Abstract
Background
Posttraumatic stress disorder (PTSD) is associated with reduced executive functioning and verbal memory performance, as well as abnormal task-specific activity in prefrontal (PFC) and anterior cingulate cortices (ACC). The current study examined how PTSD symptoms and neuropsychological performance in combat veterans relates to 1) medial PFC and ACC activity during cognitive inhibition, and 2) task-independent PFC functional connectivity.
Methods
Thirty-nine male combat veterans with varying levels of PTSD symptoms completed the multisource interference task during functional magnetic resonance imaging. Robust regression analyses were used to assess relationships between percent signal change (PSC: Incongruent-Congruent) and both PTSD severity and neuropsychological performance. Analyses were conducted voxel-wise and for PSC extracted from medial PFC and ACC regions of interest. Resting state scans were available for veterans with PTSD. Regions identified via task-based analyses were used as seeds for resting state connectivity analyses.
Results
Worse PTSD severity and neuropsychological performance related to less medial PFC and rostral ACC activity during interference processing, driven partly by increased activation to congruent trials. Worse PTSD severity related to reduced functional connectivity between these regions and bilateral, lateral PFC (Brodmann area 10). Worse neuropsychological performance related to reduced functional connectivity between these regions and the inferior frontal gyrus.
Conclusions
PTSD and associated neuropsychological deficits may result from difficulties regulating medial PFC regions associated with “default mode”, or self-referential processing. Further clarification of functional coupling deficits between default mode and executive control networks in PTSD may enhance understanding of neuropsychological and emotional symptoms and provide novel treatment targets.
Keywords: posttraumatic stress disorder, fMRI, cognition, trauma
Introduction
The anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) are implicated in both emotional and cognitive processing. Ventral and rostral aspects of ACC and mPFC (including pregenual and subgenual regions), have been implicated in emotion regulation (with ventral regions more specifically implicated in inhibition of fear during extinction), while dorsal aspects are implicated more in cognitive and attention regulation, and explicit appraisal of emotional stimuli (Etkin, Egner, & Kalisch, 2011; Stevens, Hurley, Hayman, & Taber, 2011). Posttraumatic stress disorder (PTSD) is often associated with difficulty inhibiting or regulating emotional responses to trauma (Jovanovic & Ressler, 2010). Thus, the majority of PTSD neuroimaging research has focused on responses to fear- or trauma-related stimuli. Results from this research highlight lower rostral/ventral ACC and mPFC activation (Jovanovic et al., 2013; Liberzon & Sripada, 2008; Shin & Liberzon, 2010; Shin, Rauch, & Pitman, 2006; Shin et al., 2005), thought to reflect deficiencies in down-regulating or inhibiting amygdala (processing salient external stimuli) and insula (processing of internal bodily states) hyperactivation. There is emerging evidence suggesting that PTSD may relate to impairment in neuropsychological function more generally, rather than being circumscribed to emotion regulation (Brandes et al., 2002; Hayes, Vanelzakker, & Shin, 2012). Consistent with the dual role of the ACC and mPFC, there is an emerging body of evidence suggesting that dysregulation in these regions contributes to neuropsychological symptoms associated with PTSD.
Neuropsychological research related to PTSD has most consistently reported decreased verbal memory and executive functioning performance (Aupperle, Melrose, Stein, & Paulus, 2012; Polak, Witteveen, Reitsma, & Olff, 2012). Related to executive functioning, cognitive inhibition (the ability to disregard distracting information) has face validity concerning its importance for emotion regulation after trauma (Aupperle, Melrose, et al., 2012). The multisource interference task (MSIT) was developed specifically to assess cognitive inhibition and dorsal ACC activation when used with functional magnetic resonance imaging (fMRI; Bush, Shin, Holmes, Rosen, & Vogt, 2003). The MSIT involves presentation of three digits (i.e., 311) to which participants press a button for the target number that differs from the rest. In congruent conditions, the target location matches button position; for incongruent conditions, the target location does not match button position, requiring inhibition of the response to the number location. One study reported that combat veterans and their non-trauma exposed co-twins exhibited greater dorsal ACC activation, as well as slower reaction time, relative to those without PTSD and their twins (Shin et al., 2011). Studies using other cognitive inhibition tasks (e.g., the go-nogo task) have reported reduced activation in inferior frontal, dorsolateral PFC, and orbital frontal cortex regions (OFC; Falconer et al., 2008)), which is often associated with behavioral performance (e.g., commission errors). However, in one study, individuals with PTSD exhibited similar counting Stroop task performance and dorsal ACC activation compared to healthy controls (Shin et al., 2007). These findings provide evidence that prefrontal activity may relate to impaired cognitive inhibition, and thus may represent a risk factor for development of PTSD. Critically, it remains unclear whether the group differences in task-related brain activity merely reflect task-specific performance differences between groups versus a more general task-independent PFC circuitry dysfunction.
