Abstract
Background and aims
HIV-infected persons with substance use disorders are least likely to benefit from advances in HIV treatment. Integration of extended-release naltrexone (XR-NTX) into HIV clinics may increase engagement in the HIV care continuum by decreasing substance use. We aimed to compare 1) XR-NTX treatment initiation, 2) retention, and 3) safety of XR-NTX versus treatment as usual (TAU) for treating opioid use disorder (OUD) and/or alcohol use disorder (AUD) in HIV clinics.
Design
Non-blinded randomized trial of XR-NTX versus pharmacotherapy TAU
Setting
HIV primary care clinics in Vancouver, BC, Canada and Chicago, IL, USA.
Participants
51 HIV-infected patients seeking treatment for OUD (n=16), AUD (n=27) or both OUD and AUD (n=8).
Measurements
Primary outcomes were XR-NTX initiation (receipt of first injection within 4 weeks of randomization) and retention at 16 weeks. Secondary outcomes generated point estimates for change in substance use, HIV viral suppression (HIV RNA pcr < 200 copies/mL), and safety.
Findings
Two-thirds (68%) of participants assigned to XR-NTX initiated treatment, and 88% of these were retained on XR-NTX at 16 weeks. In comparison, 96% of TAU participants initiated treatment, but only 50% were retained on medication at 16 weeks. Mean days of opioid use in past 30 days decreased from 19 to 10 for TAU (n=12) and from 18 to 13 for XR-NTX (n=10). Mean heavy drinking days decreased from 18 to 7 for TAU (n=11) and 13 to 6 for XR-NTX (n=12). Among those with OUD, HIV suppression improved from 67% to 80% for XR-NTX and 58% to 75% for TAU. XR-NTX was well-tolerated, with no precipitated withdrawals and 1 serious injection site reaction.
Conclusions
Extended-release naltrexone (XR-NTX) is feasible and safe for treatment of opioid use disorder and alcohol use disorder in HIV clinics. Treatment initiation appears to be lower and retention greater for XR-NTX compared with treatment as usual. (clinicaltrials.gov NCT01908062).
Keywords: extended-release naltrexone, Alcohol, HIV, Opioid-Related Disorders, randomized clinical trial
INTRODUCTION
Opioid and alcohol use disorders are common in HIV-infected individuals and contribute to gaps in the HIV care continuum (1–3). Untreated opioid and alcohol use disorders are associated with decreased receipt of antiretroviral therapy (ART) (3, 4), decreased ART adherence (5, 6), decreased HIV viral suppression (7, 8), and decreased survival (9).
Treatment of substance use disorders can increase engagement in HIV care (10, 11), potentially narrowing gaps in the HIV care continuum by improving linkage to care, receipt of ART, retention in care, and HIV viral suppression. Opioid agonist therapy with methadone (12) and sublingual buprenorphine/naloxone (BUP/NX) (13) for the treatment of opioid use disorder (OUD) improves HIV outcomes (11, 14–16). U.S. HIV treatment guidelines recommend opioid agonist therapy for engaging people who inject drugs in HIV treatment (17). Access to opioid agonist therapy, however, is suboptimal due to a shortage of waivered buprenorphine providers (18) and, in the case of methadone, federal prohibitions in the U.S. on office-based treatment. Patient acceptance of and retention on opioid agonist therapy is limited, in part due to frequent dosing requirements for both medications.
Pharmacotherapy for alcohol use disorders (AUD) is uncommon in HIV clinics but associated with decreased HIV RNA levels in alcohol-dependent, HIV-infected Veterans treated with oral naltrexone in addiction treatment settings (19). Oral naltrexone’s effectiveness, both for OUD and for AUD, is also limited by patient acceptability and daily dosing requirements.
Long-acting opioid antagonist treatment with extended-release naltrexone (XR-NTX) is effective in treating OUD (20–23) and AUD (24, 25). XR-NTX is a deep muscle injection that lasts 28 days, eliminating the need for daily dosing. Integration of XR-NTX for treatment of AUD into primary care clinics decreases alcohol use (26), but less is known about its use for treatment of OUD in primary care. XR-NTX also offers an alternative to agonist therapy for some HIV-infected patients who prefer a non-narcotic treatment option or once a month dosing. Given its long duration of action, XR-NTX has the potential to facilitate engagement in the HIV care continuum for patients with opioid and/or alcohol use disorder (OUD/AUD), but has not been tested in HIV clinics.
The CTN-0055 CHOICES pilot study aims were to compare 1) treatment initiation, 2) treatment retention in pharmacotherapy, 3) treatment retention in counseling, and 4) safety of XR-NTX versus TAU for treatment of patients with OUD/AUD in HIV clinics. The overall purpose of the study was to inform development of a multi-site comparative effectiveness trial of XR-NTX versus TAU in HIV clinics for improving engagement in the HIV care continuum.
