Abstract
Two novel biscembranoids, sarelengans A and B (1 and 2), five new cembranoids, sarelengans C–G (3–7), along with two known cembranoids (8 and 9) were isolated from the South China Sea soft coral Sarcophyton elegans. Their structures were determined by spectroscopic and chemical methods, and those of 1, 4, 5, and 6 were confirmed by single crystal X-ray diffraction. Compounds 1 and 2 represent the first example of biscembranoids featuring a trans-fused A/B-ring conjunction between the two cembranoid units. Their unique structures may shed light on an unusual biosynthetic pathway involving a cembranoid-∆8 rather than the normal cembranoid-∆1 unit in the endo-Diels-Alder cycloaddition. Compounds 2 and 3 exhibited potential inhibitory effects on nitric oxide production in RAW 264.7 macrophages, with IC50 values being at 18.2 and 32.5 μM, respectively.
Keywords: Sarcophyton elegans, biscembranoids, cembranoids, NO inhibition
1. Introduction
Biscembranoids are a group of unique tetraterpenoids exclusively occurring in the soft corals of the genera of Sarcophyton, Sinularia, and Lobophytum (family Alcyoniidae) [1,2,3,4,5]. Biosynthetically they are proposed to be derived from the Diels-Alder cycloaddition between two cembranoid monomers, forming a 14/6/14 (A/B/C) carbon ring system [6,7,8,9,10]. Since the first biscembranoid named methyl isosartortuoate was reported from the Chinese soft coral Sarcophyton tortuosum [1], more than 80 biscembranoids have been isolated so far [11]. The structural diversity of these biscembranoids mainly arise from the oxygenation and cyclization of the cembranoid-diene part (ring C), with maintaining of the basic structural features of cembranoid-dienophile moiety (ring A). Due to their significant biological activities (e.g., cytotoxic [2] and antibacterial [8] properties) and appealing architectures, biscembranoids have attracted broad interests from both natural product [12,13] and synthetic chemists [14,15] over the last decades.
Sarcophyton elegans is a common soft coral species found on the sea shore of the South China Sea. Previous chemical investigations of S. elegans have led to the isolation of several cembranoids, tetracyclic diterpenoids, steroids, and biscembranoids, some of which showed antibacterial and cytotoxic activities [8,16,17,18]. In our early work aiming at the discovery of antitumor agents from this species, two cembranoids with anti-tumor cell migration properties were isolated [19]. In our screening program aimed at the discovery of novel nitric oxide (NO) inhibitors from natural resources [20,21], the EtOAc fraction of the ethanolic extract of S. elegans showed a certain inhibitory activity against the lipopolysaccharide (LPS)-induced NO production in RAW 264.7 macrophages. Subsequent chemical investigation led to the isolation of two new biscembranoids (1 and 2), five new cembranoids (3–7), and two known compounds (8 and 9). Compounds 1 and 2 represent the first example of A/B ring trans-fused biscembranoids. Bioassay verified that compounds 2 and 3 were responsible for the NO inhibitory activities of the EtOAc fraction, with IC50 values being 18.2 and 32.5 μM, respectively. Herein, details of the isolation, structural elucidation, and NO inhibitory activities of these compounds are described.
2. Results and Discussion
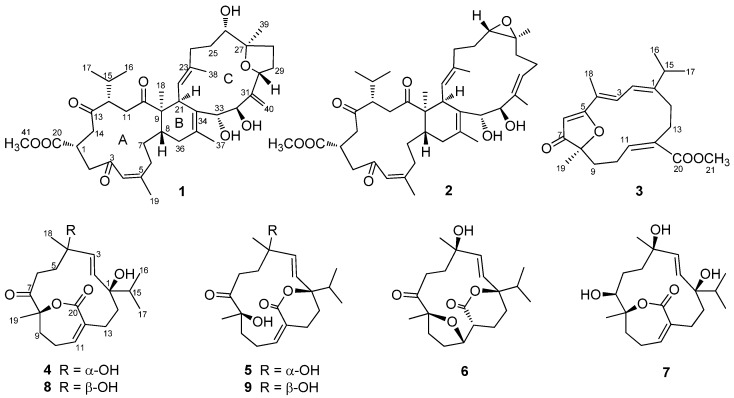
The frozen sample of S. elegans was chopped and exhaustively extracted with 95% EtOH at room temperature (rt). After removal of solvent in vacuo, the residue was suspended in H2O and then partitioned sequentially with petroleum ether (PE) and EtOAc. Various column chromatographic separations of the EtOAc extract afforded compounds 1–9 (Figure 1).
Figure 1.
Structures of compounds 1–9.
Compound 1, a colorless crystal, had the molecular formula C41H60O9, as established by HRESIMS at m/z 719.4121 [M + Na]+ (calcd. 719.4130), corresponding to 12 degrees of unsaturation (DOUs). The IR absorption bands at 3408 and 1710 cm−1 indicated the presence of the hydroxyl and carbonyl groups, respectively. Detailed analysis of the 1H-NMR revealed the presence of seven methyl groups [δH 0.92 (3H × 2, d, J = 6.7 Hz), 1.07 (3H, s), 1.08 (3H, s), 1.54 (3H, s), 1.70 (3H, s), and 1.86 (3H, d, J = 1.2 Hz)], one methoxy group [δH 3.63 (3H, s)], four oxymethine protons [δH 3.47 (1H, m), 3.89 (1H, dd, J = 10.2, 4.2 Hz), 4.07 (1H, d, J = 9.7 Hz), and 4.39 (1H, d, J = 9.7 Hz)], a terminal double bond [δH 5.21 (1H, s) and 5.47 (1H, s)], two olefinic protons [δH 4.88 (1H, d, J = 10.6 Hz) and 5.96 (1H, d, J = 1.2 Hz)], and a series of aliphatic methylene or methine multiplets. The 13C-NMR spectrum, in combination with DEPT experiments, resolved 41 carbon resonances attributable to three ketone groups (δC 215.5, 215.4 and 203.4), a methyl ester group (δC 176.3 and 52.3), a terminal double bond (δC 151.6 and 112.8), two trisubstituted double bonds (δC 154.1, 136.9, 128.2, and 127.4), a tetrasubstituted double bond (δC 132.7 and 130.2), two sp3 quaternary carbons (one oxygenated), nine sp3 methines (four oxygenated), 10 sp3 methylenes, and seven methyls. As eight of the 12 DOUs were accounted for by three ketones, an ester carbonyl group, and four double bonds, the remaining DOUs required that 1 was tetracyclic. The aforementioned data are characteristic of a biscembranoid, closely related to those reported in the literature [5,12,22].
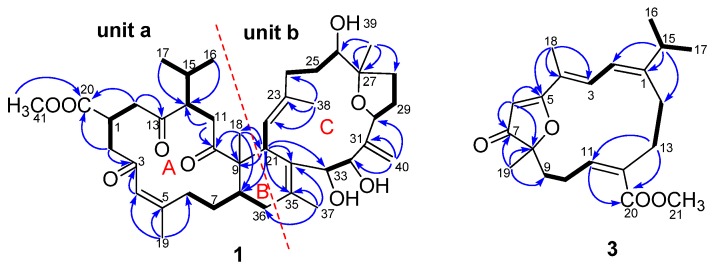
Detailed 2D NMR studies (HSQC, 1H–1H COSY, and HMBC experiments) further confirmed the presence of two highly oxygenated cembranoid units (a and b) in 1 (Figure 2). Three fragments, C-2−C-1−C-14, C-6−C-7−C-8, and C-11−C-12−C-15−C-16 (C-17), were first established in unit a by the 1H–1H COSY correlations. The connectivities of these fragments, three ketones, one double bond, one quaternary carbon, and one methyl ester were achieved by HMBC correlations of H3-18/C-8, C-9, and C-10, H3-19/C-4, C-5, and C-6, H2-2(H-4)/C-3, H2-11/C-10, and C-12, and H2-14/C-13, and C-20, which generated a methyl sarcoate moiety (ring A) commonly found in biscembranoids [23].