Resting state functional MRI (Friston, Frith, Liddle, & Frackowiak, 1993) provides a unique opportunity for assessing the functional coupling between PFC circuitry and the wider brain, independent of specific task demands or performance differences. The resting state literature in PTSD has implicated stronger functional connectivity between amygdala and insula (Rabinak et al., 2011; Sripada et al., 2012), and reduced functional connectivity between the amygdala and both dorsal and rostral ACC (Sripada et al., 2012). Increased PTSD severity has also been associated with decreased functional connectivity between posterior cingulate cortex and other default mode network (DMN) regions (i.e., mPFC, precuneus and inferior parietal; Bluhm et al., 2009; King et al., 2016; Lanius et al., 2010). The DMN has been implicated in internally oriented thought processes (Mason et al., 2007) and may compete with goal-directed processes (Anticevic et al., 2012; S. M. Smith et al., 2012). Research in healthy controls suggests that greater functional connectivity between DMN and regions involved in higher levels of executive function (i.e., lateral prefrontal cortex; Greicius, Krasnow, Reiss, & Menon, 2003) may support successful switching between internal and goal-directed processes (Daniels et al., 2010). However, it remains unclear how PFC and ACC functional connectivity specifically relate to PTSD symptoms and neuropsychological function.
Having a clear understanding of how ACC and mPFC activation and functional connectivity relate to emotional and cognitive symptoms of PTSD could help to identify neurobiological targets for PTSD treatment. In the current study, we investigate how PTSD symptoms and neuropsychological function of combat veterans relate to activation during the MSIT. To assess more general PFC pathophysiology, we examined how task-independent ACC and mPFC functional connectivity during rest relates to PTSD symptoms and neuropsychological function. It is difficult to specify directional hypotheses given inconsistencies in previous study results. However, based on previous MSIT findings, we hypothesized that greater dorsal ACC and mPFC activation (perhaps reflecting a compensatory response; (Shin et al., 2011), and lower activation within the rostral/ventral ACC and mPFC (Falconer et al., 2008; Jovanovic et al., 2013; Liberzon & Sripada, 2008; Shin & Liberzon, 2010) would relate to worse PTSD symptoms. We also hypothesized that lower activation in dorsal ACC and mPFC regions would relate to worse neuropsychological function. Lastly, we hypothesized that worse PTSD symptoms and neuropsychological performance would relate to decreased functional connectivity between ACC/mPFC and executive control network PFC regions (i.e., inferior frontal gyrus and lateral PFC).
Methods
Subjects
Subjects included 41 male veterans who served in combat since the onset of Operation Iraqi Freedom. Two subjects were excluded from analyses due to various issues with the quality of MRI data (see supplemental material), leaving 39 subjects for analyses (mean age = 32.03, SD = 7.43). For full exclusion criteria, see supplemental information. Briefly, veterans with substance or alcohol use disorder within six months, schizophrenia or bipolar disorder, medical conditions affecting the hemodynamic response, moderate to severe head injury or neurological disorder, metal or devices contraindicated for fMRI, or use of most current psychiatric medications were excluded. Stable doses (≥ 6 weeks) of selective serotonin reuptake inhibitors and/or sleep medications were allowed. This study was approved by the University of Missouri – Kansas City and the University of Kansas Medical Center Institutional Review Boards. All subjects provided written informed consent.
Clinical and Neuropsychological Assessment
Total score on the PTSD checklist – Military Version (PCL; Weathers, Huska, & Keane, 1991) was used to estimate symptom severity (secondary to combat-related trauma) over the past month. PTSD diagnosis was confirmed via the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995) for DSM-IV-TR. Twelve subjects met full diagnostic criteria for PTSD, and eight met partial criteria (see supplemental information; Stein, Walker, Hazen, & Forde, 1997). CAPS and PCL measures were conducted on a separate scan session approximately one week prior to the scan session. The PCL was used as the primary measure for symptom severity due to high test-retest reliability that has been confirmed at this delay duration (Wilkins, Lang, & Norman, 2011).
Subjects completed a battery of neuropsychological tests, including the Delis-Kaplan Executive Function System Color-Word Interference Test (word reading, color naming, inhibition, and inhibition/switching completion times), Tower Test (Total Achievement Score; Delis, Kramer, Kaplan, & Holdnack, 2004), Symbol Digit Modalities Test (total correct; Smith, 1982), Auditory Verbal Learning Test (trials 1–5 total correct; Strauss, Sherman, & Spreen, 2006), Trail Making Test (time to completion for Trails A and B; Reitan, 1979; Wagner, Helmreich, Dahmen, Lieb, & Tadic, 2011), and Neuropsychological Assessment Battery Digits (forward and backward combined Total Score; Reitan & Wolfson, 1985). Raw scores were converted into standard scores based upon previously published normative data (see supplemental information; Table S1), which were converted into z-scores. Z-scores were averaged to attain an overall mean score for neuropsychological performance. Although a composite score does not provide specificity of domain function, it provides a representation of overall cognitive ability (Elias et al., 1997). The Wechsler Test of Adult Reading (WTAR; Holdnack, 2001) was used to estimate premorbid IQ.
fMRI Procedures
Scanning was conducted on a Siemens 3.0 Tesla Skyra MRI scanner. A T1-weighted anatomical scan was acquired using a 3D MPRAGE sequence (TR/TE = 2300/2 ms, flip angle = 8°, FOV = 256 mm, matrix = 256×256, 1 mm slices). Scans were acquired in 43 contiguous axial slices at a 40° angle to the anterior commissure-posterior commissure line. The MSIT was completed during one gradient echo blood oxygenation level dependent (BOLD) scan acquired in 35 axial slices (TR/TE = 2000/25 ms, flip angle = 90°, matrix = 80×80, slice = 3.5 mm, 326 volumes). See introduction, Figure 1 and supplemental information for description of the MSIT task. The task included 6 congruent and 6 incongruent blocks. Each block consisted of 24 trials and the task lasted 652 seconds. Subjects with partial or full PTSD (N = 18) completed a resting state scan at the end of the scan session, consisting of one 6-minute gradient echo BOLD scan acquired in 43 contiguous axial slices (TR/TE = 2500/25 ms, flip angle = 90°, matrix = 80×80, slice = 3.0 mm, .5 skip; in-plane resolution = 3×3 mm; 144 volumes). Subjects were instructed to lay quietly with their eyes open, while a fixation cross was presented on the screen.
