Abstract
Purpose
The purpose of this study was to evaluate accuracy and repeatability of T1 and T2 estimates of a Magnetic Resonance Fingerprinting (MRF) method using the ISMRM/NIST MRI system phantom.
Methods
The ISMRM/NIST MRI system phantom contains multiple compartments with standardized T1, T2 and proton density values. Conventional inversion-recovery spin echo and spin echo methods were used to characterize the T1 and T2 values in the phantom. The phantom was scanned using the MRF-FISP method over 34 consecutive days. The mean T1 and T2 values were compared to the values from the spin echo methods. The repeatability was characterized as the coefficient of variation (CV) of the measurements over 34 days.
Results
T1 and T2 values from MRF-FISP over 34 days showed a strong linear correlation with the measurements from the spin echo methods (R2 = 0.999 for T1, R2 = 0.996 for T2). The MRF estimates over the wide ranges of T1 and T2 values have less than 5% variation, except for the shortest T2 relaxation times where the method still maintains less than 8% variation.
Conclusion
MRF measurements of T1 and T2 are highly repeatable over time and across wide ranges of T1 and T2 values.
Keywords: MR Fingerprinting, quantitative imaging, relaxation time, repeatability, NIST system phantom
Introduction
Quantitative relaxometry shows promise for characterization and follow-up of disease in multiple clinical settings, such as neoplasm (1,2), multiple sclerosis (3,4), stroke (5), characterizing iron overload in liver (6), myocardial infarction (7), as well as monitoring treatment responses (8,9). However, the differences in T1 and T2 values between healthy and diseased tissues or between disease stages could be very small. To use quantitative relaxometry clinically, any variation in T1 and T2 measurement must be smaller than the differences between healthy and diseased tissues. Ideally the acquisition for measuring T1 and T2 values should be fast and accurate. It is also critical that measurements are highly repeatable, an important issue for tissue classification based on T1 or T2 values (10).
While many advances have been made to accelerate relaxometry (11–14), there are few studies (15,16) that assessed the repeatability of relaxometry methods. One reason is that these studies require phantoms with appropriate ranges of accurately known T1 and T2 values. These values should be stable over extended periods. An MRI system phantom was recently developed through the collaboration between the ISMRM Ad Hoc Committee on Standards for Quantitative Magnetic Resonance and the National Institute of Standards and Technology (NIST). The phantom has compartments containing solutions with a wide range of T1 and T2 values, and the solutions are well-characterized by NIST (17).
Magnetic Resonance Fingerprinting (MRF) is accurate and efficient in the simultaneous quantification of T1 and T2 by acquiring the transient-state signal with pseudo-random acquisition parameters (18–21). However, for these metrics to have clinical utility, the T1 and T2 values must be repeatable so that any observed difference in measured relaxivity between tissues or temporal change in measurement within a tissue can be assumed to be due to differences in physiology rather than scanner instability or methodological error. In this study, the repeatability of MRF derived T1 and T2 measurements in the ISMRM/NIST MRI system phatom is accessed over a period of 34 days.
Methods
ISMRM/NIST MRI System Phantom
The ISMRM/NIST MRI system phantom has multiple layers of sphere arrays that are designed to have a range of specific T1, T2 and proton density values. The spheres in the T1 array are filled with NiCl2 doped water, while the T2 spheres are filled with MnCl2 doped water. All solutions in the various compartments of the phantom are well-characterized by NIST and monitored by NIST for stability and accuracy (http://collaborate.nist.gov/mriphantoms/bin/view/MriPhantoms/MRISystemPhantom).
Gold Standard T1 and T2 Measurements by Spin Echo Methods
To characterize the T1 and T2 values in the system phantom, an inversion recovery spin echo (IR-SE) method and a multiple single-echo spin echo method were used on a Siemens 3T Skyra scanner (Siemens AG Healthcare, Erlangen, Germany).
