Abstract
Purpose
To classify the appearance of the optic disc seen on fundus photographs of healthy subjects and patients with or suspected glaucoma whose diagnosis was based upon visual fields (VF) and spectral domain optical coherence tomography (sdOCT) results.
Patients and Methods
One eye of 100 patients with or suspected glaucoma and 62 healthy subjects were prospectively tested with 24-2 and 10-2 VF and macular and disc sdOCT cube scans. All eyes with or suspected glaucoma had a 24-2 mean deviation (MD) better than −6.0 dB and an abnormal appearing disc on stereophotographs. The retinal ganglion cell plus inner plexiform layer (RGC+) from the macular scans and the retinal nerve fiber layer (RNFL) from the macular and disc scans were segmented and converted to probabilities plots. An eye was considered “glaucoma” if the sdOCT probability plots showed an abnormality in a region that corresponded to a defect seen on the 24-2 and/or 10-2 VF total deviation plot. Similarly, an eye was considered “suspect” only if both the sdOCT and VF plots were normal. Healthy subjects (normal VFs and sdOCT) were classified as “controls” and used as reference for comparisons. Glaucoma specialists reviewed the stereophotographs and classified eyes based on the presence of signs suggestive of glaucomatous optic neuropathy (GON).
Results
The pattern of clinical signs of GON seen on stereophotographs was statistically different between glaucoma (P<0.001) and suspects (P<0.001) vs. controls and explained up to 68% of the total variance of the diagnosis based upon sdOCT and VFs. Vertical cup-to-disc (VCDR)>0.6, focal neuroretinal rim thinning, focal RNFL loss, and violation of the ISNT rule had the best performance to differentiate glaucoma and suspects from controls. Compared to the suspect group, glaucoma eyes (abnormal sdOCT and VF tests) were more likely to have VCDR>0.6 (92 vs. 69%, P=0.003), diffuse rim (53 vs. 9%, P<0.001) and RNFL (61 vs. 26%, P<0.001) thinning, and beta-zone parapapillary atrophy (68 vs. 17%, P<0.001).
Conclusions
Focal and diffuse signs of glaucoma damage seen on stereophotographs often match damage shown on VFs and sdOCT. In addition, damage shown on VFs and sdOCT is often missed during clinical evaluation. Longitudinal studies ought to differentiate focal signs of glaucoma damage seen on stereophotography from false-positives or very early loss.
Keywords: glaucoma, optical coherence tomography, visual fields, optic disc, retinal ganglion cell
INTRODUCTION
Glaucoma is a progressive optic neuropathy and leading cause of irreversible blindness worldwide.[1] However, blindness from glaucoma is preventable and early detection and treatments are the main factors associated with better visual function during patients’ lifespan.[1,2] Nonetheless, studies have shown that despite new advances in glaucoma diagnosis, rates of blindness today remain excessively high, ranging from 13.5 to 26.5% in at least one eye in 10 years. [3,4]
Glaucoma diagnosis is based on the evaluation of the structure and function of the optic nerve, which is typically performed with imaging techniques and perimetry. Optic nerve head stereophotography and standard automated perimetry (SAP) are the most commonly used methods in clinical practice, and also the ones considered gold-standard for diagnosis and follow-up. However, patients can develop structural changes to the optic nerve and retinal nerve fiber layer (RNFL) before SAP results reveal any significant abnormality. [5–8] Therefore, the analysis of stereophotographs is widely used as a clinical method for detection of early glaucoma and progression. [9–11] The main limitations of this technique are its high intra- and inter- grader variability in interpreting the features of glaucomatous optic neuropathy (GON), even among specialists. [12–14] Some of these features of GON are: focal or diffuse neuroretinal rim thinning, RNFL defects, disc hemorrhage, and beta-zone parapapillary atrophy (PPA), [15] although clinicians do not always agree regarding the presence or magnitude of these findings. Objective imaging technologies have thus been developed to circumvent these limitations; among them, spectral domain optical coherence tomography (sdOCT) has gained large acceptance among ophthalmologists given its excellent reproducibility [16] and diagnostic performance [17–19]. The potential utility of sdOCT as an objective tool to enhance glaucoma diagnosis and minimize glaucoma-related visual morbidity was recently tested, suggesting a promising role in community based glaucoma screening.[20]
Notably, the vast majority of studies that assessed the ability of sdOCT to diagnose early glaucoma employed the clinical evaluation of the optic nerve and RNFL photographs as reference to determine the balance between sensitivity and specificity of this technology.[17–19] Yet, it is possible that the different features used to define GON could influence the diagnostic performance of these techniques. Given that the optic nerve/ RNFL complex was deemed abnormal based on clinical findings and sdOCT confirmed these abnormalities in the majority of cases in these studies, it is reasonable to assume that the two methods are strongly correlated and represent the same underlying domain (i.e.: glaucoma). To date, studies have not investigated the opposite scenario: how do clinicians classify the optic disc when the reference diagnosis is based on abnormal structural and functional tests? In particular, assuming that the sdOCT and SAP results are abnormal and suggestive of glaucoma, what are the features of GON and their frequency when described by glaucoma-trained specialists using stereophotography? We investigated how clinicians classified the optic discs of patients in which ancillary diagnostic tests were suggestive of glaucoma.
MATERIALS AND METHODS
This study was approved by the institutional review boards of Columbia University and the New York Eye and Ear Infirmary of Mount Sinai. Participants gave informed, written consent to participate and the study was performed in accordance with the tenets set forth in the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act (HIPAA) regulations.
