Abstract
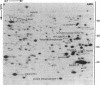
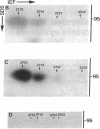
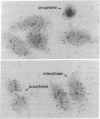
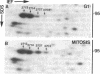
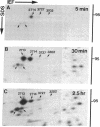
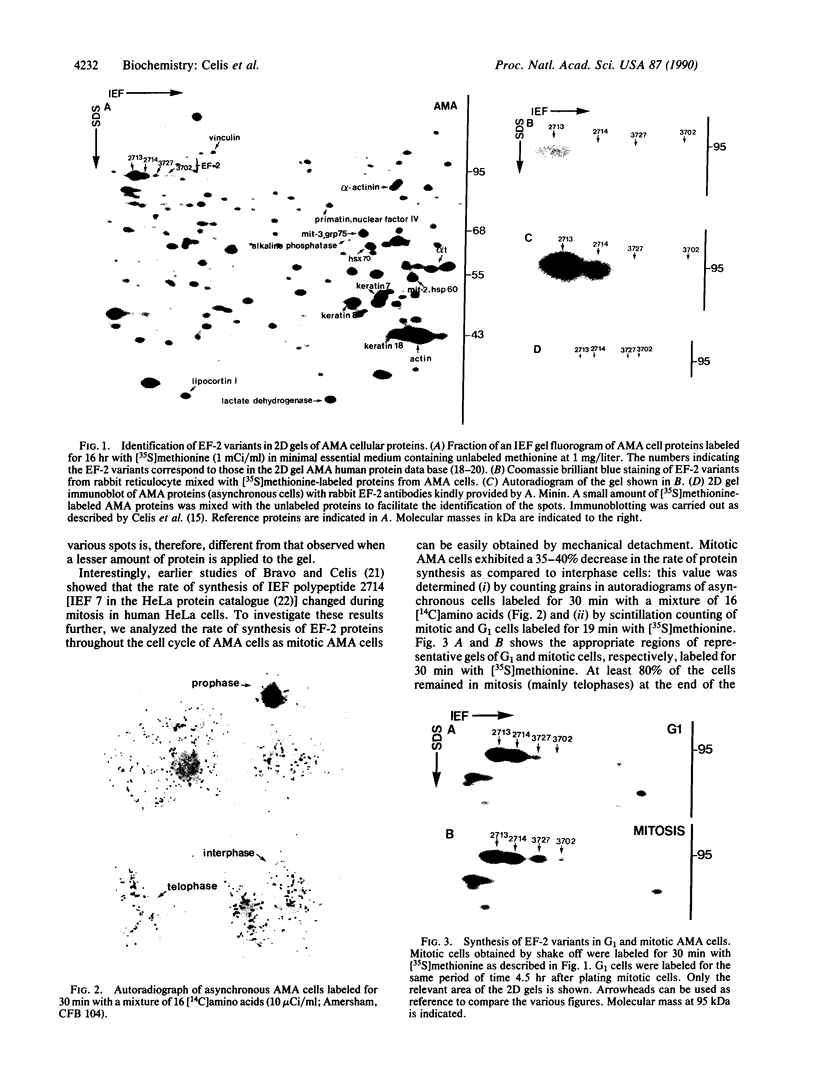
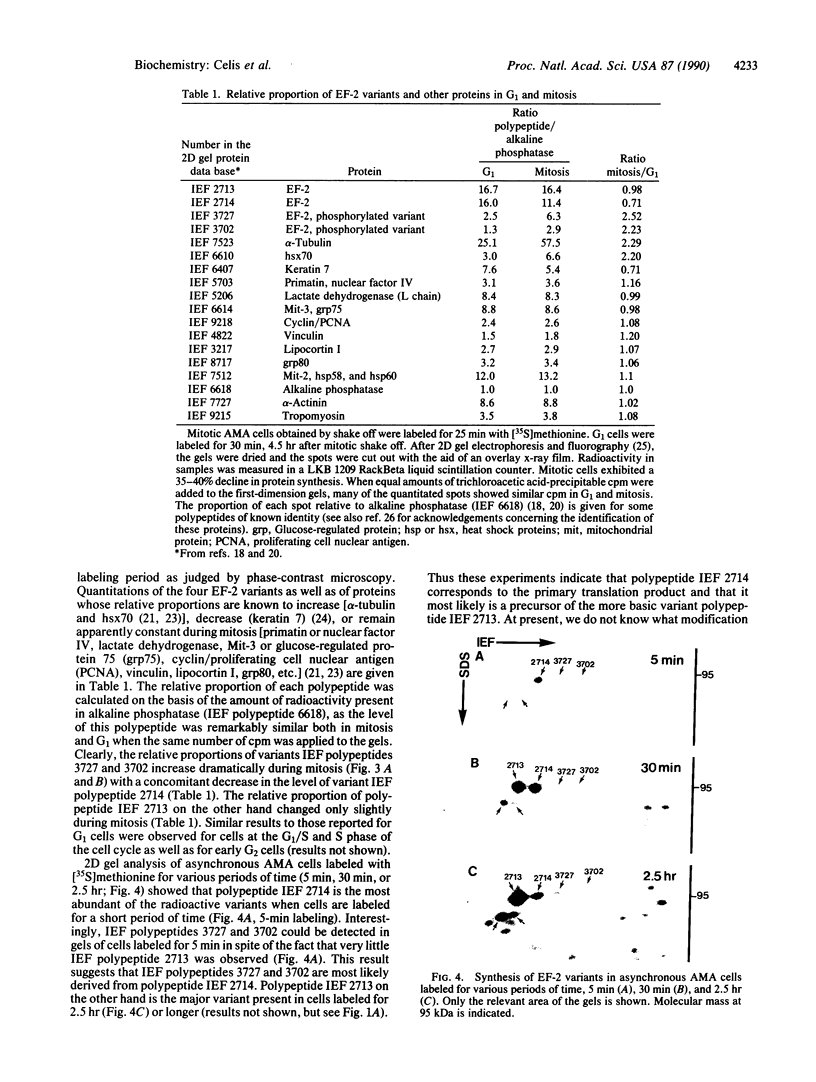
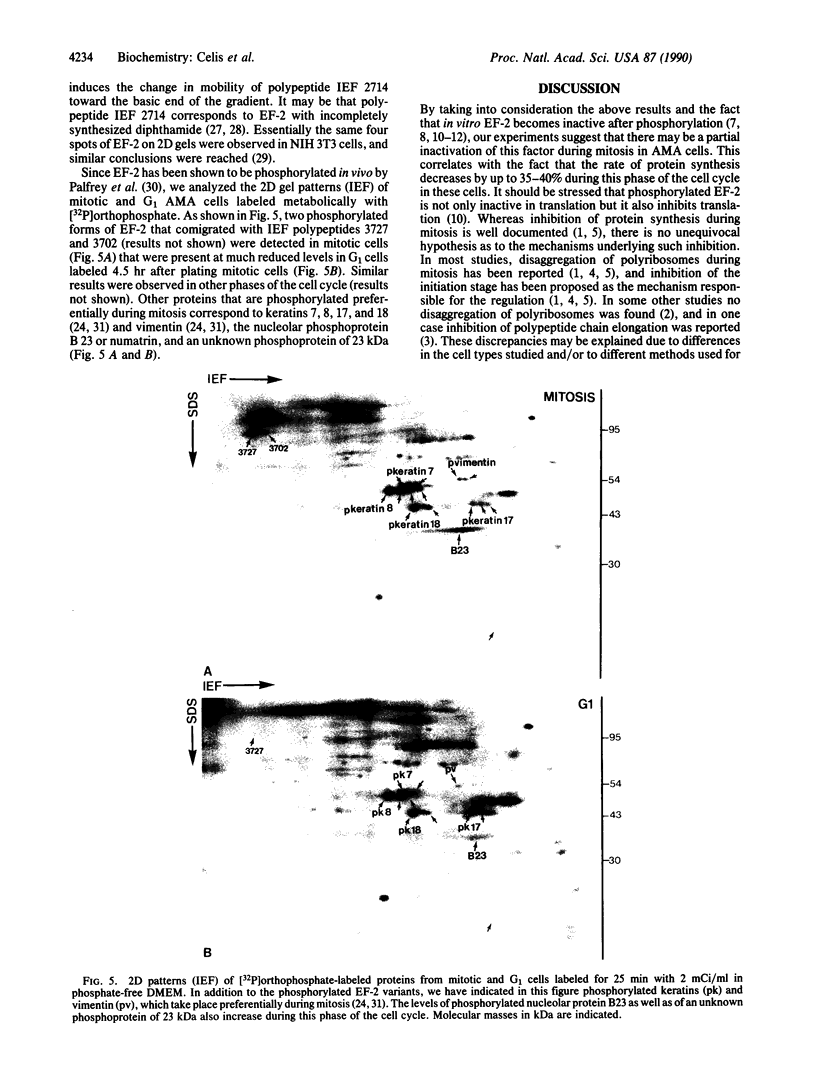
Elongation factor 2 was identified in the two-dimensional gel patterns of asynchronous human amnion cells (AMA) by comigration with purified rabbit reticulocyte elongation factor 2 and by two-dimensional gel immunoblot analysis using a specific rabbit polyclonal antibody. In all, four polypeptides were identified corresponding to isoelectric focusing polypeptides 2713 (95.0 kDa), 2714 (94.8 kDa), 3727 (94.8 kDa), and 3702 (93.6 kDa) (listed in order of decreasing pI values) in the computerized comprehensive two-dimensional gel data base of human AMA proteins. The relative proportion of two of these variants (isoelectric focusing polypeptides 3727 and 3702), which are phosphorylated, increased dramatically during mitosis. Since phosphorylation is known to render elongation factor 2 inactive in translation, this observation may partly explain the decline in the rate of protein synthesis observed during cell division.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonneau A. M., Sonenberg N. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. J Biol Chem. 1987 Aug 15;262(23):11134–11139. [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Up-dated catalogue of HeLa cell proteins: percentages and characteristics of the major cell polypeptides labeled with a mixture of 16 14C-labeled amino acids. Clin Chem. 1982 Apr;28(4 Pt 2):766–781. [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Larsen P. M., Fey S. J., Celis A. Phosphorylation of keratin and vimentin polypeptides in normal and transformed mitotic human epithelial amnion cells: behavior of keratin and vimentin filaments during mitosis. J Cell Biol. 1983 Nov;97(5 Pt 1):1429–1434. doi: 10.1083/jcb.97.