T1 measurements from the T1 array were acquired by the IR-SE method with seven inversion times (TI) of 21 ms, 100 ms, 200 ms, 400 ms, 800 ms, 1600 ms and 3200 ms with a repetition time (TR) of 10000 ms, an echo time (TE) of 12 ms, a matrix size of 128 × 128, a field of view (FOV) of 17 cm, and a slice thickness of 5 mm. The scan time for each TI measurement was 21.3 minutes. The total scan time for the gold standard T1 measurement was near 2.5 hours.
T2 measurements from the T2 array were obtained using a multiple single-echo spin echo method with seven TEs of 12 ms, 22 ms, 42 ms, 62 ms, 102 ms, 152 ms, and 202 ms, a TR of 10000 ms, a matrix size of 128 × 128, a FOV of 21 cm, and a slice thickness of 5 mm. The scan time of each TE measurement was 21.3 minutes. The total scan time the gold standard T2 measurement was near 2.5 hours.
To calculate T1 values, pixel-based nonlinear least-squares curve fitting was used to fit the magnitude of the IR-SE images to S(TI) = a − be−TI/T1. To calculate T2 values, the magnitude values from the multiple single-echo spin echo images were fit to S(TE) = ae −TE/T2.
MR Fingerprinting Repeatability Measurements
The phantom was scanned on a Siemens Skyra 3T (Siemens AG Healthcare, Erlangen, Germany) with a 20-channel head-neck receiver array for 34 consecutive days to evaluate the repeatability of T1 and T2 estimates from the MRF method. For the daily measurement, the phantom was placed in the magnet for 30 minutes before the acquisition, to decrease the effects of motion on the measurements. The default global system adjustment was performed to adjust the B0 shims and calibrate the RF power before MRF scans. No extra B0 and B1 mapping methods were performed in this study. A FISP-based MRF acquisition (19) was used to scan two slices, one through each of the T1 and T2 arrays, with an in-plane spatial resolution of 1.2 × 1.2 mm2 and a slice thickness of 5 mm. Flip angles were varied between 5° – 75° and repetition times ranged from 12 to 15 ms (19). A total of 3000 frames were acquired for each slice, resulting in a scan time of 45 seconds per slice.
To compare the MRF method with the gold standard methods, the T1 IR-SE method, the T2 spin echo method, and MRF acquisitions through the T1 and T2 arrays were each repeated 5 times. The scan parameters were the same as described in the previous sections. The long acquisition time prohibited performing this measurement every day. The 5 repeated measurements were performed continuously, and the total acquisition time was about 25 hours.
MRF Reconstruction and Pattern Recognition
A dictionary containing a set of signal evolutions was generated by Bloch simulations. The dictionary resolution, denoted as min:step:max was [10:10:100, 120:20:1000, 1040:40:2000, 2050:100:3000] ms for T1 and [2:2:10, 15:5:100, 110:10:300, 350:50:800] ms for T2. The dictionary had a total of 4,141 entries that excluded unrealistic T2 > T1 combinations.
The undersampled spiral data were reconstructed using NUFFT (22) with a separately measured spiral trajectory (23,24). The coil sensitivity map was estimated using the Walsh method (25) and derived from the average of the first 1000 coil-uncombined images. Pattern matching was performed by taking a complex dot product between the measured signal time course of each pixel and all entries of the dictionary. T1 and T2 values were derived from the entry that was maximally correlated against the acquired signal, and thus represented the closest dictionary entry to the acquired signal time course. NUFFT and pattern matching were implemented in the Siemens Image Calculation Environment (ICE, Siemens AG Healthcare, Erlangen, Germany). Twenty-five seconds were needed to reconstruct 3000 frames, estimate the coil sensitivity map, and combine the multiple coil images. The pattern matching process required 15 seconds using the current dictionary, for a 256 × 256 matrix acquisition.
Results
T1 values estimated from IR-SE and T2 values from the multiple single-echo spin echo technique are reported in Table 1. The mean and the standard deviation of each sphere were calculated from 50 pixels in a circular region-of-interest (ROI) that was manually drawn on the T1 or T2 map to exclude edge pixels.
