Abstract
Background
The association between periodontitis and systemic diseases has been increasingly recognized. However, the data on the association between periodontitis and psoriasis are still limited.
Objectives
To summarize all available data on the association between periodontitis and the risk of psoriasis.
Methods
Two investigators independently searched published studies indexed in MEDLINE and EMBASE databases from inception to July 2016 using a search strategy that included terms for psoriasis and periodontitis. Studies were included if the following criteria were met: (1) Case-control or cohort study comparing the risk of psoriasis in subjects with and without periodontitis ;(2) subjects without periodontitis were used as comparators in cohort studies while participants without psoriasis were used as controls in case-control studies; and (3) effect estimates and 95% confidence intervals (CI) were provided. Point estimates and standard errors from each study were extracted and combined together using the generic inverse variance technique described by DerSimonian and Laird.
Results
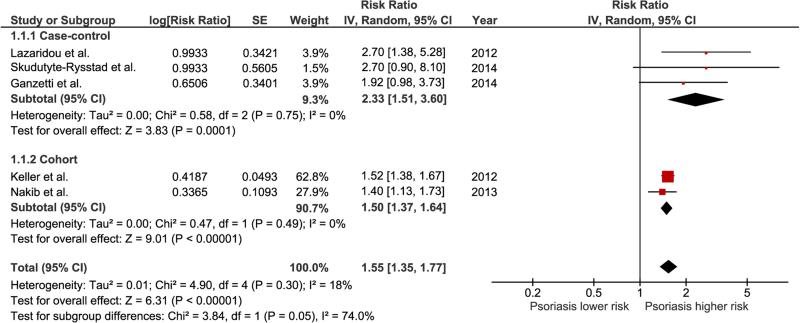
Two cohort studies and three case-control studies met the inclusion criteria and were included in the meta-analysis. The pooled risk ratio of psoriasis in patients with periodontitis versus comparators was 1.55 (95% CI, 1.35–1.77). The statistical heterogeneity was insignificant with an I2 of 18%. Subgroup analysis according to study design revealed a significantly higher risk among patients with periodontitis with a pooled RR of 1.50 (95% CI, 1.37 – 1.64) for cohort studies and a pooled RR of 2.33 (95% CI, 1.51 – 3.60) for case-control studies.
Conclusions
Patients with periodontitis have a significantly elevated risk of psoriasis.
Keywords: Psoriasis, Periodontitis, Meta-analysis
Introduction
Psoriasis is a chronic inflammatory skin disease characterized by inflammation of the dermis and epidermis associated with markedly thickened epidermis and atypical keratinocyte differentiation. It is a common disease with an estimated prevalence of 2-4% in the general population. 1 The etiology of psoriasis is unknown but epidemiologic studies have identified several risk factors including smoking, high body mass index, sedentary life-style and excessive alcohol consumption. 2-4
Periodontitis is a chronic inflammatory disease of the gingiva as a result of an exaggerated inflammatory response against polymicrobial colonization in the dental plaque. 5 It is a common disease that affects approximately one-third of adults over 30 years of age and up to half of adults over 50 years of age. 6-7
Over the past several years, the association between periodontitis and immune-mediated inflammatory diseases has been increasingly recognized. It is hypothesized that bacterial colonization in the oral cavity could trigger an exaggerated immune response in a susceptible host, leading to a perpetual inflammatory process associated with autoimmune disorders.8 This association has been most extensively studied in rheumatoid arthritis (RA), where the odds of having RA among those with periodontitis is 2 to 8 times higher than those without periodontitis. 9-13
Patients with periodontitis may also have a higher risk of psoriasis; however, the data on this association are limited. The aim of this systematic review and meta-analysis is to further analyze the potential association between periodontitis and psoriasis.
