 2009), peri-rhinal cortex (Wiig and Burwell, 1998, Barker and Warburton, 2011), and medial prefrontal cortex (Rogers et al., 1992, Chudasama and Muir, 1997, Barker and Warburton, 2011). In pharmacological studies, NOP performance has also been shown to be dependent upon cholinergic signaling. Regional modulation of cholinergic activity in the frontal cortex or hippocampus (Dunnett et al., 1990) or septum (Torres et al., 1994, Steckler et al., 1995) have been shown to contribute to impairments in NOP performance.
In the previous report by Naninck and colleagues, the author argue that ELS-associated changes in dentate gyrus (DG) neurogenesis may mediate the effects of ELS on cognitive functioning in the novel object placement task. Such effects could be consistent with prior work demonstrating involvement of hippocampal as well as para-hippocampal regions in object-location memory tasks (Wiig and Burwell, 1998, Liu and Bilkey, 2001, Lee et al., 2005, Parron et al., 2006, Bachevalier and Nemanic, 2008, Assini et al., 2009). The current study did not directly assess rates of neurogenesis in DG, but instead included proxy measures of gene expression for markers of cell proliferation and differentiation in whole hippocampus. In all groups, a significant decrease in the expression of markers of cell proliferation and differentiation were found across early postnatal development, consistent with decreasing rates of neurogenesis over the early postnatal period. ELS led to a more rapid developmental decline in expression of these markers in both male and female mice. However, the majority of the effects of ELS on Ki-67 and DCX expression dissipated by P21, the start of behavioral testing. During the adolescent and adult period, no effects of treatment or treatment*sex interactions were observed for gene expression, despite ongoing impairments in behavioral performance in ELS reared males, but intact performance in ELS reared female mice. Ki-67 and DCX expression change over development and across the life-cycle of the cell, with Ki-67 being expressed during the process of cell division and DCX being expressed during the process of cellular differentiation. In our previous work, declines in DCX gene expression were mirrored by a decrease in density of DCX-immunoreactive cells, suggesting that gene expression can serve as a proxy measure for quantification of cell number. Based upon these data, markers of cell proliferation and differentiation do not appear to predict impairments in performance on this task. It should be noted, that in the work of Naninck, the authors quantified rates of neurogenesis in the same animals that had performed the NOP task, but in the current study, measures of Ki-67 and DCX were collected from behaviorally naïve animals. Further, in the work by Naninck, the authors observed elevations in rates of neurogenesis in both sexes, but sex selective effects on survival, which were correlated with performance. It is possible that gene expression levels for markers of proliferation and differentiation in the whole hippocampus, collected here, may not provide adequate sensitivity to detect changes in region specific (e.g. DG) rates of neurogenesis or differences in rates of survival of newly born cells. Therefore, more intensive study would be required to fully resolve this issue.
In the current report, ELS led to significant and persistent impairments in the NOP task in male mice, but transient effects on NOP performance in ELS reared females. Multiple possible factors could contribute to deficits in NOP performance, including stress associated changes in object interest (e.g. neophobia), elevations in anxiety-like behavior, stress effects on locomotion and object exploration, or stress associated changes in spatial memory processing. Here, extensive analysis of rodent behavior was carried out across different phases of the task to identify whether deficits in performance were related to altered cognitive ability or some other variable, such as elevated anxiety or differences in object exploration. In the current analysis of the data, observed deficits in performance do not appear to be due to stress associated changes in locomotor function, object interest, or willingness to approach the objects. Thus, the observed deficits are likely due to effects on processing of spatial information. In the current study, a 25-min interval was employed between the T1 exploration and T2 recognition phases of the task. This interval was chosen, as rodents show greater difficultly with task performance over extended delays, especially at younger ages (Westbrook et al., 2014). By using a 25-min delay, this allowed for a task that was both sufficiently difficult and accessible to young animals. Additional studies should be carried out, using longer delays. Such studies may provide additional insights into disruption in spatial memory function in control and ELS reared male and female animals and identify further deficits in memory consolidation at these young ages.