Table 1.
The mean and standard deviation (SD) of T1 values estimated from inversion recovery spin echo measurements and T2 values estimated from multiple single-echo spin echo measurements. The mean and standard deviation of each sphere were calculated from 50 pixels within a circular region-of-interest (ROI) that was manually drawn on the T1 or T2 map.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 (ms) | Mean | 2038 | 1482 | 996 | 717 | 505 | 358 | 253 | 181 | 127 | 90 | 64 | 45 | 32 | 21 |
| SD | 126 | 41 | 23 | 20 | 8 | 6 | 4 | 8 | 3 | 2 | 1 | 2 | 4 | 7 | |
|
| |||||||||||||||
| T2 (ms) | Mean | 581 | 406 | 292 | 203 | 143 | 97 | 71 | 51 | 37 | 26 | 20 | 14 | 13 | 11 |
| SD | 22 | 15 | 16 | 10 | 9 | 3 | 7 | 5 | 5 | 3 | 5 | 2 | 10 | 6 | |
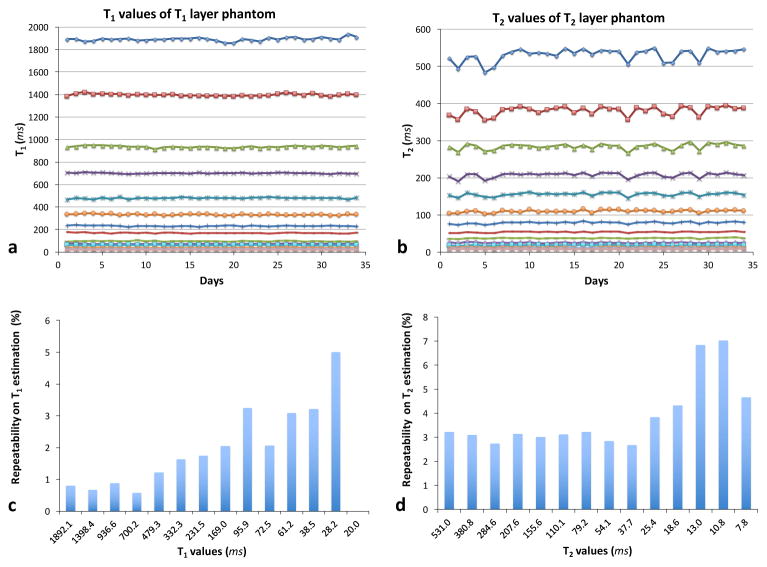
Figure 1 shows T1 (a) and T2 (b) values of each sphere over 34 consecutive days of measurement. The T1 and T2 values were averaged over 70 pixels in a circular ROI drawn on T1 and T2 maps. Maps from MRF have higher spatial resolution compared to those from spin echo methods, allowing more pixels to be included in the ROI. The repeatability of T1 (c) and T2 (d) estimates from the MRF-FISP method is characterized as the coefficient of variation (CV), defined as the ratio of the standard deviation to the mean T1 and T2 values over 34 days. Over the wide ranges of T1 and T2 values, MRF estimates have less than 5 % variation, with the exception of T2 relaxation times shorter than 13 ms, which shows a variation of 4.3–7.0 % (Figure 1c and 1d). The short T2 relaxation times are on the order of the TR used for the MRF measurement.
Figure 1.
T1 (a) and T2 (b) values of each sphere over 34 consecutive days. The repeatability of MRF-FISP T1 (c) and T2 (d) estimates is the standard deviation normalized by the mean T1 and T2 values of 34 days.