Figure 2.
1H−1H COSY (correlation spectroscopy) ( ) and key HMBC (heteronuclear multiple-bond correlation spectroscopy) (
) and key HMBC (heteronuclear multiple-bond correlation spectroscopy) ( ) correlations of compounds 1 and 3.
) correlations of compounds 1 and 3.
As for unit b, four spin systems of C-21–C-22, C-24–C-25–C-26, C-28–C-29–C-30, and C-32–C-33 were readily recognized from the 1H–1H COSY spectrum. The connections of these fragments, three double bonds, and one sp3 quaternary carbon were achieved by HMBC correlations, generating the framework of a 14-membered carbon ring C. Although no HMBC correlation was observed between H-30 and C-27, the presence of the oxygen bridge between C-30 and C-27 were deduced by comparison of its 13C-NMR data with those of bisglaucumlide H [24] bearing the similar tetrahydrofuran rings, as well as on consideration of the unsaturation degrees of the molecule. The connection of units a and b was finally achieved by HMBC correlations of H3-18/C-8, C-9, and C-21, H-21/C-8, C-9, C-18, and C-35, and H3-37/C-34, C-35, and C-36, which formed a six-membered carbon ring B bridging these two parts.
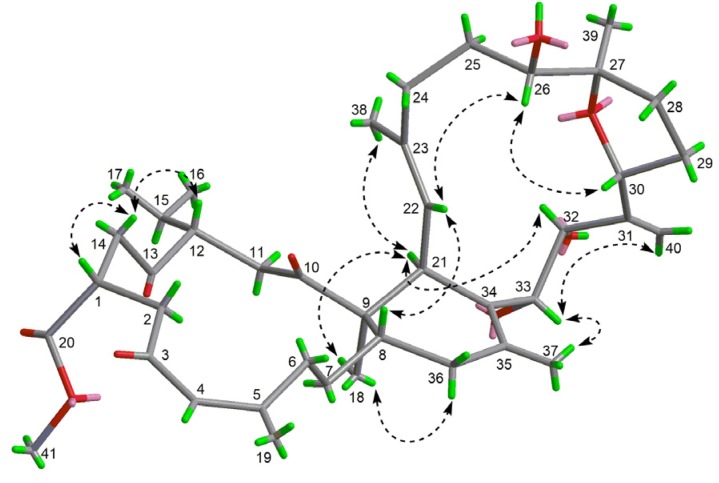
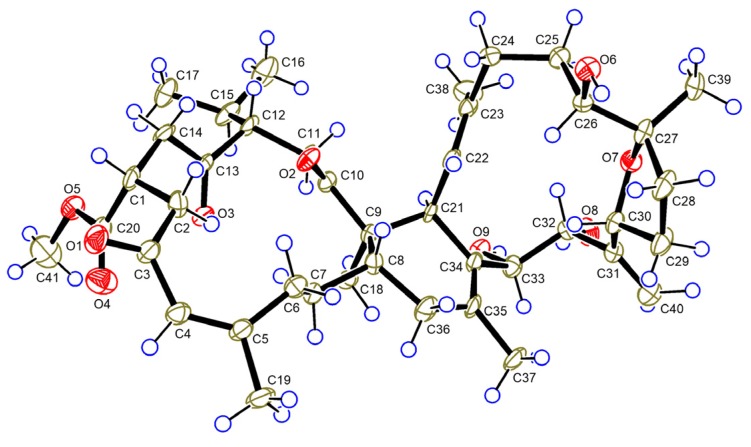
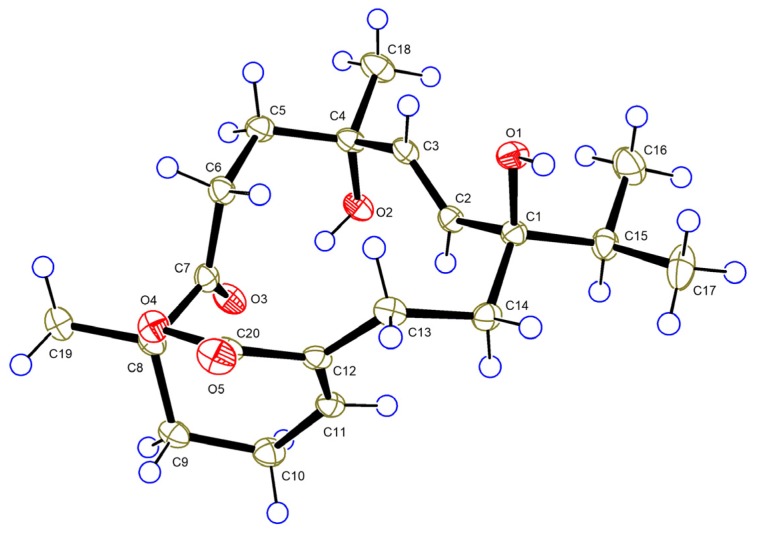
The relative configuration of 1 was established based on a NOESY experiment and analysis of coupling constants. The strong NOE interactions of H-8/H-22 and H3-18/H2-36b indicated that H-8 and CH3-18 occupied the axial bonds of ring B in a chair conformation (Figure 3). Thus, ring A and B were trans-fused. The NOE correlations of H-14a/H-1 and H-12 indicated that these protons were cofacial on ring A and were arbitrarily assigned as β-orientation. The NOE correlations of H-22/H-8 and H-26 and H-26/H-30 suggested that these protons were cofacial on ring C and were assigned as β-orientation, while correlations of H-21/H3-18 and H-32 suggested that these protons were α-oriented. The trans-relationship of H-33 and H-32 was implied by the large coupling constant between these two protons (J = 9.7 Hz) [25]. Finally, the structure of 1 including the absolute configuration was confirmed by a single crystal X-ray crystallographic analysis using anomalous scattering of Cu Kα radiation, Flack parameter = 0.04 (13) (Figure 4). Thus, compound 1 was given a trivial name sarelengan A.
Figure 3.
Key NOE (nuclear overhauser effect) correlations of 1.
Figure 4.
ORTEP diagram of 1.
Compound 2, a colorless oil, had the molecular formula C41H60O8, as determined by HRESIMS ion at m/z 703.4193 [M + Na]+ (calcd. 703.4180). The 1D NMR spectra of 2 exhibited most of the structural features found in 1, with major difference being the absence of a tetrahydrofuran ring and the replacement of a terminal double bond by a trisubstituted double bond in 2. The relatively upfield-shifted carbon signals of an oxymethine (δC 64.0, C-26) and a quaternary carbon (δC 61.2, C-27) suggested the presence of an epoxy ring in 2 [26]. The epoxy ring was located at C-26 and C-27 by HMBC correlations of H3-39/C-26 and C-27, as well as 1H–1H COSY correlation of H2-25/H-26. The trisubstituted double bond was located at C-30 and C-31 by HMBC correlations from H3-40 to C-30 and C-31, and 1H–1H COSY correlation of H2-29/H-30. The planar structure of 2 was further confirmed by detailed analyses of its 2D NMR data. The configuration of 2 was assigned to be the same as that of 1 by comparison of their NOESY and 13C-NMR data. In particular, the NOESY correlations of H-8/H-22 and H3-18/H-36b confirmed the trans-fused A/B ring, while the trans-relationship of H-32 and H-33 was assigned by NOE correlations of H-33/H3-37 and H-32/H-21, as well as the large coupling constant (J = 10.0 Hz) between H-32 and H-33. The NOE correlations of H-22/H-8 and H-26/H-22 and H-28b indicated that H-26 was β-oriented and in a trans-position of CH3-39 on the epoxy ring (S56, Supplementary Materials), which was further confirmed by comparison of the NMR data of C-26 and C-27 in 2 with those of known analogue sarcophytolide H [13]. The E geometry of Δ30 was assigned by NOE correlation of H-30/H-32. Thus, compound 2 was determined as depicted and was given the trivial name sarelengan B.