A principle goal of much of the work investigating ELS effects on cognitive development in animal models, is to provide model systems of early adversity that phenocopy traits associated with pathological outcomes in humans, and to then leverage those models to understand the neurobiological substrates of dysfunction. Here, ELS in the form of maternal bedding restriction resulted in persistent impairments in spatial skills in male mice, but only transient effects in female animals. This work is consistent with prior work assessing cognitive function following rearing in a bedding restriction stress paradigm (Naninck et al., 2015), and suggests replicability of this model across labs. In addition, this model may provide a useful tool for understanding the possible neurobiological substrates of risk, and possibly resilience, for cognitive deficits following early adversity, as well as a tool to better understand developmental changes in behavioral deficits and their relation to changes in symptom expression in human exposed to early adversity. However, it should be noted that previous work in other model systems of early life stress (including data from multiple strains of mice and rats) have yielded somewhat conflicting results. Differences in outcome could be the consequence of differences in the timing of stress induction, the mode and severity of the stressor, the species used, or the timing and form of behavioral testing. Specifically, in models of prenatal stress, some have observed selective impairments in female BalbC mouse offspring (Wang et al., 2011) while others found a male bias in impairments in various species of rats (Mueller and Bale, 2007, Salomon et al., 2011, Schulz et al., 2011, Wang et al., 2016) using the NOP or Morris water maze tasks in adolescence or adulthood. Still other studies using either mice and rats as model systems, failed to find any sex differences (Benoit et al., 2015, Nazeri et al., 2015) or showed ELS to be associated with improvements in cognitive performance (Zuena et al., 2008, Barbie-Shoshani et al., 2016). Studies investigating the effects of early postnatal stress manipulations on cognitive outcomes have been less numerous and have similarly resulted in disparate findings, that appear to depend upon the specific timing and form of stress used. For example, rats receiving low licking and grooming early in life showed improvements in spatial memory performance when tested as adults (Barha et al., 2007). However, maternal separation or maternal deprivation, which involve greater handling of subjects, were associated with female selective effects, with effects being observed in adolescence and adulthood (Wang et al., 2011, Marco et al., 2013). Given that behavioral outcomes may be highly dependent upon the form, timing, and severity of stress, as well as the age at testing, a greater understanding of how manipulation of these variables can drive disparate outcomes will be important in understanding risk factors for negative outcomes and the importance of the timing of these events on brain and behavioral development. Furthermore, in the context of translational research, care should be taken when considering the timing, duration, and method of stress, as well as the timing of testing of behavioral performance when considering models systems approaches to understanding pathology.
4.1. Conclusions
Here, a comprehensive developmental approach was used to assess the impact of ELS, in the form of maternal bedding restriction, on the development of spatial abilities in a mouse model. The current results significantly add to our understanding of the effects ELS on the development of spatial learning in this model, provide greater insights into sex selective disruptions in function, and provide clarity with regard to the timing of emergence of these effects. More work will be required to understand the neurobiological underpinnings of these effects, and the factors that confer risk and resilience in male and females.