METHODS
Design
The CTN-0055 CHOICES study (clinicaltrials.gov NCT01908062) was an open-label, randomized, pilot trial of XR-NTX versus treatment as usual (TAU) for treatment of OUD/AUD in HIV-infected patients. The study was conducted under the direction of the National Institute on Drug Abuse’s (NIDA) Clinical Trials Network (CTN) and approved by Institutional Review Boards at Oregon Health and Science University and pilot sites.
Setting
Study pilot sites a) provided HIV primary care, and had b) sufficient potential participants to achieve study enrollment goals, c) providers willing to be trained in use of XR-NTX for management of OUD/AUD, d) prior experience participating in research studies, e) the capacity to prescribe ART to participants regardless of CD4 count, and f) access to addiction counseling services as part of usual care. Two large outpatient HIV clinics were selected as study pilot sites: The Ruth M. Rothstein Core Center in Chicago, IL and The John Ruedy Immunodeficiency Clinic (IDC) at St. Paul’s Hospital in Vancouver, B.C.
Participants
Participants were recruited during HIV clinic visits and through clinic-affiliated outreach programs between June 2014 and March 2015, with last follow-up in August 2015. Prospective participants completed a brief pre-screening questionnaire requiring verbal consent for assessment for appropriateness for screening (e.g. HIV-infected, wanting to decrease opioid and alcohol use, and interest in study participation), and then provided written consent to complete full screening. NIDA set the pilot enrollment target of 50 participants within 12 months to demonstrate feasibility of enrollment. Failure to enroll 50 participants within 12 months would have been considered evidence that a scale-up trial was not feasible.
Eligible HIV-infected participants 1) met DSM-5 criteria for moderate or severe OUD/AUD, and were 2) willing to be randomized to XR-NTX or TAU, 3) willing to establish or continue ongoing HIV care at the site, 4) willing to initiate or continue ART, regardless of CD4 count, 5) at least 18 years old, 7) able to provide written informed consent, 8) able to communicate in English, and 9) if female, willing to take measures to avoid becoming pregnant. Potential participants were excluded for 1) disabling or terminal medical illness, 2) aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than 5 times upper limit of normal, 3) prothrombin time with international normalized ratio (INR) > 1.5 or platelet count <100k, 4) known allergy or sensitivity to naloxone, naltrexone, polylactide-co-glycolide, carboxymethylcellulose, or other components of the diluents, 5) anticipated surgery during study participation, 6) chronic pain requiring ongoing opioid analgesics, 7) pending legal action, 8) currently pregnant or breastfeeding, 9) body habitus that precludes safe intramuscular injection of XR-NTX, 10) receiving methadone or buprenorphine maintenance therapy in the past 4 weeks, 11) having taken an investigational drug in another study, 12) an ECG finding that, in the judgement of the study clinician, precludes safe study participation, or 13) having received treatment with XR-NTX for OUD/AUD in the past 3 months.
Intervention
Eligible participants were randomized in a 1:1 ratio via computer-generated randomized allocation by an independent NIDA data management contractor to receive non-blinded XR-NTX versus TAU, with blocking by site. Participants assigned to XR-NTX underwent medically supervised withdrawal, as needed. A urine drug screen negative for opioids, including buprenorphine and methadone, followed by a negative naloxone challenge, was required prior to the first dose of XR-NTX. Naloxone used for challenge is an off-label use. Participants received XR-NTX (Vivitrol®) 380mg intramuscular injection, provided by the manufacturer and injected by study clinician at treatment initiation and at 4, 8, and 12 weeks, in alternating gluteal muscles (total 16 weeks treatment exposure). Participants assigned to TAU were prescribed the local standard of care for OUD and AUD in their communities. All participants were referred to local counseling resources and attended monthly medical management appointments with treating providers. Research visits occurred every 4 weeks for collection of blood and urine samples, safety and other study assessments.
Measures
The main Independent variable was treatment assignment (XR-NTX versus TAU). Primary outcomes included 1) patient self-report of acceptance of opioid antagonist therapy and willingness to participate in a trial of XR-NTX vs. TAU, 2) participant recruitment rate (number randomized per month per site), 3) treatment initiation (receipt of at least one dose of XR-NTX or other medication-assisted TAU within 4 weeks of randomization), and 4) retention on treatment, (percent of assigned treatment received over 16 weeks, among those initiating treatment). Secondary outcomes, measured at 16 weeks, included change in past 30-day opioid and alcohol use (Addiction Severity Index-lite self-report, urine toxicology screening, and urine ethylglucuronide testing) and HIV-1 RNA viral suppression (plasma HIV-1 RNA pcr < 200 copies/mL). Adverse events and XR-NTX injection site reactions were monitored at each research visit. Other patient safety measures included change in AST and ALT, fatal and non-fatal opioid overdose, and precipitated opioid withdrawal due to XR-NTX. Sociodemographic data was assessed by self-report. All data was entered into a centralized database managed by an independent data management center.