Figure 1. Multisource interference task.
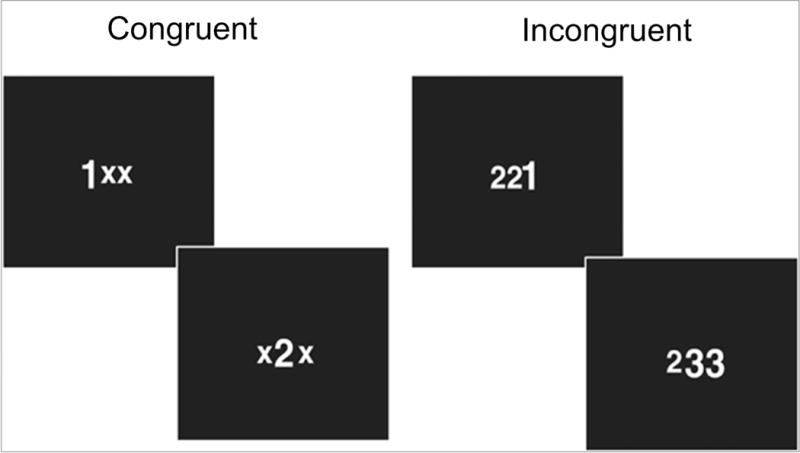
Example trials of the multi-source interference task (MSIT) are provided for the following conditions (a) congruent, in which the target number corresponds with the target location (e.g. the target, “1”, is in the first position), and (b) incongruent, in which the target number does not correspond with target location (e.g. the target, “1”, is in the third position).
Statistical Analyses
Images were preprocessed using AFNI software (Cox, 1996). Anatomical segmentation of T1-weighted structural scans for resting state analysis was performed using FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). Statistical analyses were conducted using R statistical package (cran.org).
Preprocessing methods are described in supplemental information. Briefly, for MSIT analyses, EPI images were aligned to high-resolution anatomical images. Voxel time series were corrected for non-simultaneous slice acquisition and three-dimensional motion. Multiple regression models included regressors for: 1) Congruent and 2) Incongruent trials, 3) residual motion (roll, pitch, and yaw), 4) white matter mask to control for physiological noise, and 5) baseline and linear trends. Percent signal change (PSC) was calculated by dividing the regressor of interest by the baseline. Data were spatially blurred (6 mm FWHM), normalized to Talairach space, and resampled to 4 mm3. Whole-brain, voxel-wise task effects were assessed using two-tailed t-tests (results in supplemental information; Table S2). Huber robust regressions (Huber, 1973) were implemented to investigate the relationship between voxel-wise PSC (incongruent-congruent) and both PTSD symptoms and neuropsychological function. These analyses were conducted across the whole-brain and for PSC extracted from mPFC and ACC regions of interest (See Figure 2 and supplemental information for ROI construction). Given recent evidence that traditional methods of fMRI multiple comparison corrections do not adequately control false-positive inferences (Anders Eklund, Nichols, & Knutsson, 2015), we employed the spatial autocorrelation function (acf) option for AFNI programs 3dFWHMx (estimating intrinsic smoothness based on residuals) and 3dClustSim (estimating probability of false positives), yielding a more stringent cluster threshold to counter concerns about cluster thresholding methods and false positive rates (A. Eklund, Nichols, & Knutsson, 2016). Cluster size corrections were achieved with voxel-wise p < .005 and a minimum cluster size of 640 mm3 for whole-brain (see supplemental information for voxel wise results within ROIs). Spearman’s correlation analyses were used to examine relationships between PSC extracted from ventral, rostral, and dorsal aspects of the mPFC and ACC ROIs separately, considered significant at p < .05.
Figure 2. Regions of interests.
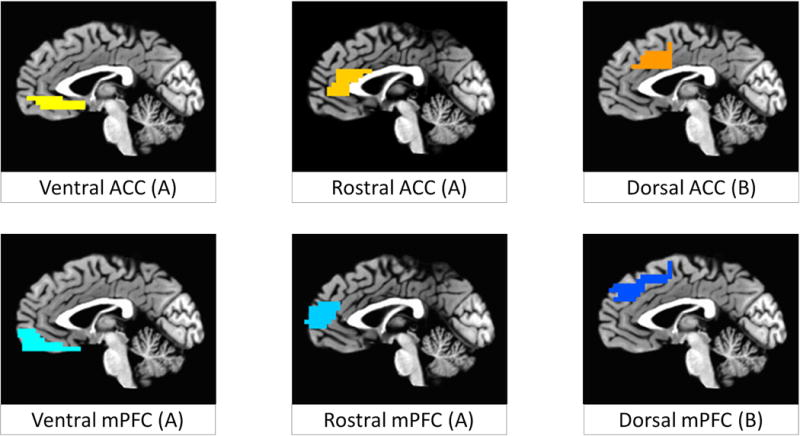
Regions of interest used for analyses of ventral, rostral, and dorsal aspects of the anterior cingulate cortex (ACC; yellow to orange, respectively), and medial prefrontal cortex (mPFC; light to dark blue, respectively), displayed at x = −1. Letters in parentheses indicate which regions were combined for purposes of correction of voxel-wise multiple comparisons (results included in supplemental information). Regions were combined for (A) ventral and rostral aspects of the medial PFC and ACC and for (B) dorsal aspects of medial PFC and ACC.