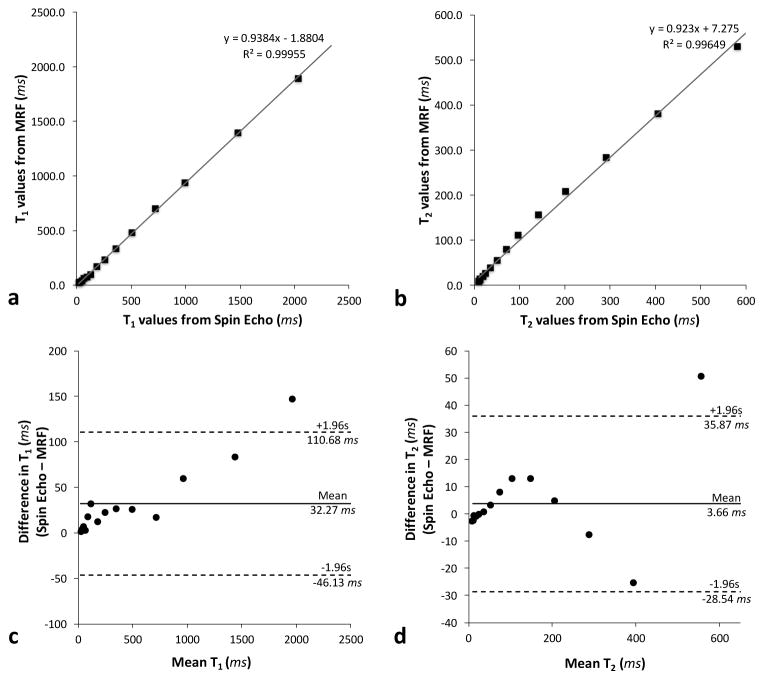
Figure 2a shows the mean T1 values obtained from MRF over 34 consecutive days plotted against those obtained from the gold standard IR-SE method. Figure 2b shows the mean T2 values from MRF plotted against the values from the multiple single-echo spin echo method. The results show a strong linear correlation (R2 = 0.999 for T1, R2 = 0.996 for T2). The linear fits have slopes of 0.94 for T1 values, 0.92 for T2, and y-intercepts of -1.88 ms for T1, and 7.28 ms for T2.
Figure 2.
Correlation plots (a–b) and Bland-Altman plots (c–d) comparing T1 and T2 values averaged over 34 consecutive days of MRF measurements to the T1 and T2 values obtained from the inversion recovery spin echo and spin echo methods, respectively.
Bland-Altman analysis was performed to assess the agreement between T1 and T2 values calculated from the MRF method and the values calculated from the spin echo methods. Figure 2c shows the Bland-Altman plot of T1 values acquired with the IR-SE and the mean T1 values obtained from MRF over 34 days. The mean bias for T1 was 32.27 ms, and the 95% limits of agreement ranged from −46.13 ms to 110.68 ms. One data point with the longest T1 value was outside of the limits of agreement. Figure 2d shows the Bland-Altman plot of T2 values calculated from the multiple single-echo spin echo method and the mean T2 values obtained from MRF over 34 days. The mean bias for T2 was 3.66 ms, and the 95% limits of agreement ranged from −28.54 ms to 35.87 ms. Similarly, one data point with the longest T2 value was outside of the limits of agreement.
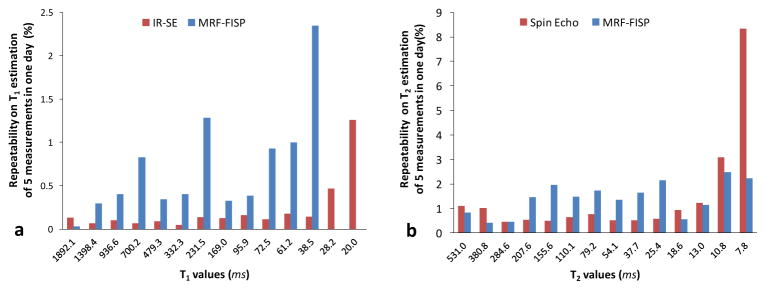
The repeatabilities of the IR-SE method, spin echo method, and MRF method are shown in Figure 3. Over 5 repetitions, the IR-SE for T1 estimation varied less than 0.2% for T1 values larger than 30 ms and less than 1.3% for smaller T1 values. The MRF results for T1 estimation varied less than 1.3% for T1 values larger than 40 ms and less than 2.3% for smaller T1 values. For T2 values larger than 20 ms, the variation of the spin echo method was less than 1.2 %, and the variation of MRF was less than 2.1%.
