Abstract
The oceans encompass a wide range of habitats and environmental conditions, which host a huge microbial biodiversity. The unique characteristics of several marine systems have driven a variety of biological adaptations, leading to the production of a large spectrum of bioactive molecules. Fungi, fungi-like protists (such as thraustochytrids) and bacteria are among the marine organisms with the highest potential of producing bioactive compounds, which can be exploited for several commercial purposes, including cosmetic and cosmeceutical ones. Mycosporines and mycosporine-like amino acids, carotenoids, exopolysaccharides, fatty acids, chitosan and other compounds from these microorganisms might represent a sustainable, low-cost and fast-production alternative to other natural molecules used in photo-protective, anti-aging and skin-whitening products for face, body and hair care. Here, we review the existing knowledge of these compounds produced by marine microorganisms, highlighting the marine habitats where such compounds are preferentially produced and their potential application in cosmetic and cosmeceutical fields.
Keywords: marine bacteria, marine fungi, cosmetics and cosmeceuticals, marine bioactive compounds
1. Introduction
The oceans host a huge biodiversity, with more 250,000 species described and up to 8.5 million species still to be discovered [1], but estimates on microbial diversity are largely unknown [2]. In recent decades, the exploration of the oceans has allowed the discovery of a multitude of previously unknown habitats characterized by extreme conditions [3]. These environments host a variety of organisms adapted to these conditions and producing a wide range of active biomolecules [4,5]. More than 25,000 new biologically active compounds have been identified in the past fifty years, with an increment of 5% per year and 1378 new molecules identified in 2014 alone [6]. Among marine organisms, microorganisms, including fungi, fungi-like protists (such as thraustochytrids) and bacteria, have attracted great attention as potential leading compound producers [6,7,8].
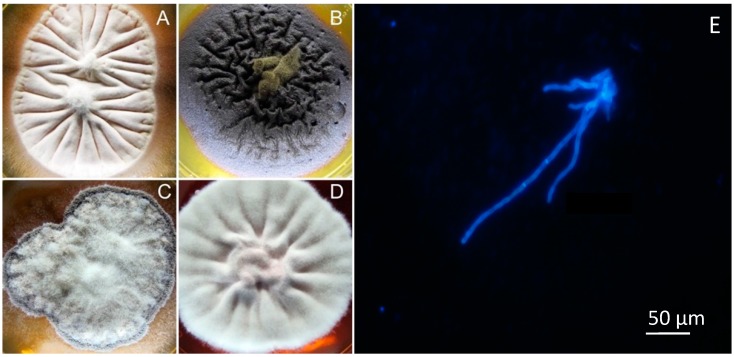
Fungi are abundant and ecologically relevant members of marine microbial assemblages (Figure 1). They were believed to be rare in marine environments, but recent studies based on molecular and metagenomics approaches have revealed an unexpected diversity from coastal to deep-sea ecosystems [9,10,11]. Deep-sea fungi have been less described in terms of their abundance, diversity and ecological role, but are potentially important and productive sources of bioactive molecules [12].
Figure 1.
Marine Fungi, Penicillium sp. (A,C); Cladosporium sp. (B); Aspergillus sp. (D) and fungal hyphae in marine sediment samples stained with Calcofluor (E).
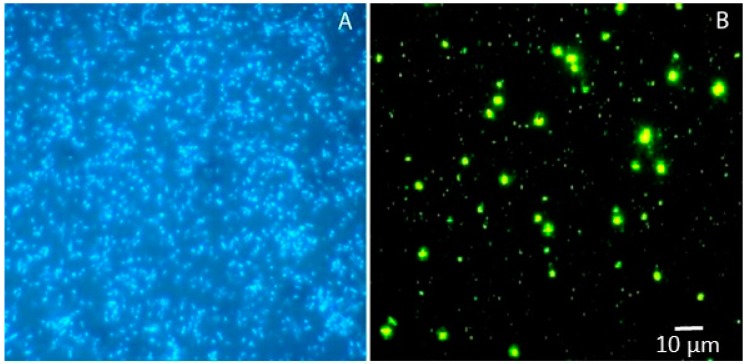
Bacteria are typically the most abundant (ca. 1029 cells, Figure 2) and diverse members of the microbial assemblages in the oceans, where they are key players in biogeochemical processes and fluxes of energy and matter [13]. Several bacterial species are distributed across all marine ecosystems worldwide, and are currently exploited for a number of biotechnological applications.
Figure 2.
Marine bacteria in seawater samples stained with DAPI (A) and SYBR Green I (B).
A multiplicity of compounds from marine and marine bacteria and fungi such as polyketides, alkaloids, peptides, proteins, lipids, mycosporines and mycosporine-like amino acids, glycosides, isoprenoids and hybrids have great potential in cosmeceutical and cosmetics since they exhibit photo-protective, anti-aging, anti-microbial, anti-oxidant and moisturizing activities [7,14]. Such compounds show specific chemical structures and activities up to two orders of magnitudes higher than those reported by species inhabiting terrestrial systems [8,15].
The global market for cosmetic and cosmeceutical products is forecasted to grow at a rate of 4.3% by 2022 with a value of USD 430 billion (https://www.alliedmarketresearch.com). Photo-protective, skin-care and hair-care products drive this trend of increasing demand. In addition, consumers’ demand is turning to natural products given health concerns and popular trends, forcing research to discover new compounds from the abundant and alternative source represented by marine organisms [16].
Bioactive compounds produced by marine microbes are still largely unexplored and unexploited [2]. Therefore, the identification of marine microbial-derived molecules for (bio)technological and industrial purposes has huge potential for new discoveries [7]. Since bioactive compounds from marine photosynthetic microorganisms (e.g., cyanobacteria and microalgae) have been extensively reported in literature, the present review provides an overview of the different bioactive compounds produced from marine and marine heterotrophic bacteria, fungi and fungi-like protists such as thraustochytrids, and their potential applications in the cosmeceutical and cosmetic industry. We also highlight the untapped potential of these microorganisms as source of photo-protective, moisturizing, anti-wrinkle, skin-whitening products and other commonly active ingredients and/or adjuvants included in the composition of personal care products (i.e., anti-oxidant, anti-microbial and preservatives).
2. Photo-Protective Compounds
There is evidence that prolonged human exposure to UVA (320–400 nm) and UVB (280–320 nm) radiation may result in acute and/or chronic effects on the skin and on overall human health [17,18]. Growing awareness of the risks associated with skin exposure to UV radiation over recent decades has led to increased production and consumption of solar products worldwide, reaching unexpected levels [19].
Several marine organisms have evolved a set of mechanisms to protect themselves from the harmful effects of UV radiation, producing UV-absorbing compounds such as scytonemins (exclusively in cyanobacteria), mycosporines, mycosporine-like amino acids (MAAs), carotenoids and melanin [20,21]. These compounds offer a great potential for the development of novel UV filters to be included in sunscreen products. Generally, common sunscreen products contain organic and/or inorganic filters [22]. However, recent investigations have proved that traditional UV-filters and other ingredients (e.g., preservatives) might have harmful effects not only on human skin but also on marine life [19,23]. This evidence has stimulated new research on alternative and possibly eco-friendly photo-protective compounds. Marine organisms are a reliable source of photo-protective compounds. In particular, photosynthetic organisms have been thoroughly investigated as sources of several compounds including mycosporines, mycosporine-like amino acids and several other UV filters such as carotenoids and scytonemin [24,25]. Despite the large contribution of heterotrophic microorganisms to marine biodiversity and biomass, the identification of UV filters produced by these microbial components has received much less attention. Here, we report the main bacterial and fungal-derived photo-protective compounds, so far studied (Table 1).
Table 1.
Main categories of cosmetic products containing bioactive compounds extracted from microorganisms (bacteria, fungi and thraustochytrids) from different marine habitats.
