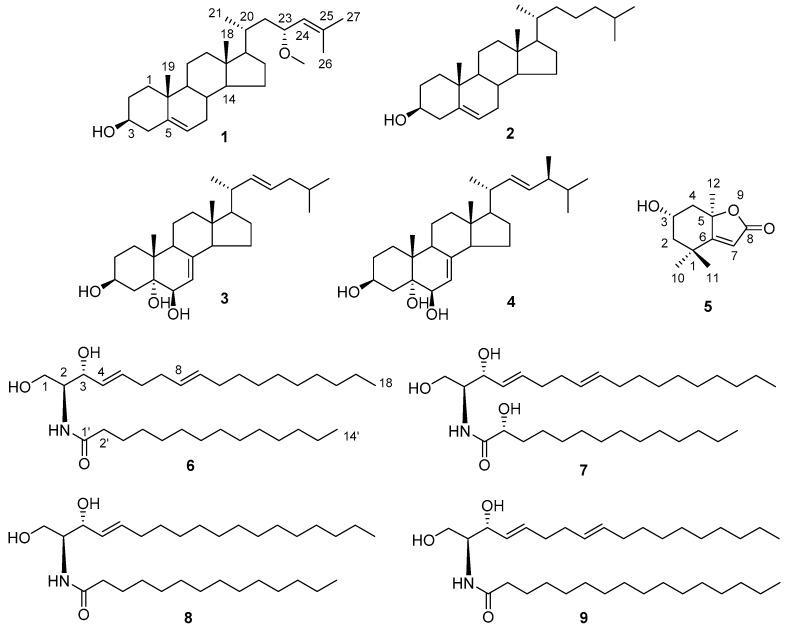
Abstract
A new sterol, (23R)-methoxycholest-5,24-dien-3β-ol (1), two new ceramides, (2S,3R,4E,8E)-2-(tetradecanoylamino)-4,8-octadecadien-l,3-diol (6) and (2S,3R,2′R,4E,8E)-2-(tetradecanoylamino)-4,8-octadecadien-l,3,2′-triol (7), together with three known sterols (2–4), a lactone (5) and two ceramides (8,9), were isolated from the marine bryozoan Cryptosula pallasiana, collected at Huang Island of China. The structures of the new compounds were elucidated by extensive spectroscopic analyses, chemical methods and quantum electronic circular dichroism (ECD) calculations. Among the isolated compounds, sterol 1 possessed a rare side chain with a methoxy group at C-23, and a double bond between C-24 and C-25. Ceramides 6 and 7 possessed 14 carbons in their long-chain fatty acid base (FAB), which were different from the normal ceramides with 16 carbons in the FAB. Moreover, compounds 5 and 8 were isolated for the first time from marine bryozoans. Compounds 1–9 were evaluated for their cytotoxicity against human tumor cell lines HL-60, Hep-G2 and SGC-7901. The results showed that lactone 5 appears to have strong cytotoxicity against the test tumor cell lines, with IC50 values from 4.12 μM to 7.32 μM, and sterol 1 displayed moderate cytotoxicity with IC50 values between 12.34 μM and 18.37 μM, while ceramides 6–9 showed weak cytotoxicity with IC50 ranging from 21.13 μM to 58.15 μM.
Keywords: marine bryozoan, Cryptosula pallasiana, sterol, ceramides, cytotoxicity
1. Introduction
Marine bryozoans are important sources for the discovery of new bioactive secondary metabolites. There are three reports containing 19 new metabolites isolated from this understudied phylum in the last year alone [1]. Modern phytochemical and pharmacological investigations of marine bryozoans demonstrated that secondary metabolites, including macrolides, alkaloids, sterols, as well as halogen-containing compounds, etc., exhibited remarkable cytotoxic activities on tumor cell lines, such as human myeloid leukemia HL-60, human leukemia U937, murine lymphocytic leukemia P388, and so on [2,3]. Cryptosula pallasiana is an encrusting colonial marine bryozoan, belonging to the class Gymnolaemata, order Cheilostomata and family Cryptosulidae. In our previous investigation on the bioactive compounds from this marine bryozoan, 16 alkaloids, 13 sterols, three aromatic compounds and two glycerol derivatives have been identified [4,5,6]. In the course of our ongoing discovery of new biologically active secondary metabolites from marine bryozoans, C. pallasiana, collected from the coasts of Huang Island in Qingdao City, Shandong Province of China, was investigated. We report herein the isolation and structure elucidation of three new compounds including one sterol (1) and two ceramides (6 and 7), along with six known secondary metabolites including three sterols (2–4), one lactone (5) and two ceramides (8,9). The structures of the known compounds were identified by comparison of their physical and spectroscopic data with those reported in the literature (Figure 1). In addition, the cytotoxicity of the isolated compounds against human tumor cell lines HL-60, Hep-G2 and SGC-7901 are also described.
Figure 1.
Structures of compounds 1–9 from marine bryozoan C. pallasiana.
2. Results
The 95% EtOH extract of C. pallasiana was concentrated and partitioned with petroleum ether, CCl4 and n-BuOH, respectively. The resulting CCl4 portion was separated by column chromatography (CC) and high-performance liquid chromatography (HPLC) to afford a new sterol (1) and two new ceramides (6 and 7), along with six known compounds (2–5, 8 and 9) (Figure 1). The known compounds were identified as cholest-5-en-3β-ol (2) [7], (22E)-cholesta-7,22-dien-3β,5α,6β-triol (3) [8], (24S,22E)-methylcholesta-7,22-dien-3β,5α,6β-triol (4) [8], loliolide (5) [9], (2S,3R,4E)-2-(tetradecanoylamino)-4-octadecen-l,3-diol (8) [10] and (2S,3R,4E,8E)-2-(hexadecanoylamino)-4,8-octadecadien-l,3-diol (9) [11], respectively, by comparison of their spectroscopic data (see supplementary information) with those reported in the literature.