Statistical Analysis
We used descriptive statistics to report participant characteristics and the four primary feasibility outcomes. We assessed differences in primary outcomes by treatment assignment using Chi-square tests for categorical variables and t-tests for continuous variables. Secondary outcomes are reported with descriptive statistics. Participants were analyzed as members of the treatment arm to which they were assigned for all outcomes (i.e. intent-to-treat design).
RESULTS
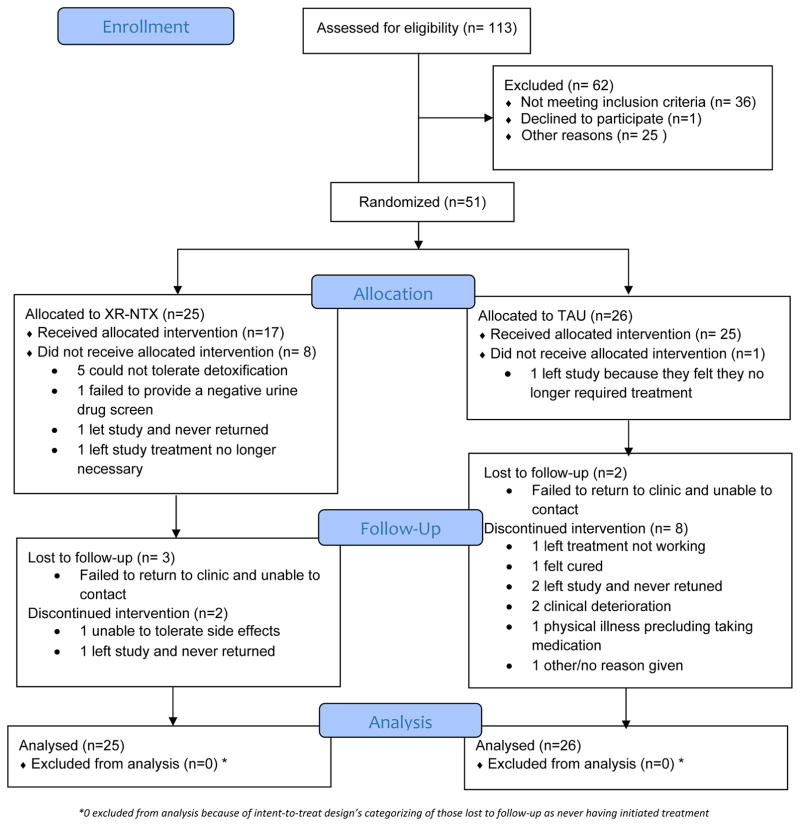
Research assistants pre-screened 113 individuals, 100 of whom were eligible for screening (Figure 1). The most common reasons for exclusion at pre-screening were current buprenorphine or methadone maintenance treatment and chronic pain requiring ongoing opioid analgesics. Of 78 participants consented for screening, 51 were randomized (45% of pre-screened; 65% of screened). The most common causes for exclusion during screening were serious medical, psychiatric, or substance use disorder. Three eligible participants declined randomization.
Figure 1.
CONSORT Flow Diagram
Forty-three percent of randomized participants were women, 47% Black, 45% disabled, and 61% had a high school education or greater (Table 1). Mean age was 46 (SD 10) years. Twenty-seven participants (53%) had only AUD, 16 (31%) had only OUD, and 8 (16%) met DSM-5 criteria for both OUD and AUD. Participant characteristics were generally balanced across these groups, though women composed 37% of those with only AUD, 56% of those with only OUD, and 38% of those with both OUD and AUD (p=0.44). Participants with OUD alone, and those with OUD and AUD were combined for the analysis; therefore, 24 participants (47%) were classified as having OUD with or without AUD and 27 (53%) were classified as having AUD, alone. Ninety-four percent of participants were prescribed ART and 80% had HIV viral suppression at baseline, with a mean CD4 cell count of 620 (SD 399).
Table 1.