| Main Categories | Bioactive Compounds | Action | Source Microorganisms | Habitat | References |
|---|---|---|---|---|---|
| Photoprotective Compounds | |||||
| Mycosporine | Mycosporine–glutaminol–glucoside and mycosporine–glutamicol–glucoside | UV screening | Marine fungi Phaeotheca triangularis, Trimmatostroma salinum, Hortaea werneckii, Aureobasidium pullulans and Cryptococcus liquefaciens | Hypersaline water and polar glacial ice | [26] |
| Mycosporine—like amino acids | Shinorine, porphyra- 334 and novel MAA (mycosporine-glycine-alanine) | UV screening | Marine bacteria Pseudonocardia sp. strain P1, Micrococcus p. AK-334, Actinosynnema mirum DSM 43827 | Oceans, coastal systems,deep-sea, hypersaline, Arctic and Antarctic waters and others | [27] |
| Carotenoids | β-carotene, astaxanthin, zeaxanthin, cantaxanthin, phoenicoxanthin and echinenone | Skin photo-protection and inhibition of adverse processes induced or mediated by solar UV radiation | Marine bacteria genera Paracoccus and Agrobacterium; marine fungi genera Rhodotorula, Phaffia, Xanthophyllomyces | Marine coastal systems | [28,29,30,31,32] |
| Thraustochytrids, such as Thraustochytrium strains ONC-T18 and CHN-1, Thraustochytriidae sp. AS4-A1 (Ulkenia sp.) and Aurantiochytrium sp. KH105 | Seawater and sediments from tropical and temperate to polar ecosystems, in particular organically enriched systems (e.g., estuaries, leaves of mangrove forests) | [33,34,35] | |||
| Benzodiazepine alkaloids | circumdatins I, C, G | UV-A screening activity | Marine fungus of the genus Exophiala | Isolated from the surface of the marine sponge Halichondria panicea | [36] |
| Anti-Aging Products | |||||
| Polysaccharides | EPS | Emulsifying, thickening, absorption and gel formation and anti-wrinkles | Marine fungi and bacteria such as Agrobacterium sp., Alcaligenes faecalis, Xanthomonas campestris, Bacillus sp., Zymonas mobilis, Eduarsiella tarda and Aureobasidium pullulans, Alteromonas macleodii , Pseudoalteromonas sp. | Different marine environments, including extreme ecosystems. Pseudoalteromonas sp. isolated from antarctic waters | [16,37,38,39,40,41,42,43,44] |
| HE 800 | Structurally analogous to hyaluronic acid | Vibrio diabolicus | Deep-sea hydrotermal vents | [41] | |
| PUFAs | DHA, EPA and omega-3 fatty acids | Soft tissue repair, skin nourishment and stimulation of collagen production | Marine fungi (i.e., Trichoderma sp., Rhodotorula mucilaginosa AMCQ8A), bacteria (i.e., Moritella dasanensis, Vibrio sp., Pseudomonas sp. Shewanella sp. and Colwellia sp.) and thraustochytrids (in particular Schizochytrium, Aurantiochytrium and Ulkenia) | Thraustochytrids isolated from seawater and sediments from tropical and temperate to polar ecosystems, in particular organically enriched systems (e.g., estuaries, leaves of mangrove forests); bacteria and fungi isolated from coastal to deep-sea habitats | [33,45,46,47,48,49,50,51,52,53,54,55,56] |
| Antioxidant Compounds | |||||
| MAAs | Antioxidant activity, scavenging activity of superoxide anions and inhibition of lipid peroxidation | Marine fungi and bacteria | Coastal and open-ocean systems, deep-sea, hypersaline, Arctic and Antarctic ecosystems and others | [26,27,57,58,59] | |
| Carotenoids | Astaxanthin | Antioxidant activity | Marine fungi bacteria and thraustochytrids | Coastal and open-ocean systems, deep-sea, hypersaline, Arctic and Antarctic ecosystems and others | [32,60] |
| Saproxanthin and myxol | Reinforce biological membranes, decreasing permeability to oxygen and enhancing protection against oxidation | Marine bacteria family Flavobacteriaceae | Antartic marine habitats | [61] | |
| Phenols | Hydroquinone derivatives (e.g., wentiquinone, ethyl 4-(3,4-dihydroxybenzamido)-butanoate) | anti-oxidant activity | Marine fungi Acremonium sp. and Aspergillus wentii N48 | Coastal systems, isolated from brown algae | [62,63] |
| Isobenzofuranone derivative | 4,5,6-trihydroxy-7-methylphthalide | Radical scavenging activity | Marine fungus, Epicoccum sp. | Coastal systems, isolated from brown algae Fucus vesiculosus | [64] |
| Exopolysaccharides | EPS2 | Radical scavenging activity | Marine fungus Keissleriella sp. YS 4108 | Marine sediments | [65] |
| Diketopiperazine alkaloids | Golmaenone and related alkaloids | Radical scavenging activity and UV-A screening function | Marine fungus Aspergillus sp. | Isolated from the surface of the marine red alga Lomentaria catenata | [66] |
| Dioxopiperazine alkaloids | Dihydroxyisoechinulin A and related echinulin | Radical scavenging activity and UV-A screening function | Marine fungus Aspergillus sp. | Isolated from the surface of the marine red alga Lomentaria catenata | [67] |
| Skin Whitening Products | |||||
| Pyrone | 5-Hydroxy-2-(hydroxymethyl)-4H-pyran-4-one (kojic acid) and derivates (kojic acid dimethyl ether and kojic acid monomethyl ether) | Inhibition of tyrosinase | Marine fungi (i.e., Aspergillus, Penicillium and Alternaria species) | Different marine ecosystems. Alternaria sp. isolated from marine green algae | [68,69] |
| α-Pyrone derivate (6-[(E)-Hept-1-enyl]-α-pyrone) | Inhibition of tyrosinase | Marine fungus Botrytis sp. | Isolated from the surface of the marine red alga Hyalosiphonia caespitose | [70] | |
| Phomaligol A | Inhibition of tyrosinase | Marine fungus Alternaria sp. | Isolated from marine green algae | [69] | |
| 6-n-pentyl-α-pyrone and myrothenone A | Inhibition of tyrosinase | Marine-derivated fungus, genus Myrothecium | Isolated from the surface of the marine green algae Entemorpha compressa | [71] | |
| N-acyl dehydrotyrosine derivatives | Thalassotalic acids A, B and C | Inhibition of tyrosinase | Marine Gram-negative bacterium, Thalassotalea sp. PP2-459 | Isolated from a marine bivalve | [72] |
| Compound similar to the structure of homothallin II | Inhibition of tyrosinase | Marine fungus T. viridae strain H1-7 | Isolated from marine sediments | [73] | |
| Seven different compounds | Inhibition of melanin | Marine fungus T. viridae strain H1-7 | Isolated from marine sediments | [73] | |
| Dicarboxylic acid | 1,7-heptanedicarboxylic acid (azelaic acid) | Inhibition of tyrosinase | Marine fungus Malasseziales | Almost every habitat in the marine environment | [74,75,76] |
| Sesquiterpenes | 1β,5α,6α,14-tetraacetoxy-9α-benzoyloxy-7β H-eudesman-2β, 11-diol and 4α,5α-diacetoxy-9α-benzoyloxy-7βH-eudesman-1β, 2β,11, 14-tetraol | Inhibition of tyrosinase | Marine fungus Pestalotiopsis sp. Z233. | Isolated from algae Sargassum horneri | [77] |
| Alkyl halides | Methylene chloride | Inhibition of tyrosinase | Marine bacteria genus Pseudomonas | Marine sediments | [78] |
| Anthraquinones | Chrysophanol | Inhibition of tyrosinase | Marine fungus, Microsporum sp. | Isolated from the red alga Lomentaria catenata | US patent 20140056834A1 |
| Carotenoids | Astaxanthin | Depigmentation properties | Marine bacteria and fungi | Seawater, sediments and marine organisms | [32] |
| Antimicrobial Products | |||||
| Polysaccharides | Chitin, chitosan and their derivatives | Antimicrobial activity | Marine fungi such as zygomycetes, chytridiomycetes, ascomycetes, basidiomycetes | Coastal and open-ocean systems, deep-sea, hypersaline, Arctic and Antarctic ecosystems and others | [79,80] |
| Carotenoids | Astaxanthin | Antimicrobial activity, anti-wrinkle and anti-acne effects | Marine bacteria, fungi and thraustochytrids | Coastal and open-ocean systems, deep-sea, hypersaline, Arctic and Antarctic ecosystems and others | [32] |
| Parabens | 4-hydroxybenzoate alkyl esters | Preventing the growth of yeasts, molds and gram-positive bacteria | The marine bacterial strain, A4B-17, genus Microbulbifer | Isolated from an ascidian | [81] |
| Surfactants, Emulsifiers, Thickeners, Stabilizers and Moistourising | |||||
| Polysaccharides | Chitin, chitosan and their derivatives | Moisturising, emulsifying, anti-microbial and adhesive properties, water resistance and absorption promoters | Marine fungi such as zygomycetes, chytridiomycetes, ascomycetes, basidiomycetes | Coastal and open-ocean systems, deep-sea, hypersaline, Arctic and Antarctic ecosystems and others | [82,83,84,85,86,87,88] |
| Protein polysaccharide complexes, glycolipids, lipopeptides | Dissolving the active compound in other ingredients, emulsifying, skin moisturising and delivery system. | Marine fungi and bacteria such as Actinobacter, Pseudomonas, Myroides, Streptomyces, Yarrowia, Rhodotorula, Bacillus, Azotobacter, Corynebacterium | Coastal and open-ocean systems, deep-sea, hypersaline, Arctic and Antarctic ecosystems and others | [89] | |
2.1. Mycosporine and Mycosporine-Like Amino Acids
Mycosporines and mycosporine-like amino acids (MAAs) are low molecular weight water-soluble molecules with great application in several fields of cosmetic and cosmeceutical industries. These compounds are synthesized and accumulated by a wide range of organisms such as cyanobacteria, prokaryotes and fungi andalgae, whereas other marine organisms (metazoans) obtain MAAs from their feed [20,90]. Available evidence suggests that these molecules are not exclusively involved in photoprotection, but can have a role in thermal, salt and desiccation stress while in fungi are involved in sporulation and germination processes [91,92,93]. Mycosporines are composed of either an aminocyclohexenone or an aminocycloheximine ring with nitrogen or imino alcohol substituents and absorb in the range of 310–320 nm [93,94]. Mycosporine-like amino acids (MAAs) are imine derivatives of mycosporines which contain an amino-cyclohexenimine ring linked to an amino acid, amino alcohol or amino group with absorption in the range of 320–360 nm [20]. MAAs are favored to mycosporines as photo-protective due to their wide spectrum of absorbance and the ability to dissipate UV radiation without producing reactive oxygen species (ROS) [20,95,96].
Previous investigations have revealed that fungal strains isolated from hypersaline waters and polar glacial ice are able to synthesize mycosporines, as well as unidentified yet UV-absorbing compounds (possibly MAAs, [26]; Table 1). In particular, mycosporine–glutaminol–glucoside and mycosporine–glutamicol–glucoside were detected in black yeasts Phaeotheca triangularis, Trimmatostroma salinum, Hortaea werneckii and Aureobasidium pullulans, as well in a basidiomycetous yeast, the Cryptococcus liquefaciens [26]. Despite previous studies revealing that bacteria might be able to synthesize MAAs, available information for these microorganisms is very limited. MAAs have been found in microorganisms including Pseudonocardia sp. strain P1 (Actinomycetales) and Micrococcus p. AK-334, whereas in other bacteria such as Actinosynnema mirum DSM 43827 only genes involved in MAAs biosynthesis have been identified [27]. These biosynthetic gene clusters were also expressed in engineered hosts (i.e., Streptomyces avermitilis SUKA22), which were able to accumulate different types of MAAs including shinorine (mycosporine-glycine-serine) and porphyra-334 (mycosporine-glycine-threonine) and a novel MAA [27].