Acknowledgements
This work was support in part by the Robert and Nancy Carney Fund for Scientific Innovation (KGB) and the Norman Prince Neuroscience Institute- New Frontiers Award (KGB). A portion of the data described in this publication was supported by an Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ynstr.2017.04.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Aggleton J.P., Keith A.B., Sahgal A. Both fornix and anterior thalamic, but not mammillary, lesions disrupt delayed non-matching-to-position memory in rats. Behav. Brain Res. 1991;44:151–161. doi: 10.1016/s0166-4328(05)80020-8. [DOI] [PubMed] [Google Scholar]
- Aggleton J.P., Keen S., Warburton E.C., Bussey T.J. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res. Bull. 1997;43:279–287. doi: 10.1016/s0361-9230(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Aggleton J.P., Keith A.B., Rawlins J.N., Hunt P.R., Sahgal A. Removal of the hippocampus and transection of the fornix produce comparable deficits on delayed non-matching to position by rats. Behav. Brain Res. 1992;52:61–71. doi: 10.1016/s0166-4328(05)80325-0. [DOI] [PubMed] [Google Scholar]
- Assini F.L., Duzzioni M., Takahashi R.N. Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation. Behav. Brain Res. 2009;204:206–211. doi: 10.1016/j.bbr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S., Eghbal-Ahmadi M., Tabachnik E., Brunson K.L., Baram T.Z. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J., Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Barbie-Shoshani Y., Shoham S., Bejar C., Weinstock M. Sex-specific effects of prenatal stress on memory and markers of neuronal activity in juvenile rats. Dev. Neurosci. 2016;38:206–219. doi: 10.1159/000446981. [DOI] [PubMed] [Google Scholar]
- Barha C.K., Pawluski J.L., Galea L.A. Maternal care affects male and female offspring working memory and stress reactivity. Physiol. Behav. 2007;92:939–950. doi: 10.1016/j.physbeh.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Barker G.R., Warburton E.C. When is the hippocampus involved in recognition memory? J. Neurosci. official J. Soc. Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates neuroal and behavioral maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J.D., Rakic P., Frick K.M. Prenatal stress induces spatial memory deficits and epigenetic changes in the hippocampus indicative of heterochromatin formation and reduced gene expression. Behav. Brain Res. 2015;281:1–8. doi: 10.1016/j.bbr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: implications for childhood development and aging. Dev. Psychopathol. 1998;10:871–885. doi: 10.1017/s0954579498001916. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Vermetten E., Mazure C.M. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Randall P., Vermetten E., Staib L., Bronen R.A., Mazure C., Capelli S., McCarthy G., Innis R.B., Charney D.S. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse–a preliminary report. Biol. psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Peterson E.L., Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch. Gen. Psychiatry. 1997;54:81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Andreski P., Peterson E.L., Schultz L.R. Sex differences in posttraumatic stress disorder. Arch. Gen. Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Burt V.K., Stein K. Epidemiology of depression throughout the female life cycle. J. Clin. psychiatry. 2002;63(Suppl. 7):9–15. [PubMed] [Google Scholar]
- Chen Y., Dube C.M., Rice C.J., Baram T.Z. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. official J. Soc. Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Kramar E.A., Chen L.Y., Babayan A.H., Andres A.L., Gall C.M., Lynch G., Baram T.Z. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol. Psychiatry. 2013;18:485–496. doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y., Muir J.L. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacol. Berl. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- De Munck S., Portzky G., Van Heeringen K. Epidemiological trends in attempted suicide in adolescents and young adults between 1996 and 2004. Crisis. 2009;30:115–119. doi: 10.1027/0227-5910.30.3.115. [DOI] [PubMed] [Google Scholar]
- Dunnett S.B., Wareham A.T., Torres E.M. Cholinergic blockade in prefrontal cortex and hippocampus disrupts short-term memory in rats. Neuroreport. 1990;1:61–64. doi: 10.1097/00001756-199009000-00017. [DOI] [PubMed] [Google Scholar]