Figure 3.
The repeatability of T1 (a) and T2 (b) estimates from MRF-FISP method and the gold standard spin echo methods for each sphere (five repeated measurements).
Discussion
MRF estimates of the wide range of T1 and T2 values in the ISMRM/NIST MRI system phantom varied less than 5% over 34 consecutive days . The mean T1 and T2 values over 34 days also showed strong linear correlation with the results from the gold standard T1 and T2 measurements. The longest relaxation times (both T1 and T2) were outside the Bland-Altman limits of agreement. This could be due to very long T2 values in these spheres (> 500 ms) (17). Measurements of solutions with such long T2 values are more susceptible to any system imperfections, such as inaccurate flip angles and the eddy current, etc.
While the gold standard spin echo methods showed better repeatability than the MRF method, the prohibitively long acquisition time of the spin echo method precludes its use in almost all clinical situations.
All methods showed greater variation in the shortest T1 and T2 values due to the choice of acquisition parameters in current experiment. The minimum TI in the IR-SE and the MRF method was 21 ms, which limited the ability to quantify T1 values less than 21 ms accurately. The minimum TE used in the spin echo method was 12 ms, which limited the quantification of T2 values that are on the order of the minimum TE. T2 values on the order of the minimum TR used in the MRF method are the lower bound of accurate T2 estimation.
The T2 measurements had greater variation than the T1 measurements, which could be a results of the B1 variation from day to day. For the current study, the system default adjustment for the global B0 and transmit radiofrequency power setting were used in the daily scan. No additional B1 mapping was used to correct B1 variation within the field-of-view. A previous MRF study (26) showed that B1 variation affects the measured T2 values more than T1 values. Additional B1 measurement and correction can improve the accuracy of the T1 and T2 estimates and should be included in cases where less than 5% variation is required. These variations could also be a result of small temperature fluctuations from day to day: the MnCl2 solutions in the T2 array are more sensitive to temperature changes than the NiCl2 solutions in the T1 array (27). A thorough study to examine the temperature dependence of the ISMRM/NIST MRI system phantom will be needed to address this issue.
The observed variations in T1 and T2 values could be affected by the dictionary resolution. In the current study, the shortest T1 values, 21 ms and 32 ms, showed no variations. This was due to the T1 value step size (10 ms) in the current dictionary. The dictionary resolution is a trade-off between the calculation time and the expected precision. A previous study (reported in the supplementary information of (18)) showed that the accuracy of the T1 and T2 estimates was not affected by the different dictionary resolutions, but the standard deviations of the estimated T1 and T2 values were reduced when finer dictionary step sizes were used. This is a common result of almost any digital system in the presence of quantization noise; a higher precision in the quantization leads to higher precision in the final result. The repeatability observed in the current study could potentially be improved using a dictionary with a finer step size, though previous studies (18,20) have shown only minor improvements. In the current implementation of MRF, a straightforward template matching algorithm was utilized. This simple approach was used to rule out complications from the use of faster, but more complex algorithms. Higher repeatability could potentially be achieved without increasing the computation time by using a compressed dictionary (28,29) or other advanced processing algorithms.
Conclusion
Using the ISMRM/NIST MRI system phantom, MRF has high repeatability and accuracy over a period of 34 days across a wide range of T1 and T2 values.
Acknowledgments
Authors YJ, DM, VG and MAG acknowledge the NIH (1R01EB016728, 1R01EB017219, and 1R01DK098503) and Siemens Healthcare for grant support.