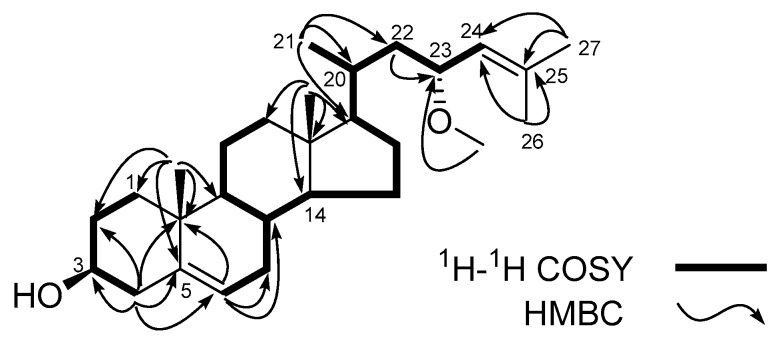
Compound 1 was obtained as a white amorphous powder. Its molecular formula was determined as C28H46O2 on the basis of the positive high-resolution electrospray ionization mass spectroscopy (HRESIMS) m/z 437.3395 [M + Na]+ (C28H46O2Na, calcd. for 437.3396). The 1H-NMR spectrum of 1 showed two tertiary methyl resonance signals at δH 0.73, s (H3-18) and 1.00, s (H3-19), three secondary methyl groups at δH 0.95, d, J = 5.9 Hz (H3-21), δH 1.67, d, J = 1.0 Hz (H3-26) and 1.74, d, J = 1.1 Hz (H3-27), one methoxy group at δH 3.21 s (OMe-23), two oxymethine protons at δH 3.52, m, (H-3) and 3.94, dt, J = 9.3, 3.1 Hz (H-23), and two tri-substituted olefinic protons at δH 5.35, t, J = 2.5 Hz (H-6) and 5.03, td, J = 9.0, 2.6, 1.3 Hz (H-24). The 13C-NMR spectrum showed 28 carbon resonances, which were assigned by distortionless enhancement by polarization transfer (DEPT) and heteronuclear single quantum coherence (HSQC) spectra to one methoxy carbon at δC 55.7 (OMe-23), five methyl (C-18, C-19, C-21, C-26 and C-27), nine methylene sp3 (C-1, C-2, C-4, C-7, C-11, C-12, C-15, C-16 and C-22), seven methine sp3 (C-3, C-8, C-9, C-14, C-17, C-20 and C-23) including two hydroxymethines C-3 (δC 71.8) and C-23 (δC 74.8), two quaternary sp3 carbons (C-10 and C-13), two methine sp2 carbons C-6 (δC 121.7) and C-24 (δC 127.0), and two quaternary sp2 carbons C-5 (δC 140.7) and C-25 (δC 134.5). These data suggested that 1 possessed a 3-hydroxy ∆5-steroid nucleus with a methoxy group and double bond in its side chain. Further structural information for compound 1 was obtained by 2D NMR analysis including 1H-1H correlation spectroscopy (COSY) and heteronuclear multiple bond correlation (HMBC) spectra (Figure 2). The observation of correlations from H-23 to H2-22 and H-24 in the 1H-1H COSY spectrum, and correlations from methoxy proton to C-23, from H2-22 to C-23, from H3-26 to C-24 and C-25, and from H3-27 to C-24 and C-25 in the HMBC spectrum indicated that the methoxy group is on C-23, and the tri-substituted double bond is between C-24 and C-25. Based on the above evidences, the planar structure of sterol 1 could be established as 23-methoxycholest-5,24-dien-3-ol.
Figure 2.
Key 1H-1H COSY and HMBC correlations of 1.
