Abstract
Objective
To examine the impact of maternal psychological distress on the development of parental perception of child vulnerability (PPCV) in mothers of VLBW infants; and to examine the impact of PPCV on neurodevelopmental outcome in VLBW infants in the 2nd year of life.
Study Design
Prospective study of 69 mothers and their VLBW infants recruited in 2011-2012 for whom maternal psychological data was collected during the NICU hospitalization. Maternal PPCV was assessed at 4 months corrected age (CA). Neurodevelopmental outcome was assessed at 20 months CA. Regression analyses modeled the development of PPCV and the impact of PPCV on neurodevelopmental outcome.
Results
PPCV at 4 months CA was predicted by maternal anxiety and history of previous fetal loss reported during the NICU stay. Higher PPCV at 4 months CA was associated with lower language scores at 20 months CA.
Conclusions
Targeted interventions aimed reducing PPCV in the NICU are supported.
Keywords: Parental Perception of Child Vulnerability (PPCV), very low birth weight (VLBW), neurodevelopmental outcome, maternal psychological distress
Background
Parental perception of child vulnerability (PPCV) refers to a parent’s impression that his or her child is at greater risk for injury, illness, behavioral and developmental disorders than what is actually likely, and impacts approximately 10% of the general population(1). Children of parents with high levels of PPCV incur overall higher healthcare utilization rates and represent twice as many emergency room visits as do children of parents with low levels of PPCV (2). PPCV occurs primarily in parents of children who have survived a serious, potentially fatal illness or a prolonged hospitalization or health problem during the first year of the child’s life (3). However, an emerging body of literature suggests that PPCV is more consistently related to parental social and psychological functioning rather than the child’s past medical acuity or current medical state (3). PPCV has been related to socioeconomic disadvantage, prior exposure to potentially traumatic events (PTE), and maternal psychological distress defined as, levels of depression, anxiety and perinatal-specific post-traumatic stress (perinatal-specific PTSD; 3,4).
Mothers of very low birth weight (VLBW; birthweight < 1500 grams) infants are at especially high risk for PPCV, with rates up to six times higher than mothers of full-term infants (1,5,6). Mothers of VLBW infants typically experience a complicated pregnancy, traumatic preterm delivery, and prolonged hospitalization for a medically fragile infant who is at actual risk for long-term health and neurodevelopmental problems. In the context of the stressful NICU hospitalization, mothers of VLBW infants are known to experience disproportionately elevated rates of psychological distress (7) when compared to post-partum mothers of healthy full-term infants. Mothers of VLBW infants also are more likely to be socioeconomically disadvantaged (8) which places them at further risk for elevated rates of psychological distress and prior exposure to PTE. It is hypothesized that this confluence of socioeconomic disadvantage and elevated psychological distress in the NICU explains elevated rates of PPCV in mothers of VLBW infants because these stressors are by their very nature associated with feelings of insecurity, vulnerability, and maintained vigilance after a threatening situation (3,4). Complicating this conceptualization is the emerging evidence that elevated psychological distress in the NICU changes over time and that mothers follow different trajectories of resilience, recovery, delayed distress or chronic distress in reaction to the stressor of the NICU hospitalization (9-11). However, to date little is known about the associations among maternal socioeconomic disadvantage, psychological distress in the NICU, trajectories of psychological distress in the NICU, PTEs and PPCV among mothers of VLBW infants.
Understanding these relationships and designing interventions to target modifiable risks for PPCV are important initiatives because PPCV is an independent risk factor for behavioral and neurodevelopmental problems in VLBW infants in the first year of life even after controlling for infant medical risk factors (1,6, 12). As many as 25% of VLBW infants experience neurodevelopmental problems requiring special education services at school age, resulting in additional educational resource use and associated costs (13, 14). Thus, the identification and targeting of modifiable risk factors for PPCV is a priority for families, healthcare and educational institutions and society in general. To date, the impact of PPCV on neurodevelopmental problems in the second year of life, an age thought to be a better predictor of long-term neurodevelopmental outcome, is unknown. Therefore, the purpose of this study was to examine: 1) the relationship among socioeconomic disadvantage, maternal psychological distress, trajectories of psychological distress in the NICU and exposure to PTE, and PPCV in mothers of VLBW infants; 2) the role of PPCV in predicting VLBW neurodevelopmental outcome in the second year of life.
METHODS
This study is a prospective, longitudinal multimethods (quantitative, qualitative) study of 69 mothers and their VLBW infants recruited from a 57 bed level IV NICU in an urban academic center from 2011-2012. Qualitative results have been previously published (15, 16). Eligible infants were enrolled in a larger, NIH - funded cohort study (NR010009, Meier, PI), for which inclusion criteria have been previously reported (7). The larger NIH-funded cohort study examined the associations between infant health and cost outcomes as a function of the dose and exposure period of human milk during the NICU hospitalization. This sub-study enrolled 69 mothers of infants from the larger NIH-funded cohort and aimed to investigate the associations between socioeconomic disadvantage, infant medical variables, NICU-based and post-discharge maternal psychological distress, maternal visitation to the NICU, and provision of HM in the NICU, PPCV post-NICU discharge and VLBW neurodevelopmental outcomes post-NICU discharge. The present investigation reports socioeconomic disadvantage, infant medical variables, NICU-based psychological distress, trajectories of NICU-based psychological distress defined as resilient, recovered, delayed distress or chronic distress, post-NICU PPCV and post-NICU VLBW neurodevelopmental outcome. Analyses of maternal psychological distress measures collected at two post-NICU discharge time points are underway.