The potential for cosmetics of mycosporines and MAAs, especially extracted from microalgae, is well known [25,91,97,98,99,100] and proved by several patents. However, only very few UV-screening and anti-aging products containing mycosporines and MAAs are commercially available (such as the MAA produced by the red alga Porphyra umbilicalis) [24,25,101], and to our knowledge no cosmetic containing such compounds from fungi and bacteria has been developed so far. Diverse synthetic analogues of MAAs (including analogues of mycosporine-glycine) have been tested for commercial purposes but most of them were not sufficiently stable for commercial application as sunscreen products [101].
2.2. Carotenoids
Carotenoids are the most common pigments in nature [102] and have several applications as colorants, food supplements and cosmetics/nutraceuticals; they are also used for medical and biotechnological purposes [103]. More than 750 carotenoids have been described, but lycopene, β-carotene, astaxanthin, zeaxanthin and lutein are the most important from a commercial point of view [28]. These pigments have diverse biological functions, therefore fit into a wide range of cosmetic and cosmeceutical applications [22,28]. Marine carotenoids have significant anti-oxidant and anti-inflammatory effects and may contribute to skin photo-protection and inhibit adverse processes induced or mediated by solar UV radiation. It has been suggested, indeed, that routine consumption or topical treatment of carotenoids such as lycopene, β-carotene and lutein may provide efficient protection against the harmful effects of solar UV radiation [101].
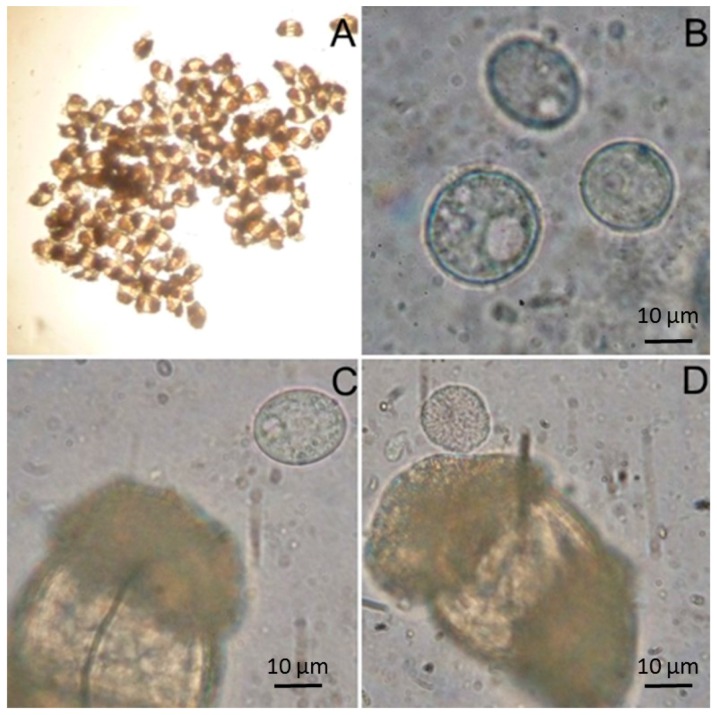
Despite carotenoids being photo-protective compounds, they are more used for their anti-oxidant properties in sunscreen formulations [22,104,105]. Besides photosynthetic organisms, heterotrophic bacteria and marine fungi (especially pigmented yeasts), thraustochytrids (generally defined as fungi-like protists) are also a relevant source of carotenoids [28,29,30,33]. However, these microorganisms have not been examined as extensively as the photosynthetic organisms (i.e., algae) for the production of carotenoids [106]. Among marine heterotrophic microorganisms, bacteria such as the genera Paracoccus and Agrobacterium have been reported as promising astaxanthin producers [28,31,32] (Table 1). Astaxanthin is also produced by several yeast species belonging to the genera Rhodotorula, Phaffia, Xanthophyllomyces [32]. Although the production from yeasts and bacteria is lower compared to algae, yeasts have higher growth rates and easier cultivation conditions [29,107]. Thraustochytrids have a wide geographical distribution from the polar to tropical regions, and they include planktonic and benthonic forms inhabiting various habitats such as sediments of mangroves, estuaries and deep-sea ecosystems (Figure 3, [34]). These fungi-like protists such as Thraustochytrium strains ONC-T18 and CHN-1, Thraustochytriidae sp. AS4-A1 (Ulkenia sp.) and Aurantiochytrium sp. KH105 synthesize different carotenoids including β-carotene, astaxanthin, zeaxanthin, cantaxanthin, phoenicoxanthin and echinenone [33]. Engineering approaches have allowed the increase in production of carotenoids (even nine-fold increased astaxanthin content production) such as in Aurantiochytrium sp. SK4 [35]. From this perspective, the development of genetic tools and genome sequencing of thraustochytrids are fundamental to expand our knowledge of these promising sources of carotenoids to be employed in cosmetic products.
Figure 3.
Marine thraustrochytrids associated with pollen grains (A,C,D) and free (B).
2.3. Benzodiazepine Alkaloids
Benzodiazepine alkaloids, e.g., circumdatins A–H, are widespread compounds produced by terrestrial and marine fungi. Circumdatin I, C and G have been isolated from the mycelium of a marine fungus of the genus Exophiala. These compounds showed high UV-A screening activity, exhibiting ED50 (i.e., effective dose for 50% of the tests) values of 98, 101 and 105 μM, and were more efficient of oxybenzone (ED50, 350 μM) than is currently used sunscreen filter [36].
3. Anti-Aging Products
Skin aging involves changes in skin physical properties creating visible signs on the skin surface due to the degradation of the extracellular matrix in both the epidermal and dermal layers. Anti-aging products are among the most marketed cosmetics/cosmeceuticals worldwide and the global anti-aging market is expected to reach USD 216.52 billion in 2021, growing at 7.5% (CAGR) from 2016 to 2021 (www.zionmarketresearch.com). Such personal care products, including face, hair and body treatments are widely used to contrast cutaneous dryness, roughness, the depth of wrinkles and loss of skin tone. Generally, all anti-aging formulations contain moisturizing substances. The maintenance of hydration, indeed, is pivotal for keeping skin functions. The external application of lipid compounds that have the ability to limit water loss or molecules that produce bonds with water may have the potential of mimicking the natural hydrating mechanisms of the skin. Among these substances, marine organisms produce several high molecular weight molecules such as polysaccharides, fatty acids (PUFA, sophorolipids, rhamnolipids and mannosylerythritol) and proteins (collagene) that are widely used in skin care (facial care, facial cleansing, body care, baby care) for their softening and smoothening effects on the skin. Several bioactive substances with anti-wrinkling action of marine origin are already produced on a large scale. Among these, exopolysaccharides (EPSs) and fatty acids are of great importance for anti-aging products (Table 1).
3.1. Exopolysaccharides
Among the bioactive substances with anti-wrinkling action of marine origin, polysaccharides of microbial origins, especially EPSs, are the most used. EPSs are high molecular weight carbohydrate polymers that in nature are involved in a variety of mechanisms, from attachment to intra- and inter-specific communication and competition [37]. EPSs are produced not only by bacteria but also by other microorganisms such as fungi and microalgae. However, bacteria are amenable to the largest production [38]. EPSs constitute a class of products with properties including emulsifying, thickening, absorption and gel formation [39,40].
In recent years, there has been a growing interest in isolating new EPSs particularly from extreme environments such as deep-sea hydrothermal vents, cold seeps, polar and hypersaline ecosystems [37,41,42,43]. Among the most important producers of EPS there are several taxa of bacteria and molds including Agrobacterium sp., Alcaligenes faecalis, Xanthomonas campestris, Bacillus sp., Zymonas mobilis and Aureobasidium pullulans [44] (Table 1).
EPSs (HYD657) secreted by the marine bacterium Alteromonas macleodii have already found application in cosmetics and are commercially available [16]. Similarly, a mixture of EPSs from Pseudoalteromonas sp. isolated from Antarctic waters is included in the formulation of anti-aging products. This mixture, obtained through fermentation, enhances the synthesis of collagen I, contributing to the amelioration of skin structural properties [16]. Other anti-aging products containing EPSs include those based on extracts from marine microbes Pseudoalteromonas antarctica and Halomonas eurihalina. Recently, the Vibrio diabolicus, a deep-vent marine bacterium, has been discovered to produce an exo saccharide (HE 800) structurally analogous to hyaluronic acid with unique functions that stimulate collagen structuring [41].
3.2. Fatty Acids
Fatty acids are known not only as dietary supplements, but they also have a broad spectrum of topical applications in cosmetics and cosmeceuticals thanks to their role in soft tissue repair and skin nourishment through stimulation of collagen production as well as anti-inflammatory and wound healing [108]. Among the different fatty acids, polyunsaturated fatty acids (PUFA), and specifically the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have been linked to several health benefits [109]. Most marine animals obtain long-chain PUFAs from their diets (i.e., as products of photosynthetic processes) and few are known to produce these compounds de novo (microalgae, bacteria, thraustochytrids and fungi). The main source of omega-3 fatty acids for human consumption is wild fish [45,46,110]. However, due to its decline and the consequent increase in price, to satisfy current demand of DHA and EPA research has started to focus on alternatives to fish oil such as oil from plants, algae, bacteria and fungi. The term “single-cell oils” (SCOs) refers to oils produced by single-cell microorganisms such as yeasts and molds [47]. SCOs produced by microorganisms offer many advantages compared to fish oil, including the higher growth rate and oil content and the presence of a number of natural anti-oxidants such as carotenoids that prevent omega-3 fatty acids oxidation [48]. Among marine microorganisms, thraustochytrids, fungi and bacteria have received lower attention although have great potential to produce these fatty acids. Thraustochytrids have been isolated from marine environments through the classic technique of the pine pollen grains as bait (Figure 3) [34], and quantified in the world’s oceans [10]. Since the 1990s they have been used for the industrial production of DHA due to their high production per unit of biomass and fast growth rate [49,50,51,52,111]. These microorganisms can accumulate more than 50% of their weight as lipid drops, with a concentration of DHA higher than 25% of the total lipids. The lipids of thraustochytrids contain specifically eicosapentaenoic acid (EPA), docosapentaenoic (DPA) and docosahexaenoic acid (DHA) and have a higher level of oxidative stability than that of fish oil. The development of refined techniques has been important for the cultivation, isolation and identification of thraustochytrids for industrial purposes. In particular, there is evidence that species belonging to Schizochytrium, Aurantiochytrium and Ulkenia isolated from several marine ecosystems, including sandy beaches and mangrove forests, are the major producers of DHA [52]. DHA-rich oils from thraustochytrids are currently on the market particularly for applications in nutraceuticals and aquaculture [33]. However, they also have a great potential for cosmetic and cosmeceutical applications.