Participant characteristics overall, and by treatment group.
| Characteristic | TAU (N=26) | XR-NTX (N=25) | Total (N=51) |
|---|---|---|---|
|
| |||
| Female Gender | 8 (31%) | 14 (56%) | 22 (43%) |
|
| |||
| Age, Mean (SD) | 45 (12) | 47 (8.8) | 46 (10) |
|
| |||
| Hispanic Ethnicity | 3 (12%) | 1 (4%) | 4 (8%) |
|
| |||
| Race | |||
| Black/African American | 12 (46%) | 12 (48%) | 24 (47%) |
| White | 9 (35%) | 4 (16%) | 13 (26%) |
| Other | 5 (19%) | 9 (36%) | 14 (27%) |
|
| |||
| Education completed | |||
| < High school | 8(31%) | 12 (48%) | 20 (39%) |
| High school/GED | 7(27%) | 8(32%) | 15(29%) |
| ≥ Some college | 11(42%) | 5(20%) | 16 (32%) |
|
| |||
| Married or living with partner | 2 (8%) | 6 (24%) | 8 (16%) |
|
| |||
| Employment | |||
| Working now | 4 (15%) | 3 (12%) | 7 (14%) |
| Looking for work, unemployed | 6 (23%) | 5 (20%) | 11 (22%) |
| Disabled | 10 (38%) | 13 (52%) | 23 (45%) |
| Other | 6 (23%) | 4 (16%) | 10 (20%) |
|
| |||
| Substance Use Disorder | |||
| Opioid use disorder alone | 9 (35%) | 7 (28.0%) | 16 (31%) |
| Alcohol use disorder alone | 14(54%) | 13 (52%) | 27 (53%) |
| OUD+AUD | 3(11%) | 5(20%) | 8(16%) |
|
| |||
| ASI drug score | .37(SD=.33) | .44(SD=.39) | .41(SD=.36) |
|
| |||
| ASI alcohol score | .33(SD=.32) | .41(SD=.32) | .37(SD=.32) |
|
| |||
| Baseline CD4 Count, mean (SD) | 564 (246) | 683 (418) | 620 (339) |
|
| |||
| Baseline ART, n (%) | 25 (96%) | 23 (92%) | 48 (94%) |
|
| |||
| Baseline HIV Viral Suppression, n (%) | 21 (81%) | 20 (80%) | 41 (80%) |
Feasibility
Patient acceptance of opioid antagonist therapy
Patients being approached for study participation were interested in XR-NTX treatment. Prospective study participants (n=113) were asked about their interest in cutting back or quitting substance use and their willingness to participate in a trial of XR-NTX during pre-screening. Ninety-eight percent of 60 prospective participants interested in reducing opioid use and 99% of 82 prospective participants interested in reducing alcohol use were definitely or maybe willing to consider enrolling in a clinical trial of XR-NTX.
Rate of participant recruitment
Fifty-one of 113 pre-screened individuals (45.5%) were randomized. The trial achieved 155% actual versus expected randomizations, reaching randomization targets 4 months ahead of schedule (Figure 1). The study enrolled a mean 3.2 participants per month per site (1.5 and 1.7 participants per month per site for OUD and AUD, respectively).
Treatment initiation
Overall, 68% of participants assigned to XR-NTX initiated treatment within 4 weeks of randomization compared with 96% of those assigned to TAU (p=.011) (Table 2). XR-NTX initiation was greater for those with AUD-only compared with those with OUD (92% versus 42%, p=.011). TAU participants with OUD +/− AUD (n=12) primarily received office-based buprenorphine/naloxone (84%). Pharmacotherapy for TAU participants with AUD (n=14) consisted of oral naltrexone (50%), gabapentin (29%), acamprosate (14%), and disulfiram (7%). Among participants with OUD, TAU pharmacotherapy exceeded XR-NTX initiation (100% versus 42%, p=.002), but among those with AUD-only, pharmacotherapy initiation was comparable (93% versus 92%, p=1.00). The leading reason for not initiating XR-NTX was inability to tolerate opioid detoxification (Figure 1). All but one TAU participant received pharmacotherapy for OUD/AUD treatment.
Table 2.
Treatment Initiation and Retention.