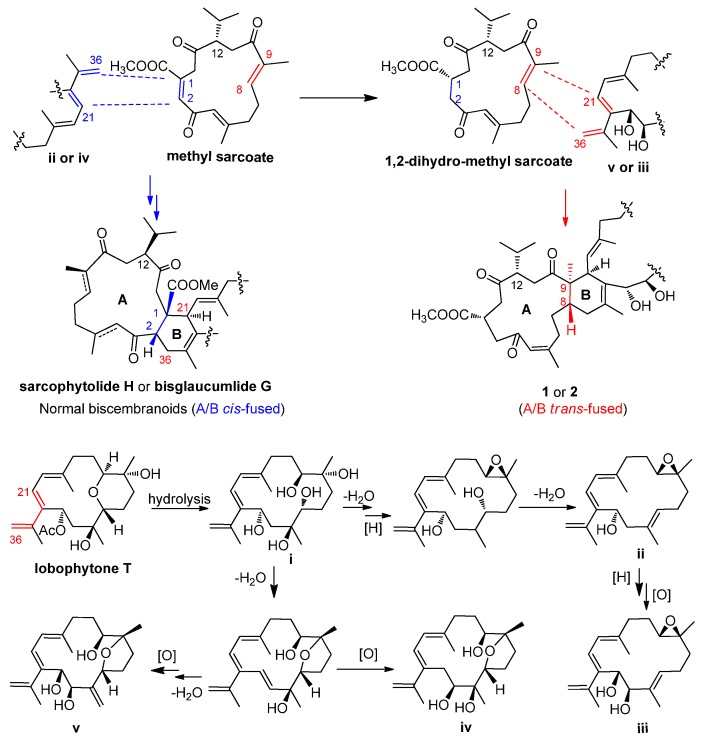
It is worth noting that compounds 1 and 2 possessed a trans-fused A/B ring system, differing from those of the previously reported biscembranoids [8,10], suggesting that the coupling between two monemeric cembranoids in 1 and 2 may involve an unusual biosynthetic pathway. As shown in Scheme 1, the well-known soft coral metabolite, methyl sarcoate, was considered as the cembranoid-dienophile precursor, which underwent biohydrogenation to produce a dienophile intermediate 1,2-dihydro-methyl sarcoate. Lobophytone T isolated from Lobophytum pauciflorum [22], was served as the cembranoid-diene precursor, which underwent hydrolysis to generate intermediate i. Dehydration of i followed by a series redox reactions generated the epoxy intermediates (ii and iii) and the tetrahydrofuran-containing intermidiates (iv and v), respectively. The endo-Diels-Alder cycloaddition between methyl sarcoate-Δ1 and ii- or iv-Δ21(34), Δ35 generates the “normal biscembranoids” (A/B ring cis-fused), sarcophytolide H [13] or bisglaucumlide G [24], respectively, while the endo-Diels-Alder cycloaddition between methyl 1,2-dihydro-methyl sarcoate -Δ8 and v- or iii-Δ21(34), Δ35 produces the A/B ring trans-fused scaffold of 1 or 2, respectively.
Scheme 1.
Proposed biogenesis of compounds 1 and 2.
Compound 3, a colorless oil, had the molecular formula C21H28O4 as determined by HRESIMS at m/z 367.1871 [M + Na]+ (calcd. 367.1880), implying eight DOUs. The 1H-NMR data of 3 showed two methyl groups [δH 1.43 (3H, s) and 1.96 (3H, d, J = 1.1 Hz)], an isopropyl group [δH 1.12 (3H × 2, d, J = 6.8 Hz), 2.52 (1H, m)], four olefinic protons [δH 5.64 (1H, s), 5.99 (1H, t, J = 6.4 Hz), 6.29 (1H, d, J = 12.1 Hz), and 7.42 (1H, d, J = 12.1, 1.1 Hz)], and a series of aliphatic methylene or methine multiplets. The 13C-NMR spectrum, in combination with DEPT experiments, resolved 21 carbon resonances attributable to one ketone group (δC 209.6), a methyl ester group (δC 169.2 and 51.5), four trisubstituted double bonds (δC 187.7, 164.1, 152.1, 136.6, 130.1, 121.7, 119.3, and 100.4), one sp3 oxygenated quaternary carbon, one sp3 methine, four sp3 methylenes and four methyls. Aforementioned information indicated that 3 possessed structural features of a cembranoid. The gross structure of 3 was further established by analysis of its 2D NMR data (Figure 2). In the 1H–1H COSY spectrum four partial structures, C-2–C-3, C-9–C-10–C-11, C-13–C-14, and C-16–C-15–C-17 were established by a series of 1H–1H COSY correlation (Figure 2). The connectivities of the four structural fragments, the double bonds, the methyls, and the methyl ester were achieved by analysis of the HMBC correlations. In particular, HMBC correlations from H-15 to C-1, C-2, and C-14, from H3-18 to C-3, C-4, and C-5, from H3-19 to C-7, C-8, and C-9, from H-6 to C-5, C-7, and C-8, and from H2-13 to C-11 and C-20 constructed a 14-membered carbon ring. As the above-mentioned structure elucidation already accounted for seven of the eight DOUs, the remaining one DOU required the presence of an additional ring. The severely down-field shifted carbon signals of C-5 (δC 187.7) and C-8 (δC 91.3) implied the presence of an ether bond between C-8 and C-5 to form a furan-3(2H)-one ring. This was further supported by comparison of its 13C-NMR data with those of synthetic 2,2,5-trimethyl-3(2H)-furanone [27]. The configuration of C-8 was tentatively assigned as S based on the biogenetic consideration of this compound class, while the 1E, 3E, 5Z, 11Z configurations were determined by NOESY correlations of H-2/H3-16, H-2/H3-18, H-6/H3-18, and H-11/H2-13, respectively. Thus, compound 3 was determined as shown in Figure 1 and was given the trivial name sarelengan C.
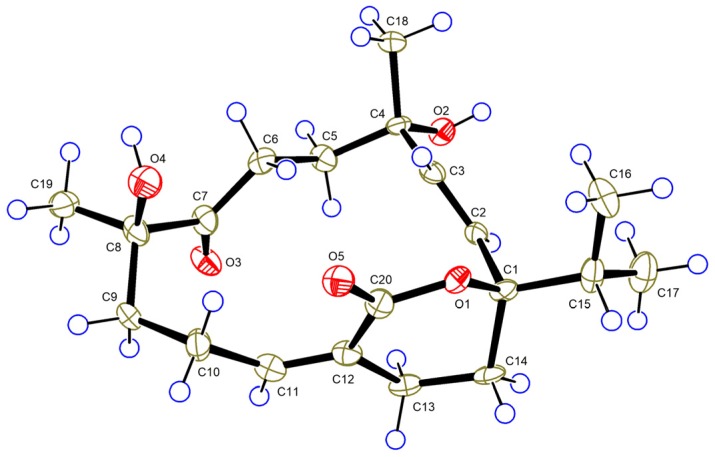
Compound 4, a colorless crystal, had the molecular formula C20H30O5, as established by HRESIMS. The NMR data of 4 were very similar to those of sartrolide E (8) [28], with slight differences arising from the carbon signals of C-5 (δC 34.3 in 4; δC 32.8 in 8) and C-18 (δC 31.4 in 4; δC 29.9 in 8), indicating that 4 was a C-4 epimer of 8. Detailed 2D analysis further supported that they shared the same planar structure. The structure of 4 including the absolute configuration (1R, 4S, and 8R) was secured by a single crystal X-ray crystallographic analysis using anomalous scattering of Cu Kα radiation, Flack parameter = 0.08 (10) (Figure 5). Thus, compound 4 was given the trivial name sarelengan D.