Also, yeast species isolated from seawater (e.g., Rhodotorula mucilaginosa AMCQ8A) are capable of producing high biomass with high lipid yield [53,54]. Similarly, several oleaginous marine bacteria have been reported to produce important PUFAs (Table 1), such as the marine Moritella dasanensis [55]. PUFA-producing bacterial isolates are known to be associated with high-pressure, low-temperature, deep-sea habitats. In literature, species belonging to Shewanella and Colwellia genera have been reported to produce DHA and EPA [56]. However, to our knowledge, their use in cosmetic and cosmeceutical sectors has not been addressed yet.
3.3. Antioxidant Compounds
Antioxidant compounds are added to prevent oxidation of ingredients in cosmetic formulations. These compounds also have a fundamental role in protecting the skin from oxidation induced by reactive oxygen species (ROSs) due to natural oxidation occurring within the cells, stimulated by UV radiation and loss of skin moisture. At present, several synthetic anti-oxidants have been used in cosmetic and cosmeceutical products such as butylated hydroxyanisole, butylated hydroxytoluene, tertiary butyl hydroquinone and propyl gallate [31]. Since synthetic compounds might be toxic [79], natural anti-oxidants have been investigated to be used in cosmetics. Marine anti-oxidants include mycosporines, MAAs, carotenoids and other compounds that may serve multiple functions within cosmeceutical formulation [28,29,57,100].
MAAs may protect the skin not only against UV radiation but also exhibit a high anti-oxidant activity, scavenging superoxide anions and inhibiting lipid peroxidation [57,58,59,95]. The properties of MAAs as UV screens and ROS scavengers suggest that they could be used in sunscreen products [96]. Their roles as UV-absorbing and anti-oxidant compounds in human fibroblast cells have been rarely investigated [58]. However, previous studies revealed that mycosporine-glycine has strong anti-oxidant, anti-inflammatory and anti-aging activity providing new insights into the application of MAAs in the cosmetic/cosmeceutical sectors.
Carotenoids are known for their powerful anti-oxidant activity [112,113]. Astaxanthin is among the strongest anti-oxidants due to its structure and better biological activity than other anti-oxidants [60,113,114]. In addition, thanks to the discovery of new species, novel and rare carotenoids are being screened [87]. Two rare carotenoids with relevant anti-oxidant action (i.e., saproxanthin and myxol) have been isolated from new strains of marine bacteria belonging to the family Flavobacteriaceae [61] (Table 1). Saproxanthin or myxol addition to cosmetics might help to reinforce biological membranes, decreasing permeability to oxygen and enhancing protection against oxidation. The anti-oxidant activities of saproxanthin and myxol were even greater than those of the commonly used zeaxanthin and β-carotene [61]. However, these new and rare marine carotenoids require a thorough evaluation before their implementation within cosmeceutical products [105].
Marine fungi are an excellent source of anti-oxidant compounds. The marine fungus Acremonium sp., was found to produce four novel hydroquinone derivatives with significant anti-oxidant activity [62]. Similarly, the Epicoccum sp. isolated from the alga Fucus vesiculosus was found to produce an isobenzofuranone derivative (4,5,6-trihydroxy-7-methylphthalide) with high a,a-diphenyl-picrylhydrazyl (DPPH) radical scavenging effects [64]. Recently, eight secondary anti-oxidant metabolites were identified in Aspergillus wentii EN-48 isolated from brown algae [63]. The activities of these anti-oxidants were found to be considerably higher than the synthetic ones commonly used such as butylated hydroxytoluene [63]. Another interesting anti-oxidant is represented by the exopolysaccharide EPS2 isolated from the marine filamentous fungus Keissleriella sp. YS 4108 that displayed profound scavenging activities of superoxide radicals [65]. Other anti-oxidant compounds arediketopiperazine alkaloid, golmaenone and related alkaloid, as well as dihydroxy isoechinulin A and related echinulin, which have been isolated from the culture broth of the marine fungus Aspergillus sp. [66,67]. Golmaenone and related alkaloid exhibited a significant radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) with IC50 (i.e., the concentration which shows 50% inhibition) values of 20 μM, similarly to ascorbic acid (IC50, 20 μM). Furthermore, these compounds also displayed UV-A screening function with ED50 values of 90 and 170 μM, thus were more efficient than oxybenzone (ED50, 350 μM) [66].
4. Skin-Whitening Products
Public interest in skin-whitening cosmetics is increasing notably and this market is forecasted to reach USD 23 billion by 2020 [115]. Skin whitening refers to the use of natural or synthetic substances that provide an even pigmentation by reducing the melanin concentration in the skin. This practice may be driven by dermatological needs such as skin hyperpigmentation due to autoimmune conditions, exposure to UV radiation, genetic factors and hormonal changes that can induce overproduction of melanin in the skin [116]. Nowadays, skin whitening is more often practiced for aesthetic ends for a whiter and paler skin tone, as it is synonymous with youth, whereas darker skin is associated with lower social classes [117].
Melanin biosynthesis can be reduced by the inhibition of the tyrosinase enzyme, the inhibition of melanocytes’ metabolism and proliferation [118]. Numerous natural compounds from marine organisms (e.g., hydroquinones, kojic acid, azelaic acid and electron-rich phenols) have already been employed as skin whiteners and in particular as tyrosinase inhibitors, although several of these have been proven to have negative effects on human health [119,120]. Several compounds, including those from marine and marine bacteria and fungi, have been investigated for their employment in cosmetic products [116]. However, few microbial taxa have been investigated for the production of these inhibitors [121]. In recent years, research focused on marine microorganisms producing skin-whitening compounds such as kojic acid, methylene chloride, azelaic acid and others.
Kojic acid (5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one) is a water-soluble fungal secondary metabolite produced by Aspergillus and Penicillium species (Table 1). Other marine and marine fungi have been observed to produce such a compound, which owns anti-oxidant, anti-microbial and anti-inflammatory properties, and have significant tyrosinase inhibiting activity [68,122,123]. Two derivatives of kojic acid, kojic acid dimethyl ether and kojic acid monomethyl ether, as well as phomaligol A, were identified from broth of marine fungi Alternaria sp. isolated from marine green algae with tyrosinase inhibitory activity [69]. Similarly, azelaic acid (1,7-heptanedicarboxylic acid) is a reliable inhibitor of tyrosinase [74,75,124] produced by the fungus Malassezia sp., which inhabits almost every habitat in the marine environment [76]. However, there is no direct evidence on the industrial potential of these marine fungi as source of azelaic acid. Also, the fungal strain H1-7 of Trichoderma sp. has been found to produce tyrosinase inhibitors [125]. A competitive inhibitor of tyrosinase (5.4 × 105 U·mL−1) similar to the structure of homothallin II was isolated from T. viridae strain H1-7 from marine sediments. It inhibited the enzyme by binding to the copper active site. In addition, T. viridae strain H1-7 produced seven different melanogenesis inhibitors, but not all of them showed inhibition of tyrosinase [73]. The marine fungus Botrytis sp., isolated from the surface of the marine red alga Hyalosiphonia caespitosem, was found to produce an α-Pyrone derivate (6-[(E)-Hept-1-enyl]-α-pyrone) characterized by anti-tyrosinase activity (IC50 = 4.5 μM) [70]. Two compounds, 6-n-pentyl-α-pyrone and myrothenone A, from marine fungus Myrothecium sp. MFA 58, exhibited stronger activity than kojic acid (IC50 = 7.7 μM), with IC50 values of 0.8 and 6.6 μM, respectively [71]. Sesquiterpenes (i.e., 1β,5α,6α,14-tetraacetoxy-9α-benzoyloxy-7β H-eudesman-2β,11-diol and 4α,5α-diacetoxy-9α-benzoyloxy-7βH-eudesman-1β,2β,11, 14-tetraol) with tyrosinase inhibitory activities were isolated from the marine fungus Pestalotiopsis sp. Z233. [77]. Other compounds such as 2,4-dihydroxybenzoic acid, caffeic acid, benzene-acetic acid-α,4-dihydroxy, benzeneacetic acid-2-hydroxy, benzenepropanoic acid-α-hydroxy were isolated from the mycelia of Aspergillus unguis SPMD-EGY [126]. A recent patent based on chrysophanol as skin whitening extracted from the marine fungus Microsporum sp. was also developed (U.S. patent 20140056834A1).
So far, bacteria have been relatively less studied for their potential role in the production of skin-whitening compounds. However, a novel species of the marine bacteria Pseudomonas was found to produce methylene chloride, which reduced the pigmentation of human melanocytes and cultured skin cells by inhibiting the expression of tyrosinase [78]. In addition, tyrosinase inhibitors were reported from the marine bacterium Thalassotalea sp. PP2-459 isolated from a bivalve. The tyrosinase inhibitors identified as thalassotalic acid A, B and C, with IC50 values of 130, 470 and 280 μM, respectively. Thalassotalic acids are N-acyl dehydrotyrosine derivatives produced by this bacterium, thalassotalic acid A being comparable to the inhibitory activity of arbutin and could be used as a whitening agent or in preventing browning of foods. They suggest that the presence of a carboxylic acid and a straight aliphatic chain increased enzyme inhibition within this structural class of inhibitors [72].
In addition, the ketocarotenoid astaxanthin owns interesting depigmentation properties. There is evidence, indeed, that astaxanthin, which is also produced by marine yeasts and other taxa, can reduce melanin production by 40% in skin cells protecting skin from flakes and age spots [32]. To our knowledge, skin-whitening compounds used in cosmetic products are mostly extracted from terrestrial organisms although the huge number of marine skin whitening molecules offers new opportunities for the cosmetic market.