Resting state preprocessing was conducted similarly, except that local white matter and ventricle masks were used as nuisance regressors (see supplemental information). Using the AFNI program 3dTfitter, a predicted time course for each voxel was constructed from the following nuisance variables: an average ventricle time series, a local average white-matter time series, and six parameter estimates for head motion. This predicted time course was subtracted from the voxel’s actual time series to yield a residual time series for analyses. We conducted additional analyses to determine that PTSD symptoms did not relate to resting state motion (see supplemental information).
Time course residuals from anatomical ROIs (described above) that related to PTSD symptom severity (rostral ACC) or neuropsychological performance (mPFC) via task-based MSIT analyses were used as seeds in resting state analyses. These ROI time courses served as predictors in separate regression analyses to produce a map of the correlations (r-values) with each voxel across the whole brain. Huber robust regression analyses were implemented to identify relationships between z-transformed r-values and both PTSD symptom severity and neuropsychological performance. Resting state analyses were conducted voxel-wise across the whole brain and within middle frontal gyrus and inferior frontal gyrus ROIs. Cluster size corrections for multiple comparisons were achieved with voxel-wise p < .005 and a minimum cluster size of 256 mm3 for left and right middle frontal/inferior frontal gyrus ROIs, and 640 mm3 for the remainder of the brain. Lastly, we conducted post-hoc analyses exploring the relationship between task-based ACC and mPFC activation and resting state z-values for identified clusters, results of which are reported in supplemental information.
Results
On average, subjects reported moderate PTSD symptoms (M = 35.90, SD = 14.99) and 14.63 years of education (SD = 1.92). Subjects were found to have average estimated FSIQ (M = 105.77, SD = 7.16) and neuropsychological performance (mean z score = .17, SD = .53). See supplemental information for descriptive statistics on individual neuropsychological tasks. There was a trend for individuals with worse PTSD symptoms to perform worse on neuropsychological testing (ρ = −0.28, p = .088). Estimated FSIQ was associated with neuropsychological performance (ρ = 0.44, p = .005).
MSIT Behavioral Data
During incongruent relative to congruent trials, subjects were less accurate (t(37) = 0.–4.42, p < .001), and slower to respond (t(36) = 21.57, p < .001). Subjects with higher levels of neuropsychological performance were more accurate (ρ = 0.34, p = .039) during incongruent trials, and faster during incongruent (ρ = −0.70, p < .001) and congruent trials (ρ = −0.32, p = .010). Those with higher estimated FSIQ were faster during congruent trials (ρ = −0.32, p = .047). Neither accuracy nor speed during incongruent and congruent trials related to PTSD symptom severity (all p’s > .10).
MSIT Task Effects
Using PSC extracted from ACC and mPFC ROIs, congruent trials elicited greater ventral ACC (t(38) = 3.80, p = .001), rostral ACC (t(38) = 5.46, p < .001), and rostral mPFC (t(38) = 8.61, p < .001) activation (see Figure 3). Incongruent trials were found to elicit greater dorsal ACC activation (t(38) = −2.59, p = .014). Voxel-wise whole-brain results were similar, with incongruent trials eliciting greater dorsal ACC and dmPFC activation and congruent trials eliciting greater rostral/ventral ACC and mPFC activation (see supplemental information).
Figure 3. Task effects on medial prefrontal and anterior cingulate regions of interest.
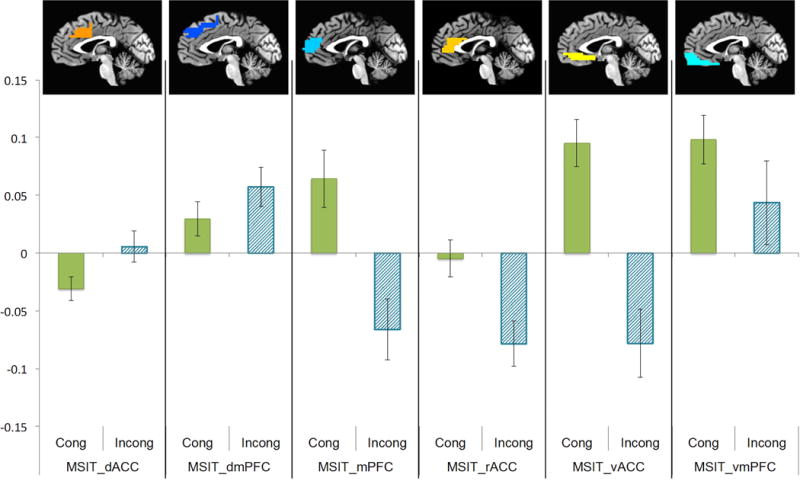
Average percent signal change extracted from a priori regions of interest are shown for incongruent and congruent trials of the multisource interference task (MSIT). Activation was greater for incongruent versus congruent trials in dorsal aspects, including dorsal ACC (t(38) = −2.59, p = .014). Activation was greater for congruent than incongruent trials in rostral and ventral aspects, including medial PFC (t(38) = 8.61, p < .001), rostral ACC (t(38) = 5.46, p < .001), and ventral ACC (t(38) = 3.80, p = .001).