Participants
For this sub-study, a non-random sample of 69 mothers were recruited to participate if the mothers were English-speaking, ≥ 18 years of age, had a VLBW infant recently admitted to the NICU, and the infant was deemed likely to survive by the attending neonatologist. Of 100 eligible mothers, 72 mothers initially signed consent, 17 refused and/or were not approached, and 11 had immediate plans to transport the infants from the NICU to a hospital closer to the mother’s home. The study was approved by the institutional review board in the university where data were collected, and written informed consent was obtained for all participants.
Design
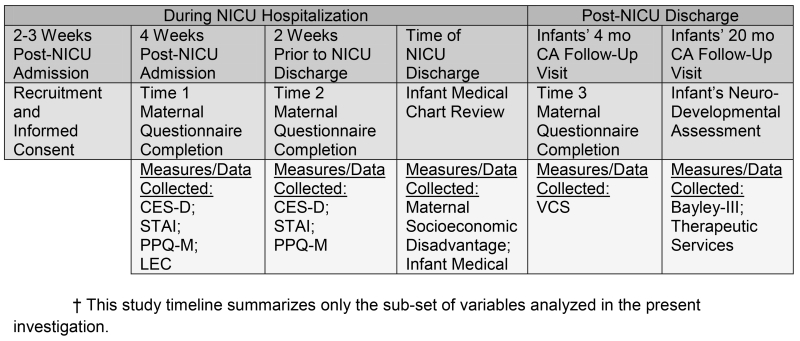
This longitudinal study enrolled mothers within 2-4 weeks after their infants’ NICU admission, and collected maternal and infant data through the infants’ reaching 20 months of age, corrected for prematurity (CA) . Figure 1 depicts the timeline for measures that were collected at serial time points. For this study, socioeconomic disadvantage and infant medical data during the NICU hospitalization were obtained from the larger NIH-funded cohort study database.
Figure 1. Study Timeline.
NICU-based depression, anxiety, perinatal-specific PTSD and previous exposure to PTEs were collected via maternal self-report questionnaire at the following time points: T1 (mean 28.1 days after birth, N=69) and T2 (mean 14.8 days prior to discharge, N=64). Post-NICU assessment of PPCV and VLBW neurodevelopmental outcome occurred at routine clinical visits to the pre-existing Neonatal High Risk Follow-up Clinic, a multidisciplinary clinic that monitors growth, neurologic and developmental status of infants cared for in the NICU. PPCV was measured during the infants’ visits at 4 months CA (N=52/69) by maternal self-report questionnaire. VLBW neurodevelopmental outcome was measured by formal, standardized neurodevelopmental assessment at the 20 month CA follow-up visit (N=39/69).
Measures
Maternal Socioeconomic Disadvantage and Infant Medical Data
Maternal age, education level, employment status, race/ethnicity, relationship status, insurance status, eligibility for Women, Infant and Children (WIC) program, primipara status,maternal history of prior fetal loss, and number of other children born prematurely were collected by medical record review at the time of infants’ NICU discharge. Access to social support also was identified as part of demographic data collection by asking mothers to identify the total number of caretakers available for the infant. Infant medical data collected included: birthweight (BW), gestational age (GA), gender, days of mechanical ventilation, suspected or confirmed presence of late-onset sepsis, necrotizing enterocolitis stages 2-3 (17), presence of severely abnormal head ultrasound (grades 3 and 4 intraventricular hemorrhage and/or periventricular leukomalacia), average daily dose of human milk (ADDHM; mL per kilogram per day averaged over the duration of the NICU stay, 18), infant receiving any human milk at time of NICU discharge (yes/no), and length of the NICU hospitalization (days).
Maternal Psychological Distress and History of PTE
These measures were collected during the NICU hospitalization, as follows.
The Center for Epidemiological Studies-Depression Scale (CES-D), administered in the NICU at T1 and T2, is a 20-item measure that assesses the frequency of depressive symptoms (19). The CES-D has moderate test-retest reliability over 6-8 weeks (rxx=.59, 19) and been commonly used in this study’s population with good internal reliability (α =.81-.90, 10). CESD clinical cutoff scores used by previous investigations (CESD > 16, 10) were used to define elevated depressive symptomatology.