- Eacott M.J., Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J. Neurosci. official J. Soc. Neurosci. 2004;24:1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Frick K.M., Gresack J.E. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Gater R., Tansella M., Korten A., Tiemens B.G., Mavreas V.G., Olatawura M.O. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Archives general psychiatry. 1998;55:405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L., Telzer E.H., Humphreys K.L., Goff B., Shapiro M., Flannery J., Lumian D.S., Fareri D.S., Caldera C., Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014;25:2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin B.L. Development of sex differences in depressive and co-occurring anxious symptoms during adolescence: descriptive trajectories and potential explanations in a multiwave prospective study. J. Clin. Child. Adolesc. Psychol. 2009;38:460–472. doi: 10.1080/15374410902976288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heun-Johnson H., Levitt P. Early-life stress paradigm transiently alters maternal behavior, dam-pup interactions, and offspring vocalizations in mice. Front. Behav. Neurosci. 2016;10:142. doi: 10.3389/fnbeh.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski S.A., Schreiber W.B., Westbrook S.R., Brennan L.E., Stanton M.E. Determinants of novel object and location recognition during development. Behav. Brain Res. 2013;256:140–150. doi: 10.1016/j.bbr.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita G.P. Psychosocial and cultural contributions to depression in women: considerations for women midlife and beyond. J. Manag. Care Pharm. 2007;13:S12–S15. doi: 10.18553/jmcp.2007.13.9-a.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Klauer T., Filipp S.H., Hellhammer D.H. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr. Scand. 2003;108:163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Lee I., Hunsaker M.R., Kesner R.P. The role of hippocampal subregions in detecting spatial novelty. Behav. Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P.M., Meaney M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu P., Bilkey D.K. The effect of excitotoxic lesions centered on the hippocampus or perirhinal cortex in object recognition and spatial memory tasks. Behav. Neurosci. 2001;115:94–111. doi: 10.1037/0735-7044.115.1.94. [DOI] [PubMed] [Google Scholar]
- Luine V.N., Jacome L.F., Maclusky N.J. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Marco E.M., Valero M., de la Serna O., Aisa B., Borcel E., Ramirez M.J., Viveros M.P. Maternal deprivation effects on brain plasticity and recognition memory in adolescent male and female rats. Neuropharmacology. 2013;68:223–231. doi: 10.1016/j.neuropharm.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Avishai-Eliner S., Baram T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014;56:1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Heins K., Zhuo X., Mei Y.T., Regev L., Baram T.Z., Stern H. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl. Psychiatry. 2016;6:e702. doi: 10.1038/tp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B.R., Bale T.L. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Naninck E.F., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S.E., Lucassen P.J., Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Nazeri M., Shabani M., Ghotbi Ravandi S., Aghaei I., Nozari M., Mazhari S. Psychological or physical prenatal stress differentially affects cognition behaviors. Physiol. Behav. 2015;142:155–160. doi: 10.1016/j.physbeh.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Olino T.M., Klein D.N., Lewinsohn P.M., Rohde P., Seeley J.R. Latent trajectory classes of depressive and anxiety disorders from adolescence to adulthood: descriptions of classes and associations with risk factors. Compr. Psychiatry. 2010;51:224–235. doi: 10.1016/j.comppsych.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C., Poucet B., Save E. Cooperation between the hippocampus and the entorhinal cortex in spatial memory: a disconnection study. Behav. Brain Res. 2006;170:99–109. doi: 10.1016/j.bbr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Pratchett L.C., Pelcovitz M.R., Yehuda R. Trauma and violence: are women the weaker sex? Psychiatr. Clin. North Am. 2010;33:465–474. doi: 10.1016/j.psc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Raineki C., Moriceau S., Sullivan R.M. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol. psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C.J., Sandman C.A., Lenjavi M.R., Baram T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D.C., Wright P.W., Roberts J.C., Reavill C., Rothaul A.L., Hunter A.J. Photothrombotic lesions of the frontal cortex impair the performance of the delayed non-matching to position task by rats. Behav. Brain Res. 1992;49:231–235. doi: 10.1016/s0166-4328(05)80169-x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P., Gutierrez Y.A., Martin A.M., Mallett H.A., Alleva E., Levine S. Maternal regulation of the adrenocortical response in preweanling rats. Physiol. Behav. 