5. Additives and Other Active Ingredients of Cosmetic Products
Cosmetic and cosmeceutical products include, besides active ingredients (as described above), excipients and additives such as thickening agents, stabilizers, preservatives, colorants and perfumes. While the active ingredients, such as photo-protective compounds, are the main compounds that determine the function of the products, excipients have the purpose of dissolving the active compound in other ingredients. They regulate the delivery of the active ingredients as well as the aesthetical presentation of the product. Stabilizers maintain the stability of the cosmetic product during its lifetime and thickeners increase the viscosity of the product maintaining a proper texture of the cosmeceutical product, which is needed to distribute the active ingredients.
5.1. Antimicrobial Compounds and Preservatives
Preservatives are added to cosmetic products to prevent or delay their alteration and to protect them from microbial contamination. 4-hydroxybenzoate alkyl esters, called parabens, have been largely used as preservatives in food and cosmetic industries. The marine bacterial strain, A4B-17, belonging to the genus Microbulbifer isolated from an ascidian, was found to produce 4HBA and its esters. Such compounds were effective in preventing the growth of yeasts, molds and gram-positive bacteria [81]. Among anti-microbial compounds of marine origin, chitosan is widely used in cosmetics and cosmeceuticals. Chitosan is a polysaccharide of basic nature made mostly of glucosamine and a variable number of GlcNAc residues bound through β-1,4-linkages obtained from partial deacetylation of chitin [79]. Chitin is among the most abundant natural polysaccharides [127]. This polymer characterizes the exoskeleton of marine arthropods or the cell walls of fungi [121,122,123].
Chitosan shows anti-microbial activity against bacteria, viruses and fungi [80]. In addition to anti-microbial activity, chitosan and derivatives have several beneficial properties and have numerous applications in cosmeceuticals [128,129]. Unlike other disinfectants, chitosan has a higher anti-microbial and broader spectrum activity and lower toxicity towards humans. However, the actual mechanism of chitosan is not yet fully understood [130], and possible action mechanisms of chitosan and its derivatives have been proposed. Low molecular weight chitosan could penetrate cell walls of bacteria and then combine with DNA-inhibiting transcription [80,131,132]. High molecular weight chitosan could, instead, interact with cell surfaces and consequently alter cell permeability, impeding essential solutes transport into the cell [133,134]. Antifungal activity is attributed to the ability of chitosan to form a permeable film at the interface and has two functions: direct interference of fungal growth and activation of several defense processes [135].
Fungi could be a useful source of chitin and chitosan [128,136]. In fact, chitin constitutes 22%–44% of cell walls of fungi depending on the life stage or morphology, and types and amount of polysaccharides change greatly among taxa. Generally, Zygomycetes contain chitin/chitosan, Chytridiomycetes contain chitin/beta-glucan, Ascomycetes contain chitin/mannan/beta-glucan, and Basidiomycetes contain chitin/beta-glucan [137]. Chitin and chitosan from fungi lack proteins that could cause allergy reactions as in the case of crustacean-derived chitosan [80]. In addition, advances in fermentation technology suggest that the cultivation of selected fungi is less expensive and easier compared to other sources, hence reducing time and costs required for the chitosan production.
Carotenoids also have interesting anti-microbial properties [28]. Astaxanthin, for example, is of particular interest for anti-microbial activity, anti-wrinkle and anti-acne effects and can be used in products for skin conditioning to avoid dryness and decrease swelling under the eyes [32].
5.2. Surfactants, Emulsifiers, Thickeners, Stabilizers and Moisturising
Surfactants and emulsifiers are amphipathic compounds having both a hydrophilic and a hydrophobic part [138]. These compounds include protein polysaccharide complexes, glycolipids and lipopeptides from a wide range of marine and marine bacteria and fungi. Marine microorganisms including Acinetobacter, Arthrobacter, Pseudomonas, Halomonas, Myroides, Corynebacteria, Bacillus, Alteromonas sp. have been studied for production of biosurfactants and bioemulsifiers [89] (Table 1). Among these, the family of compounds derived from chitin display properties as emulsifiers because they are good polymer matrices for the delivery of bioactive compounds, especially of hydrophilic nature. Previous investigations provide evidence that chitosan has a greater water-binding capacity compared to methyl cellulose commonly used in cosmetic and cosmeceutical formulations, suggesting the suitability of high molecular weight chitosan as skin moisturizer and as delivery system in cosmeceutical preparations for anti-aging products [82]. Chitosan in fiber or film state, is mainly applied for improving the epithelial layer and increasing granular density of skin [83]. Indeed, it stimulates fibroblast production that in addition to their moisturizing and anti-microbial properties provide them with remarkable healing properties [84]. A chitosan derivative, carboxymethyl chitosan (CMCS), containing active hydroxyl, carboxyl and amine groups is soluble in water at neutral pH [85] and has anionic functionality, high viscosity, large hydrodynamic volumes, cation-binding characteristics, large osmotic pressures and gel-forming capabilities [83]. Due to all these characteristics, chitosan and chitosan derivatives are very attractive candidates for applications as absorption promoters and hydrating agents, anti-microbial and anti-oxidant agents, delivery system and stabilizers [86]. Chitin nanofibrils are also able to associate with other compounds such as vitamins, carotenoids and collagen, facilitating the penetration into the skin [84]. Due to the proprieties of bio-adhesivity, film formation, stiffness and curl retention to synthetic polymers, chitosan is also used as a hair care ingredient for shampoo, hair gel, hair colorants, hair sprays, permanent wave agents, hair colorants, styling lotions, hair sprays and hair tonics [87]. In addition, since some derivatives of chitin and chitosan (e.g., glyceryl chitosan) form foam and have an emulsifying action, they can be used directly in shampoo [138]. Even carotenoids, such as astaxanthin, have application in hair care products to protect hair from sunlight exposure and chemical damage. Marine-derived exopolysaccharides can also contribute to the cosmeceutical industry as thickening or gelling agents [31]. Marine bacterial EPS have been identified as novel thickening agents, which have the potential to be used as cosmeceutical ingredients.
6. Conclusions and Future Perspectives
Natural products represent the future of cosmetic and cosmeceutical industry. From this perspective, the biological properties of marine natural products have received increased attention. A wide variety of marine molecules, including those deriving from micro- and macro-algae and by-products of the fishing industry, are already on the cosmetic and cosmeceutical market. Conversely, molecules produced by marine bacteria and fungi with potential for these applications are still far from being fully exploited. In the present review, we have highlighted the alternative biomolecules produced by marine bacteria, fungi and fungi-like protists (thraustochytrids) and their advantages compared to other compounds commonly used in cosmetic products. For example, mycosporines and mycosporine-like amino acids produced by marine and marine fungi and bacteria are potentially very efficient natural UV-filters, with strong anti-oxidant activity. Even PUFA and carotenoids produced by marine thraustochytrids might have an important role in cosmetic applications. Similarly, anti-microbial compounds such as chitosan and derivatives extracted from marine fungi and bacteria offer a valid alternative to other synthetic preservatives (e.g., BHA, BHT), being not harmful for skin and environmental health. The natural and biodegradable surfactants extracted from marine microorganisms may reduce the use of synthetic surfactants, thus reducing the impacts on the marine environment.
The natural products described in this review are extracted from microorganisms inhabiting a wide spectrum of marine habitats. Molecules such as carotenoids, mycosporines and mycosporine-like amino acids are obtained preferentially from organisms subjected to strong light radiation, such as in tropical systems or in shallow water hypersaline pounds. Similarly, compounds with strong anti-oxidant potential are mainly obtained from microorganisms inhabiting extreme systems, such as hydrothermal vents. So far, most of these molecules have been identified in the more accessible and better explored portion of the oceans, such as shallow water ecosystems. However more than 95% of this realm, mostly represented by deep sea, is still uncharted. Therefore, the oceans still have enormous potential for the discovery and development of new compounds and bioactive molecules of microbial origin for technological and cosmetic purposes [2]. The fast discovery rate of previously unknown deep-sea habitats hints at a bright future for identifying new, sustainable and eco-friendly microbial molecules for human well-being.
Acknowledgments
This study was conducted within the frame of the projects MERCES (Marine Ecosystem Restoration in Changing European Seas), funded by the European Union’s Horizon 2020 research and innovation program (grant agreement No. 689518), and the Flagship Project RITMARE—The Italian Research for the Sea—coordinated by the Italian National Research Council and funded by the Italian Ministry of Education, University and Research within the National Research Program 2011–2013.