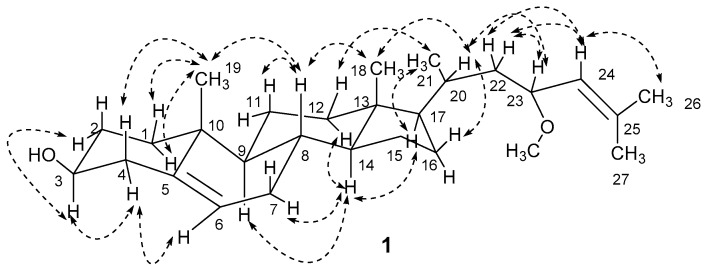
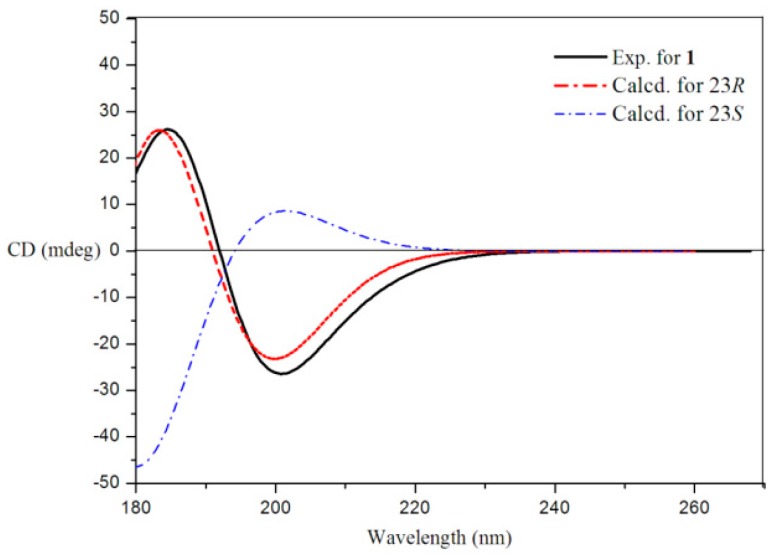
The stereochemistry of 1 was established by extensive analysis of nuclear overhauser effect spectroscopy (NOESY) spectrum and quantum electronic circular dichroism (ECD) calculations. The 1H-NMR assignment of α and β protons for compound 1 (Table 1) was based on careful analysis of the NOESY spectrum as well as comparison with the reference [5] (Figure 3). The NOESY correlations from H3-19 to H-1α and H-1β, from H-3 to H-4α and H-2α, and from H-4α to H-6, suggested that the A-ring exists in a chair conformation. The 3-hydroxyl group was oriented in an equatorial position and therefore in a β-orientation, which was confirmed by the chemical shift of C-3 (δC 71.8 > 70.0) [12]. Similarly, the presence of a cross-peak between H-14 and H-17 implied that the C-17 side chain was in a β-position. The correlations from H3-21 to H-12β and H-17, and from H-20 to H-16β and H3-18 indicating the α-orientation of the methyl group at C-20, and determined the C-20S configuration for 1. The C-23R configuration was determined by ECD calculations of 23R and 23S for 1 using the time-dependent density functional theory (TD-DFT) method at the B3LYP/6-31G (d) level [13,14,15,16]. The preliminary conformational distribution search was performed by HyperChem 7.5 software (Hypercube, Inc., Gainesville, FL, USA). The corresponding minimum geometries were fully optimized by using DFT at the B3LYP/6-31G (d) levels implemented in the Gaussian 09 program package. The ECD calculations were performed after optimization of the selected conformers at the B3LYP/6-31G (d) [17,18]. The result showed that the experimental circular dichroism (CD) curve of 1 was consistent with the calculated ECD curve of 23R, while opposite to that of 23S (Figure 4). The NOESY correlations from H-23 to H-20, H-22α and H3-26, and from H-24 to H-22α, H-22β and H3-27, indicated the α-orientation of the methoxy group at C-23, which also confirmed the 23R configuration. Thus, sterol 1 was unambiguously elucidated as (23R)-methoxycholest-5,24-dien-3β-ol.
Table 1.
1H- and 13C-NMR (500 and 125 MHz, CDCl3) data of 1 a.
| No. | δC | δH mult., J in Hz | No. | δC | δH mult., J in Hz |
|---|---|---|---|---|---|
| 1 | 37.3 | α 1.86, m | 13 | 42.5 | – |
| β 1.08, m | 14 | 56.9 | 1.04, m | ||
| 2 | 31.7 | α 1.84, m | 15 | 24.3 | α 1.57, m |
| β 1.50, m | β 1.07, m | ||||
| 3 | 71.8 | 3.52, m | 16 | 28.2 | α 1.83, m |
| 4 | 42.3 | α 2.28, m | β 1.30, m | ||
| β 2.23, m | 17 | 56.8 | 1.01, m | ||
| 5 | 140.7 | – | 18 | 12.0 | 0.73, s |
| 6 | 121.7 | 5.35, t (2.5) | 19 | 19.4 | 1.00, s |
| 7 | 31.9 | α 1.50, m | 20 | 32.5 | 1.66, m |
| β 1.97, m | 21 | 18.7 | 0.95, d (5.9) | ||
| 8 | 31.9 | 1.50, m | 22 | 42.5 | α 0.92, m |
| β 1.68, m | |||||
| 9 | 50.1 | 0.93, m | 23 | 74.8 | 3.94, dt (9.3, 3.1) |
| 10 | 36.5 | – | 24 | 127.0 | 5.03, td (9.0, 2.6, 1.3) |
| 11 | 21.1 | α 1.43, m | 25 | 134.5 | – |
| β 1.49, m | 26 | 18.2 | 1.67, d (1.0) | ||
| 12 | 39.8 | α 1.16, m | 27 | 25.9 | 1.74, d (1.1) |
| β 2.03, m | 23-OCH3 | 55.7 | 3.21, s |
a Assignments based on DEPTs and HSQC.
Figure 3.
Key NOESY correlations ( ) for 1.
) for 1.
Figure 4.
Experimental and calculated ECD spectra for 1.