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Lauridsen J. B., Basse B. Cell cycle-associated change in the expression of the proliferation-sensitive and heat-shock protein hs x 70 (IEF14): increased synthesis during mitosis. Exp Cell Res. 1988 Jul;177(1):176–185. doi: 10.1016/0014-4827(88)90035-3. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Ratz G. P., Celis A., Madsen P., Gesser B., Kwee S., Madsen P. S., Nielsen H. V., Yde H., Lauridsen J. B. Towards establishing comprehensive databases of cellular proteins from transformed human epithelial amnion cells (AMA) and normal peripheral blood mononuclear cells. Leukemia. 1988 Sep;2(9):561–601. [PubMed] [Google Scholar]
- Celis J. E., Ratz G. P., Madsen P., Gesser B., Lauridsen J. B., Hansen K. P., Kwee S., Rasmussen H. H., Nielsen H. V., Crüger D. Computerized, comprehensive databases of cellular and secreted proteins from normal human embryonic lung MRC-5 fibroblasts: identification of transformation and/or proliferation sensitive proteins. Electrophoresis. 1989 Feb;10(2):76–115. doi: 10.1002/elps.1150100204. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Ratz G. P., Madsen P., Gesser B., Lauridsen J. B., Kwee S., Rasmussen H. H., Nielsen H. V., Crüger D., Basse B. Comprehensive, human cellular protein databases and their implication for the study of genome organization and function. FEBS Lett. 1989 Feb 27;244(2):247–254. doi: 10.1016/0014-5793(89)80538-1. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Bodley J. W. Biosynthesis of diphthamide in Saccharomyces cerevisiae. Partial purification and characterization of a specific S-adenosylmethionine:elongation factor 2 methyltransferase. J Biol Chem. 1988 Aug 25;263(24):11692–11696. [PubMed] [Google Scholar]