The Spielberger State-Trait Anxiety Inventory (STAI), administered in the NICU at T1 and T2, is comprised of a 20-item subscale of “state” or acute/situational anxiety and 20-item subscale of “trait” or generalized subscale (20). The STAI has demonstrated test-retest reliability (rxx) of .65-.75 over 8 weeks, good internal consistency among mothers of VLBW infants (α = .89) and is frequently used with this population (10). STAI cutoff scores used by previous investigations (State scores >40-50 , 21) were used to define elevated anxious symptomatology.
The Modified Perinatal Posttraumatic Stress Disorder Questionnaires (PPQ-M), administered in the NICU at T1 and T2, is a 14-item questionnaire, administered at T1 and T2, designed to measure frequency of perinatal-specific PTSD that is well-validated in the NICU with good test-retest reliability over 2-4 weeks (rxx=.92) and good internal consistency (α =.90) (22, 23). The PPQ-M’s clinical cutoff score of 19 was used to define elevated perinatal-specific PTSD symptomatology (23).
The Life Events Checklist (LEC), administered in the NICU at only T1, is a 17-item scale that quantifies cumulative lifetime exposure to potentially traumatic events that was administered. The LEC provides a total score that incorporates both previous history of exposure to and proximity of exposure to a potentially traumatic event (i.e., it happened to you personally, you witnessed it) (24). The LEC is well-cited in the PTSD literature, has temporal stability ranging from .37-.84and correlates well to other measures of post-traumatic stress.
Consistent with previous literature (9,11), by category of distress (depression, anxiety or perinatal-specific PTSD), mothers’ trajectories of distress were classified as resilient if their scores were below the aforementioned cutoffs for T1 and T2; recovered if their scores were above the cutoff at T1 and below at T2;delayed distress if scores were below cutoff at T1 and above at T2; and, chronic distress if scores were above the cutoff for T1 and T2.
Parental Perception of Child Vulnerability
The Vulnerable Child Scale (VCS), administered at VLBW infants’ 4 month CA follow-up visit, is a 16-item questionnaire that measures the frequency and strength of parents’ perception that their child is vulnerable. The measure has good test-retest reliability (rXX=.96) and internal consistency (α =.75, 25). Lower scores represent greater vulnerability. For ease of interpretation, the items were reverse coded so that higher scores represented greater vulnerability. One item inquiring about discipline practices, which was considered inappropriate for a 4 month old CA infant, was omitted from analyses. The VCS was scored both continuously (total sum) and categorically (using a cut-off score). A cut-off score of 19 or higher was established to determine the frequency of mothers’ with elevated vs. non-elevated PPCV. The cut-off score was determined based on previously established cut-off scores (1,6).
Neurodevelopmental Outcome
The Bayley Scales of Infant and Toddler Development (Bayley-III, 26), a normed, standardized measure of neurodevelopmental outcome, was administered by trained licensed clinical psychologists during the infants’ 20 months CA follow-up visit. Outcome measures on the Bayley-III included the Cognitive, Language and Motor Indices (mean 100±15) and the Cognitive, Receptive Language, Expressive Language, Fine Motor and Gross Motor Subscales (mean 10±3).
Statistical Analysis
Measures of central tendency and frequencies were used to provide descriptive statistics. Bivariate correlation analyses determined which variables were associated with attendance at 4-month and 20-month CA follow-up visit, and with PPCV at 4 months CA and neurodevelopmental outcome at 20 months CA. Maternal socioeconomic, infant medical and maternal psychological variables associated with PPCV at 4 months CA and neurodevelopmental outcome at 20 months CA at p-values of <.25 were selected as covariates and entered into multiple regression analyses predicting PPCV at 4 months CA and neurodevelopmental outcome at 20 months CA . Variables with p-values <.50 were retained in final multiple regression analyses. When determining the impact of PPCV at 4 months CA on neurodevelopmental outcome at 20 months CA, multiple regression models were only calculated for neurodevelopmental outcome variables correlated with PPCV at 4 months CA at p-values <.05. This decision was made to minimize experiment-wise type I error. Post-hoc analyses of power achieved in multiple regressions were calculated using the known sample size,α= .05, number of predictors, and regression correlation coefficients.
Results
Of the 69 mothers initially enrolled, 52/69 (75%) completed the 4 month CA visit and 39/69 (57%) completed the 20 month CA visit. At the 4 month CA visit, infants of older gestational age at birth (p=.009) and those whose mothers had higher rate of unemployment (p=.011) were less likely to return for follow up. At 20 months CA the only difference between those who did and did not return to the clinic was a higher rate of maternal unemployment (p=.049) for those who did not return.