Figure 5.
ORTEP diagram of 4.
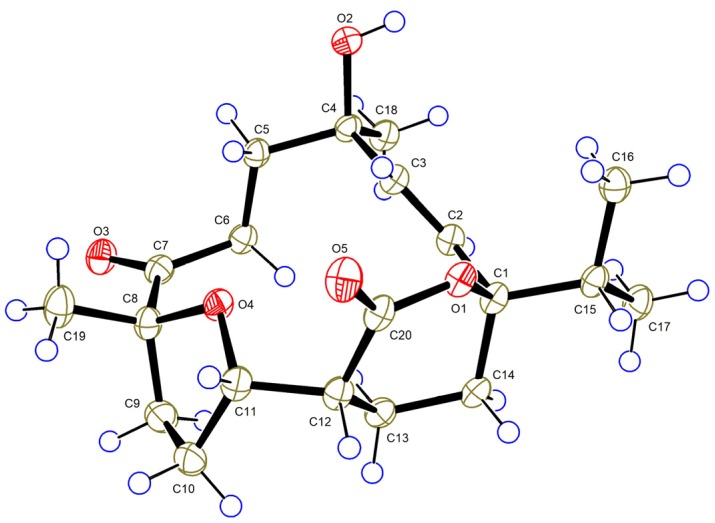
Compound 5, a colorless crystal, gave a [M + Na]+ ion at m/z 373.1984 in the HRESIMS corresponding to the molecular formula C20H30O5. The 1D NMR spectra of 5 showed high similarity to those of sarcophelegan B (9) [19], except for the slight difference of carbon signals of C-5 (δC 38.1 in 5; δC 36.5 in 9) and C-18 (δC 26.9 in 5; δC 29.3 in 9), indicating that 5 was a C-4 epimer of 9. Detailed 2D analysis further supported that they shared the same planar structure. The structure of 5 including the absolute configuration (1R, 4S, and 8R) was secured by a single crystal X-ray crystallographic analysis using anomalous scattering of Cu Kα radiation, Flack parameter = −0.01 (10) (Figure 6). Thus, compound 5 was given the trivial name sarelengan E.
Figure 6.
ORTEP diagram of 5.
Compound 6, a colorless crystal, exhibited a molecular formula of C20H30O5 as determined by HRESIMS. The 1H and 13C-NMR data of 6 showed high similarity to those of sarcophelegan B (9) [19], except for the replacement of the Δ11 in 9 by an oxymethine (δH 4.87; δC 80.8) and a methine (δH 2.69; δC 44.1) in 6. Since 6 possessed the same DOUs as that of 9, the formation of an oxygen bridge between C-8 and the oxymethine (C-11) was suggested. This was supported by the downfield-shifted carbonyl at C-20 (δC 175.3 in 6; δC 168.8 in 9) and C-8 (δC 90.0 in 6; δC 79.7 in 9), although no direct HMBC correlation from H-11 to C-8 was available. The structure of 6 was further confirmed by converting 9 to 6 in a basic condition and the absolute configuration (1S, 4R, 8R, 11S, and 12R) was assigned by a single crystal X-ray diffraction analysis with Flack parameter = −0.05 (13) (Figure 7). Thus, compound 6 was given a trivial name sarelengan F.
Figure 7.
ORTEP diagram of 6.
Compound 7 had a molecular formula of C20H32O5 as established by HRESIMS data. The 1D NMR data of 7 were very similar to those of sartrolide D [28], a known cembranoid isolated from Sarcophyton trocheliophorum, with the only difference being due to the chemical shift of C-7 (δC 66.1 in 7; δC 72.7 in sartrolide D), indicating that 7 was a C-7 epimer of sartrolide D. Detailed 2D analysis further supported that they shared the same planar structure. The β-orientation of 7-OH was supported by the NOE correlations of H-2/H-7 and H-15. Thus, compound 7 was given the trivial name sarelengan G.
The known compounds, sartrolide E (8) [28] and sarcophelegan B (9) [19], were identified by comparison of their NMR data and optical rotation values with those reported in the literature.
All compounds were evaluated for their inhibitory effects on the NO production in LPS-induced RAW 264.7 macrophages using the Griess assay, and quercetin was used as the positive control (IC50 = 20.0 μM). Compounds 2 and 3 showed moderate inhibitory activities with IC50 values being at 18.2 and 32.5 μM, respectively, while other compounds were inactive (inhibition <50% at 50 μM). To investigate whether the NO inhibition was caused by the cytotoxicity, the effects of compounds 2 and 3 on LPS-induced RAW 264.7 macrophages viability were measured using the MTT method. The results showed that 2 and 3 (up to 80 μM) had no significant cytotoxicity towards the cells in 24 h incubation.
3. Materials and Methods
3.1. General Experimental Procedures
X-ray data were collected using an Agilent Xcalibur Nova X-ray diffractometer (Agilent, Santa Clara, CA, USA). Melting points were measured on an X-4 melting instrument (SRI International, Silicon Valley, CA, USA) and are uncorrected. Optical rotations were measured on a Rudolph Autopol I automatic polarimeter (Perkin-Elmer, Waltham, MA, USA), UV spectra on a Shimadzu UV-2450 spectrophotometer (Shimadzu, Kyoto, Japan), and IR spectra were determined on a Bruker Tensor 37 infrared spectrophotometer (Bruker, Karlsruhe, Germany). NMR spectra were measured on a Bruker AM-400 spectrometer (Bruker, Karlsruhe, Germany) at 25 °C. ESIMS was measured on a Finnigan LCQ Decainstrument (Thermo Finnigan, San Jose, CA, USA), and HRESIMS were performed on a Waters Micromass Q-TOF spectrometer (Waters, Milford, MA, USA). A Shimadzu LC-20 AT equipped with an SPD-M20A PDA detector (Shimadzu, Kyoto, Japan) was used for HPLC. A YMC-pack ODS-A column (250 × 10 mm, S-5 μm, 12 nm) (YMC, Tokyo, Japan) were used for semi-preparative HPLC separation. Silica gel (300–400 mesh, Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), C18 reversed-phase (RP-C18) silica gel (12 nm, S-50 μm, YMC Co., Ltd., Kyoto, Japan), and Sephadex LH-20 gel (Amersham Biosciences, Piscataway, NJ, USA) were used for column chromatography (CC). All solvents were of analytical grade (Guangzhou Chemical Reagents Company, Ltd., Guangzhou, China).
3.2. Animal Material
The soft coral S. elegans were collected from the Xisha Islands in the South China Sea, in October 2014, at a depth of 8–10 m of water. The biological material was frozen immediately until used and was identified by Cheng-Qi Fan from East China Sea Fisheries Research Institute. A voucher specimen (accession number: HLRZ201410) has been deposited at the School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, China.
3.3. Extraction and Isolation
The frozen samples (500 g, wet weight) of S. elegans were extracted with EtOH (3 × 2 L) at rt to give 40 g of crude extract. The extract was suspended in H2O (200 mL) and partitioned sequentially to give dried PE (5 g) and EtOAc (9 g) extracts. The EtOAc extract was subjected to silica gel CC eluted with a CH2Cl2/MeOH gradient (200:1→20:1) to afford Fr. I–VI. Separation of the Fr. II (1 g) by Sephadex LH-20 eluted with MeOH led to Fr. IIa−IIb. Fr. IIa was purified on a semi-preparative reversed-phase (RP) HPLC system equipped with a YMC column (CH3CN/H2O, 8:2, 3 mL/min) to give 3 (4 mg, tR 10 min). Fr. IIb was purified using RP-HPLC (CH3CN/H2O, 7:3, 3 mL/min) to give 5 (11 mg, tR 8 min). Fractionation of Fr. III (2.5 g) by Sephadex LH-20 eluted with EtOH led to Fr. IIIa−IIIc. Fr. IIIa (1 g) was chromatographed over a RP-C18 column eluted with MeOH/H2O (4:6→10:0) to afford Fr. IIIa1−IIIa3. Fr. IIIa1 was loaded onto a Sephadex LH-20 column and eluted with CH2Cl2-MeOH (1:1) to give 6 (20 mg). Fr. IIIa2 was purified using RP-HPLC (CH3CN/H2O, 6:4, 3 mL/min) to give 8 (31 mg, tR 8.5 min) and 9 (16.8 mg, tR 9 min). Fr. VI (2 g) was subjected to silica gel CC (PE/EtOAc, 2:1→1:4) to give Fr. VIa–VId. Fr. VIa was purified using RP-HPLC (CH3CN/H2O, 4:6, 3 mL/min) to give 4 (35 mg, tR 10 min) and 7 (4 mg, tR 12 min). Fr. VIc was purified using RP-HPLC (MeOH/H2O, 8:2, 3 mL/min) to give 1 (3 mg, tR 10.5 min). Fr. VId was purified using RP-HPLC (MeOH/H2O, 7:3, 3 mL/min) to give 2 (12 mg, tR 11 min).