MSIT: Relationship to PTSD Symptoms
Anatomical ROI results (for incongruent-congruent trials) indicated that worse PTSD symptoms related to less rostral ACC activation (ρ = −0.35, p = .031). This relationship was driven, at least partially, by those with greater PTSD exhibiting greater activation during congruent trials (see Figure 4). Although there was a trend for rostral mPFC (ρ = −0.30, p = .064), PTSD symptoms were not significantly related to other ROIs (p’s > .40). No clusters met corrected thresholds for whole-brain analysis.
Figure 4. Relationships between PTSD symptoms and neuropsychological performance and activation of the rostral ACC and medial PFC regions of interest.
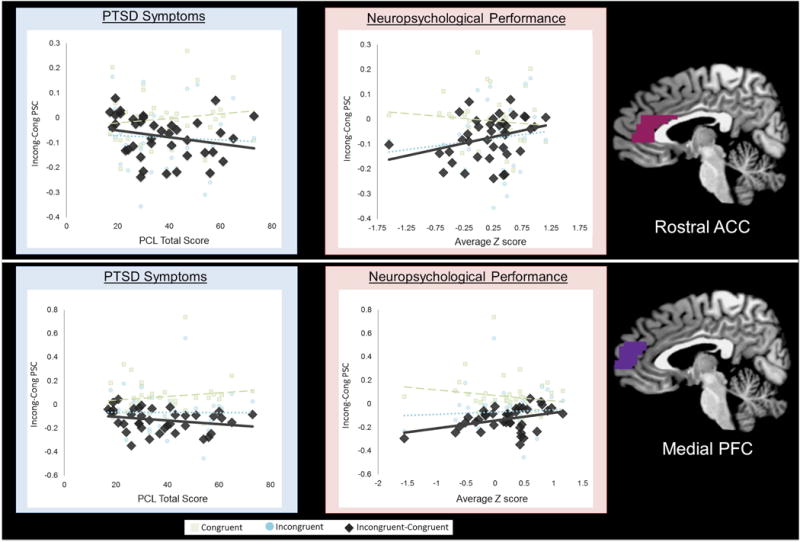
Extracted percent signal change from regions of interest indicate that worse PTSD symptoms (rostral ACC: ρ = −0.35, p = 0.031; medial PFC: NS. ρ = −0.30, p = .064) or neuropsychological performance (rostral ACC: r = 0.32, p = .048; medial PFC:r = 0.34, p = 0.032) relate to less differential activation (Incongruent-Congruent) during the multi source interference task, which was partially driven by increased activation during congruent trials.
MSIT: Relationship to Neuropsychological Performance
Anatomical ROI results indicated that worse neuropsychological performance related to less rostral mPFC (ρ = 0.34, p = .032) and rostral ACC (ρ = .32, p = .048) activation during the MSIT (incongruent-congruent trials). As shown in Figure 4, this relationship was partly driven by worse neuropsychological performance relating to greater activation during congruent trials. Rostral mPFC PSC predicted neuropsychological performance above and beyond that of full-scale IQ (β = 1.78, p = .014), while the rostral ACC did not (β = 0.91, p = .299). Although there was a trend relationship with dmPFC (β = .29, p = .076), PTSD symptoms were not significantly related to other ROIs (p’s > .50). No clusters met corrected thresholds for whole-brain analysis.
Resting State: Relationship to PTSD Symptoms
Given identified relationships with PTSD symptoms or neuropsychological performance, the rostral mPFC and rostral ACC ROIs were used as seeds for resting analyses. Voxel-wise ROI analyses revealed that worse PTSD symptoms related to less functional connectivity between the rostral mPFC ROI and right anterior lateral PFC (BA 10; 640 mm3; x,y,z = 31,56,9; t = −2.539), as well as between the rostral ACC ROI and a similar right anterior lateral PFC cluster (BA 10; 320 mm3; x,y,z = 27,57,11; t = −3.71; see Figure 5). The former remained significant in whole-brain analyses (BA 10; 768 mm3; x,y,z = 34,55,8; t = 15.51). In addition, worse PTSD symptoms related to decreased functional connectivity between the rostral mPFC ROI and a cluster within the cerebellum (left declive; 768 mm3; x,y,z = −14,−57,20; t = 20.26).
Figure 5. Relationships between PTSD symptoms and neuropsychological performance and functional connectivity with the rostral ACC and medial PFC regions of interest.