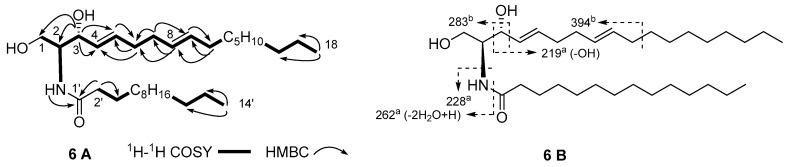
Compound 6 was obtained as a white amorphous powder. The (+)-HRESIMS showed a pseudomolecular ion peak at m/z 530.4545 [M + Na]+ (calcd. for C32H61NO3Na, 530.4549), which, together with the pseudomolecular ion peak at m/z 506 [M − H]− in the (‒)-ESIMS spectrum, enabled the determination of the molecular formula for 6 as C32H61NO3. A careful analysis of the 1H- and 13C-NMR spectra of 6 (Table 2) by DEPT and HSQC experiments revealed the presence of an amide group δC 174.0 (C-1′), δH 6.25, d, J = 7.5 Hz (NH), a nitrogen- or oxygen-bearing methine δC 54.5 (C-2), δH 3.95, dd, J = 11.3, 3.5 Hz (H-2), an oxymethylene sp3 δC 62.5 (C-1), δH 3.70, dd, J = 11.3, 3.3 Hz and 3.90, m (H2-1), an oxymethine sp3 δC 74.7 (C-3), δH 4.32, m (H-3), and four methine sp2 δC 129.2 (C-4), δH 5.54, dd, J = 15.4, 6.4 Hz (H-4); δC 133.6 (C-5), δH 5.78, dt, J = 15.4, 6.6 Hz (H-5); δC 131.4 (C-8), δH 5.42, dt, J = 15.2, 6.3 Hz (H-8) and δC 129.0 (C-9), δH 5.37, dt, J = 15.2, 6.4 Hz (H-9), which together with a series of alkyl proton signals (δC 29.2–29.7, δH 1.26, br s), indicated an existence of a sphinga-4,8-diene skeleton for 6 [19]. The 1H-1H COSY correlations from H-2 to NH, H2-1 and H-3, and the HMBC correlations from NH to C-1′, from H2-2′ to C-1′ and C-3′, and from H-3 to C-1 and C-2 confirmed the sphingosine skeleton. That the two double bonds were placed on C-4 and C-8 was supported by 1H-1H COSY correlations of H-3/H-4, H-4/H-5, H-5/H2-6, H2-6/H2-7, H2-7/H-8, H-8/H-9 and H-9/H2-10, as well as by HMBC correlations from H-3 to C-4 and C-5, from H-8 to C-6 and C-7, and from H2-10 to C-8 and C-9 (Figure 5A). That the sphingoid long chain base (LCB) and the long chain fatty acid base (FAB) of the amide portion contained 18 and 14 carbons, respectively, was evidenced by the electrospray ionization mass spectroscopy (ESI-MS) fragments at m/z 219, 228, 262, 283, 394 (Figure 5B) [20]. The number of the carbon for the FAB was further validated through electron impact mass spectrometry (EI-MS) molecular ion at m/z 242 [M]+ for the methyl tetradecanoate obtained after methanolysis of 6 with 5% HCl-MeOH. Therefore, the planar structure and the key connectivities of 6 were established.
Table 2.
1H- and 13C-NMR (500 and 125 MHz, CDCl3) of 6 and 7 a.
| No. | 6 | 7 | ||
|---|---|---|---|---|
| δC | δH mult., J in Hz | δC | δH mult., J in Hz | |
| 1 | 62.5 | 3.70, dd (11.3, 3.3) | 62.2 | 3.72, dd (11.0, 3.0) |
| 3.90, m | 3.89, m | |||
| 2 | 54.5 | 3.95, dd (11.3, 3.5) | 54.4 | 3.92, br d (11.0) |
| 3 | 74.7 | 4.32, m | 74.7 | 4.30, m |
| 4 | 129.2 | 5.54, dd (15.4, 6.4) | 129.2 | 5.52, dd (15.5, 6.0) |
| 5 | 133.6 | 5.78, dt (15.4, 6.6) | 133.5 | 5.75, dt (15.5, 6.1) |
| 6 | 32.3 | 1.97, q (6.7) | 32.4 | 1.96, q (6.5) |
| 7 | 32.1 | 2.12, m | 32.2 | 2.11, m |
| 8 | 131.4 | 5.42, dt (15.2, 6.3) | 131.3 | 5.41, m |
| 9 | 129.0 | 5.37, dt (15.2, 6.4) | 129.0 | 5.38, m |
| 10 | 32.6 | 2.08, m | 32.2 | 2.07, m |
| 11~15 | 29.2–29.7 | 1.26, br s | 29.2–29.7 | 1.26, br s |
| 16 | 31.9 | 1.26, br s | 32.0 | 1.26, br s |
| 17 | 22.7 | 1.26, br s | 22.7 | 1.26, br s |
| 18 | 14.1 | 0.88, t (6.8) | 14.1 | 0.87, t (7.0) |
| 1′ | 174.0 | – | 175.0 | – |
| 2′ | 36.9 | 2.23, t (7.5) | 72.4 | 4.12, dd (7.8, 3.4) |
| 3′ | 25.8 | 1.64, m | 34.8 | 2.16, t (3.3) |
| 4′ | 29.2–29.7 | 1.26, br s | 31.8 | 1.26, br s |
| 5′ | 29.2–29.7 | 1.26, br s | 25.1 | 1.63, m |
| 6′~11′ | 29.2–29.7 | 1.26, br s | 29.2–29.7 | 1.26, br s |
| 12′ | 31.9 | 1.26, br s | 31.9 | 1.26, br s |
| 13′ | 22.7 | 1.26, br s | 22.7 | 1.26, br s |
| 14′ | 14.1 | 0.88, t (6.8) | 14.1 | 0.87, t (7.0) |
| NH | – | 6.25, d (7.5) | – | 6.20, d (7.8) |
a Assignments based on DEPTs and HSQC.
Figure 5.
Key 1H-1H COSY and HMBC correlations (A), and main ESI-MS fragment ions (B) of 6. (a positive fragment ions; b negative fragment ions).