Maternal socioeconomic variables are summarized in Table 1. Descriptive statistics reveal a generally socioeconomically disadvantaged group of participants. The majority of mothers were black (54%) and low income (67% WIC eligible). Approximately 1 in 8 (13%) were unemployed. On average mothers had completed high school (mean highest grade level completed=13.4). Infant medical variables are summarized in Table 2. Mean birth weight and gestational age of infants were 957 ± 243 grams and 27.5 ± 2 weeks, respectively. Table 3 summarizes maternal psychological variables, PTE and PPCV. At T1, elevated depression, anxiety, and perinatal-specific PTSD were found in 33%, 55% and 25% of mothers, respectively. Overall, this group of mothers had experienced an average of 2.75 PTEs prior to the birth of their preterm infant, with the most common being witness to unexpected death (60%) and motor vehicle accident (50%). Other types of events such as unwanted sexual experience (16%), assault with a weapon (15%) and physical assault (14%) were not uncommon. At 4 months CA 13% of mothers had PPCV. Table 4 summarizes trajectories of NICU-based maternal psychological distress. Consistent with previous literature, resiliency was the most prevalent trajectory across type of distress with chronic distress and recovered distress being the next most prevalent trajectories (9, 11). Neurodevelopmental outcome at 20 months CA is summarized in Table 5. Consistent with previous literature, language scores are lower than cognitive or motor scores and mark the domain of poorest performance. Table 5 also summarizes bivariate correlations between PPCV at 4 months CA and neurodevelopmental outcome at 20 months CA. Bivariate analyses revealed that PPCV at 4 months was negatively associated with expressive language in the infant at 20 months CA (p=.012). PPCV at 4 months was not significantly associated with any other neurodevelopmental outcome variables at 20 months.
Table 1. Maternal Socioeconomic Data.
| N=69 | M±SD [Range], N (%) |
|---|---|
| Maternal Age (Years) | 27 ± 6 [18-40] |
| Maternal Education (Highest Grade) | 13.4 ± 2.4 [10-20] |
| Maternal Unemployment | 9 (13%) |
| WIC Eligibility | 46 (67%) |
| Maternal Race | |
| Black | 38 (54%) |
| Non-Hispanic White | 18 (26%) |
| Hispanic | 12 (17%) |
| Marital Status | |
| Married and/or Living with Partner | 32 (51%) |
| Partnered, not Living Together | 20 (32%) |
| Single | 10 (16%) |
| Primipara | 23 (34%) |
| History of Prior Preterm Birth | 7 (10%) |
| History of Prior Fetal Loss | 28 (41%) |
| Total Number of Infant’s Caretakers† | 2.27±1.12 [1-5] |
maternal report of total number of caretakers available for infant, includes family and friends beyond child’s other parent
Table 2. Infant Characteristics and Medical Data.
| N=69 | M±SD [Range], N (%) |
|---|---|
| Birthweight (grams) | 957±243 [470-1470] |
| Gestational Age (weeks) | 27.5±2 [23.2-32.30] |
| Male Sex | 34 (49%) |
| Severely Abnormal HUS | 5 (8%) |
| Sepsis | 20 (30%) |
| Necrotizing Enterocolitis (Stage 2-3) | 5 (8%) |
| Days on Mechanical Ventilation | 14.3±20.2 [0-60] |
| Average HM dose mL/Kg Over Course of NICU Hospitalization | 56.10±53.27 [0-152.50] |
| Receiving any HM at NICU Discharge | 27 (31.93%) |
| Length of NICU Stay | 91±37.1 [30-179] |
Table 3. Maternal Psychological Distress in the NICU and PPCV at 4 months CA.
| T1, N=69 T2, N=64 T3/Infant 4 month CA visit, N=52 |
N (%) |
|---|---|
| Elevated Depression T1 | 23 (33%) |
| Elevated Depression T2 | 15 (23%) |
| Elevated Anxiety T1 | 38 (55%) |
| Elevated Anxiety T2 | 23 (36%) |
| Elevated Perinatal-Specific PTSD T1 | 17 (25%) |
| Elevated Perinatal-Specific PTSD T2 | 16 (25%) |
|
| |
| M±SD [Range] | |
|
| |
| Mean Parental Child Vulnerability at 4 months CA | 11.0±26.03 [2-26] |
Table 4. Trajectories of NICU-based Maternal Psychological Distress.
| Depression N=64 |
Anxiety N=64 |
Perinatal-Specific PTSD N=64 |
|
|---|---|---|---|
| Resilient | 42 (66%) | 24 (38%) | 41 (64%) |
| Recovered | 7 (11%) | 18 (28%) | 7 (11%) |
| Delayed | 1 (2%) | 4 (6%) | 5 (8%) |
| Chronic | 14 (22%) | 18 (28%) | 11 (7%) |
Table 5. Neurodevelopmental Outcome at 20 mo CA and Bivariate Correlations between PPCV at 4 months CA and Neurodevelopmental Outcome at 20 mo CA.