Author Contribution
C.C., R.D. and A.D. conceived the study. G.B. and F.M. collected available bibliographic information. C.C., G.B., F.M., A.D. and R.D. wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mora C., Tittensor D.P., Adl S., Simpson A.G.B., Worm B. How Many Species Are There on Earth and in the Ocean? PLoS Biol. 2011;9:e1001127-8. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corinaldesi C. New perspectives in benthic deep-sea microbial ecology. Front. Mar. Sci. 2015;2 doi: 10.3389/fmars.2015.00017. [DOI] [Google Scholar]
- 3.Danovaro R., Snelgrove P.V.R., Tyler P.A. Challenging the paradigms of deep-sea ecology. Trends Ecol. Evol. (Amst.) 2014;29:465–475. doi: 10.1016/j.tree.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Panno L., Bruno M., Voyron S., Anastasi A., Gnavi G., Miserere L., Varese G.C. Diversity, ecological role and potential biotechnological applications of marine fungi associated to the seagrass Posidonia oceanica. New Biotechnol. 2013;30:685–694. doi: 10.1016/j.nbt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Raghukumar S. Marine biotechnology: An approach based on components, levels and players. Indian J. Mar. Sci. 2011;40:609–619. [Google Scholar]
- 6.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2016;33:382–431. doi: 10.1039/C5NP00156K. [DOI] [PubMed] [Google Scholar]
- 7.Imhoff J.F., Labes A., Wiese J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011;29:468–482. doi: 10.1016/j.biotechadv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Bhatnagar I., Kim S.-K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs. 2010;8:2673–2701. doi: 10.3390/md8102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton A.D., Dutkiewicz S., Flierl G., Bragg J. Patterns of diversity in marine phytoplankton. Science. 2010;327:1509–1511. doi: 10.1126/science.1184961. [DOI] [PubMed] [Google Scholar]
- 10.Scheckenbach F., Hausmann K., Wylezich C., Weitere M., Arndt H. Large-scale patterns in biodiversity of microbial eukaryotes from the abyssal sea floor. Proc. Natl. Acad. Sci. USA. 2010;107:115–120. doi: 10.1073/pnas.0908816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manohar C.S., Raghukumar C. Fungal diversity from various marine habitats deduced through culture-independent studies. FEMS Microbiol. Lett. 2013;341:69–78. doi: 10.1111/1574-6968.12087. [DOI] [PubMed] [Google Scholar]
- 12.Le Calvez T., Burgaud G., Mahe S., Barbier G., Vandenkoornhuyse P. Fungal Diversity in Deep-Sea Hydrothermal Ecosystems. Appl. Environ. Microbiol. 2009;75:6415–6421. doi: 10.1128/AEM.00653-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stal L.J., Cretoiu M.S. In: The Marine Microbiome. Stal L.J., Cretoiu M.S., editors. Springer; Cham, Germany: 2016. [Google Scholar]
- 14.Jin L., Quan C., Hou X., Fan S. Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi. Mar. Drugs. 2016;14:76. doi: 10.3390/md14040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson B.S. New dimensions in natural products research: Cultured marine microorganisms. Curr. Opin. Biotechnol. 1995;6:284–291. doi: 10.1016/0958-1669(95)80049-2. [DOI] [Google Scholar]
- 16.Martins A., Vieira H., Gaspar H., Santos S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs. 2014;12:1066–1101. doi: 10.3390/md12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittié L., Fisher G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002;1:705–720. doi: 10.1016/S1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 18.Young A.R., Claveau J., Rossi A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017;76:S100–S109. doi: 10.1016/j.jaad.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Quiles D., Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015;83:158–170. doi: 10.1016/j.envint.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Carreto J.I., Carignan M.O. Mycosporine-Like Amino Acids: Relevant Secondary Metabolites. Chemical and Ecological Aspects. Mar. Drugs. 2011;9:387–446. doi: 10.3390/md9030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Q., Garcia-Pichel F. Microbial Ultraviolet Sunscreens. Nat. Rev. Microbiol. 2011;9:791–802. doi: 10.1038/nrmicro2649. [DOI] [PubMed] [Google Scholar]
- 22.Morabito K., Shapley N.C., Steeley K.G., Tripathi A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int. J. Cosmet. Sci. 2011;33:385–390. doi: 10.1111/j.1468-2494.2011.00654.x. [DOI] [PubMed] [Google Scholar]
- 23.Danovaro R., Bongiorni L., Corinaldesi C., Giovannelli D., Damiani E., Astolfi P., Greci L., Pusceddu A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008;116:441–447. doi: 10.1289/ehp.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallela R., Na-Young Y., Kim S.K. Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms. Mar. Drugs. 2010;8:1189–1202. doi: 10.3390/md8041189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rastogi R.P., Richa, Sinha R.P., Singh S.P., Häder D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010;37:537–558. doi: 10.1007/s10295-010-0718-5. [DOI] [PubMed] [Google Scholar]
- 26.Kogej T., Gostinčar C., Volkmann M., Gorbushina A.A., Gunde-Cimerman N. Mycosporines in Extremophilic Fungi—Novel Complementary Osmolytes? Environ. Chem. 2006;3:105–106. doi: 10.1071/EN06012. [DOI] [Google Scholar]
- 27.Miyamoto K.T., Komatsu M., Ikeda H. Discovery of Gene Cluster for Mycosporine-Like Amino Acid Biosynthesis from Actinomycetales Microorganisms and Production of a Novel Mycosporine-Like Amino Acid by Heterologous Expression. Appl. Environ. Microbiol. 2014;80:5028–5036. doi: 10.1128/AEM.00727-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vílchez C., Forján E., Cuaresma M., Bédmar F., Garbayo I., Vega J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs. 2011;9:319–333. doi: 10.3390/md9030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mata-Gómez L.C., Montañez J.C., Méndez-Zavala A., Aguilar C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014;13:12. doi: 10.1186/1475-2859-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sy C., Dangles O., Borel P., Caris-Veyrat C. Interactions between Carotenoids from Marine Bacteria and Other Micronutrients: Impact on Stability and Antioxidant Activity. Mar. Drugs. 2015;13:7020–7039. doi: 10.3390/md13117020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S.K. Marine Cosmeceuticals: Trends and Prospects. CRC Press; Boca Raton, FL, USA: 2011. [Google Scholar]
- 32.Ambati R.R., Phang S.M., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aasen I.M., Ertesvåg H., Heggeset T.M.B., Liu B., Brautaset T., Vadstein O., Ellingsen T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biotechnol. 2016;100:4309–4321. doi: 10.1007/s00253-016-7498-4. [DOI] [PubMed] [Google Scholar]
- 34.Raghukumar S. Ecology of the marine protists, the Labyrinthulomycetes (Thraustochytrids and Labyrinthulids) Eur. J. Protistol. 2002;38:127–145. doi: 10.1078/0932-4739-00832. [DOI] [Google Scholar]
- 35.Suen Y.L., Tang H., Huang J., Chen F. Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of Vitreoscilla hemoglobin. J. Agric. Food Chem. 2014;62:12392–12398. doi: 10.1021/jf5048578. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D., Yang X., Kang J.S., Choi H.D.M., Son B.W. Circumdatin I, a New Ultraviolet-A Protecting Benzodiazepine Alkaloid from a Marine Isolate of the Fungus Exophiala. J. Antibiot. 2008;61:40–42. doi: 10.1038/ja.2008.108. [DOI] [PubMed] [Google Scholar]
- 37.Poli A., Anzelmo G., Nicolaus B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs. 2010;8:1779–1802. doi: 10.3390/md8061779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nwodo U., Green E., Okoh A. Bacterial Exopolysaccharides: Functionality and Prospects. IJMS. 2012;13:14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suresh Kumar A., Mody K., Jha B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007;47:103–117. doi: 10.1002/jobm.200610203. [DOI] [PubMed] [Google Scholar]
- 40.Freitas F., Alves V.D., Reis M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011;29:388–398. doi: 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Courtois A., Berthou C., Guézennec J., Boisset C., Bordron A. Exopolysaccharides isolated from hydrothermal vent bacteria can modulate the complement system. PLoS ONE. 2014;9:e94965. doi: 10.1371/journal.pone.0094965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cambon-Bonavita M.-A., Raguénès G., Jean J., Vincent P., Guezennec J. A novel polymer produced by a bacterium isolated from a deep-sea hydrothermal vent polychaete annelid. J. Appl. Microbiol. 2002;93:310–315. doi: 10.1046/j.1365-2672.2002.01689.x. [DOI] [PubMed] [Google Scholar]
- 43.Colliec Jouault S., Chevolot L., Helley D., Ratiskol J., Bros A., Sinquin C., Roger O., Fischer A.-M. Characterization, chemical modifications and in vitro anticoagulant propertiesof an exopolysaccharide produced by Alteromonas infernus. Biochim. Biophys. Acta (BBA) Gen. Subj. 2001;1528:141–151. doi: 10.1016/S0304-4165(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 44.Donot F., Fontana A., Baccou J.C., Schorr-Galindo S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012;87:951–962. doi: 10.1016/j.carbpol.2011.08.083. [DOI] [Google Scholar]
- 45.Hutchings J.A., Reynolds J.D. Marine Fish Population Collapses: Consequences for Recovery and Extinction Risk. BioScience. 2004;54:297–309. doi: 10.1641/0006-3568(2004)054[0297:MFPCCF]2.0.CO;2. [DOI] [Google Scholar]
- 46.Adarme-Vega T.C., Thomas-Hall S.R., Schenk P.M. Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 2014;26:14–18. doi: 10.1016/j.copbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Ratledge C., Wynn J. Food Biotechnology. 2nd ed. Volume 20051848 CRC Press; Boca Raton, FL, USA: 2005. Microbial Production of Oils and Fats. Food Science and Technology. [Google Scholar]