References
- 1.Just M, Thelen M. Tissue characterization with T1, T2, and proton density values: results in 160 patients with brain tumors. Radiology [Internet] 1988;169:779–85. doi: 10.1148/radiology.169.3.3187000. [DOI] [PubMed] [Google Scholar]
- 2.Roebuck JR, Haker SJ, Mitsouras D, Rybicki FJ, Tempany CM, Mulkern RV. Carr-Purcell-Meiboom-Gill imaging of prostate cancer: quantitative T2 values for cancer discrimination. Magn Reson Imaging [Internet] 2009;27:497–502. doi: 10.1016/j.mri.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manfredonia F, Ciccarelli O, Khaleeli Z, Tozer DJ, Sastre-Garriga J, Miller DH, Thompson AJ. Normal-appearing brain t1 relaxation time predicts disability in early primary progressive multiple sclerosis. Arch Neurol [Internet] 2007;64:411–5. doi: 10.1001/archneur.64.3.411. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos K, Tozer DJ, Fisniku L, Altmann DR, Davies G, Rashid W, Thompson AJ, Miller DH, Chard DT. TI-relaxation time changes over five years in relapsing-remitting multiple sclerosis. Mult Scler [Internet] 2010;16:427–33. doi: 10.1177/1352458509359924. [DOI] [PubMed] [Google Scholar]
- 5.Bernarding J, Braun J, Hohmann J, Mansmann U, Hoehn-Berlage M, Stapf C, Wolf KJ, Tolxdorff T. Histogram-based characterization of healthy and ischemic brain tissues using multiparametric MR imaging including apparent diffusion coefficient maps and relaxometry. Magn Reson Med [Internet] 2000;43:52–61. doi: 10.1002/(sici)1522-2594(200001)43:1<52::aid-mrm7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood [Internet] 2005;105:855–61. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 7.Ghugre NR, Ramanan V, Pop M, Yang Y, Barry J, Qiang B, Connelly KA, Dick AJ, Wright GA. Quantitative tracking of edema, hemorrhage, and microvascular obstruction in subacute myocardial infarction in a porcine model by MRI. Magn Reson Med [Internet] 2011;66:1129–41. doi: 10.1002/mrm.22855. [DOI] [PubMed] [Google Scholar]
- 8.McSheehy PMJ, Weidensteiner C, Cannet C, Ferretti S, Laurent D, Ruetz S, Stumm M, Allegrini PR. Quantified tumor T1 is a generic early-response imaging biomarker for chemotherapy reflecting cell viability. Clin Cancer Res [Internet] 2010;16:212–25. doi: 10.1158/1078-0432.CCR-09-0686. [DOI] [PubMed] [Google Scholar]
- 9.Weidensteiner C, Allegrini PR, Sticker-Jantscheff M, Romanet V, Ferretti S, McSheehy PMJ. Tumour T1 changes in vivo are highly predictive of response to chemotherapy and reflect the number of viable tumour cells--a preclinical MR study in mice. BMC Cancer [Internet] 2014;14:88. doi: 10.1186/1471-2407-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson [Internet] 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt P, Griswold MA, Jakob PM, Kotas M, Gulani V, Flentje M, Haase A. Inversion recovery TrueFISP: quantification of T(1), T(2), and spin density. Magn Reson Med [Internet] 2004;51:661–7. doi: 10.1002/mrm.20058. [DOI] [PubMed] [Google Scholar]
- 12.Warntjes JBM, Dahlqvist O, Lundberg P. Novel method for rapid, simultaneous T1, T*2, and proton density quantification. Magn Reson Med [Internet] 2007;57:528–37. doi: 10.1002/mrm.21165. [DOI] [PubMed] [Google Scholar]
- 13.Doneva M, Börnert P, Eggers H, Stehning C, Sénégas J, Mertins A. Compressed sensing reconstruction for magnetic resonance parameter mapping. Magn Reson Med [Internet] 2010;64:1114–20. doi: 10.1002/mrm.22483. [DOI] [PubMed] [Google Scholar]