| Bayley-III Index & Subscale Scores at 20 mo CA | M±SD [Range] | Bivariate Correlation with PPCV at 4 months CA |
|---|---|---|
| Cognitive Index | 97.05±11.63 [70-125] | r =−.08, p=.69 |
| Cognitive | 9.41±2.32 [4-15] | r =−.08, p=.69 |
| Language Index | 84.92±15.46 [50-112] | r =−.35, p=.07 |
| Receptive Language | 7.05±2.66 [3-13] | r =−.11, p=.57 |
| Expressive Language | 7.64±3.01 [1-12] | r =−.46, p=.012 |
| Motor Index | 90.15±12.45 [49-110] | r =−.34, p=.07 |
| Fine Motor | 9.13±2.41 [2-13] | r (29)=−.29, p=.13 |
| Gross Motor | 7.59±2.27 [1-11] | r (29)=−.33, p=.08 |
Multivariate Analyses
Predictors of Maternal PPCV at 4 Months Corrected Age
In bivariate analyses maternal depression at T1, anxiety at T1, perinatal-specific PTSD at T1, cumulative history of PTE, and history of fetal loss were positively associated with PPCV at 4 months CA. Trajectory of distress did not relate to PPCV at 4 months CA (depression F (2, 36)=1.73, p=.19; anxiety F (3, 35)=.37, p=.78; perinatal-specific PTSD F (3, 35)=.66, p=.58). History of NEC diagnosis (stages 2-3) and greater average daily dose of HM (ADDHM) throughout the course of the NICU hospitalization was negatively associated with PPCV at 4 months CA. Maternal depression and perinatal-specific PTSD were not retained in the final regression model as both independent predictors had p-values in excess of .50 in preliminary regression models.
In the final multiple regression model only maternal anxiety and history of previous fetal loss remained significant predictors of PPCV at 4 months CA (p<.05, Table 6). We tested an alternate multiple regression model that included infant GA and maternal unemployment as covariates given their correlation with attendance at the 4 month appointment; however, infant GA and maternal unemployment were not significant predictors of PPCV and as such these variables were not included in the final model.
Table 6. Predictors of Parental Perception of Child Vulnerability at 4 Months Corrected Age.
| Unstandardized | Unstandardized 95% CI | Beta | p-value | |
|---|---|---|---|---|
|
| ||||
| Step 1 | ||||
| Trait Anxiety at T1 | .28 | .46, .11 | .52 | .003 |
| PTE at T1 | .12 | .29, −.05 | .23 | .15 |
| History of Previous Fetal Loss | 4.18 | 7.75, .60 | .36 | .024 |
| Necrotizing Enterocolitis (Stage 2-3) |
−7.28 | 3.50, −18.06 | −.22 | .18 |
| ADDHM- NICU stay (mL/kG per day) |
−.02 | .03, −.05 | −.20 | .23 |
|
| ||||
| Final F (5, 26)=4.51, p=.004; R2=.46 | ||||
Predictors of Neurodevelopmental Outcome at 20 Months Corrected Age
As noted above, bivariate analyses revealed that PPCV at 4 months was negatively associated with expressive language in the infant at 20 months CA (p=.012). The only maternal socioeconomic and infant medical factors which related to expressive language outcome were infant GA at birth, infant birth weight, length of NICU hospitalization and maternal education level. High correlations between infant GA at birth and infant birth weight (r=.70, p<.001) caused problems with multicollinearity in regression models and only infant GA at birth was retained in the final model. Length of NICU hospitalization was not retained in the final regression model (p>.50).
In final multiple regression analyses only lower levels of PPCV at 4 mo CA (p=.014) significantly predicted better expressive language at 20 months CA (Table 7). We tested an alternate multiple regression model that also included significant predictors of PPCV, maternal anxiety and history of fetal loss as covariates. The inclusion of maternal anxiety and history of fetal loss did not change the association between PPCV and expressive language at 20 months CA, and, maternal anxiety and history of fetal loss did not significantly predict expressive language at 20 months CA so these maternal variables were not retained in the final model. Finally, we tested an alternate multiple regression model that included maternal unemployment as a covariate given the correlation between maternal unemployment and attendance to the 20 month appointment; maternal unemployment was not a significant predictor of expressive language and as such was not retained in the final model.
Table 7. Predictors of Expressive Language at 20 Months Corrected Age.
| Unstandardized | Unstandardized 95% CI |
Beta | p- value |
|
|---|---|---|---|---|
| Step 1 | ||||
| Gestational Age (weeks) | .27 | (−.25, .80) | .18 | .30 |
| Maternal Education Level | .28 | (−.05, .61) | .29 | .10 |
| Step 2 | ||||
| PPCV at 4 months CA | −.21 | (−.05, −.37) | −.45 | .014 |
| Final F (3, 25)=3.94, p=.02; R2=.32; Step 2 ΔR2=.19 | ||||
Discussion
The “Vulnerable Child Syndrome” was first defined in 1964 and later redefined as PPCV in the mid-1990’s by researchers who noted that parents of preterm infants were most susceptible to this persistent preoccupation with the vulnerability of their children (1,9,27). Investigations of PPCV in preterm infants have since established that PPCV is a disproportionate problem among parents of preterm infants. Very recent investigations have confirmed that this topic remains highly relevant and that PPCV is strongly related to parental levels of psychological functioning in parents of preterm infants (3,4,6,7). To our knowledge, the present study is the first to reveal an adverse impact of PPCV on language development in preterm infants in the second year of life. This further reinforces the importance of a maintained interest in this established, powerful construct. These findings also have important clinical implications for the identification of mothers at risk for PPCV and identification of modifiable factors contributing to neurodevelopmental outcome in their infants.