| Treatment Initiation Within 4 Weeks | ||||
|---|---|---|---|---|
| Total (n=51) | AUD alone (n= 27) | OUD ± AUD (n= 24) | P value (AUD vs OUD) | |
| TAU (n=26) | 25/26 (96%) | 13/14 (93%) | 12/12 (100%) | 1.00 |
| XR-NTX (n=25) | 17/25 (68%) | 12/13 (92%) | 5/12 (42%) | 0.011 |
| Total (n=51) | 42/51 (82%) | 25/27 (93%) | 17/24 (71%) | 0.066 |
| P value (TAU vs XR-NTX) | 0.011 | 1.00 | 0.002 | -- |
| Retention on Pharmacotherapy at 16 Weeks | ||||
| Total (n=41) | AUD alone (n=24) | OUD ± AUD (n=17) | P value (AUD vs OUD) | |
| TAU-MAT (n=24) | 12/24 (50%) | 6/12 (50%) | 6/12 (50%) | 1.00 |
| XR-NTX (n=17) | 15/17 (88%) | 10/12 (83%) | 5/5 (100%) | 1.00 |
| Total (n=41) | 27/41 (66%) | 16/24 (67%) | 11/17 (65%) | 0.896 |
| P value (TAU vs XR-NTX) | 0.018 | 0.190 | 0.100 | -- |
| Retention in Counseling at 16 Weeks | ||||
| Total (n=34) | AUD alone (n=19) | OUD ± AUD (n=15) | P value (AUD vs OUD) | |
| TAU-Counseling (n=17) | 6/17 (35%) | 2/7 (20%) | 4/10 (40%) | 1.00 |
| XR-NTX (n=17) | 15/17 (88%) | 10/12 (83%) | 5/5 (100%) | 1.00 |
| Total (n=34) | 21/34 (62%) | 12/19 (63%) | 9/15 (60%) | 0.851 |
| P value (TAU vs XR-NTX) | 0.004 | 0.040 | 0.040 | -- |
Treatment retention
Of those initiating treatment (n=41), 88% were retained on XR-NTX at 16 weeks compared with 50% retention on TAU pharmacotherapy (Table 2). All but one participant who initiated XR-NTX received all four possible doses without treatment interruption. Treatment retention was higher for XR-NTX than TAU pharmacotherapy, both for those with AUD (83% versus 50%, p=.190) and those with OUD (100% versus 50%, p=.100). Likewise, retention in counseling was higher for XR-NTX than TAU overall (88% versus 35%, p=.004), and regardless of AUD (83% versus 20%, p=.040) and OUD (100% versus 40%, p=.040) status.
Secondary outcomes
Point estimates of secondary outcomes were collected to inform a potential large scale trial and not powered for hypothesis testing (Table 3). Among participants with OUD, the mean number of days of opioid use and the percent of UDS positive for opioids decreased in both treatment arms. Among participants with AUD, the mean number of days of drinking to intoxication and the percent of UDS positive for urine ethylglucuronide decreased in both treatment groups.
Table 3.
Secondary Outcomes.
| TAU | XR-NTX | |||
|---|---|---|---|---|
| Baseline | 16 weeks | Baseline | 16 weeks | |
| Opioid Use | ||||
| Mean days of opioid use in past 30 days1 | 17.3 (SD=13.14) n=12 | 4.1 (SD=5.43) n=11 | 20.3(SD=12.29) n=12 | 7.7 (SD=11.32) n=11 |
| UDS positive for opioids1 | 9 (75.0%) n=12 | 7 (58.3%) n=12 | 9 (75.0%) n=12 | 4 (40.0%) n=12 |
| Alcohol Use | ||||
| Mean days of alcohol use in past 30 days2 | 15.6 (SD=9.95) n=14 | 5.7 (SD=8.40) n=12 | 12.5 (SD=11.02) n=13 | 2.8 (SD=3.05) n=12 |
| Urine ethylglucuronide positive, n (%)2 | 7 (50.0%) n=14 | 4 (30.8%) n=14 | 6 (54.5%) n=13 | 3 (25.0%) n=13 |
| HIV Outcomes | ||||
| Prescribed ART3 | 25 (96.2%) n=26 | 26 (100%) n=26 | 23 (92.0%) n=25 | 24 (96.0%) n=25 |
| HIV Viral Suppression3 | 21 (80.8%) n=26 | 20 (87.0%) n=23 | 20 (80.0%) n=25 | 17 (81.0%) n=21 |
Among participants with opioid use disorder retained at 16 weeks
Among participants with alcohol use disorder retained at 16 weeks
Among all participants completing study assessments
Overall, 48 of 51 (94.1%) participants were receiving ART at baseline (Table 3). Two of the 3 participants not prescribed ART at baseline were prescribed ART during study participation. The single participant who was not prescribed ART by 16 weeks was an HIV elite suppressor (HIV RNA pcr < 200 copies/mL without ART) and declined ART initiation. Overall, 80% of participants had HIV viral suppression at baseline, and 84% were suppressed at 16 weeks. Among those with OUD, HIV viral suppression increased from 67% to 80% for XR-NTX and 58% to 75% for TAU. Among those with AUD-only, HIV viral suppression changed from 92% to 82% for XR-NTX and 100% to 100% for TAU.