That the geometry of C-4/C-5 and C-8/C-9 double bonds are trans was based on the vicinal coupling constants J4,5 = 15.4 Hz and J8,9 = 15.2 Hz, as well as the chemical shifts of C-6 (δC 32.3) and C-7 (δC 32.1) [21]. The absolute stereochemistry of 6 was determined as d-(−)-erythro-2S,3R configuration based on the chemical shifts of C-2 (δC 54.5) and C-3 (δC 74.7), which were in good agreement with those reported for N-palmitoyl-d-erythro-(2S,3R)-octadecasphinga-4(E)-en (C-2 for 54.5 and C-3 for 74.7) [22,23]. Based on the above analyses, the structure of 6 was determined as (2S,3R,4E,8E)-2-(tetradecanoylamino)-4,8-octadecadien-l,3-diol.
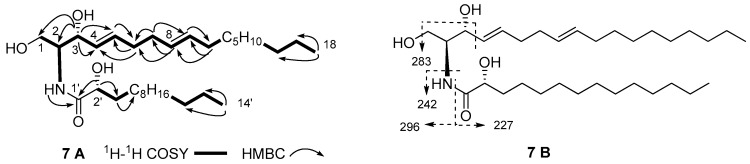
The molecular formula of compound 7 was assigned as C32H61NO4 on the basis of (+)-HRESIMS pseudomolecular ion peak at m/z 546.4496 [M]+ (calcd. for C32H61NO4Na, 546.4498) and the 1H- and 13C-NMR spectra (Table 2). The 13C-NMR spectrum of 7 showed a close resemblance to that of 6, except for the additional hydroxyl group on C-2′ in the FAB, since 7 showed different chemical shifts of C-1′ (δC 175.0), C-2′ (δC 72.4) and C-3′ (δC 34.8) in its FAB. HMBC correlations of H-2′ (δH 4.12) to C-1′ and C-3′ confirmed that the hydroxyl group was on C-2′ (Figure 6A). A series of fragment ions at m/z 227, 242, 283 and 296 in the (‒)-ESIMS spectrum (Figure 6B), deduced the lengths of LCB and FAB to be 18 and 14 carbons, respectively. Additionally, the length of FAB was further validated through (+)-EIMS molecular ion at m/z 258 [M]+ of (R)-methyl 2-hydroxytetradecanoate obtained from methanolysis of 7. Comparisons of the optical rotation of 7 ( +9.5°) with (2S,3R,2′R,4E)-2′-hydroxy-C16-ceramide ([α]D +5.2°) and its 2′S-isomer ([α]D −16.1°), suggested that 7 and (2S,3R,2′R,4E)-2′-hydroxy-C16-ceramide have the same absolute configurations for core chiral centers C-2, C-3 and C-2′ [24]. Thus, compound 7 was determined as (2S,3R,2′R,4E,8E)-2-(tetradecanoylamino)-4,8-octadecadien-l,3,2′-triol.
Figure 6.
Key 1H-1H COSY and HMBC correlations (A), and main ESI-MS fragments (B) of 7.
Compounds 1–9 were evaluated for their cytotoxicity against human myeloid leukemia cell line HL-60, human hepatocellular carcinoma HepG-2, and human gastric carcinoma SGC-7901 using an MTT assay, and results are shown in Table 3. Compound 1 displayed a moderate cytotoxicity to the test cell lines with IC50 values between 12.34 μM and 18.37 μM, while 2–4 did not show any cytotoxicity. Since 5 exhibited strong cytotoxicity against the test tumor cell lines with IC50 values between 4.12 μM and 7.32 μM, it can be considered as a potential for the further development of an anticancer agent. Compounds 6–9 showed weak cytotoxicity against all the test tumor cell lines with IC50 values ranging from 21.13 μM to 58.15 μM; however, they showed stronger cytotoxicity against HepG-2 and SGC-7901 than HL-60, suggesting a selectivity of these ceramides against HepG-2 and SGC-7901. Furthermore, ceramide 7, having the 2′-OH group in its FAB, displayed stronger cytotoxicity than its dehydroxy counterparts, indicating that the 2′-OH group in the FAB may play an important role in the expression of cytotoxicity for ceramides [24,25].
Table 3.
Cytotoxicity of compounds 1–9 against HL-60, HepG2 and SGC7901 tumor cell lines in vitro (IC50, μM) a.
| Compound | HL-60 | HepG-2 | SGC-7901 |
|---|---|---|---|
| 1 | 17.64 ± 0.32 | 12.34 ± 0.12 | 18.37 ± 0.17 |
| 2 | >100 | >100 | >100 |
| 3 | >100 | >100 | >100 |
| 4 | >100 | >100 | >100 |
| 5 | 6.29 ± 0.11 | 4.12 ± 0.15 | 7.32 ± 0.26 |
| 6 | 32.26 ± 0.23 | 26.69 ± 0.21 | 27.14 ± 0.30 |
| 7 | 25.32 ± 0.17 | 21.13 ± 0.13 | 22.74 ± 0.16 |
| 8 | 35.72 ± 0.36 | 28.53 ± 0.24 | 30.31 ± 0.14 |
| 9 | 58.15 ± 0.28 | 46.21 ± 0.17 | 45.79 ± 0.12 |
| Adriamycin b | 2.51 ± 0.14 | 2.73 ± 0.23 | 2.65 ± 0.17 |
a IC50 values are means from three independent experiments in which each compound concentration was tested in three replicate wells; b Adriamycin as a positive control.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer 343 polarimeter. 1D and 2D NMR spectra were obtained in CDCl3 or CD3OD on a Bruker AVANCE-500 spectrometer, with tetramethylsilane (TMS) as an internal standard. Chemical shifts (δ) were expressed in ppm and coupling constants in Hz. EI-MS spectrum was obtained on a Finnigan MAT 212 mass spectrometer. ESI-MS and HR-ESI-MS spectra were taken on a Micromass Quattro mass spectrometer (Micromass, Manchester, UK). CD spectrum was recorded on JASCOJ-720 spectrophotometer (JASCO Corporation, Tokyo, Japan). Separation and purification were performed by CC on silica gel H (10–40 μm, Qingdao Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (Pharmacia Inc., New Jersey, NJ, USA) and reversed-phase Si gel (Lichroprep RP-18, 40–63 μm, Merck Inc., Darmstadt, Germany). Semi-preparative HPLC was carried out on a Dionex P680 liquid chromatograph equipped with a UV 170 UV/Vis detector at 206 nm using a YMC-Pack R&D ODS-A column (250 mm × 20 mm i.d., 5 μm, YMC, Kyoto, Japan). Compounds detection in the thin-layer chromatography (TLC) plate was achieved by spraying the silica gel plates (Qingdao Marine Chemical Inc., Qingdao, China) with 15% H2SO4 in ethanol followed by heating.