Our findings reveal that the negative impact of PPCV on language functioning at 20 months CA persisted even after adjusting for variables such as GA and maternal education level, which has recently been shown to be a particularly powerful socioeconomic predictor of neurodevelopment (28). Although prior studies have demonstrated adverse outcome in relation to PPCV, these studies were older and reported on behavioral problems or adaptive skills, rather than specific neurodevelopmental functioning (6,12). Allen et al. reported on a predominately non-Hispanic white cohort of 116 preterm infants born in 1996-1998 and found that higher PPCV was predictive of suboptimal infant adaptive functioning at 1 year of age (12). However, these researchers did not detect differences in cognitive or motor neurodevelopmental performance as assessed by a previous version of the Bayley in regression analyses. De Ocampo et al followed a lower-income, predominately black cohort of 90 preterm infants and found that after controlling for neonatal medical problems, socioeconomic variables, and developmental functioning, PPCV was the only significant predictor of behavioral difficulties in children at early childhood (6). The present investigation follows a contemporary cohort of socioeconomically disadvantaged mothers and their VLBW infants and is the only study on PPCV to have used the Bayley-III which examines specific language domains (26).
We speculate that PPCV was specifically associated with language development given the hypothesized associations among PPCV and either controlling or overly passive parenting styles (1) and the important role of parent-child relationships in facilitating language development (29). It has long been established that sensitive parent-child interactions characterized by “in-tune,” synchronized parenting behaviors allow parents to more accurately attend to a young child’s language efforts, provide more appropriate verbal responses and elaborations, and therefore reinforce more optimal language development (29). PPCV is theorized to be associated with less sensitive mother-child interactions (1) that would result in less synchronous interactions, and therefore less optimal opportunities for language development. Our findings may, therefore, relate to a better diagnostic ability to detect language difficulties prior to school age in this group of children at-risk for poorer language outcomes.
Consistent with the extant literature, we found that maternal anxiety level in the NICU predicted PPCV in mothers of VLBW infants after NICU discharge (2,7). Unlike previous literature that has revealed that chronic or extreme distress trajectories are associated with PPCV (10), this study did not find a significant association between distress trajectories and PPCV. This may be related to the study’s relatively smaller sample size and highlights the need for replication and extension studies.
The rate of elevated PPCV detected in this study appears somewhat low relative to previous estimates of PPCV occurring in 10% of the general population and occurring at much higher rates in mothers of VLBW infants (1,5,6). This is the first study to report the prevalence of elevated PPCV in a contemporary preterm cohort and may indicate that with advances in neonatology, NICU family-centered care, and psychosocial supports in the NICU, mothers continue to experience the various degrees of PPCV but fewer mothers pass the threshold of having acutely elevated PPCV. Furthermore, this single-center study’s NICU has an established breastfeeding peer counselor program (15). Although this program was originally designed to support lactation, previous studies have shown that mothers benefit from the psychosocial support provided by the peers counselors (15). It is conceivable that this program positively influences both mothers’ psychological distress and eventual PPCV. Review of other relevant data proposes an alternate hypothesis. A recent multi-center study of maternal psychological distress in the NICU that included 2 Midwestern sites in the same city as the present study’s site with comparable sample demographics revealed relatively low levels of maternal psychological distress among their Midwestern site mothers (10). This investigation concluded that these “inner city,” mothers experienced so much chronic stress that the birth of a preterm infant did not significantly increase their distress (10, pg. 161). That is, it is possible that the chronic, daily stress of the generally low income mothers in the present sample dominates over the stress of the NICU and eventual development of PPCV and explains the relatively low prevalence of elevated PPCV. Nonetheless, the aforementioned neurodevelopmental impact of PPCV was revealed by quantifying PPCV continuously, which suggests that all levels of PPCV are meaningful and that future studies should quantify PPCV both continuously (total score) and dichotomously (elevated vs. non-elevated).
Aside from maternal anxiety, prior history of fetal loss was the only other significant predictor of maternal PPCV, which was not predicted by perinatal-specific PTSD or cumulative exposure to other prior potentially traumatic events. This was the first study to assess the impact of perinatal-specific PTSD on maternal PPCV. We speculated that perinatal-specific PTSD would predict PPCV because of the striking similarity of symptoms. Some of the hallmark symptoms of perinatal-specific PTSD, e.g., hypervigilance and intrusive thoughts and guilt surrounding the experience of the NICU hospitalization, were thought to relate to symptoms of PPCV, e.g., medical hypervigilance, intrusive thoughts about medical vulnerability and guilt when needing to set limitations on toddler behavior. Interestingly, our previous investigations have revealed that prior history of fetal loss is an important predictor of perinatal-specific PTSD early in the NICU hospitalization (7). Previous investigations have highlighted the importance of presence of any prior potentially traumatic event (3), but have not specifically investigated prior reproductive traumatic events. It is possible that there is a more complex mechanism through which reproductive trauma, such as a prior fetal loss, and reproductive or perinatal-specific PTSD impact eventual development of PPCV that we were unable to fully disentangle given the sample size.