Safety
Fourteen participants experienced a total of 29 adverse events (38% mild, 55% grade 2 moderate, and 7% severe). The majority (62%) were not related to study treatment. Leading adverse events were gastrointestinal disorders (11 participants) followed by psychiatric disorders/insomnia (3 participants) and nervous system disorders such as headache and migraine (3 participants). Two serious adverse events occurred: 1 suicidal ideation and 1 ankle fracture, both of which led to inpatient admissions. Neither were related to study treatment. Four participants receiving XR-NTX experienced a total of 7 injection site reactions. In three participants, reactions were mild and self-limited and XR-NTX was continued. One participant developed a delayed hypersensitivity reaction to XR-NTX (27) and switched to oral naltrexone. There were no precipitated withdrawals.
Mean AST and ALT were unchanged between screening and 16 weeks, overall, and by treatment group (AST: 32.3 to 32.2 for TAU and 36.8 to 38.8 for XR-NTX; ALT: 33.0 to 30.8 for TAU and 35.0 to 40.1 for XR-NTX). Two participants (3.9%) reported at least one opioid overdose during the study: one assigned to TAU with 2 non-fatal overdoses; one assigned to XR-NTX with 1 non-fatal overdose prior to initiating XR-NTX). There were no fatal overdoses.
DISCUSSION
Creative solutions for improving engagement in HIV care for patients with substance use disorders are urgently needed. The current study demonstrates integration of XR-NTX into HIV clinics was feasible and safe for the treatment of OUD/AUD. These findings support the need for a multi-site trial to assess the capacity of integrated addiction treatment in HIV clinics to improve engagement and retention in the HIV care continuum.
After being informed of the pros and cons of opioid antagonist therapy, nearly all prospective participants who were interested in cutting back opioid and/or alcohol use were willing to consider participating in a clinical trial of XR-NTX. The high willingness to consider opioid antagonist therapy was likely influenced by the fact that these people were being approached by a research assistant from a clinical trial for which they hoped to qualify. One pilot site independently surveyed 657 community-based people who injected opioids to assess willingness to try XR-NTX, as part of its site application process (28), 52% of whom expressed willingness to receive XR-NTX for treatment of OUD. Daily heroin injection was associated with increased willingness to try XR-NTX (OR 1.53, 95% CI 1.02, 3.12).(28) Together, these data call into question the common clinical assumption that people with OUD would not be interested in treatment with an opiate receptor blocker.
Nearly all participants assigned to TAU initiated pharmacotherapy as did those assigned to XR-NTX with AUD-only. Fewer than half of participants with OUD initiated XR-NTX within four weeks of randomization, and pharmacodynamics may have played a role. Because naltrexone’s binding affinity at the opioid mu receptor exceeds that of full or partial opioid agonists, naltrexone initiation requires patients to be opioid-free in order to avoid precipitated withdrawal. Medically supervised withdrawal from opioids is typically required for persons with OUD, and this may take several weeks depending on the severity of physical dependence and methods used (i.e. buprenorphine taper vs. non-opioid medication withdrawal support). The CTN-0055 CHOICES study randomized participants prior to detoxification and provides a benchmark for XR-NTX treatment initiation in community-dwelling outpatients with OUD. Both of the two clinical trials of XR-NTX for OUD that led to FDA-approval required successful residential detoxification prior to randomization and did not report treatment initiation rates (20, 22).
Another reason for lower XR-NTX initiation rates may have been the study’s conservative requirements for a UDS negative for all opioids, including buprenorphine. Providers were generally encouraged to avoid use of buprenorphine or methadone for detoxification unless the participant failed non-opioid detoxification, often resulting in prolonged time from last use of illicit opioids to XR-NTX injection. In a recent pilot study, streamlined induction procedure using cross-tapered dosing of buprenorphine with escalating very low doses of oral naltrexone achieved 70% XR-NTX initiation at 8 days (29). Similar protocols adapted for use in HIV clinics could improve XR-NTX initiation. Additional implementation studies are needed to identify ways to address barriers and maximize uptake of XR-NTX in outpatient settings.
Most (88%) participants who initiated XR-NTX received all four doses over 16 weeks. The high retention rate among participants with OUD (100%) exceeds that reported in two previous clinical trials of XR-NTX for OUD conducted in specialty addiction treatment settings. In a U.S.-based study, 68% of participants with OUD were retained on XR-NTX at 8 weeks.(20) In a study conducted in Russia among people with no other pharmacotherapy options for OUD, 57.9% were retained on XR-NTX at 24 weeks (22). Similarly, the high retention rate participants with AUD (82%) exceeds that reported in a large trial of XR-NTX for AUD (62.5% retention and receipt of all doses of XR-NTX at 24 weeks) (24) and a demonstration study of XR-NTX for AUD in primary care (62% retained in treatment at 12 weeks) (26). The high rate of retention on XR-NTX in CTN-0055 may be related to XR-NTX treatment integration into HIV clinics where participants receive ART in a patient-centered medical home model. Further implementation research is required to explore contributors to XR-NTX retention, particularly for individuals less engaged in HIV primary care.