- 48.Armenta R.E., Valentine M.C. Single-Cell Oils as a Source of Omega-3 Fatty Acids: An Overview of Recent Advances. J. Am. Oil Chem. Soc. 2012;90:167–182. doi: 10.1007/s11746-012-2154-3. [DOI] [Google Scholar]
- 49.Xie Y., Wang G. Mechanisms of fatty acid synthesis in marine fungus-like protists. Appl. Microbiol. Biotechnol. 2015;99:8363–8375. doi: 10.1007/s00253-015-6920-7. [DOI] [PubMed] [Google Scholar]
- 50.Sijtsma L., de Swaaf M.E. Biotechnological production and applications of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. Appl. Microbiol. Biotechnol. 2004;64:146–153. doi: 10.1007/s00253-003-1525-y. [DOI] [PubMed] [Google Scholar]
- 51.Burja A.M., Radianingtyas H., Windust A., Barrow C.J. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: Screening of strains and optimization of omega-3 production. Appl. Microbiol. Biotechnol. 2006;72:1161–1169. doi: 10.1007/s00253-006-0419-1. [DOI] [PubMed] [Google Scholar]
- 52.Cohen Z., Ratledge C. Single Cell Oils, Microbial and Algal Oils. Elsevier; Amsterdam, The Netherlands: 2015. [Google Scholar]
- 53.Kot A.M., Błażejak S., Kurcz A., Gientka I., Kieliszek M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016;100:6103–6117. doi: 10.1007/s00253-016-7611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A., Singh D., Barrow C.J., Puri M. Exploring potential use of Australian thraustochytrids for the bioconversion of glycerol to omega-3 and carotenoids production. Biochem. Eng. J. 2013;78:11–17. doi: 10.1016/j.bej.2013.04.028. [DOI] [Google Scholar]
- 55.Kim H.J., Park S., Lee J.M., Park S., Jung W., Kang J.-S., Joo H.M., Seo K.-W., Kang S.-H. Moritella dasanensis sp. nov., a psychrophilic bacterium isolated from the Arctic ocean. Int. J. Syst. Evolut. Microbiol. 2008;58:817–820. doi: 10.1099/ijs.0.65501-0. [DOI] [PubMed] [Google Scholar]
- 56.Abd Elrazak A., Ward A.C., Glassey J. Polyunsaturated fatty acid production by marine bacteria. Bioprocess Biosyst. Eng. 2013;36:1641–1652. doi: 10.1007/s00449-013-0936-0. [DOI] [PubMed] [Google Scholar]
- 57.Wada N., Sakamoto T., Matsugo S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants. 2015;4:603–646. doi: 10.3390/antiox4030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suh S.-S., Hwang J., Park M., Seo H., Kim H.-S., Lee J., Moh S., Lee T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs. 2014;12:5174–5187. doi: 10.3390/md12105174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De la Coba F., Aguilera A.J., Lòpez F.F., De Málaga U. Use of a Mycosporin-Type Amino Acid (Shinorine) as an Antioxidant. WO2007026038 A3. WIPO Patent. 2007 May 24;
- 60.Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 61.Shindo K., Kikuta K., Suzuki A., Katsuta A., Kasai H., Yasumoto-Hirose M., Matsuo Y., Misawa N., Takaichi S. Rare carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl. Microbiol. Biotechnol. 2007;74:1350–1357. doi: 10.1007/s00253-006-0774-y. [DOI] [PubMed] [Google Scholar]
- 62.Abdel-Lateff A., König G.M., Fisch K.M., Höller U., Jones P.G., Wright A.D. New antioxidant hydroquinone derivatives from the algicolous marine fungus Acremonium sp. J. Nat. Prod. 2002;65:1605–1611. doi: 10.1021/np020128p. [DOI] [PubMed] [Google Scholar]
- 63.Li X., Li X.-M., Xu G.-M., Li C.-S., Wang B.-G. Antioxidant metabolites from marine alga-derived fungus Aspergillus wentii EN-48. Phytochem. Lett. 2014:120–123. doi: 10.1016/j.phytol.2013.11.008. [DOI] [Google Scholar]
- 64.Abdel-Lateff A., Fisch K.M., Wright A.D., König G.M. A new antioxidant isobenzofuranone derivative from the algicolous marine fungus Epicoccum sp. Planta Med. 2003;69:831–834. doi: 10.1055/s-2003-43209. [DOI] [PubMed] [Google Scholar]
- 65.Sun C., Wang J.-W., Fang L., Gao X.-D., Tan R.X. Free radical scavenging and antioxidant activities of EPS2, an exopolysaccharide produced by a marine filamentous fungus Keissleriella sp. YS 4108. Life Sci. 2004;75:1063–1073. doi: 10.1016/j.lfs.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 66.Li Y., Li X., Kim S., Kang J.S., Choi H.D., Rho J.R., Son B.W. Golmaenone, a New Diketopiperazine Alkaloid from the Marine-Derived Fungus Aspergillus sp. Chem. Pharm. Bull. 2004;52:375–376. doi: 10.1248/cpb.52.375. [DOI] [PubMed] [Google Scholar]
- 67.Li Y., Li X., Kang J.S., Choi H.D., Son B.W. New Radical Scavenging and Ultraviolet-A Protecting Prenylated Dioxopiperazine Alkaloid Related to Isoechinulin A from a Marine Isolate of the Fungus Aspergillus. J. Antibiot. 2004;57:337–340. doi: 10.7164/antibiotics.57.337. [DOI] [PubMed] [Google Scholar]
- 68.Lee G.T., Lee S.Y., Jeong J.H., Jo B.K., Li X.F., Son B.W. Screening of tyrosinase inhibiting activity from the marine-derived fungus. Pigment Cell Res. 2003;16:604. doi: 10.1034/j.1600-0749.2003.83100.x. [DOI] [Google Scholar]
- 69.Li X., Jeong J.H., Lee K.T., Rho J.R., Choi H.D., Kang J.S., Son B.W. Gamma-pyrone derivatives, kojic acid methyl ethers from a marine—Derived fungus Alternaria sp. Arch. Pharm. Res. 2003;26:532–534. doi: 10.1007/BF02976876. [DOI] [PubMed] [Google Scholar]
- 70.Zhang D., Li X., Kang J.S., Choi H.D., Son B.W. A New α-Pyrone Derivative, 6-[(E)-Hept-1-enyl]-α-pyrone, with Tyrosinase Inhibitor Activity from a Marine Isolate of the Fungus Botrytis. ChemInform. 2007;38:1–3. doi: 10.1002/chin.200740195. [DOI] [Google Scholar]
- 71.Li X., Kim M.K., Lee U., Kim S.-K., Kang J.S., Choi H.D., Son B.W. Myrothenones A and B, Cyclopentenone Derivatives with Tyrosinase Inhibitory Activity from the Marine-Derived Fungus Myrothecium sp. Chem. Pharm. Bull. 2005;53:453–455. doi: 10.1248/cpb.53.453. [DOI] [PubMed] [Google Scholar]
- 72.Deering R.W., Chen J., Sun J., Ma H., Dubert J., Barja J.L., Seeram N.P., Wang H., Rowley D.C. N-acyl dehydrotyrosines, tyrosinase inhibitors from the marine bacterium Thalassotalea sp. PP2-459. J. Nat. Prod. 2016;79:447–450. doi: 10.1021/acs.jnatprod.5b00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuchiya T., Yamada K., Minoura K., Miyamoto K., Usami Y., Kobayashi T., Hamada-Sato N., Imada C., Tsujibo H. Purification and determination of the chemical structure of the tyrosinase inhibitor pro-duced by Trichoderma viride strain H1–7 from a marine environment. Biol. Pharm. Bull. 2008;31:1618–1620. doi: 10.1248/bpb.31.1618. [DOI] [PubMed] [Google Scholar]
- 74.Baliña L.M., Graupe K. The treatment of melasma. 20% azelaic acid versus 4% hydroquinone cream. Int. J. Dermatol. 1991;30:893–895. doi: 10.1111/j.1365-4362.1991.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 75.Rendon M., Berneburg M., Arellano I., Picardo M. Treatment of melasma. J. Am. Acad. Dermatol. 2006;54:S272–S281. doi: 10.1016/j.jaad.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 76.Amend A.S. From dandruff to deep-sea vents: Malassezia-like fungi are ecologically hyper-diverse. PLoS Pathog. 2014;10:e1004277. doi: 10.1371/journal.ppat.1004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu B., Wu X., Sun M., Li M. Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233. Mar. Drugs. 2013;11:2713–2721. doi: 10.3390/md11082713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang H.Y., Yoon T.-J., Lee G.J. Whitening effects of marine pseudomonas extract. Ann. Dermatol. 2011;23:144–149. doi: 10.5021/ad.2011.23.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Friedman M., Junja V.K. Review of Antimicrobial and Antioxidative Activities of Chitosans in Food. J. Food Prot. 2016 doi: 10.4315/0362-028X-73.9.1737. [DOI] [PubMed] [Google Scholar]
- 81.Peng W., Adachi K., Chen C., Kasai H., Kanoh K., Shizuri Y., Misawa N. Discovery of a Marine Bacterium Producing 4-Hydroxybenzoate and Its Alkyl Esters, Parabens. App. Environ. Microbiol. 2006:5556–5561. doi: 10.1128/AEM.00494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen R.H., Heh R.S. Skin hydration effects, physico-chemical properties and vitamin E release ratio of vital moisture creams containing water-soluble chitosans. Int. J. Cosmet. Sci. 2000;22:349–360. doi: 10.1111/j.1467-2494.2000.00036.x. [DOI] [Google Scholar]
- 83.Ito I., Osaki T., Ifuku S., Saimoto H., Takamori Y., Kurozumi S., Imagawa T., Azuma K., Tsuka T., Okamoto Y., et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014;101:464–470. doi: 10.1016/j.carbpol.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 84.Kumirska J., Weinhold M.X., Thöming J., Stepnowski P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers. 2011;3:1875–1901. doi: 10.3390/polym3041875. [DOI] [Google Scholar]
- 85.Mourya V.K., Inamdar N.N., Tiwari A. Carboxymethyl chitosan and its applications. Adv. Mater. Lett. 2010;1:11–33. doi: 10.5185/amlett.2010.3108. [DOI] [Google Scholar]
- 86.Gautier S., Xhauflaire-Uhoda E., Gonry P., Piérard G.E. Chitin-glucan, a natural cell scaffold for skin moisturization and rejuvenation. Int. J. Cosmet. Sci. 2008;30:459–469. doi: 10.1111/j.1468-2494.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 87.Senevirathne M., Ahn C.-B., Kim S.-K., Je J.-Y. Marine Cosmeceuticals: Trends and Prospects. Trends and Prospects; CRC Press; Boca Raton, FL, USA: 2011. Cosmeceutical Applications of Chitosan and Its Derivatives; pp. 169–178. [Google Scholar]
- 88.Kim K.-S., Bak S.-S. Marine Cosmeceuticals: Trends and Prospects. CRC Press; Boca Raton, FL, USA: 2011. Hair Biology and Care Product Ingredients from Marine Organisms. [Google Scholar]