Sarelengan A (1). Colorless crystal; mp 210–212 °C; +130.0 (c 0.20, MeOH); UV (MeOH) λmax (log ε) 217 (4.05) nm; IR (KBr) νmax 3408, 2968, 2931, 1710, 1698, 1379, 1190, 987, 734 cm−1; 1H and 13C-NMR data, see Table 1; HRESIMS m/z 719.4121 [M + Na]+ (calcd. for C41H60O9Na, 719.4130).
Table 1.
1H (400 MHz) and 13C (100 MHz) NMR (nuclear magnetic resonance) data for 1 and 2 in methanol-d4 (δ in ppm).
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, Multi. (J in Hz) | δC, Type | δH, Multi. (J in Hz) | |
| 1 | 36.7, CH | 3.15, m | 36.5, CH | 3.14, m |
| 2 | 43.0, CH2 | a 3.06, dd (17.6, 4.6) | 43.3, CH2 | a 3.07, dd (14.0, 4.4) |
| b 2.86, m | b 2.89, m | |||
| 3 | 203.4, C | 202.5, C | ||
| 4 | 127.4, CH | 5.96, d (1.2) | 126.7, CH | 6.03, d (1.1) |
| 5 | 154.1, C | 156.0, C | ||
| 6 | 34.1, CH2 | a 2.80, m | 33.2, CH2 | a 2.54, m |
| b 2.07, m | b 2.36, m | |||
| 7 | 36.7, CH2 | 1.05, m | 35.6, CH2 | 1.06, m |
| 8 | 33.2, CH | 2.30, m | 33.0, CH | 2.33, m |
| 9 | 55.9, C | 56.4, C | ||
| 10 | 215.5, C | 214.7, C | ||
| 11 | 41.1, CH2 | a 2.99, dd (18.0, 10.6) | 41.8, CH2 | 2.95, m |
| b 2.61, dd (18.0, 3.3) | ||||
| 12 | 54.8, CH | 2.51, m | 55.8, CH | 2.35, m |
| 13 | 215.4, C | 215.5, C | ||
| 14 | 44.1, CH2 | a 3.44, dd (18.1, 3.7) | 42.0, CH2 | a 3.18, dd (17.9, 3.8) |
| b 2.87, m | b 2.74, dd (17.9, 6.2) | |||
| 15 | 31.2, CH | 1.70, m | 31.4, CH | 1.72, m |
| 16 | 21.0, CH3 | 0.92, d (6.7) | 21.1, CH3 | 0.96, d (6.7) |
| 17 | 21.2, CH3 | 0.92, d (6.7) | 21.2, CH3 | 0.90, d (6.7) |
| 18 | 16.8, CH3 | 1.07, s | 16.5, CH3 | 1.13, s |
| 19 | 26.1, CH3 | 1.86, d (1.2) | 27.4, CH3 | 1.90, d (1.1) |
| 20 | 176.3, C | 176.6, C | ||
| 21 | 41.6, CH | 3.68, d (10.6) | 42.0, CH | 3.40, d (11.2) |
| 22 | 128.2, CH | 4.88, d (10.6) | 126.4, CH | 4.89, d (11.2) |
| 23 | 136.9, C | 133.1, C | ||
| 24 | 38.3, CH2 | 2.00, m | 35.5, CH2 | a 2.22, m |
| b 1.99, m | ||||
| 25 | 32.2, CH2 | a 1.79, m | 26.6, CH2 | 1.41, m |
| b 1.43, m | ||||
| 26 | 76.2, CH | 3.47, m | 64.0, CH | 2.90, m |
| 27 | 84.4, C | 61.2, C | ||
| 28 | 37.1, CH2 | a 2.08, m | 39.4, CH2 | a 1.99, m |
| b 1.69, m | b 1.01, m | |||
| 29 | 29.4, CH2 | 1.76, m | 24.3, CH2 | a 2.22, m |
| b 1.98, m | ||||
| 30 | 79.3, CH | 3.89, dd (10.2, 4.2) | 130.6, CH | 5.14, dd (9.1, 4.8) |
| 31 | 151.6, C | 135.6, C | ||
| 32 | 72.0, CH | 4.07, d (9.7) | 78.7, CH | 3.80, d (10.0) |
| 33 | 76.4, CH | 4.39, d (9.7) | 71.8, CH | 4.66, d (10.0) |
| 34 | 130.2, C | 130.7, C | ||
| 35 | 132.7, C | 132.7, C | ||
| 36 | 38.9, CH2 | a 2.45, m | 38.7, CH2 | a 2.55, m |
| b 1.62, m | b 1.65, m | |||
| 37 | 20.2, CH3 | 1.54, s | 19.6, CH3 | 1.71, s |
| 38 | 18.1, CH3 | 1.70, s | 18.9, CH3 | 1.59, s |
| 39 | 20.3, CH3 | 1.08, s | 16.3, CH3 | 1.30, s |
| 40 | 112.8, CH2 | a 5.47, s | 11.4, CH3 | 1.61, s |
| b 5.21, s | ||||
| 41 | 52.3, CH3 | 3.63, s | 52.3, CH3 | 3.64, s |
Crystal data for sarelengan A (1). C41H60O9·C2H3N, (M = 737.98 g/mol): monoclinic, space group P21 (no. 4), a = 13.28203 (9) Å, b = 9.73686(7) Å, c = 16.15823 (13) Å, β = 100.9378 (7)°, V = 2051.70 (3) Å3, Z = 2, T = 103 (2) K, μ (Cu Kα) = 0.664 mm−1, Dcalc = 1.1945 g/cm3, 35174 reflections measured (5.58° ≤ 2θ ≤ 144.78°), 7856 unique (Rint = 0.0889, Rsigma = 0.0626) which were used in all calculations. The final R1 was 0.0557 (I ≥ 2σ (I)) and wR2 was 0.1463 (all data). Flack parameter = 0.04 (13). Crystallographic data for the structure of 1 have been deposited in the Cambridge Crystallographic Data Centre (deposition number: CCDC 1520904).
Sarelengan B (2). Colorless oil; +154.7 (c 0.30, MeOH); UV (MeOH) λmax (log ε) 209 (3.97) nm; IR (KBr) νmax 3408, 2928, 1736, 1698, 1438, 1373, 1284, 1037, 735 cm−1; 1H and 13C-NMR data, see Table 1; HRESIMS m/z 703.4193 [M + Na]+ (calcd. for C41H60O8 Na, 703.4180).
Sarelengan C (3). Colorless oil; +110.5 (c 0.40, MeOH); UV (MeOH) λmax (log ε) 222 (3.87), 354 (4.13) nm; IR (KBr) νmax 2956, 2925, 1691, 1544, 1438, 1377, 1137, 794 cm−1; 1H and 13C-NMR data, see Table 2 and Table 3; HRESIMS m/z 367.1871 [M + Na]+ (calcd. for C21H28O4Na, 367.1880).