- Eremenko T., Volpe P. Polysome translational state during the cell cycle. Eur J Biochem. 1975 Mar 17;52(2):203–210. doi: 10.1111/j.1432-1033.1975.tb03988.x. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Levenson R. M., Nairn A. C., Blackshear P. J. Insulin rapidly induces the biosynthesis of elongation factor 2. J Biol Chem. 1989 Jul 15;264(20):11904–11911. [PubMed] [Google Scholar]
- Mackie K. P., Nairn A. C., Hampel G., Lam G., Jaffe E. A. Thrombin and histamine stimulate the phosphorylation of elongation factor 2 in human umbilical vein endothelial cells. J Biol Chem. 1989 Jan 25;264(3):1748–1753. [PubMed] [Google Scholar]
- Nairn A. C., Nichols R. A., Brady M. J., Palfrey H. C. Nerve growth factor treatment or cAMP elevation reduces Ca2+/calmodulin-dependent protein kinase III activity in PC12 cells. J Biol Chem. 1987 Oct 15;262(29):14265–14272. [PubMed] [Google Scholar]
- Nairn A. C., Palfrey H. C. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987 Dec 25;262(36):17299–17303. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palfrey H. C., Nairn A. C., Muldoon L. L., Villereal M. L. Rapid activation of calmodulin-dependent protein kinase III in mitogen-stimulated human fibroblasts. Correlation with intracellular Ca2+ transients. J Biol Chem. 1987 Jul 15;262(20):9785–9792. [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. The tumour promoter okadaic acid inhibits reticulocyte-lysate protein synthesis by increasing the net phosphorylation of elongation factor 2. Biochem J. 1989 Aug 15;262(1):69–75. doi: 10.1042/bj2620069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanov A. G. Ca2+/calmodulin-dependent phosphorylation of elongation factor 2. FEBS Lett. 1987 Apr 20;214(2):331–334. doi: 10.1016/0014-5793(87)80081-9. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Natapov P. G., Shestakova E. A., Severin F. F., Spirin A. S. Phosphorylation of the elongation factor 2: the fifth Ca2+/calmodulin-dependent system of protein phosphorylation. Biochimie. 1988 May;70(5):619–626. doi: 10.1016/0300-9084(88)90245-3. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Shestakova E. A., Natapov P. G. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988 Jul 14;334(6178):170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Shestakova E. A., Riazanov A. G. Vliianie fosforilirovaniia faktora élongatsii 2 na ego aktivnost' v beskletochnoi sisteme transliatsii. Dokl Akad Nauk SSSR. 1987;297(6):1495–1498. [PubMed] [Google Scholar]
- Sitikov A. S., Simonenko P. N., Shestakova E. A., Ryazanov A. G., Ovchinnikov L. P. cAMP-dependent activation of protein synthesis correlates with dephosphorylation of elongation factor 2. FEBS Lett. 1988 Feb 15;228(2):327–331. doi: 10.1016/0014-5793(88)80025-5. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Agol V. I. Translational barrier in central region of encephalomyocarditis virus genome. Modulation by elongation factor 2 (eEF-2). Eur J Biochem. 1983 Jun 1;133(1):145–154. doi: 10.1111/j.1432-1033.1983.tb07440.x. [DOI] [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res. 1963 Apr;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]
- Tarnowka M. A., Baglioni C. Regulation of protein synthesis in mitotic HeLa cells. J Cell Physiol. 1979 Jun;99(3):359–367. doi: 10.1002/jcp.1040990311. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Howard J. B., Bodley J. W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem. 1980 Nov 25;255(22):10710–10716. [PubMed] [Google Scholar]