- 89.Satpute S.K., Banat I.M., Dhakephalkar P.K., Banpurkar A.G., Chopade B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010;28:436–450. doi: 10.1016/j.biotechadv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Sinha R.P., Singh S.P., Häder D.-P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007;89:29–35. doi: 10.1016/j.jphotobiol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 91.Shick J.M., Dunlap W.C. Mycosporine-Like Amino Acids and Related Gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- 92.Volkmann M., Gorbushina A.A. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol. Lett. 2006;255:286–295. doi: 10.1111/j.1574-6968.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 93.Libkind D., Pérez P., Sommaruga R., Diéguez M.D.C., Ferraro M., Brizzio S., Zagarese H., Broock M.V. Constitutive and UV-inducible synthesis of photoprotective compounds (carotenoids and mycosporines) by freshwater yeasts. Photochem. Photobiol. Sci. 2004;3:281–286. doi: 10.1039/B310608J. [DOI] [PubMed] [Google Scholar]
- 94.Volkmann M., Whitehead K., Rütters H., Rullkötter J., Gorbushina A.A. Mycosporine-glutamicol-glucoside: A natural UV-absorbing secondary metabolite of rock-inhabiting microcolonial fungi. Rapid Commun. Mass Spectrom. 2003;17:897–902. doi: 10.1002/rcm.997. [DOI] [PubMed] [Google Scholar]
- 95.Řezanka T., Temina M., Tolstikov A.G., Dembitsky V.M. Natural microbial UV radiation filters—Mycosporine-like amino acids. Folia Microbiol. 2004;49:339–352. doi: 10.1007/BF03354663. [DOI] [PubMed] [Google Scholar]
- 96.Suh H.-J., Lee H.-W., Jung J. Mycosporine glycine protects biological systems against photodynamic damage by quenching singlet oxygen with a high efficiency. Photochem. Photobiol. 2003;78:109–113. doi: 10.1562/0031-8655(2003)078<0109:MGPBSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 97.Conde F.R., Churio M.S., Previtali C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000;56:139–144. doi: 10.1016/S1011-1344(00)00066-X. [DOI] [PubMed] [Google Scholar]
- 98.Torres A., Hochberg M., Pergament I., Smoum R., Niddam V., Dembitsky V.M., Temina M., Dor I., Lev O., Srebnik M., et al. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. FEBS J. 2004;271:780–784. doi: 10.1111/j.1432-1033.2004.03981.x. [DOI] [PubMed] [Google Scholar]
- 99.Whitehead K., Hedges J.I. Photodegradation and photosensitization of mycosporine-like amino acids. J. Photochem. Photobiol. B Biol. 2005;80:115–121. doi: 10.1016/j.jphotobiol.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Oren A., Gunde-Cimerman N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007;269:1–10. doi: 10.1111/j.1574-6968.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 101.Cardozo K.H.M., Guaratini T., Barros M.P., Falcão V.R., Tonon A.P., Lopes N.P., Campos S., Torres M.A., Souza A.O., Colepicolo P., et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;146:60–78. doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 102.Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids. Birkhäuser; Basel, Switzerland: 2012. [Google Scholar]
- 103.Kirti K., Amita S., Priti S., Mukesh Kumar A., Jyoti S. Colorful World of Microbes: Carotenoids and Their Applications. Adv. Biol. 2014;2014:1–13. doi: 10.1155/2014/837891. [DOI] [Google Scholar]
- 104.Junghans A., Sies H., Stahl W. Macular Pigments Lutein and Zeaxanthin as Blue Light Filters Studied in Liposomes. Arch. Biochem. Biophys. 2001;391:160–164. doi: 10.1006/abbi.2001.2411. [DOI] [PubMed] [Google Scholar]
- 105.Miyashita K. Function of marine carotenoids. Forum Nutr. 2009;61:136–146. doi: 10.1159/000212746. [DOI] [PubMed] [Google Scholar]
- 106.Maoka T. Carotenoids in Marine Animals. Mar. Drugs. 2011;9:278–293. doi: 10.3390/md9020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bumbak F., Cook S., Zachleder V., Hauser S., Kovar K. Best practices in heterotrophic high-cell-density microalgal processes: Achievements, potential and possible limitations. Appl. Microbiol. Biotechnol. 2011;91:31–46. doi: 10.1007/s00253-011-3311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ziboh V.A., Miller C.C., Cho Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000;71:361S–366S. doi: 10.1093/ajcn/71.1.361s. [DOI] [PubMed] [Google Scholar]
- 109.McCusker M.M., Grant-Kels J.M. Healing fats of the skin: The structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin. Dermatol. 2010;28:440–451. doi: 10.1016/j.clindermatol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 110.Covington M.B. Omega-3 fatty acids. Am. Fam. Physician. 2004;70:133–140. [PubMed] [Google Scholar]
- 111.Raghukumar C. Marine fungal biotechnology: An ecological perspective. Fungal Divers. 2008;31:19–35. [Google Scholar]
- 112.Alvarez-Rivera G., Llompart M., Garcia-Jares C., Lores M. Identification of unwanted photoproducts of cosmetic preservatives in personal care products under ultraviolet-light using solid-phase microextraction and micro-matrix solid-phase dispersion. J. Chromatogr. A. 2015;1390:1–12. doi: 10.1016/j.chroma.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 113.Llewellyn C., Galley E., Council N.E.R. Personal Care Compositions. WO0239974. Patent. 2002 May 23;
- 114.Gammone M., Riccioni G., D’Orazio N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs. 2015;13:6226–6246. doi: 10.3390/md13106226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yuan J.-P., Peng J., Yin K., Wang J.-H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- 116.Shindo K., Misawa N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs. 2014;12:1690–1698. doi: 10.3390/md12031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017;31:403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burger P., Landreau A., Azoulay S., Michel T., Fernandez X. Skin Whitening Cosmetics: Feedback and Challenges in the Development of Natural Skin Lighteners. Cosmetics. 2016;3:36. doi: 10.3390/cosmetics3040036. [DOI] [Google Scholar]
- 119.Hunter M. The Persistent Problem of Colorism: Skin Tone, Status, and Inequality. Sociol. Compass. 2007;1:237–254. doi: 10.1111/j.1751-9020.2007.00006.x. [DOI] [Google Scholar]
- 120.Yun E.J., Lee S., Kim J.H., Kim B.B., Kim H.T., Lee S.H., Pelton J.G., Kang N.J., Choi I.-G., Kim K.H. Enzymatic production of 3,6-anhydro-l-galactose from agarose and its purification and in vitro skin whitening and anti-inflammatory activities. Appl. Microbiol. Biotechnol. 2012;97:2961–2970. doi: 10.1007/s00253-012-4184-z. [DOI] [PubMed] [Google Scholar]
- 121.Cristaudo A., D’Ilio S., Gallinella B., Mosca A., Majorani C., Violante N., Senofonte O., Morrone A., Petrucci F. Use of potentially harmful skin-lightening products among immigrant women in Rome, Italy: A pilot study. Dermatology (Basel) 2013;226:200–206. doi: 10.1159/000348706. [DOI] [PubMed] [Google Scholar]
- 122.Rana J., Diwakar G., Qiang H., Li T., Scholten J., Llc A.B.G.I. Topical Composition and Method for Skin Lightening. WO2013169634 A2. U.S. Patent. 2013 Nov 14;
- 123.Chang T.-S. An updated review of tyrosinase inhibitors. IJMS. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balboa E.M., Conde E., Soto M.L., Pérez-Armada L., Domínguez H. Cosmetics from Marine Sources. In: Kim S.-K., editor. Springer Handbook of Marine Biotechnology. Springer; Berlin/Heidelberg, Germany: 2015. pp. 1015–1042. [Google Scholar]
- 125.Hong J.-H., Jang S., Heo Y., Min M., Lee H., Lee Y., Lee H., Kim J.-J. Investigation of Marine-Derived Fungal Diversity and Their Exploitable Biological Activities. Mar. Drugs. 2015;13:4137–4155. doi: 10.3390/md13074137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fitton A., Goa K.L. Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs. 1991;41:780–798. doi: 10.2165/00003495-199141050-00007. [DOI] [PubMed] [Google Scholar]
- 127.Tharanathan R.N., Kittur F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003;43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 128.Farka V. Fungal cell walls: Their structure, biosynthesis and biotechnological aspects. Acta Biotechnol. 1990;10:225–238. doi: 10.1002/abio.370100303. [DOI] [Google Scholar]
- 129.Ruiz-Herrera J. Fungal Cell Wall. CRC Press; Boca Raton, FL, USA: 2012. [Google Scholar]
- 130.Nadarajah K., Kader J., Mazmira M., Paul D.C. Production of Chitosan by Fungi. Pak. J. Biol. Sci. 2001;4:263–265. [Google Scholar]
- 131.Ruocco N., Costantini S., Guariniello S., Costantini M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules. 2016;21:551. doi: 10.3390/molecules21050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sudarshan N.R., Hoover D.G., Knorr D. Antibacterial action of chitosan. Food Biotechnol. 1992;6:257–272. doi: 10.1080/08905439209549838. [DOI] [Google Scholar]
- 133.Choi B.-K., Kim K.-Y., Yoo Y.-J., Oh S.-J., Choi J.-H., Kim C.-Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents. 2001;18:553–557. doi: 10.1016/S0924-8579(01)00434-4. [DOI] [PubMed] [Google Scholar]
- 134.Eaton P., Fernandes J.C., Pereira E., Pintado M.E., Xavier Malcata F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy. 2008;108:1128–1134. doi: 10.1016/j.ultramic.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 135.Bai R.-K., Huang M.-Y., Jiang Y.-Y. Selective permeabilities of chitosan-acetic acid complex membrane and chitosan-polymer complex membranes for oxygen and carbon dioxide. Polym. Bull. 1988;20:83–88. doi: 10.1007/BF00262253. [DOI] [Google Scholar]
- 136.Ghormade V., Pathan E.K., Deshpande M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017 doi: 10.1016/j.ijbiomac.2017.01.112. [DOI] [PubMed] [Google Scholar]
- 137.Abdel-Mohsen A.M., Jancar J., Massoud D., Fohlerova Z., Elhadidy H., Spotz Z., Hebeish A. Novel chitin/chitosan-glucan wound dressing: Isolation, characterization, antibacterial activity and wound healing properties. Int. J. Pharm. 2016;510:86–99. doi: 10.1016/j.ijpharm.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 138.McClements D.J., Gumus C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016;234:3–26. doi: 10.1016/j.cis.2016.03.002. [DOI] [PubMed] [Google Scholar]