Given the small number of participants enrolled to assess feasibility, the pilot study was not power for hypothesis testing of secondary HIV or substance use outcomes. The study’s secondary analyses must be regarded as hypothesis-generating only, and need to be validated in a fully powered study. Still, XR-NTX decreased use of opioids and, to a lesser degree, alcohol in this intent-to-treat analysis, consistent with previous studies demonstrating its effectiveness for treating OUD/AUD (22, 24). Greater retention on XR-NTX compared with TAU suggests that greater treatment exposure over longer follow-up periods may indeed improve long-term substance use outcomes that mediate improved HIV outcomes. The majority of participants were already prescribed ART at baseline, consistent with expansion of universal ART coverage in these cities. HIV viral suppression was consequently high at baseline, with the exception of participants with OUD, who experienced an increase in the percent suppressed at 16 weeks. Future trials testing the ability of XR-NTX to improve engagement and retention in HIV care should also consider sustained viral suppression at 12 months, retention in HIV care, and mortality as outcomes.
XR-NTX was safe for use in HIV clinics. XR-NTX injections were generally well-tolerated, apart from one participant who experienced a delayed hypersensitivity reaction, likely to the microspherule drug delivery mechanism (27). Unchanged liver enzymes levels are consistent with recent studies supporting the lack of hepatotoxicity in patients receiving XR-NTX and led to removal of the previous FDA black box hepatotoxicity warning (19, 21, 30).
The current study should be interpreted in view of several limitations. First, the pilot trial was not powered to assess XR-NTX effects on HIV outcomes. The study’s successful demonstration of HIV clinic integration feasibility merits development of a fully powered, multisite trial designed to assess the effect of XR-NTX on HIV care engagement, retention, HIV viral suppression, and mortality risk. Second, the short-term nature of a pilot study precluded assessment of continued use of XR-NTX and opioid overdose risk after the 16-week treatment period. Future trials should include follow-up periods that determine the proportion of participants who continue on XR-NTX treatment covered by healthcare insurance, and the long-term risk of opioid overdose among those participants who choose not to continue opioid antagonist therapy. Third, pilot HIV clinics were chosen partly on the basis of their previous successes in implementation, as evidenced by high rates of pharmacotherapy initiation among those assigned to TAU that are likely atypical of many other HIV clinics. HIV clinics with less implementation experience should be included in a multi-site scale-up trial and may demonstrate larger differences between XR-NTX and TAU for substance use and HIV outcomes. Finally, study results should not be interpreted to support XR-NTX replacement of opioid agonist therapy for OUD, which has more than 40 years of data supporting its safety and effectiveness. XR-NTX should be viewed, rather, as a potential addition to an expanding menu of effective treatment options that can be tailored to patient preferences.
In conclusion, the CTN-0055 CHOICES randomized trial demonstrates that XR-NTX treatment of OUD/AUD is acceptable, feasible, and safe for integrating into HIV clinics. The findings underscore the need for a multi-site trial to test the potential of XR-NTX for improving engagement in the HIV care continuum. Use of long-acting addiction pharmacotherapies such as XR-NTX may improve the capacity for HIV-infected patients with substance use disorders to better engage in HIV treatment and close gaps in the HIV care continuum. Such interventions are urgently needed to achieve the UNAIDS 90-90-90 (90% diagnosed, 90% treated with ART, and 90% with HIV viral suppression) by 2020.
Acknowledgments
Funding: The study was funded by the U.S. National Institutes of Health, National Institute on Drug Abuse (NIDA, UG1DA015815 and UG1DA013732). The manufacturer donated extended-release naltrexone to the NIDA for use in this trial. We thank all the study participants for their time and effort. We are grateful to CTN on their technical assistance, and EMMES for data management. We acknowledge HIV clinics in Vancouver, B.C. and Chicago, IL for recruitment and data collection.
The authors wish to thank Sarann Bielavitz and Rana Leed for assistance with human subjects and manuscript preparation.
Footnotes
Author Contributions: P.T.K., P.V.R., K.A., E.W., R.L., J.L.S., V.A., R.N.M., and D.M., designed and implemented the study. L.E.K., managed and oversaw the study implementation. N.L.O., and D.H., conducted the analysis. All authors significantly contributed to data interpretation and revision of the manuscript and approved the article.
Conflict of Interest: Dr. McCarty served as the Principal Investigator on research service agreements with Alkermes, Inc. and Purdue Pharma. The other authors have no disclosures or conflicts.