Methods
Search strategy
Two investigators (P.U. and K.W.) independently searched published studies indexed in MEDLINE and EMBASE databases from inception to July 2016 using a search strategy that included terms for psoriasis and periodontitis as described in supplementary material 1. Bibliographies of selected review articles were also manually reviewed. Studies were included if the following criteria were met: (1) Case-control or cohort study (either prospective or retrospective) comparing the risk of psoriasis in subjects with and without periodontitis; (2) subjects without periodontitis were used as comparators in cohort studies while participants without psoriasis were used as controls in case-control studies; and (3) odds ratio (OR), relative risk (RR), hazard ratio (HR) or standardized incidence ratio (SIR) with 95% confidence intervals (CI) or sufficient raw data to calculate these ratios were provided.
Retrieved studies were independently reviewed by each investigator noted above. Any disagreements in the determination of study eligibility were resolved by mutual consensus. Included studies were appraised for their quality using the Newcastle-Ottawa quality assessment scale 14 which assessed the quality of the study's methodology in three domains including the selection of the participants; the comparability between the two groups; and the methods used to identify and verify the exposures of interest and the outcomes of interest for case-control studies and cohort studies, respectively.
Data extraction
A standardized data collection form was used to extract the following information: first author's name, study's name, published journal, year of publication, year when the study was conducted, study design, country of origin, method used to identify and diagnose psoriasis and periodontitis, follow up (for cohort study), demographic data of participants, confounders that were adjusted, and adjusted effect estimates with the corresponding 95% CIs. This data extraction was independently performed by P.U. and K.W. The extracted data were cross-checked; any discrepancies were evaluated by referring back to the original study.
Statistical analysis
Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom) was used to calculate the pooled estimate effect. Point estimates and standard errors from each study were extracted and combined together using the generic inverse variance technique described by DerSimonian and Laird.15 As this meta-analysis included data from two different study designs, a high between-study variance was expected and a random-effect model, rather than a fixed-effect model, was utilized. As the outcome of interest in this study was relatively uncommon, OR of a case-control study was used as an estimate for RR to calculate the pooled estimate with RR from a cohort study. Cochran's Q test and I2 statistic were used to determine the statistical heterogeneity. A value of I2 of 0% to 25% represented insignificant heterogeneity, more than 25% but less than or equal to 50% represented low heterogeneity, more than 50% but less than or equal to 75% represented moderate heterogeneity, and more than 75% represented high heterogeneity.16 Publication bias was assessed by visualization of a funnel plot and Egger's regression test using Comprehensive Meta-analysis 3.0 software (Englewood, New Jersey, United States).17
Results
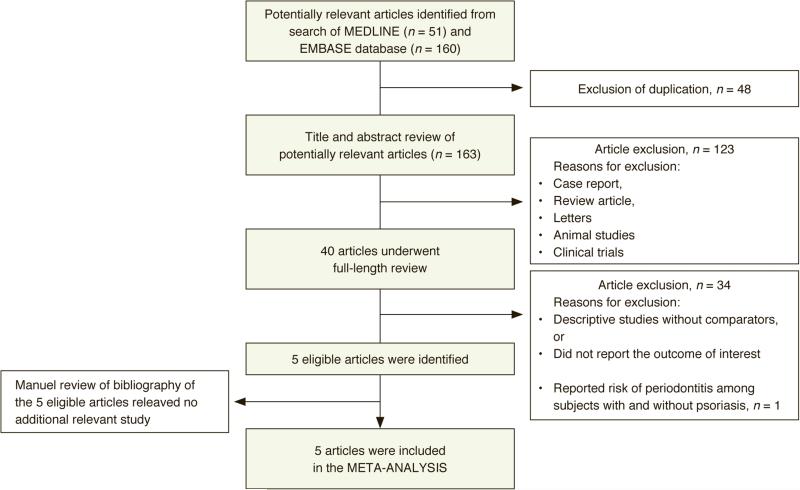
Our search strategy yielded 163 potentially relevant articles (160 articles from EMBASE and 51 articles from MEDLINE). After exclusion of 48 duplicate articles, 163 articles underwent title and abstract review. 123 articles were excluded at this stage because they were not observational studies (but rather were case reports, reviews, correspondences, randomized controlled trials etc.), leaving 40 articles for full-length article review. 34 of these 40 articles were excluded because they were descriptive studies without comparison groups or did not report the outcomes of interest. One study was excluded because it compared the risk of periodontitis among subjects with and without psoriasis (and did not compare the risk of psoriasis in subjects with and without periodontitis).18 Therefore, five studies (two cohort studies and three case-control studies) met the pre-defined eligibility criteria and were included in the meta-analysis.19-23 The bibliographies of these five studies were manually reviewed for additional relevant studies but no further studies were identified. The study review process is outlined in Figure 1. The characteristics and quality assessment of the included cohort studies and case-control studies are described in Tables 1 and 2, respectively.