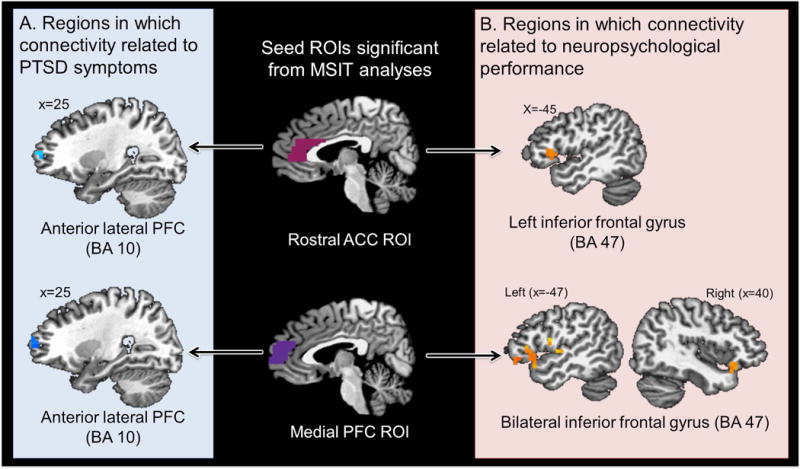
(A) Worse PTSD symptoms related to decreased functional connectivity between the medial PFC ROI and a cluster within the right anterior lateral PFC (BA 10; 640 mm3; x,y,z = 31,56,9; t = −2.539), as well as between the rostral ACC ROI and a similar right anterior lateral frontal cluster (BA 10; 320 mm3; x,y,z = 27,57,11; t = −3.71). (B) Worse neuropsychological performance related to reduced functional connectivity between the medial PFC ROI and bilateral inferior frontal gyrus (left BA 47; 1728 mm3; x,y,z = −47,18,−7; t = −2.67; right BA 47; 1280 mm3; x,y,z = 35,19,−10; t = −2.83) and other regions not pictured (e.g., insula, temporal lobe, and cerebellum) as well as reduced functional connectivity between the rostral ACC ROI and left inferior frontal gyrus (BA 47; 1216 mm3; x,y,z = −46,19,8; t = −2.540).
Resting State: Relationship to Neuropsychological Performance
Worse neuropsychological performance related to weaker functional connectivity between the rostral mPFC ROI and bilateral inferior frontal cortex (which remained significant in whole-brain analyses; see Figure 5) as well as clusters within the temporal lobe, dorsal mid insula, occipital lobe and cerebellum (see Table 1). Worse neuropsychological performance also related to decreased functional connectivity between the rostral ACC and left inferior frontal gyrus (see Figure 5), which remained significant in whole-brain analyses (BA 47; 1216 mm3; x,y,z = −46,19,8; t = −2.540).
Table 1.
Results from regression analyses examining the relationships between neuropsychological performance and resting state functional connectivity with the medial PFC region of interest.
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Side | Region | BA | Volumec | x | y | z | t |
| Incongruent>Congruent | |||||||
| left | Superior temporal gyrus | 22 | 2304 | −57 | −24 | 4 | −2.82 |
| left | Inferior frontal gyrus | 47 | 1728 | −47 | 18 | −1 | −2.67 |
| right | Inferior frontal gyrus | 47 | 1280 | 35 | 19 | −10 | −2.83 |
| left | Occipital lobe, lingual gyrus | 18 | 1152 | −6 | −72 | 1 | −2.53 |
| right | Cerebellum, anterior lobe | – | 1088 | 12 | −46 | −4 | −2.27 |
| left | Dorsal mid insula | 13 | 960 | −41 | −5 | 13 | −2.15 |
| left | Cerebellum, posterior lobe | – | 768 | 0 | −63 | −35 | −2.01 |
| left | Middle temporal gyrus | 38 | 768 | −38 | 3 | −35 | −2.49 |
| left | Sub-lobar | 13 | 704 | −29 | 17 | −10 | −3.78 |
| left | Middle frontal gyrus | 47 | 640 | −43 | 41 | −6 | −1.79 |
Results shown from whole-brain, voxel-wise, robust regression analysis examining functional connectivity using the medial prefrontal cortex region of interest as a seed region.
All coordinates are reported according to stereotaxic array of Talairach and Tournoux.
Volume is reported as mm3.
Discussion
The current study presents three key findings. First, in partial support of our first hypothesis, PTSD symptom severity and general neuropsychological performance related to differences in rostral mPFC and ACC activity during the MSIT. As these findings were partially driven by levels of activation to the easier, congruent trials, they may reflect difficulties in the acute regulation or control of the DMN to changing task demands. Second, congruent with our hypothesis, PTSD symptom severity and neuropsychological dysfunction related to more general task-independent PFC dysfunction, characterized by reduced functional connectivity between both rostral ACC and mPFC and lateral prefrontal regions implicated in executive control. Third, worse neuropsychological performance was related to weaker functional connectivity between the rostral mPFC and a broadly distributed collection of brain regions including the insula, temporal lobes and cerebellar regions.
The rostral ACC and mPFC have been associated with the DMN, and are also thought to be involved in a number of different functions, including emotion regulation and signaling of safety (Grady et al., 2010; Greicius et al., 2003; Mason et al., 2007). Our findings are consistent with those of a previous study assessing inhibition in victims of domestic violence (Aupperle et al., 2016); these findings were also driven by PTSD being associated with greater PFC activation to the easier trials (non-stop trials of the Stop Signal task). We therefore believe that both the earlier findings and the current results point to difficulties down-regulating PFC regions associated with the DMN and/or emotional processing during goal-directed cognitive tasks, specifically when the cognitive demand of the task is relatively low. The present results are complemented by relationships to overall neuropsychological performance. In our study, veterans with poorer neuropsychological performance exhibited less activity within the mPFC and rostral ACC (anatomical ROI analyses) during a cognitive inhibition task. Again, increased activation to the easier (congruent) trials contributed to the results. These findings are consistent with previous work reporting greater mPFC activity during emotional processing for individuals with PTSD relates to worse neuropsychological performance (Aupperle, Allard, et al., 2012). The relationship with both neuropsychological function and PTSD symptoms supports the functional significance of activity in these regions.