- 14.Ehses P, Seiberlich N, Ma D, Breuer FA, Jakob PM, Griswold MA, Gulani V. IR TrueFISP with a golden-ratio-based radial readout: fast quantification of T1, T2, and proton density. Magn Reson Med [Internet] 2013;69:71–81. doi: 10.1002/mrm.24225. [DOI] [PubMed] [Google Scholar]
- 15.Siversson C, Tiderius C-J, Neuman P, Dahlberg L, Svensson J. Repeatability of T1-quantification in dGEMRIC for three different acquisition techniques: Two-dimensional inversion recovery, three-dimensional look locker, and three-dimensional variable flip angle. J Magn Reson Imaging [Internet] 2010;31:1203–1209. doi: 10.1002/jmri.22159. [DOI] [PubMed] [Google Scholar]
- 16.Hannila I, Lammentausta E, Tervonen O, Nieminen MT. The repeatability of T2 relaxation time measurement of human knee articular cartilage. MAGMA [Internet] 2015;28:547–53. doi: 10.1007/s10334-015-0494-3. [DOI] [PubMed] [Google Scholar]
- 17.Russek SE, Boss M, Jackson EF, Jennings DL, Evelhoch JL, Gunter JL, Sorensen AG. Characterization of NIST/ISMRM MRI System Phantom. Proceeding 20th Annu. Meet. ISMRM; Melbourne, Victoria. Aust. 2012; p. 2456. [Google Scholar]
- 18.Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature [Internet] 2013;495:187–92. doi: 10.1038/nature11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med [Internet] 2015;74:1621–31. doi: 10.1002/mrm.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Ma D, Jerecic R, Duerk J, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using the quick echo splitting NMR imaging technique. Magn Reson Med [Internet] 2016 doi: 10.1002/mrm.26173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton JI, Jiang Y, Chen Y, Ma D, Lo WC, Griswold M, Seiberlich N. MR fingerprinting for rapid quantification of myocardial T1, T2, and proton spin density. Magn Reson Med. 2016 doi: 10.1002/mrm.26216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fessler JA, Sutton BP. Nonuniform fast fourier transforms using min-max interpolation. IEEE Trans Signal Process [Internet] 2003;51:560–574. doi: 10.1109/TSP.2002.807005. [DOI] [Google Scholar]
- 23.Zhang Y, Hetherington HP, Stokely EM, Mason GF, Twieg DB. A novel k-space trajectory measurement technique. Magn Reson Med [Internet] 1998;39:999–1004. doi: 10.1002/mrm.1910390618. [DOI] [PubMed] [Google Scholar]
- 24.Duyn JH, Yang Y, Frank JA, van der Veen JW. Simple correction method for k-space trajectory deviations in MRI. J Magn Reson [Internet] 1998;132:150–3. doi: 10.1006/jmre.1998.1396. [DOI] [PubMed] [Google Scholar]
- 25.Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med [Internet] 2000;43:682–90. doi: 10.1002/(sici)1522-2594(200005)43:5<682::aid-mrm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Jiang Y, Pahwa S, Ma D, Lu L, Twieg MD, Wright KL, Seiberlich N, Griswold MA, Gulani V. MR Fingerprinting for Rapid Quantitative Abdominal Imaging. Radiology [Internet] 2016;279:278–286. doi: 10.1148/radiol.2016152037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keenan K, Stupic K, Boss M, et al. Multi-Site, Multi-Vendor Comparison of T1 Measurement Using ISMRM/NIST System Phantom. Proceeding 24th Annu. Meet. ISMRM; Singapore. 2016. p. 3290. [Google Scholar]
- 28.McGivney DF, Pierre E, Ma D, Jiang Y, Saybasili H, Gulani V, Griswold MA. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans Med Imaging [Internet] 2014;33:2311–22. doi: 10.1109/TMI.2014.2337321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauley SF, Setsompop K, Ma D, Jiang Y, Ye H, Adalsteinsson E, Griswold MA, Wald LL. Fast group matching for MR fingerprinting reconstruction. Magn Reson Med [Internet] 2015;74:523–528. doi: 10.1002/mrm.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]