Figure 1.
Literature review process
| Keller et al.19 | Nakib et al.20 | |
|---|---|---|
| Country of origin | Taiwan | United States |
| Study design | Retrospective cohort | Prospective cohort |
| Year of publication | 2012 | 2014 |
| Cases | Cases of periodontitis were identified from the Longitudinal Health Insurance Database which included random sample of one million enrollees under the Taiwan National Health Insurance Program. The study included subjects with first diagnostic code of periodontitis between 1 January 2001 and 31 December 2004. Subjects in this study needed to have at least 2 diagnostic codes of periodontitis in the database. | Cases of periodontitis were identified from Nurses' Health Study cohort which was established in 1976. Subjects with self-reported history of periodontitis in the 1998 survey and were free of psoriasis at that time were included. |
| Comparators | Comparators were sex, age and urbanization-matched. They were randomly recruited from the same database. | Comparators were the rest of subjects in Nurses' Health Study cohort who did not have psoriasis and periodontitis. |
| Identification and verification of psoriasis | Presence of diagnostic code for psoriasis was used to identify the diagnosis of psoriasis. No further verification was made. | Self-reported physician–diagnosed psoriasis though health questionnaires that were mailed to the subjects every 2 years |
| Number of subjects (cases/comparators) | 115,365 /115,365 | 11,358 /70,020 |
| Percentage of female (cases/comparators) | 52.1/52.1 | 100.0/100.0 |
| Mean age in years (cases/comparators) | 39.2/39.2 | 64.7/64.6 |
| Follow up | Medical records were tracked for 5 years after index date | Until last health questionnaire in 2008 |
| Confounder assessed | Income, urbanization and geographic region | Age, body mass index, smoking, alcohol and physical activity |
| Quality assessment (Newcastle – Ottawa scale) | Selection: 4 stars Comparability: 2 stars Outcome: 3 stars |
Selection: 3 stars Comparability: 1 star Outcome: 3 stars |
| Lazaridou et al.21 | Skudutyte-Rysstad et al.22 | Ganzetti et al.23 | |
|---|---|---|---|
| Country of origin | Greek | Norway | Italy |
| Study design | Case-control | Case-control | Case-control |
| Year of publication | 2012 | 2014 | 2014 |
| Cases | Cases of psoriasis were consecutively recruited from outpatient clinic of the study hospital (Hospital of skin and venereal disease in Thessaloniki, Greek) from January 2011 to May 2011. Diagnosis required histopathological confirmation. Patients who were receiving systemic therapy for psoriasis were excluded. | Cases of psoriasis were consecutively recruited from outpatient clinic of the study hospital (Oslo-University Hospital-Rikshospitalet, Norway). Diagnosis was made by study physicians. Patients with concomitant other autoimmune disorders, malignancy and pregnancy were excluded. | Cases of psoriasis were consecutively recruited from outpatient clinic of the study hospital. Diagnosis was made by study physicians. Cases never received biologic agents prior to enrollment. |
| Controls | Controls were sex and age-matched. They were recruited from the same outpatient clinic. Controls did not have psoriasis or other autoimmune skin diseases. | Controls were randomly selected from the Norwegian National Population Register. Controls did not have psoriasis. | Controls were sex and age-matched. They were recruited from the same outpatient clinic. |