Table 2.
1H-NMR (400 MHz) data of 3–7 (δ in ppm, J in Hz).
| No. | 3 a | 4 a | 5 a | 6 a | 7 b |
|---|---|---|---|---|---|
| 2 | 6.29, d (12.1) | 5.45, d (15.5) | 5.69, d (15.9) | 5.50, d (16.2) | 5.57, d (15.7) |
| 3 | 7.42, dd (12.1, 1.1) | 5.53, d (15.5) | 5.64, d (15.9) | 5.56, d (16.2) | 5.66, d (15.7) |
| 5 | a 1.93, m | a 1.75, m | 1.74, m | a 1.89, m | |
| b 1.79, m | b 1.59, ddd (13.4, 10.7, 7.0) | b 1.68, m | |||
| 6 | 5.64, s | 2.65, m | a 2.79, ddd (20.4, 10.9, 7.0) | a 2.65, m | 1.54, m |
| b 2.35, ddd (20.4, 10.7, 2.4) | b 2.35, m | ||||
| 7 | 4.37, m | ||||
| 9 | a 2.09, m | a 2.72, m | a 1.92, m | a 2.06, m | a 2.26, m |
| b 2.04, m | b 2.18, m | b 1.81, m | b 1.74, m | b 1.99, m | |
| 10 | a 2.74, m | 2.41, m | a 3.37, m | a 2.04, m | a 2.58, m |
| b 2.64, m | b 2.18, m | b 1.72, m | b 2.49, m | ||
| 11 | 5.99, t (6.4) | 6.09, m | 5.73, m | 4.87, m | 6.34, t (4.3) |
| 12 | 2.69, m | ||||
| 13 | a 2.73, m | 2.37, m | 2.46, m | 1.82, m | a 2.99, m |
| b 2.13, m | b 2.13, m | ||||
| 14 | 2.48, t (6.2) | 1.73, m | a 2.05, ddd (13.9, 6.6, 2.1) | a 2.04, m | a 2.00, m |
| b 1.77, m | b 1.82, m | b 1.73, m | |||
| 15 | 2.52, m | 1.72, m | 1.85, m | 1.89, m | 1.80, m |
| 16 | 1.12, d (6.8) | 0.92, d (6.8) | 0.96, d (6.7) | 0.98, d (6.8) | 0.84, d (6.8) |
| 17 | 1.12, d (6.8) | 0.92, d (6.8) | 0.98, d (6.7) | 1.00, d (6.8) | 0.89, d (6.8) |
| 18 | 1.96, d (1.1) | 1.29, s | 1.26, s | 1.27, s | 1.31, s |
| 19 | 1.43, s | 1.43, s | 1.23, s | 1.31, s | 1.32 s |
| 21 | 3.66, s |
a in methanol-d4; b in CDCl3.
Table 3.
13C-NMR (100 MHz) data of 3–7 (δ in ppm).
| No. | 3 a | 4 a | 5 a | 6 a | 7 b |
|---|---|---|---|---|---|
| 1 | 164.1, C | 78.2, C | 88.1, C | 89.6, C | 76.1, C |
| 2 | 119.3, CH | 131.8, CH | 128.7, CH | 129.8, CH | 134.6, CH |
| 3 | 136.6, CH | 134.9, CH | 139.8, CH | 139.9, CH | 132.4, CH |
| 4 | 121.7, C | 73.6, C | 73.5, C | 74.0, C | 75.2, CH |
| 5 | 187.7, C | 34.3, CH2 | 38.1, CH2 | 36.9, CH2 | 34.3, CH2 |
| 6 | 100.4, CH | 34.3, CH2 | 36.1, CH2 | 34.5, CH2 | 25.7, CH2 |
| 7 | 209.6, C | 212.0, C | 217.8, C | 215.1, C | 66.1, CH |
| 8 | 91.3, C | 88.1, C | 79.8, C | 90.0, C | 83.6, C |
| 9 | 40.5, CH2 | 37.4, CH2 | 42.1, CH2 | 36.7, CH2 | 34.8, CH2 |
| 10 | 25.8, CH2 | 24.8, CH2 | 25.9, CH2 | 27.5, CH2 | 27.4, CH2 |
| 11 | 152.1, CH | 133.8, CH | 150.9, CH | 80.8, CH | 141.0, CH |
| 12 | 130.1, C | 137.5, C | 125.2, C | 44.1, CH | 134.1, C |
| 13 | 36.5, CH2 | 26.5, CH2 | 25.1, CH2 | 17.9, CH2 | 31.0, CH2 |
| 14 | 31.6, CH2 | 33.0, CH2 | 29.1, CH2 | 28.2, CH2 | 39.7, CH2 |
| 15 | 38.6, CH | 38.8, CH | 38.3, CH | 38.0, CH | 36.3, CH |
| 16 | 21.8, CH3 | 18.1, CH3 | 17.5, CH3 | 17.6, CH3 | 17.2, CH3 |
| 17 | 21.4, CH3 | 17.0, CH3 | 16.9, CH3 | 17.0, CH3 | 16.0, CH3 |
| 18 | 12.2, CH3 | 31.4, CH3 | 26.9, CH3 | 24.4, CH3 | 33.5, CH3 |
| 19 | 20.9, CH3 | 28.0, CH3 | 28.3, CH3 | 24.0, CH3 | 21.6, CH3 |
| 20 | 169.2, C | 172.1, C | 168.9, C | 175.3, C | 168.4, C |
| 21 | 51.5, CH3 |
a in methanol-d4; b in CDCl3.
Sarelengan D (4). Colorless crystal; mp 186–188 °C; +34.9 (c 0.75, MeOH); UV (MeOH) λmax (log ε) 210 (3.67) nm; IR (KBr) νmax 3398, 2927, 1697, 1437, 1377, 1283, 1206, 1035, 734 cm−1; 1H and 13C-NMR data, see Table 2 and Table 3; HRESIMS m/z 373.1979 [M + Na]+ (calcd. for C20H30O5Na, 373.1985).
Crystal data for sarelengan D (4). C20H30O5, (M = 350.46 g/mol): monoclinic, space group P21 (no. 4), a = 7.06058 (10) Å, b = 8.04934 (10) Å, c = 16.4003 (2) Å, β = 90.0131 (11)°, V = 932.08 (2) Å3, Z = 2, T = N/A K, μ (Cu Kα) = 0.716 mm−1, Dcalc = 1.2486 g/cm3, 8797 reflections measured (13.66° ≤ 2θ ≤ 144.14°), 3369 unique (Rint = 0.0135, Rsigma = 0.0126) which were used in all calculations. The final R1 was 0.0254 (I ≥ 2σ (I)) and wR2 was 0.0645 (all data). Flack parameter = 0.08 (10). Crystallographic data for the structure of 4 have been deposited in the Cambridge Crystallographic Data Centre (deposition number: CCDC 1520903).
Sarelengan E (5). Colorless crystal; mp 177–178 °C; −6.4 (c 0.90, MeOH); UV (MeOH) λmax (log ε) 230 (3.66) nm; IR (KBr) νmax 3418, 2965, 2928, 1685, 1616, 1450, 1177, 791 cm−1; 1H and 13C-NMR data, see Table 2 and Table 3; HRESIMS m/z 373.1984 [M + Na]+ (calcd. for C20H30O5Na, 373.1985).
Crystal data for sarelengan E (5). C20H30O5, (M = 350.46 g/mol): triclinic, space group P1 (no. 1), a = 9.6159 (2) Å, b = 9.7162 (2) Å, c = 21.2262 (4) Å, α = 102.7736 (18)°, β = 99.6112 (18)°, γ = 96.8219 (18)°, V = 1881.77 (7) Å3, Z = 4, T = N/A K, μ (Cu Kα) = 0.709 mm−1, Dcalc = 1.2369 g/cm3, 35900 reflections measured (4.36° ≤ 2θ ≤ 144.66°), 11571 unique (Rint = 0.0249, Rsigma = 0.0249) which were used in all calculations. The final R1 was 0.0372 (I ≥ 2σ (I)) and wR2 was 0.1183 (all data). Flack parameter = −0.01 (10). Crystallographic data for the structure of 5 have been deposited in the Cambridge Crystallographic Data Centre (deposition number: CCDC 1520902).