References
- 1.Bertholet N, Cheng DM, Samet JH, Quinn E, Saitz R. Alcohol consumption patterns in HIV-infected adults with alcohol problems. Drug Alcohol Depend. 2010;112(1–2):160–3. doi: 10.1016/j.drugalcdep.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chander G, Josephs J, Fleishman JA, Korthuis PT, Gaist P, Hellinger J, et al. Alcohol use among HIV-infected persons in care: results of a multi-site survey. HIV Med. 2008;9(4):196–202. doi: 10.1111/j.1468-1293.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korthuis PT, Fiellin DA, McGinnis KA, Skanderson M, Justice AC, Gordon AJ, et al. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J Acquir Immune Defic Syndr. 2012;61(2):171–8. doi: 10.1097/QAI.0b013e31826741aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8(1):33–7. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- 5.Hicks PL, Mulvey KP, Chander G, Fleishman JA, Josephs JS, Korthuis PT, et al. The impact of illicit drug use and substance abuse treatment on adherence to HAART. AIDS Care. 2007;19(9):1134–40. doi: 10.1080/09540120701351888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112(3):178–93. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu ES, Metzger DS, Lynch KG, Douglas SD. Association between alcohol use and HIV viral load. J Acquir Immune Defic Syndr. 2011;56(5):e129–30. doi: 10.1097/QAI.0b013e31820dc1c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. Can Med Assoc J. 2003;169(7):656–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Korthuis PT, McGinnis KA, Kraemer KL, Gordon AJ, Skanderson M, Justice AC, et al. Quality of HIV Care and Mortality Rates in HIV-Infected Patients. Clin Infect Dis. 2016;62(2):233–9. doi: 10.1093/cid/civ762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas GM, Chaudhry A, Hsu J, Woodson T, La uB, Olsen Y, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Ann Intern Med. 2010;152(11):704–11. doi: 10.1059/0003-4819-152-11-201006010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6(9):1049–56. [PubMed] [Google Scholar]
- 13.Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O’Connor PG, et al. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. J Subst Abuse Treat. 2008;35(1):87–92. doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S33–8. doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korthuis PT, Fiellin DA, Fu R, Lum PJ, Altice FL, Sohler N, et al. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S83–90. doi: 10.1097/QAI.0b013e31820bc9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korthuis PT, Tozzi MJ, Nandi V, Fiellin DA, Weiss L, Egan JE, et al. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S39–45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 18.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. Am J Public Health. 2015;105(8):e55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetrault JM, Tate JP, McGinnis KA, Goulet JL, Sullivan LE, Bryant K, et al. Hepatic Safety and Antiretroviral Effectiveness in HIV-Infected Patients Receiving Naltrexone. Alcohol Clin Exp Res. 2012;36(2):318–24. doi: 10.1111/j.1530-0277.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(2):210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krupitsky E, Nunes EV, Ling W, Gastfriend DR, Memisoglu A, Silverman BL. Injectable extended-release naltrexone (XR-NTX) for opioid dependence: long-term safety and effectiveness. Addiction. 2013;108(9):1628–37. doi: 10.1111/add.12208. [DOI] [PubMed] [Google Scholar]
- 22.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, Jr, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med. 2016;374(13):1232–42. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 25.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889–900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- 26.Lee JD, Grossman E, Huben L, Manseau M, McNeely J, Rotrosen J, et al. Extended-release naltrexone plus medical management alcohol treatment in primary care: findings at 15 months. J Subst Abuse Treat. 2012;43(4):458–62. doi: 10.1016/j.jsat.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Ahamad K, Korthuis PT, Lum PJ, Johnson C, Wood E. A Delayed Injection-Site Reaction in a Patient Receiving Extended Release Naltrexone. Subst Abus. 2016:0. doi: 10.1080/08897077.2016.1138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahamad K, Milloy MJ, Nguyen P, Uhlmann S, Johnson C, Korthuis TP, et al. Factors associated with willingness to take extended release naltrexone among injection drug users. Addict Sci Clin Pract. 2015;10:12. doi: 10.1186/s13722-015-0034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannelli P, Wu LT, Peindl KS, Swartz MS, Woody GE. Extended release naltrexone injection is performed in the majority of opioid dependent patients receiving outpatient induction: a very low dose naltrexone and buprenorphine open label trial. Drug Alcohol Depend. 2014;138:83–8. doi: 10.1016/j.drugalcdep.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell MC, Memisoglu A, Silverman BL. Hepatic safety of injectable extended-release naltrexone in patients with chronic hepatitis C and HIV infection. J Stud Alcohol Drugs. 2012;73(6):991–7. doi: 10.15288/jsad.2012.73.991. [DOI] [PubMed] [Google Scholar]