| Identification and verification of periodontitis | Diagnosis of periodontitis was made by study dentists who were blinded to skin status. The evaluation was done by the measurement of the periodontal parameters according to standard method. Radiologic confirmation of bone loss was also done. | Diagnosis of periodontitis was made by study dentists who were blinded to skin status. The evaluation was done by the measurement of the periodontal parameters according to standard method. Periodontitis was defined according to the definition by CDC. | Diagnosis of periodontitis was made by study dentists. |
| Number of subjects (cases/controls) | 100/100 | 50/121 | 60/45 |
| Percentage of female (cases/controls) | 57.0/57.0 | 24.0/50.0 | 42.0/47.0 |
| Mean age in years (cases/controls) | 57.2/57.2 | 44.4/48.6 | 44.4/48.7 |
| Confounder assessed | None | Propensity score, smoking and dental visits | None |
| Quality assessment (Newcastle– Ottawa scale) | Selection: 2 stars Comparability: 1 star Outcome: 3 stars |
Selection: 3 stars Comparability: 1 stars Outcome: 3 stars |
Selection: 2 stars Comparability: 1 star Outcome: 3 stars |
The five studies included 312,584 subjects. One study was conducted in Asia (Taiwan) while the rest were conducted in Western countries (United States, Norway, Greece and Italy). All of the included studies demonstrated good to high quality with a Newcastle-Ottawa score ranging from 6 to 9.
The pooled risk ratio of psoriasis in patients with periodontitis versus comparators was 1.55 (95% CI, 1.35–1.77). The statistical heterogeneity was insignificant with an I2 of 18%. Subgroup analysis according to study design revealed a significantly higher risk of psoriasis among patients with periodontitis with a pooled RR of 1.50 (95% CI, 1.37 – 1.64) for cohort studies and a pooled RR of 2.33 (95% CI, 1.51 – 3.60) for case-control studies. Figure 2 demonstrates the forest plots of the overall analysis as well as subgroup analysis.
Figure 2.
Forest plot of this meta-analysis. The sizes of the boxes used for each study represent the weight of that study used for calculation of the pooled effect. The diamonds at the bottom of this forest plot represent the pooled risk ratios with 95 % CIs (the center of the diamond represents the pooled risk ratio, and the lateral lips of diamond represent the associated 95 % CI)
To confirm the robustness of the pooled effect estimate, two sensitivity analyses were performed. The first sensitivity analysis was conducted by using a fixed-effect model rather than a random-effect model, given the low statistical heterogeneity. The pooled effect estimate from this sensitivity analysis was not significantly different from the original analysis (pooled OR 1.53; 95% CI, 1.40 – 1.66). Additionally, jack-knife sensitivity analysis was performed. This analysis excluded one study from the pooled analysis at a time to investigate if one study had an exaggerated effect on the pooled results. The effect estimates were not significantly affected by this sensitivity analysis as the new pooled RR ranged from 1.51 to 1.80 and remained statistically significant.
Evaluation of publication bias
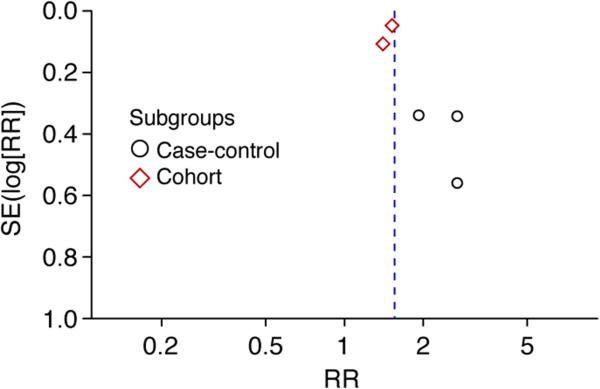
Visualization of the funnel plot revealed evidence of asymmetry with more studies on the right side of the plot which suggested that publication bias in favor of positive studies may have been present. In addition, there was evidence of publication bias from Egger's regression test with a p-value of 0.076.17 Figure 3 depicts the results of the funnel plot analysis.