3.2. Animal Material
The marine bryozoan Cryptosula pallasiana was collected in March 2009 from Huang Island, Qingdao City, Shandong Province of China, was identified by one of us (H.-W. Lin). A voucher specimen (No: QD-0903-1) was deposited in Marine Laboratory, Changzheng Hospital, Second Military Medical University.
3.3. Extraction and Isolation
The fresh animal C. pallasiana (about 20 kg) was exhaustively extracted with 95% EtOH at room temperature. Followed the extraction procedure we described previously, CCl4 fraction (about 12.9 g) was further separated by CC on Sephadex LH-20 with CHCl3/MeOH (1:1) as an eluting solvent to afford three fractions (Frs. A–C) [5]. Fr. A (5.56 g) was subjected to a reversed-phase silica gel column eluting with a mixture of MeOH/H2O (4:1) and MeOH to afford Fr. A1 and Fr. A2, respectively. Fr. A2 was submitted to a silica gel column and eluted with petroleum ether/EtOAc (15:1, 10:1, 5:1, 1:1) to give 12 fractions (Frs. A2-1–A2-12). The isolation of 16 alkaloids, 13 sterols, three aromatic compounds and two glycerol derivatives from Fr. B, Fr. C, and Fr. A2-4 to Fr. A2-9 have been reported previously [4,5,6]. Fr. A2-3 (183.0 mg) was purified by semi-preparative HPLC to give 1 (2.5 mg, tR = 67.2 min) and 2 (20.9 mg, tR = 73.4 min), using MeOH/H2O (19:1) as mobile phase at a flow rate of 8.0 mL/min. Fr. A2-11 (114.7 mg) was purified by semi-preparative HPLC to obtain 3 (6.2 mg, tR = 47.7 min) and 4 (20.9 mg, tR = 54.4 min), using MeOH/H2O (9:1) as mobile phase at a flow rate of 8.0 mL/min. Fr. A1 (2.4 g) was eluted with CHCl3/MeOH (1:1) on Sephadex LH-20, and then further purified by semi-preparative HPLC to give 5 (4.0 mg, tR = 39.6 min), using MeOH/H2O (1:3) as mobile phase at a flow rate of 8.0 mL/min. Fr. A2-10 (150.0 mg) was eluted with CHCl3/MeOH (1:1) on Sephadex LH-20, and then further purified by semi-preparative HPLC to afford 6 (9.3 mg, tR = 49.5 min), 7 (7.4 mg, tR = 46.3 min), 8 (8.5 mg, tR = 51.4 min) and 9 (7.1 mg, tR = 54.7 min), using MeOH/H2O (19:1) as mobile phase at a flow rate of 8.0 mL/min.
3.3.1. (23R)-Methoxycholest-5,24-dien-3β-ol (1)
White amorphous powder, −22.0° (c 0.05, CHCl3); 1H- and 13C-NMR data see Table 1; (+)-ESI-MS m/z: 437 [M + Na]+, 415 [M + H]+, 385, 349, 318, 274, 256; (+)-HRESIMS m/z: 437.3395 [M + Na]+ (calcd. for C28H46O2Na, 437.3396).
3.3.2. (2S,3R,4E,8E)-2-(Tetradecanoylamino)-4,8-octadecadien-l,3-diol (6)
White amorphous powder, −5.7° (c 0.10, CHCl3); 1H- and 13C-NMR data see Table 2; (+)-ESIMS m/z: 530 [M + Na]+, 437, 330, 302, 274, 262, 256, 228, 219; (‒)-ESIMS m/z: 552 [M + COOH]−, 542 [M + Cl]−, 506 [M − H]−, 447, 394, 341, 283, 255, 218, 186, 143, 126; (+)-HRESIMS m/z: 530.4545 [M + Na]+ (calcd. for C32H61NO3Na, 530.4549).
3.3.3. (2S,3R,2′R,4E,8E)-2-(Tetradecanoylamino)-4,8-octadecadien-l,3,2′-triol (7)
White amorphous powder, +9.5° (c 0.10, CHCl3); 1H- and 13C-NMR data see Table 2; (+)-ESIMS m/z: 546 [M + Na]+, 437, 330, 274, 262, 256; (‒)-ESIMS m/z: 558 [M + Cl]−, 522 [M − H]−, 340, 296, 283, 242, 227; (+)-HRESIMS m/z: 546.4496 [M + Na]+ (calcd. for C32H61NO4Na, 546.4498).