Resting state analyses provided information crucial for interpreting the task-specific findings above by allowing us to examine task-independent PFC functional connectivity. Results suggest that worse PTSD symptoms related to reduced functional connectivity between mPFC and rostral ACC seed regions (associated with DMN) and anterior lateral PFC regions (associated with executive control network). Additionally, neuropsychological performance was associated with reduced functional connectivity between mPFC and rostral ACC seed regions and the inferior frontal gyrus (also associated with executive control network). Given that the default mode and executive control networks are thought to produce opposing activations (i.e., as one increases activation, the other decreases), reduced functional coupling between these regions may suggest more widespread dysregulation in cognitive and emotional processing (Whitfield-Gabrieli & Ford, 2012). Specifically, these results may point to deficiencies in recruiting lateral PFC, and inferior frontal regions associated with executive control, thus impairing the ability to acutely regulate the DMN and switch from an internal focus to a goal-directed state. (This hypothesis was partially supported by post-hoc analyses reported in supplemental information, indicating that task-based activation within medial PFC and rACC related to connectivity of these regions to lateral PFC during resting state). Furthermore, our findings suggest that mPFC is associated with widespread, decreased functional connectivity with regions spanning the inferior frontal cortex, temporal and occipital lobes, cerebellar regions and insular cortex. In concert with prior research (Daniels et al., 2010), this suggests that optimal neuropsychological function relies on functional connectivity between a variety of networks. Future research could test hypotheses concerning the relationship of PTSD to difficulties switching between internal and goal-directed states by utilizing 1) tasks specifically designed to model neural activation when switching between tasks of different demand and 2) multi-modal EEG/fMRI (Yuan, Zotev, Phillips, Drevets, & Bodurka, 2012), to model neural changes that may occur more quickly and reflect switches between networks.
The above results have important implications for PTSD interventions. An intervention target for PTSD and related cognitive dysfunction could be functional connectivity between networks, or the ability to switch between networks efficiently (executive control and default mode specifically), rather than simply the level of activation in specific regions (Koush et al., 2015; Yuan et al., 2014). This could include cognitive and behavioral interventions (i.e., mindfulness or cognitive training to enhance regulation of attention), as well as neuromodulation techniques. Recent studies suggest that fMRI-based amygdala neurofeedback may beneficially impact depression (Yuan et al., 2014; Zotev et al., 2016) and PTSD (Gerin et al., 2016). Additionally, there have been recent developments supporting the use of neurofeedback with entire networks (i.e., DMN) rather than individual regions (Ruiz, Buyukturkoglu, Rana, Birbaumer, & Sitaram, 2014). These techniques will be useful for further testing whether efficient regulation of DMN activation or switching between networks may support optimal functioning.
Limitations
The present study lacked a non-trauma exposed control group and was cross-sectional, limiting interpretations regarding risk or resiliency factors. Subjects included all male combat veterans, limiting generalizability to other populations. The current study was unable to disentangle effects due specifically to PTSD versus overlapping symptoms (i.e., depression) or severity of combat exposure. In addition, the average z score calculated for neuropsychological function included measures of basic attention and processing speed (in addition to executive functioning and memory). However, a recent meta-analysis highlights the potential importance of considering the impact of PTSD on processing speed (Scott et al., 2015) and it is possible that the ability to inhibit interference and switch between networks could be important for supporting these basic processes. We also did not correct for multiple comparisons for our anatomical ROI analyses. Given the a priori evidence for our hypothesis about the mPFC, our conservative approach to ROI analyses (i.e., by using extracted PSC from relatively large ROI’s specified anatomically a priori), and the fact that the voxelwise analyses (which were corrected) are consistent with the ROI results, we have high confidence that the results were not identified simply due to error. However, we encourage replication in future studies to verify and further elucidate the present findings. Lastly, resting state data was only available for veterans with PTSD (N=18), limiting interpretations regarding how symptoms and neuropsychological functioning relate to functional connectivity for those with very mild levels or no PTSD symptoms.
Conclusion
This study provides evidence that both PTSD symptoms and neuropsychological performance relate to dysregulation of mPFC and rostral ACC activity and functional connectivity. Worse PTSD symptoms and neuropsychological performance related specifically to decreased functional connectivity between default mode (i.e., mPFC) and executive control (i.e., lateral PFC, inferior frontal) PFC regions. These results provide a framework for future, longitudinal research investigating the impact of PTSD treatments on default mode and executive control PFC network engagement and functional connectivity.
Supplementary Material
Acknowledgments
Dr. Martin reported receiving funding from the American Cancer Society [Principal Investigator], the University of Kansas Research Investment Council (Co-Investigator), the University of Kansas Cancer Center Pilot Program (Co-Investigator), and the National Institutes of Health (ROI DK085605 [Co-Investigator], R21 CA184834 [Co-Investigator], P30 AG035982 [Principal Investigator], UL1 TR000001 [Co-Investigator]). Dr. Aupperle reported serving as consultant for a Department of Defense Congressionally Directed Medical Research Program project (PT100018). Dr. Bruce reported that he is a paid consultant to the National Hockey League and provides unbranded talks for Novartis. Dr.’s McDowd, Simmons, and Ms. Clausen, Mr. Francisco, and Ms.