Figure 3.
Funnel plot of this meta-analysis
Discussion
To our knowledge, this systematic review and meta-analysis is the first study to summarize all available evidence on the association between the risk of developing psoriasis in patients with periodontitis. We found a 1.55-fold increased risk of psoriasis among subjects with periodontitis compared with those without periodontitis. The elevated risk was observed with both study designs (i.e., cohort and case-control), though the risk appeared to be higher among case-control studies.
As previously stated, the association between periodontitis and an increased risk of immune-mediated inflammatory diseases has been increasingly recognized and has been particularly well-studied in RA. It has been suggested that Porphyromonas gingivalis, a common gram-negative anaerobic periodontal pathogen, is the key link between RA and periodontitis because of its unique capability to produce peptidyl arginine deiminase, the major enzyme that promotes post-translational citrullination of peptides. Excessive amount of citrullinated peptides could then induce production of anti-citrullinated peptide antibody (ACPA), which is a pivotal autoantibody in the pathogenesis of synovitis in RA.12, 24 Despite this, it is unlikely that excessive citrullination of peptides is also responsible for the increased risk of psoriasis, as ACPA has no role in the pathogenesis of this inflammatory cutaneous disease.
It is known that psoriasis can be triggered or worsened by the presence of infections such as streptococcal pharyngitis or human immunodeficiency virus. Even though the exact mechanism behind the increased risk of psoriasis among patients with periodontitis is unknown, several possible explanations have been proposed. One hypothesis is related to the shared pathology between psoriasis and periodontitis, as exaggerated immune responses to the residing microbiota at the epithelial surface are observed in both conditions, which might suggest a shared genetic predisposition affecting dendritic cells and toll-like receptor expression.18, 25, 26 The other possible explanation involves the activation of Th-17 cells and the increased expression of interleukin 17 (which is one of the major players in the pathogenesis of both psoriasis and psoriatic arthritis) induced by the bacteria involved in periodontal infection and their products.25, 26
It would be informative to analyze whether patients with psoriasis associated with periodontitis have a higher (or lower) risk of developing psoriatic arthritis compared to the overall psoriatic population. However, none of the included primary studies in our meta-analysis investigated this association. Further studies to clarify this risk would be of interest.
We acknowledge that this study has some limitations. Therefore, the findings should be interpreted with caution.
First, the accuracy of diagnosis of periodontitis and psoriasis of the two cohort studies included in this meta-analysis may be limited, as the study by Keller et al.18 was an administrative database-based study that used diagnostic codes to identify the events of interest without further verification, while the study by Nakib et al.19 relied solely on self-reported health questionnaires. Second, publication bias may have been present in ourstudy, based upon the results of our funnel plot analysis and Egger's regression test. Moreover, it is possible that the apparent increased risk of psoriasis in patients with periodontitis is a result of confounding rather than true causality, as periodontal disease and psoriasis share several risk factors including smoking, obesity and diabetes mellitus,2-7 and only two out of the five studies included in our meta-analysis appropriately adjusted for those factors.19, 21 Nonetheless, the pooled RR from the sensitivity analysis that included only the two studies with adequate confounder adjustment was not significantly different from the complete analysis (pooled RR 1.54; 95% CI, 0.98 – 2.45). Finally, our study was unable to assess for a possible association between periodontitis and psoriatic arthritis.