1991;50:661–671. doi: 10.1016/0031-9384(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Raineki C., Salstein L., Perry R., Sullivan-Wilson T.A., Sloan A., Lalji B., Hammock E., Wilson D.A., Levitt P., Okutani F., Kaba H., Sullivan R.M. Neurobiology of secure infant attachment and attachment despite adversity: a mouse model. Genes Brain Behav. 2013;12:673–680. doi: 10.1111/gbb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon S., Bejar C., Schorer-Apelbaum D., Weinstock M. Corticosterone mediates some but not other behavioural changes induced by prenatal stress in rats. J. Neuroendocrinol. 2011;23:118–128. doi: 10.1111/j.1365-2826.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- Schulz K.M., Pearson J.N., Neeley E.W., Berger R., Leonard S., Adams C.E., Stevens K.E. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol. Behav. 2011;104:340–347. doi: 10.1016/j.physbeh.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K., Moriceau S., Bradstock P., Sullivan R.M. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm. Behav. 2007;52:391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M.E., Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev. Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Steckler T., Keith A.B., Wiley R.G., Sahgal A. Cholinergic lesions by 192 IgG-saporin and short-term recognition memory: role of the septohippocampal projection. Neuroscience. 1995;66:101–114. doi: 10.1016/0306-4522(94)00603-3. [DOI] [PubMed] [Google Scholar]
- Suchecki D., Mozaffarian D., Gross G., Rosenfeld P., Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- Taylor S.E., Burklund L.J., Eisenberger N.I., Lehman B.J., Hilmert C.J., Lieberman M.D. Neural bases of moderation of cortisol stress responses by psychosocial resources. J. Pers. Soc. Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Tomoda A., Andersen S.L. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann. N. Y. Acad. Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Torres E.M., Perry T.A., Blockland A., Wilkinson L.S., Wiley R.G., Lappi D.A., Dunnet S.B. Behavioural, histochemical and biochemical consequences of selective immunolesions in discrete regions of the basal forebrain cholinergic system. Neuroscience. 1994;63:95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Uysal N., Sisman A.R., Dayi A., Ozbal S., Cetin F., Baykara B., Aksu I., Tas A., Cavus S.A., Gonenc-Arda S., Buyuk E. Acute footshock-stress increases spatial learning-memory and correlates to increased hippocampal BDNF and VEGF and cell numbers in adolescent male and female rats. Neurosci. Lett. 2012;514:141–146. doi: 10.1016/j.neulet.2012.02.049. [DOI] [PubMed] [Google Scholar]
- van Rooij S.J., Cross D., Stevens J.S., Vance L.A., Kim Y.J., Bradley B., Tottenham N., Jovanovic T. Maternal buffering of fear-potentiated startle in children and adolescents with trauma exposure. Soc. Neurosci. 2016:1–10. doi: 10.1080/17470919.2016.1164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Jiao J., Dulawa S.C. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacol. Berl. 2011;216:207–218. doi: 10.1007/s00213-011-2209-4. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ma Y., Hu J., Zhang X., Cheng W., Jiang H., Li M., Ren J., Zhang X., Liu M., Sun A., Wang Q., Li X. Sex-specific effects of prenatal chronic mild stress on adult spatial learning capacity and regional glutamate receptor expression profiles. Exp. Neurol. 2016;281:66–80. doi: 10.1016/j.expneurol.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Weible A.P., Rowland D.C., Pang R., Kentros C. Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. J. Neurophysiol. 2009;102:2055–2068. doi: 10.1152/jn.00214.2009. [DOI] [PubMed] [Google Scholar]
- Weissman M.M., Bland R.C., Canino G.J., Faravelli C., Greenwald S., Hwu H.G., Joyce P.R., Karam E.G., Lee C.K., Lellouch J., Lepine J.P., Newman S.C., Rubio-Stipec M., Wells J.E., Wickramaratne P.J., Wittchen H., Yeh E.K. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Westbrook S.R., Brennan L.E., Stanton M.E. Ontogeny of object versus location recognition in the rat: acquisition and retention effects. Dev. Psychobiol. 2014;56:1492–1506. doi: 10.1002/dev.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig K.A., Burwell R.D. Memory impairment on a delayed non-matching-to-position task after lesions of the perirhinal cortex in the rat. Behav. Neurosci. 1998;112:827–838. doi: 10.1037//0735-7044.112.4.827. [DOI] [PubMed] [Google Scholar]
- Zuena A.R., Mairesse J., Casolini P., Cinque C., Alema G.S., Morley-Fletcher S., Chiodi V., Spagnoli L.G., Gradini R., Catalani A., Nicoletti F., Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One. 2008;3:e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.