3.4. Methanolysis of Compounds 6 and 7
Compounds 6 and 7 (each about 3 mg) were dissolved in 5% HCl-MeOH (3 mL), respectively, and then refluxed for 12 h at 80 °C. The reaction mixture was extracted with n-hexane (3 × 4 mL). The n-hexane portion was washed with H2O and concentrated in vacuo to yield methyl tetradecanoate and (R)-methyl 2-hydroxytetradecanoate, respectively. The H2O portion was neutralized with NH4OH, and then further concentrated in vacuo to yield the corresponding LCB with specific rotation at −3.4° (c 0.05, CHCl3).
Methyl tetradecanoate: EI-MS m/z 242 [M]+ (25), 211 [M − CH3]+ (11), 199 [M − COCH3]+ (22), 185 (8), 171 (4), 157 (7), 143 (22), 129 (9), 115 (4), 101 (9), 87 (61), 74 (100), 55 (24).
(R)-Methyl 2-hydroxytetradecanoate: −6.5° (c 0.05, CHCl3); EI-MS m/z 258 [M]+ (7), 199 [M − COCH3]+ (65), 125 (30), 111 (56), 90 (47), 83 (85), 69 (100), 55 (92).
3.5. MTT Cytotoxicity Assays
Compounds 1–9 were evaluated for their cytotoxic activity against human tumor cell lines HL-60, HepG-2 and SGC-7901 using MTT assay. The cell lines were obtained from American type culture collection (ATCC), and were seeded to RPMI-1640 medium with 10% fetal bovine serum and 100 U/mL benzyl penicillin-streptomycin solutions, at 37 °C in a humidified atmosphere with 5% CO2/air for 24 h. Then the test samples were added and incubated at 37 °C for another 72 h. The detailed procedure for MTT assay can be found in our previous published literature [5]. The cytotoxic activity was expressed as IC50 value using adriamycin as a positive control.
4. Conclusions
A new sterol (1), two new ceramides (6,7) and six known compounds (2–5,8,9) were identified from the marine bryozoan C. pallasiana. Among the isolated compounds, sterol 1 showed novelty in the C-23R methoxy group and double bond between C-24 and C-25 in its side chain. Since this is the first report of ceramides in this species, it can be considered important for its chemotaxonomic significance. Furthermore, ceramides with 14 carbons in the FAB were different from those we previously obtained from Bugula neritina [25,26]. All compounds were evaluated for their cytotoxicity, and lactone (5) was found to exhibit stronger cytotoxicity than sterols and ceramides, suggesting that it could be responsible for the cytotoxicity of C. pallasiana.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 31201552 and No. 81473132), and National High-Tech Research and Development Project (863 Project, No. 2007AA09Z401).
Abbreviations
The following abbreviations are used in this manuscript:
| CC | Column Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| MTT | Microculture Tetrazolium |
| ATCC | American Type Culture Collection |
| RPMI | Roswell Park Memorial Institute |
| LCB | Long Chain Base |
| FAB | Fatty Acid Base |
| TD-DFT | Time-Dependent Density Functional Theory |
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/4/120/s1, Figures S1–S9: HR-ESI-MS, ESI-MS, 1H-NMR, 13C-NMR, DEPT135, 1H-1H COSY, HSQC, HMBC and NOESY spectra for new compound 1, Figures S10, S12 and S13: HR-ESI-MS, 1H-NMR and 13C-NMR spectra for new compound 6, Figures S14, S16 and S17: HR-ESI-MS, 1H-NMR and 13C-NMR spectra for new compound 7, Figures S11 and S15: EI-MS spectra of methyl tetradecanoate and (R)-methyl 2-hydroxytetradecanoate obtained from methanolysis of ceramides 6 and 7, respectively, Data S18–S22: 1H-NMR and 13C-NMR data for the known compounds 2–5, Data S23 and S24: 1H-NMR, 13C-NMR and ESI-MS data for the known compounds 8 and 9.