Conclusion
Our meta-analysis demonstrated that patients with periodontitis have a significantly increased risk of psoriasis. Further investigation is needed to determine if the association between periodontitis and psoriasis is causal.
Supplementary Material
Acknowledgement
We certify that the manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere.
Funding: CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contribution:
Patompong Ungprasert: 1. Conception and design 2. Acquisition and interpretation of data 3. Drafting of the manuscript 4. Statistical analysis
Karn Wijarnpreecha: 1. Conception and design 2. Acquisition and interpretation of data 3. Drafting of the manuscript
David A. Wetter: 1. Conception and design 2. Interpretation of data 3. Critical revision of the manuscript for important intellectual content 4. Supervision
Patompong Ungprasert had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statement for all authors: We do not have any financial or non-financial potential conflicts of interest.
References
- 1.Christophers E. Psoriasis – Epidemiology and clinical spectrum. Clin Exp Dermatol. 2011;26:314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol. 2014;170:304–314. doi: 10.1111/bjd.12670. [DOI] [PubMed] [Google Scholar]
- 3.Duffy DL, Spelman LS, Martin NG. Psoriasis in Australian twins. J Am Acad Dermatol. 1993;29:428–434. doi: 10.1016/0190-9622(93)70206-9. [DOI] [PubMed] [Google Scholar]
- 4.Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529–34. doi: 10.1016/j.clindermatol.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2175–2184. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 6.Albandar JM. Periodontal diseases in North America. Periodontol 2000. 2002;29:31–69. doi: 10.1034/j.1600-0757.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- 7.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal disease. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 8.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. 2013;84:S8–S19. doi: 10.1902/jop.2013.1340010. [DOI] [PubMed] [Google Scholar]
- 9.Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–87. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 10.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–76. [PubMed] [Google Scholar]
- 11.Mikuls TR, Payne JB, Reinhardt RA, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9:38–42. doi: 10.1016/j.intimp.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leech MT, Bartold PM. The association between rheumatoid arthritis and periodontitis. Best Pract Res Clin Rheumatol. 2015;29:189–201. doi: 10.1016/j.berh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Pischon N, Pischon T, Kroger J, et al. Association among rheumatoid arthritis, oral hygiene and periodontitis. J Periodontol. 2008;79:979–86. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trial. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Raman A, Pradeep AR. Association of chronic periodontitis and psoriasis: periodontal status with severity of psoriasis. Oral Dis. 2015;21:314–9. doi: 10.1111/odi.12271. [DOI] [PubMed] [Google Scholar]
- 19.Keller JJ, Lin HC. The effects of chronic periodontitis and its treatment on the subsequent risk of psoriasis. Br J Dermatol. 2012;167:1338–44. doi: 10.1111/j.1365-2133.2012.11126.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakib S, Han J, Li T, Joshipura K, Qureshi AA. Periodontal disease and risk of psoriasis among nurses in the United States. Acta Odontol Scand. 2013;71:1423–9. doi: 10.3109/00016357.2013.766360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazaridou E, Tsikrikoni A, Fotiadou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. J Eur Acad Dermatol Venereol. 2013;27:967–72. doi: 10.1111/j.1468-3083.2012.04615.x. [DOI] [PubMed] [Google Scholar]
- 22.Skudutyte-Rysstad R, Slevolden EM, Hansen BF, Sandvik L, Preus HR. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139. doi: 10.1186/1472-6831-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganzetti G, Campanati A, Santerlli A, et al. Involvement of the oral cavity in psoriasis: results of a clinical study. Br J Dermatol. 2015;172:282–4. doi: 10.1111/bjd.13201. [DOI] [PubMed] [Google Scholar]
- 24.Mikuls TR, Payne JB, Deane KD, Thiele GM. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: The spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol. 2016;137:28–34. doi: 10.1016/j.jaci.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 2003;31:135–66. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 26.Sabat, Philipp S, Hoflich C, et al. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16:77. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.