Sarelengan F (6). Colorless crystal; mp 183–185 °C; +57.3 (c 0.67, MeOH); UV (MeOH) λmax (log ε) 207 (3.03) nm; IR (KBr) νmax 3533, 2979, 2930, 1717, 1695, 1462, 1270, 1027, 738 cm−1; 1H and 13C-NMR data, see Table 2 and Table 3; HRESIMS m/z 373.1985 [M + Na]+ (calcd. for C20H30O5Na, 373.1985).
Crystal data for sarelengan F (6). C20H30O5, (M = 350.46 g/mol): orthorhombic, space group P212121 (no. 19), a = 6.43129 (10) Å, b = 12.91548 (17) Å, c = 22.1013 (3) Å, V = 1835.81 (5) Å3, Z = 4, T = N/A K, μ (Cu Kα) = 0.727 mm−1, Dcalc = 1.2679 g/cm3, 17493 reflections measured (7.92° ≤ 2θ ≤ 144.66°), 3612 unique (Rint = 0.0661, Rsigma = 0.0432) which were used in all calculations. The final R1 was 0.0418 (I ≥ 2σ (I)) and wR2 was 0.1088 (all data). Flack parameter = −0.05 (13). Crystallographic data for the structure of 6 have been deposited in the Cambridge Crystallographic Data Centre (deposition number: CCDC 1520905).
Sarelengan G (7). Colorless oil; +10.0 (c 0.20, MeOH); UV (MeOH) λmax (log ε) 226 (3.70) nm; IR (KBr) νmax 3454, 2925, 1673, 1388, 1225, 1124, 1050, 734 cm−1; 1H and 13C-NMR data, see Table 2 and Table 3; HRESIMS m/z 375.2138 [M + Na]+ (calcd. for C20H32O5Na, 375.2142).
3.4. Chemical Transformation of 9 to 6
Compound 9 (6 mg) was treated with NaOH (1% in MeOH, 1 mL) at room temperature for 2 h. The mixture was then diluted with 5 mL of H2O, followed by the extraction of EtOAc (3 × 5 mL). The organic layer was dried and evaporated to give a residue, which was purified on a flash silica gel column eluting with CH2Cl2 to afford 6 (3.4 mg). The structure was confirmed by comparison of its NMR and optical rotation data with those of the natural product.
3.5. Cell Culture and Viability Assay
The RAW 264.7 mouse macrophage cell line was obtained from the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, Shanghai, China), and was cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco Invitrogen Corp., Carlsbad, CA, USA). The cells were supplemented with 3.0 mM glutamine, antibiotics (100 U/mL penicillin and 100 U/mL streptomycin), and 10% heat-inactivated fetal bovine serum at 37 °C under a humidified atmosphere of 5% CO2. The cell viability of the cultured cells was assessed by MTT assay. Briefly, RAW 264.7 mouse macrophage cells were incubated with 200 μL MTT solution (0.5 mg/mL in medium) for 4 h at 37 °C, and then the supernatants were removed and residues were dissolved in 100 μL DMSO. The absorbance was detected at 490 nm using a microplate reader (Molecular Devices, Silicon Valley, CA, USA) and analyzed using a Soft Max Pro 5 software (Molecular Devices, Silicon Valley, CA, USA).
3.6. Measurement of NO Production
The NO concentration was measured by the Griess reaction. Briefly, RAW 264.7 macrophage cells were cultured onto 96-well plates at a density of 5 × 104 cell/well and then incubated with or without 1.0 μg/mL LPS (Escherichia coli 055:B5, Sigma, St. Louis, MO, USA) in the absence or presence of compounds for 24 h. After that, 50 μL of culture supernatant was allowed to react with equal volume of Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylethylenediamine dihydrochloride in 5% phosphoric acid) for 10 min at rt in the dark. Then, the optical density was measured at 540 nm using a microplate reader (Molecular Devices, Silicon Valley, CA, USA). Sodium nitrite was used as a standard to calculate the nitrite concentration. Inhibition (%) = (1 − (ALPS + sample − Auntreated)/(ALPS − Auntreated)) × 100. The experiments were performed in triplicates, and the data were expressed as the mean ± standard deviation (SD) values. Quercetin was used as a positive control.
4. Conclusions
The chemical investigation of the South China Sea coral S. elegans led to the isolation of two novel biscembranoids, sarelengans A and B (1 and 2), five new cembranoids, sarelengans C–G (3–7), along with two known cembranoids (8 and 9). Compounds 1 and 2 represent the first example of biscembranoids featuring a trans-fused A/B-ring conjunction between the two cembranoid units. Their unique structures may shed light on an unusual biosynthetic pathway involving a cembranoid-∆8 rather than the normal cembranoid-∆1 unit in the endo-Diels-Alder cycloaddition. All compounds were tested for their inhibitory activity against nitric oxide (NO) production induced by lipopolysaccharide (LPS) in RAW 264.7 macrophages, and compounds 2 and 3 showed moderate inhibitory activities with IC50 values being at 18.2 and 32.5 μM, respectively. The current study expanded the members of the biscembranoid family.
Acknowledgments
The authors thank the Science and Technology Planning Project of Guangdong Province (No. 2013B021100009) and the National Natural Science Foundation of China (No. 81573302) for providing financial support for this work.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/4/85/s1, HRESIMS, 1D and 2D NMR spectra of 1–7, 1H and 13C-NMR spectra of known compounds 8 and 9.