The study presents several limitations including, as noted above, a relatively small sample size and the use of maternal self-report in the assessment of psychological distress. The statistical effects detected were large and power analyses revealed that despite the relatively small sample size the study had adequate statistical power to detect meaningful associations among variables (achieved power=.99). The fact that these associations demonstrate relatively large effect sizes further reinforces the need for additional study of these influential associations. In contrast to existing literature, certain expected infant or maternal covariates of neurodevelopment did not significantly relate to language outcome in our sample (28). Specifically, higher human milk consumption and higher maternal education level have been two established predictors of better neurodevelopmental outcome (23) that were not significant predictors of language in bivariate or regression analyses in this sample. This is hypothesized to relate to the small sample size and highlights the need for replication with a larger number of mothers.
There was also loss to follow-up of our sample with only 75% and 57% attending the follow-up clinic at 4 and 20 months CA, respectively. Infants who returned at 4 months CA were of significantly lower GA at birth but did not differ from those who attended with respect to neonatal morbidities. The only difference between those who did/did not attend at 20 months CA were rates of maternal unemployment, but even after controlling for this factor, PPCV was predictive of worse language outcome. Another significant limitation in this and a majority of the extant literature is the exclusion of fathers and their PPCV. In spite of these limitations, we have presented the only prospective analysis of correlates of PPCV and its impact on 2 year neurodevelopmental outcome in a cohort of preterm infants.
Conclusion
We have found that PPCV is an independent risk factor for adverse language outcome in VLBW Infants in the 2nd year of life and is predicted by maternal psychological functioning and prior history of fetal loss. This suggests that early identification of mothers with psychological distress, particularly those who manifest elevated anxiety levels, is pivotal as a first step in reducing PPCV in mothers of infants already at risk for neurodevelopmental impairment. Future investigations should explicitly investigate the mechanism by which PPCV impacts language development and childhood behavior. Finally, targeted interventions for mothers in the NICU with psychological distress as well as for at risk parents after discharge may help to modify the severity of PPCV and ameliorate its adverse neurodevelopmental impact.
Acknowledgements
This project was partially funded by NR010009 awarded to P. Meier, principal investigator and from an internal grant from Rush University Medical Center’s Departments of Adult Health and Gerontologic Nursing and Women, Children, and Family Nursing awarded to the manuscript’s first and second authors
Footnotes
Conflict of Interest: The authors declare no conflicts of interest
Contributor Information
Michelle Greene, Departments of Behavioral Sciences & Pediatrics, Rush University Medical Center, 1653 W Congress Parkway, 1200 Kellogg Building, Chicago, IL 60612.
Beverly Rossman, College of Nursing, Rush University Medical Center, Chicago, Illinois.
Paula Meier, College of Nursing, Rush University Medical Center, Chicago, Illinois.
Kousiki Patra, Department of Pediatrics, Rush University Medical Center, Chicago, Illinois.
References
- 1.Forsyth BWC, Horwitz SM, Leventhal JM, Burger J. The Child Vulnerability Scale: An Instrument to Measure Parental Perceptions of Child Vulnerability. J Pediatr Psychol. 1996;21(1):89–101. doi: 10.1093/jpepsy/21.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Chambers PL, Mahabee-Gittens EM, Leonard AC. Vulnerable child syndrome, parental perception of child vulnerability, and emergency department usage. Pediatr Emerg Care. 2011:10009–13. doi: 10.1097/PEC.0b013e318235bb4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tallandini MA, Morsan V, Gronchi G, Macagno F. Systematic and meta-analytic review: Triggering agents of parental perception of child’s vulnerability in instances of preterm birth. J Pediatr Psychol. 2015;40(6):545–553. doi: 10.1093/jpepsy/jsv010. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz SM, Storger-Issner A, Kerker BD, Lilo E, Leibovitz A, St.John N, Shaw RJ. A model for the development of mothers’ perceived vulnerability of preterm infants. J Dev Behav Pediatr. 2015;36:371–380. doi: 10.1097/DBP.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estroff D, Yando R, Burke K, Snyder D. Perceptions of vulnerability by mothers who had delivered preterm. J Pediatr Psychol. 1994;19(6):709–721. doi: 10.1093/jpepsy/19.6.709. [DOI] [PubMed] [Google Scholar]