Author Contributions
Xiang-Rong Tian, Yan-Qing Gao and Xiao-Lin Tian carried out the experiment, including isolation and bioactive evaluation of the related materials. First author Xiang-Rong Tian also wrote the manuscript. Jiao Li tested the CD spectrum and calculated quantum ECD. Yu-Shan Li participated in the structural elucidation. Hou-Wen Lin collected and identified the samples of Cryptosula pallasiana. As corresponding authors, Hai-Feng Tang and Zhi-Qing Ma organized and designed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine nature products. Nat. Prod. Rep. 2016;33:382–431. doi: 10.1039/C5NP00156K. [DOI] [PubMed] [Google Scholar]
- 2.Blackman A.J., Walls J.T. Bryozoan secondary metabolites and their chemical ecology. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Elsevier; Amsterdam, The Netherlands: 1995. pp. 73–112. [Google Scholar]
- 3.Pejin B., Mojovic M., Savic A.G. Novel antitumour natural products from the phylum Bryozoa. Biol. Serbica. 2013;35:3–14. [Google Scholar]
- 4.Tian X.R., Tang H.F., Li Y.S., Lin H.W., Tong X.Y., Ma N. Alkaloids from marine bryozoan Cryptpsula pallasiana. Biochem. Syst. Ecol. 2010;38:1250–1252. doi: 10.1016/j.bse.2010.12.019. [DOI] [Google Scholar]
- 5.Tian X.R., Tang H.F., Li Y.S., Lin H.W., Chen X.L., Ma N., Yao M.N., Zhang P.H. New cytotoxic oxygenated sterols from the marine bryozoan Cryptosula pallasiana. Mar. Drugs. 2011;9:162–183. doi: 10.3390/md9020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian X.R., Tang H.F., Li Y.S., Lin H.W., Zhang X.Y., Feng J.T., Zhang X. Studies on the chemical constituents from marine bryozoan Cryptosula pallasiana. Rec. Nat. Prod. 2015;9:628–632. [Google Scholar]
- 7.Xiao D., Deng S., Zeng L. Studies on the chemical constituents of the marine sponge Clathria fasciculate from the South China Sea. Chin. J. Mar. Drugs. 2002;2:1–4. [Google Scholar]
- 8.Madaio A., Piccialli V., Sica D., Corriero G. New polyhydroxysterols from the dictyoceratid sponges Hipposoingia communis, Spongia officinalis, Ircinia variabilis, and Spongionella gracilis. J. Nat. Prod. 1989;52:952–961. doi: 10.1021/np50065a007. [DOI] [Google Scholar]
- 9.Kimura J., Maki N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002;65:57–58. doi: 10.1021/np0103057. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y. Six kinds of N-acylsphingosines from the Chinese gorgonian Junceella squamata. Chin. J. Mar. Drugs. 1995;2:1–4. [Google Scholar]
- 11.Babu U.V., Bhandari S.P.S., Garg H.S. Temno-sides A and B, two new glycosphingolipids from the Sea, urchin Temnopleurus toreumaticus of the Indian coast. J. Nat. Prod. 1997;60:732–734. doi: 10.1021/np960708k. [DOI] [Google Scholar]
- 12.Calderon G.J., Castellanos L., Duque C., Echigo S., Hara N., Fujimoto Y. Ophirasterol, a new C31 sterol from the marine sponge Topsentia ophiraphidites. Steroids. 2004;69:93–100. doi: 10.1016/j.steroids.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Gross E.K.U., Dobson J.F., Petersilka M. Density functional theory of time-dependent phenomena. In: Nalewajski R.F., editor. Density Functional Theory II. Volume 181. Springer; Berlin, Germany: 1996. p. 81. [Google Scholar]
- 14.Casida M.E. In: Recent Advances in Density Functional Methods, Part I. Chong D.P., editor. World Scientific; Singapore: 1995. pp. 155–192. [Google Scholar]
- 15.Gross E.K.U., Kohn W. Density Functional Theory of Many-Fermion Systems. Volume 21. Elsevier; Amsterdam, The Netherlands: 1990. Time-dependent density functional theory; pp. 255–291. Advances in Quantum Chemistry. [Google Scholar]
- 16.Runge E., Gross E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984;52:997–1000. doi: 10.1103/PhysRevLett.52.997. [DOI] [Google Scholar]
- 17.Kong F., Wang Y., Liu P., Dong T., Zhu W. Thiodiketopiperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014;77:132–137. doi: 10.1021/np400802d. [DOI] [PubMed] [Google Scholar]
- 18.Sun K., Li Y., Guo L., Wang Y., Liu P., Zhu W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs. 2014;12:3970–3981. doi: 10.3390/md12073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chebaane K., Guyot M. Occurrence of erythro-docosasphinga-4,8-dienine, as an ester, in Anemonia sulcata. Tetrahedron Lett. 1986;27:1495–1496. doi: 10.1016/S0040-4039(00)84294-X. [DOI] [Google Scholar]
- 20.Colsch B., Afonso C., Popa I., Portoukalian J., Fournier F., Tabet J.-C., Baumann N. Characterization of the ceramide moieties of sphingoglycolipids from mouse brain by ESI-MS/MS: Identification of ceramides containing sphingadienine. J. Lipid Res. 2004;45:281–286. doi: 10.1194/jlr.M300331-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.De Hann J.W., Van de Ven L.J.M. Configurations and conformations in acyclic unsaturated hydrocarbons. A 13C-NMR study. Org. Magn. Reson. 1973;5:147–153. doi: 10.1002/mrc.1270050309. [DOI] [Google Scholar]
- 22.Shi J., Seo Y. Isolation of new ceramides from the gorgonian Acabaria undulate. J. Nat. Prod. 1995;58:948–953. [Google Scholar]
- 23.Nakagawa M., Tsuruoka A., Yoshida J., Hino T. Total synthesis of (+)-erythro-N-Lauroyldocosasphinga-4,8-dienine from Anemonia sulcata and determination of the absolute configuration. J. Chem. Soc. Chem. Commun. 1990:603–605. doi: 10.1039/c39900000603. [DOI] [Google Scholar]
- 24.Szulc Z.M., Bai A., Bielawski J., Mayroo N., Miller D.E., Gracz H., Hannun Y.A., Bielawska A. Synthesis, NMR characterization and divergent biological actions of 2′-hydroxy-ceramide/dihydroceramide stereoisomers in MCF7 cells. Bioorg. Med. Chem. 2010;18:7565–7579. doi: 10.1016/j.bmc.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian X.R., Tang H.F., Feng J.T., Li Y.S., Lin H.W., Fan X.P., Zhang X. Neritinaceramides A–E, new ceramides from the marine bryozoan Bugula neritina inhabiting South China Sea and their cytotoxicity. Mar. Drugs. 2014;12:1987–2003. doi: 10.3390/md12041987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian X.R., Tang H.F., Li Y.S., Lin H.W., Ma N., Zhang W., Yao M.N. Ceramides and cerebrosides from the marine bryozoan Bugula neritina inhabiting South China Sea. J. Asian Nat. Prod. 2009;11:1005–1012. doi: 10.1080/10286020903207050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.