Author Contributions
Gui-Hua Tang and Sheng Yin participated in the design of the research. Wei Li, Yi-Hong Zou, Man-Xi Ge, Lan-Lan Lou, Yun-Shao Xu, Abrar Ahmed, Yun-Yun Chen, and Jun-Sheng Zhang performed the experiments. Wei Li and Sheng Yin analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Su J.Y., Long K.H., Pang T.S., He C.H., Clardy J. The Structure of Methyl Isosartortuoate, a Novel Tetracyclic Tetraterpenoid from the Soft Coral Sarcophyton tortuosum. J. Am. Chem. Soc. 1986;108:177–178. doi: 10.1021/ja00261a030. [DOI] [Google Scholar]
- 2.Kusumi T., Igari M., Ishitsuka M.O., Ichikawa A., Itezono Y., Nakayama N., Kakisawa H. A Novel Chlorinated Biscembranoid from the Marine Soft Coral Sarcophyton glaucum. J. Org. Chem. 1990;55:6286–6289. doi: 10.1021/jo00313a014. [DOI] [Google Scholar]
- 3.Leone P.A., Bowden B.F., Carroll A.R., Coll J.C., Meehan G.V. Studies of Australian soft corals, XLIX. A new biscembranoid and its probable biosynthetic precursors from the soft coral Sarcophyton tortuosum. J. Nat. Prod. 1993;56:521–526. doi: 10.1021/np50094a011. [DOI] [Google Scholar]
- 4.Chen B.W., Chao C.H., Su J.H., Huang C.Y., Dai C.F., Wen Z.H., Sheu J.H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010;51:5764–5766. doi: 10.1016/j.tetlet.2010.08.027. [DOI] [Google Scholar]
- 5.Yan P.C., Deng Z.W., van Ofwegen L., Proksch P., Lin W.H. Lobophytones H–N, Biscembranoids from the Chinese Soft Coral Lobophytum pauciflorum. Chem. Pharm. Bull. 2010;58:1591–1595. doi: 10.1248/cpb.58.1591. [DOI] [PubMed] [Google Scholar]
- 6.Zeng L.M., Lan W.J., Su J.Y., Zhang G.W., Feng X.L., Liang Y.J., Yang X.P. Two New Cytotoxic Tetracyclic Tetraterpenoids from the Soft Coral Sarcophyton tortuosum. J. Nat. Prod. 2004;67:1915–1918. doi: 10.1021/np0400151. [DOI] [PubMed] [Google Scholar]
- 7.Iwagawa T., Hashimoto K., Okamura H., Kurawaki J.I., Nakatani M., Hou D.X., Fujii M., Doe M., Morimoto Y., Takemura K. Biscembranes from the Soft Coral Sarcophyton glaucum. J. Nat. Prod. 2006;69:1130–1133. doi: 10.1021/np058115+. [DOI] [PubMed] [Google Scholar]
- 8.Bishara A., Rudi A., Benayahu Y., Kashman Y. Three Biscembranoids and their Monomeric Counterpart Cembranoid, a Biogenetic Diels–Alder Precursor, from the Soft Coral Sarcophyton elegans. J. Nat. Prod. 2007;70:1951–1954. doi: 10.1021/np070129n. [DOI] [PubMed] [Google Scholar]
- 9.Jia R., Guo Y.W., Chen P., Yang Y.M., Mollo E., Gavagnin M., Cimino G. Biscembranoids and Their Probable Biogenetic Precursor from the Hainan Soft Coral Sarcophyton tortuosum. J. Nat. Prod. 2007;70:1158–1166. doi: 10.1021/np060220b. [DOI] [PubMed] [Google Scholar]
- 10.Yan P.C., Lv Y., van Ofwegen L., Proksch P., Lin W.H. Lobophytones A–G, New Isobiscembranoids from the Soft Coral Lobophytum pauciflorum. Org. Lett. 2010;12:2484–2487. doi: 10.1021/ol100567d. [DOI] [PubMed] [Google Scholar]
- 11.Sun P., Yu Q., Li J., Riccio R., Lauro G., Bifulco G., Kurtan T., Mandi A., Tang H., Li T.J., et al. Bissubvilides A and B, Cembrane–Capnosane Heterodimers from the Soft Coral Sarcophyton subviride. J. Nat. Prod. 2016;79:2552–2558. doi: 10.1021/acs.jnatprod.6b00453. [DOI] [PubMed] [Google Scholar]
- 12.Yan P.C., Deng Z.W., van Ofwegen L., Proksch P., Lin W.H. Lobophytones U–Z1, Biscembranoids from the Chinese Soft Coral Lobophytum pauciflorum. Chem. Biodivers. 2011;8:1724–1734. doi: 10.1002/cbdv.201000244. [DOI] [PubMed] [Google Scholar]
- 13.Xi Z.F., Bie W., Chen W., Liu D., van Ofwegen L., Proksch P., Lin W.H. Sarcophytolides G–L, New Biscembranoids from the Soft Coral Sarcophyton elegans. Helv. Chim. Acta. 2013;96:2218–2227. doi: 10.1002/hlca.201300086. [DOI] [Google Scholar]
- 14.Ichige T., Okano Y., Kanoh N., Nakata M. Total Synthesis of Methyl Sarcophytoate. J. Am. Chem. Soc. 2007;129:9862–9863. doi: 10.1021/ja073952e. [DOI] [PubMed] [Google Scholar]
- 15.Ichige T., Okano Y., Kanoh N., Nakata M. Total Synthesis of Methyl Sarcophytoate, a Marine Natural Biscembranoid. J. Org. Chem. 2009;74:230–243. doi: 10.1021/jo802249k. [DOI] [PubMed] [Google Scholar]
- 16.Xi Z.F., Bie W., Chen W., Liu D., van Ofwegen L., Proksch P., Lin W.H. Sarcophyolides B–E, New Cembranoids from the Soft Coral Sarcophyton elegans. Mar. Drugs. 2013;11:3186–3196. doi: 10.3390/md11093186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anjaneyulu A.S. R., Venugopal M.J. R.V., Sarada P., Rao G.V., Clardy J., Lobkovsky E. Sarcophytin, A Novel Tetracyclic Diterpenoid From The Indian Ocean Soft Coral Sarcophyton elegans. Tetrahedron Lett. 1998;39:135–138. doi: 10.1016/S0040-4039(97)10469-5. [DOI] [Google Scholar]
- 18.Moldowan J.M., Tursch B.M., Djerassi C. 24ξ-Methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate, a novel polyhydroxylated steroid from an alcyonarian. Steroids. 1974;24:387–398. doi: 10.1016/0039-128X(74)90036-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Zhang J.S., Liu Q., Tang G.H., Wang H.S., Fan C.Q., Yin S. Bioactive Cembranoids from the South China Sea Soft Coral Sarcophyton elegans. Molecules. 2015;20:13324–13335. doi: 10.3390/molecules200713324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y.Q., Zhao J.J., Li Z.Z., Tang G.H., Zhao Z.M., Yin S. Natural nitric oxide (NO) inhibitors from Chloranthus japonicus. Bioorg. Med. Chem. Lett. 2016;26:3163–3166. doi: 10.1016/j.bmcl.2016.04.081. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J.Y., Cheng B., Zheng Y.J., Dong Z., Lin S.L., Tang G.H., Gu Q., Yin S. Enantiomeric neolignans and sesquineolignans from Jatropha integerrima and their absolute configurations. RSC Adv. 2015;5:12202–12208. doi: 10.1039/C4RA15966G. [DOI] [Google Scholar]
- 22.Yan P.C., Deng Z.W., van Ofwegen L., Proksch P., Lin W.H. Lobophytones O–T, New Biscembranoids and Cembranoid from Soft Coral Lobophytum pauciflorum. Mar. Drugs. 2010;8:2837–2848. doi: 10.3390/md8112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichige T., Kamimura S., Mayumi K., Sakamoto Y., Terashita S., Ohteki E., Kanoh N., Nakata M. Synthetic studies on biscembranoids: Asymmetric total synthesis of methyl sarcoate. Tetrahedron Lett. 2005;46:1263–1267. doi: 10.1016/j.tetlet.2004.12.132. [DOI] [Google Scholar]
- 24.Iwagawa T., Hashimoto K., Yokogawa Y., Okamura H., Nakatani M., Doe M., Morimoto Y., Takemura K. Cytotoxic Biscembranes from the Soft Coral Sarcophyton glaucum. J. Nat. Prod. 2009;72:946–949. doi: 10.1021/np8003485. [DOI] [PubMed] [Google Scholar]
- 25.McMurry J.E., Rico J.G., Shih Y.N. Synthesis and stereochemistry of sarcophytol B: An anticancer cembranoid. Tetrahedron Lett. 1989;30:1173–1176. doi: 10.1016/S0040-4039(00)72708-0. [DOI] [Google Scholar]
- 26.Kurtan T., Jia R., Li Y., Pescitelli G., Guo Y.W. Absolute Configuration of Highly Flexible Natural Products by the Solid-State ECD/TDDFT Method: Ximaolides and Sinulaparvalides. Eur. J. Org. Chem. 2012;2012:6722–6728. doi: 10.1002/ejoc.201200862. [DOI] [Google Scholar]
- 27.Chimichi S., Boccalini M., Cosimelli B., Dall’Acqua F., Viola G. New 5-(2-ethenylsubstituted)-3(2H)-furanones with in vitro antiproliferative activity. Tetrahedron. 2003;59:5215–5223. doi: 10.1016/S0040-4020(03)00776-2. [DOI] [Google Scholar]
- 28.Liang L.F., Lan L.F., Taglialatela-Scafati O., Guo Y.W. Sartrolides A–G and bissartrolide, new cembranolides from the South China Sea soft coral Sarcophyton trocheliophorum Marenzeller. Tetrahedron. 2013;69:7381–7386. doi: 10.1016/j.tet.2013.06.068. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.