- 6.De Ocampo AC, Macias MM, Saylor CF, Katikaneni LD. Caretaker perception of child vulnerability predicts behavior problems in NICU graduates. Child Psychiatry Hum Dev. 2003;34(2):83–96. doi: 10.1023/a:1027384306827. [DOI] [PubMed] [Google Scholar]
- 7.Greene MM, Rossman B, Patra K, Kratovil A, Janes J, Meier PP. Depressive, Anxious and Perinatal Post-Traumatic Distress in Mothers of Very Low Birth Weight Infants. J Dev Behav Pediatr. 2015;36(5):362–370. doi: 10.1097/DBP.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention CDC Health Disparities and Inequalities Report-United States-2013. CDC Morbidity and Mortality Weekly Report. 2013;62(Supplement):3. [PubMed] [Google Scholar]; Allen EC, Manuel JC, Legault C, Naughton MJ, Pivor C, O’Shea TM. Perception of child vulnerability among mothers of former premature infants. Pediatrics. 2004;113(2):267–73. doi: 10.1542/peds.113.2.267. [DOI] [PubMed] [Google Scholar]
- 9.Bonanno GA, Westphal M, Anthony D, Mancini AD. Resilience to loss and potential trauma. Ann Rev Clin Psych. 2011;7:511–35. doi: 10.1146/annurev-clinpsy-032210-104526. [DOI] [PubMed] [Google Scholar]
- 10.Holditch-Davis D, Santos H, Levy J, et al. Patterns of psychological distress in mothers of preterm infants. Inf Behav Dev. 2015;41:154–163. doi: 10.1016/j.infbeh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WJ, Lee E, Namkoong K, Park ES, Rha D-w. Progress of PTSD symptoms following birth: a prospective study in mothers of high-risk infants. J Perinatol. 2015;35(8):575–579. doi: 10.1038/jp.2015.9. [DOI] [PubMed] [Google Scholar]
- 12.Allen EC, Manuel JC, Legault C, Naughton MJ, Pivor C, O’Shea TM. Perception of child vulnerability among mothers of former premature infants. Pediatrics. 2004;113(2):267–73. doi: 10.1542/peds.113.2.267. [DOI] [PubMed] [Google Scholar]
- 13.Stephens BE, Vohr BR. Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am. 2009;56(3):631–46. doi: 10.1016/j.pcl.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Lewit EM, Baker LS, Corman H, Shiono PH. The direct cost of low birth weight. Future Child. 1995;5(1):35–56. [PubMed] [Google Scholar]
- 15.Rossman B, Greene MM, Meier PP. The role of peer support in the development of maternal identity for “NICU Mom.”. J Obstet Gynecol Neonatal Nurs. 2015;44(1):3–16. doi: 10.1111/1552-6909.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossman B, Kratovil AL, Greene MM, Engstrom JL, Meier PP. “I have faith in my milk”: the meaning of milk for mothers of very low birth weight infants hospitalized in the neonatal intensive care unit. J Hum Lact. 2013;29(3):359–65. doi: 10.1177/0890334413484552. [DOI] [PubMed] [Google Scholar]
- 17.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1979;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigger HR, Fogg LJ, Patel A, Johnson T, Engstrom JL, Meier PP. Quality indicators for human milk use in very low-birthweight infants: are we measuring what we should be measuring? J Perinatol. 2014;34(4):287–91. doi: 10.1038/jp.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 20.Spielberger CD, Gorsuch R, Lushene R, Vagg R, Jacobs GA. State-Trait Anxiety Inventory for Adults: Manual. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 21.Rogers CE, Kidokoro H, Wallendorf W, et al. Identifying mothers of very preterm infants at-risk for postpartum depression and anxiety prior to discharge. J Perinatol. 2013;33:171–176. doi: 10.1038/jp.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMier RI, Hynan MT, Harris HB, et al. Perinatal stressors as predictors of symptoms of posttraumatic stress in mothers of infants at high risk. J Perinatol. 1996;16:276–280. [PubMed] [Google Scholar]
- 23.Callahan JL, Borja SE, Hynan MT. Modification of the Perinatal PTSD Questionnaire to enhance clinical utility. J Perinatol. 2006;26:533–539. doi: 10.1038/sj.jp.7211562. [DOI] [PubMed] [Google Scholar]
- 24.Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the life events checklist. Assessment. 2004;11:330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- 25.Perrin EC, West PD, Culley B. Is my child normal yet? Correlates of Vulnerability. Pediatrics. 1989;83(3):355–363. [PubMed] [Google Scholar]
- 26.Bayley N. Bayley Scales of Infant Development. Third edition The Psychological Corporation; San Antonio, TX: 2006. [Google Scholar]
- 27.Green M, Solnit AJ. Reactions to the threatened loss of a child: A vulnerable child syndrome. Pediatrics. 1964;34:58–66. [PubMed] [Google Scholar]
- 28.Patra K, Greene MM, Patel AL, Meier P. Maternal education level predicts cognitive, language and motor outcome in preterm infants in the second year of life. Am J Perinatol. 2016 doi: 10.1055/s-0036-1572532. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van IJzendoorn MH, Dijkstra J, Bus AG. Attachment, intelligence and language: A Meta-analysis. Soc Dev. 1995;4:115–128. [Google Scholar]