Financial Disclosures
This work is supported by The Heartland Institute for Clinical and Translational Research (UL1TR000001; Pilot Grant to Aupperle) and the University of Missouri Research Board (UMRB; Pilot Grant to Aupperle). The Hoglund Brain Imaging Center is supported by a generous gift from Forrest and Sally Hoglund and funding from the National Institutes of Health (S10 RR29577, UL1 TR000001).
Footnotes
Financial Disclosures: Thelen reported no biomedical financial interests or potential conflicts of interest.
References
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons A, Flagan T, Behrooznia M, Stein MB. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in PTSD. Archives of General Psychiatry. 2012;69(4):360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Stillman AN, Simmons AN, Flagan T, Allard CB, Thorp SR, Stein MB. Intimate Partner Violence PTSD and Neural Correlates of Inhibition. Journal of Traumatic Stress. 2016 doi: 10.1002/jts.22068. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Brandes D, Ben-Schachar G, Gilboa A, Bonne O, Freedman S, Shalev AY. PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res. 2002;110(3):231–238. doi: 10.1016/s0165-1781(02)00125-7. doi:S0165178102001257 [pii] [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8(1):60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daniels JK, McFarlane AC, Bluhm RL, Moores KA, Clark CR, Shaw ME, Lanius RA. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35(4):258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10(2):301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols T, Knutsson H. Can parametric statistical methods be trusted for fMRI based group studies? 2015 arXiv preprint arXiv:1511.01863. [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D’Agostino RB, Cupples LA, Wilson PW, Silbershatz H, Wolf PA. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20(9):1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. doi: S1364-6613(10)00252-4 [pii] 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer EM, Felmingham KL, Allen A, Clark CR, McFarlane AC, Williams LM, Bryant RA. Developing an integrated brain, behavior and biological response profile in posttraumatic stress disorder (PTSD) J Integr Neurosci. 2008;7(3):439–456. doi: 10.1142/s0219635208001873. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13(1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Gerin MI, Fichtenholtz H, Roy A, Walsh CJ, Krystal JH, Southwick S, Hampson M. Real-Time fMRI Neurofeedback with War Veterans with Chronic PTSD: A Feasibility Study. Front Psychiatry. 2016;7:111. doi: 10.3389/fpsyt.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20(6):1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Vanelzakker MB, Shin LM. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integr Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdnack HA. Wechsler Test of Audlt Reading: WTAR. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Huber PJ. Robust regression: asymptomatics, conjectures, and Monte Carlo. Annals of Statistics. 1973;1(5):799–821. [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Ressler KJ. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49(7):1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167(6):648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Liberzon I. Altered Default Mode Network (Dmn) Resting State Functional Connectivity Following a Mindfulness-Based Exposure Therapy for Posttraumatic Stress Disorder (Ptsd) in Combat Veterans of Afghanistan and Iraq. Depress Anxiety. 2016;33(4):289–299. doi: 10.1002/da.22481. [DOI] [PubMed] [Google Scholar]
- Koush Y, Meskaldji DE, Pichon S, Rey G, Rieger SW, Linden DE, Scharnowski F. Learning Control Over Emotion Networks Through Connectivity-Based Neurofeedback. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv311. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. doi: S0079-6123(07)67011-3 [pii] 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J Affect Disord. 2012;141(1):11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: TMT. Testzentrale; 1979. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Vol. 4. Reitan Neuropsychology; 1985. [Google Scholar]
- Ruiz S, Buyukturkoglu K, Rana M, Birbaumer N, Sitaram R. Real-time fMRI brain computer interfaces: self-regulation of single brain regions to networks. Biol Psychol. 2014;95:4–20. doi: 10.1016/j.biopsycho.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Schweinsburg BC. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, Pitman RK. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. The American Journal of Psychiatry. 2011;168(9):979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI, Rauch SL. Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress. 2007;20(5):701–712. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. doi:npp200983 [pii] 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amydala, medial prefrontal cortex, and hippocampal function in PTSD. Annals New York Academy of Science. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. doi: 62/3/273 [pii] 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Ugurbil K. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci U S A. 2012;109(8):3131–3136. doi: 10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37(4):241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Hazen AL, Forde DR. Full and partial posttraumatic stress disorder: findings from a community survey. Am J Psychiatry. 1997;154(8):1114–1119. doi: 10.1176/ajp.154.8.1114. [DOI] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, Hayman LA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. The Journal of neuropsychiatry and clinical neurosciences. 2011;23(2):121–125. doi: 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. USA: Oxford University Press; 2006. [Google Scholar]
- Wagner S, Helmreich I, Dahmen N, Lieb K, Tadic A. Reliability of three alternate forms of the trail making tests a and B. Arch Clin Neuropsychol. 2011;26(4):314–321. doi: 10.1093/arclin/acr024. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Huska J, Keane TM. The PTSD checklist military version (PCL-M) Boston, MA: National Center for PTSD; 1991. [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Young KD, Phillips R, Zotev V, Misaki M, Bodurka J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 2014;4(9):690–701. doi: 10.1089/brain.2014.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Zotev V, Phillips R, Drevets WC, Bodurka J. Spatiotemporal dynamics of the brain at rest-exploring EEG microstates as electrophysiological signatures of BOLD resting state networks. Neuroimage. 2012;60(4):2062–2072. doi: 10.1016/j.neuroimage.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Zotev V, Yuan H, Misaki M, Phillips R, Young KD, Feldner MT, Bodurka J. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. Neuroimage Clin. 2016;11:224–238. doi: 10.1016/j.nicl.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