One eye of 100 glaucoma patients or suspects and 62 healthy subjects were prospectively tested with macular and disc 6mm x 6mm sdOCT cube scans (Topcon Inc., 3D-OCT 2000, Tokyo), 24-2 and 10-2 SAP tests. To be included, subjects with or suspected glaucoma were required to have optic disc abnormalities based on clinical evaluation (which included not only fundus photographs but also intraocular pressure and family history), a mean deviation on 24-2 SAP better than −6.00 dB, ≤75 years old, refractive error with spherical equivalent less −6.00 diopters or better, reliable performance on both visual fields (fixation losses of 33% or better, false positives or false negatives 20% or better), open angles as viewed during gonioscopic examination and a best-corrected visual acuity equal or better than 20/40. As inclusion criteria, patients had to be experienced with VF testing for at least 2 years and VF abnormalities had to be repeatable on two or more examinations. Healthy subjects were defined based upon normal optic disc appearance, IOP < 22 mmHg, and normal 24-2 and 10-2 VF results. Patients with retinal or neurophthalmologic abnormalities or those with significant lens opacity were excluded. The mean age of the 100 patients was 54.7 years old and 51% were women. The average (range) 24-2 SAP MD was −1.76dB (−5.66dB to +1.9dB). Healthy subjects had a mean age of 55.4 years, 60% were women, and the average 24-2 MD was −0.02dB [−1.0 to 1.5dB]
Definition of Glaucomatous Optic Neuropathy
Fundus stereophotographs were analyzed by two glaucoma specialists (CGDM, PAA) simultaneously. These graders performed their analysis masked from the results of sdOCT and SAP tests (to be described further) or any other clinical data. Clinical features were classified into one or more of the following 12 non-mutually exclusive categories :[15] vertical cup–to-disc ratio (VCDR) > 0.6; inter-eye VCDR asymmetry > 0.2; small disc with significant cupping; optic disc pit; focal and diffuse neuroretinal rim thinning; disc hemorrhage; beta-zone peripapillary atrophy (bPPA); violation of the “ISNT” rule (i.e: inferior rim > superior > nasal > temporal); nasal cupping; focal and diffuse RNFL loss.
Definition of Spectral-Domain OCT Abnormalities
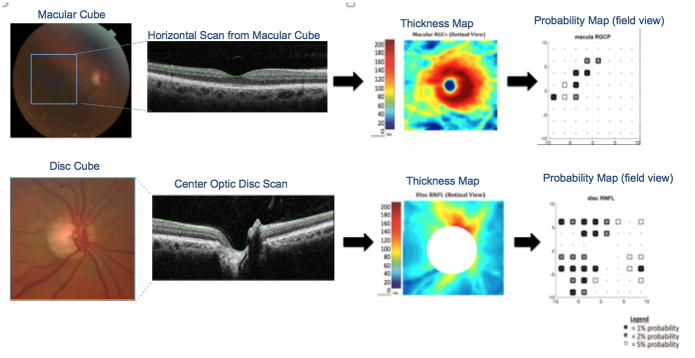
The combined retinal ganglion cell and inner plexiform layers (RGC+) of the sdOCT macular scans and the retinal nerve fiber layer (RNFL) of discs scans were segmented (Fig. 1, second column) using a using a previously validated segmentation algorithm,[21] which was manually corrected as necessary,[22] down-sampled into an 8 by 8 grid (64 locations), converted to a thickness map (Fig. 1, third column) and then to a probability map (Fig. 1, fourth column), after a point by point comparison to control values.[23] Each hemifield was classified separately and a significant defect on the probability cluster plot was defined as three abnormal points horizontally or vertically adjacent to each other in the same hemifield, representing a 2%, 2%, 1% or worse defect for macular cube or 2%, 2%, 5% for disc cube. These criteria were selected to produce an approximately 5% false positives rate in both cases.[24] Scans with poor fixation (as indicated by poor B-scan alignment or grossly off-center scans) and blink artifact (as indicated by missing B-scans) were rejected.
Figure 1.
The combined retinal ganglion cell and inner plexiform layers (RGC+) of the OCT macular scans and the retinal nerve fiber layer (RNFL) of discs scans were segmented using a computer-assisted manual segmentation technique, down-sampled into 64 pixels and converted to a thickness map and then to a probability map.
Definition of Visual Field Abnormalities
The 24-2 and 10-2 SAP VFs were obtained with the SITA-Standard Automated Perimetry strategy (Humphrey VF Analyzer; Carl Zeiss Meditec, Inc., Dublin, CA) and were performed within 6 months of the OCT. The classification of 24-2 VF and 10-2 VF was also based upon cluster criteria. Each hemifield was classified separately and considered abnormal if at least 3 contiguous test points respecting the horizontal midline were abnormal (at 5%, 5%, 1% or 5%, 2%, 2%) on either total deviation (TD) or pattern deviation (PD) probability plots.
To minimize false-positives and false-negatives, eyes were classified into three groups: “suspects” (both OCT and VFs normal in both hemifields); “glaucoma” (both the 24-2 SAP and disc cube sdOCT and/or both 10-2 SAP and macula cube sdOCT were abnormal in the same matching hemifield); “controls” (healthy subjects with normal discs, both OCT and VFs normal in both hemifields); and other (the remaining eyes) as illustrated in Fig. 2. A similar approach was employed by De Moraes et al.[25] using a combination of structural and functional tests to diagnose glaucoma. Only the group deemed “glaucoma” (i.e.: topographically consistent damage in both sdOCT and SAP) and “suspect” (no abnormality in either test) were analyzed and compared to the “control” group based upon the GON features described by the specialists (although they reviewed the photos of all 100 patients).
Figure 2.
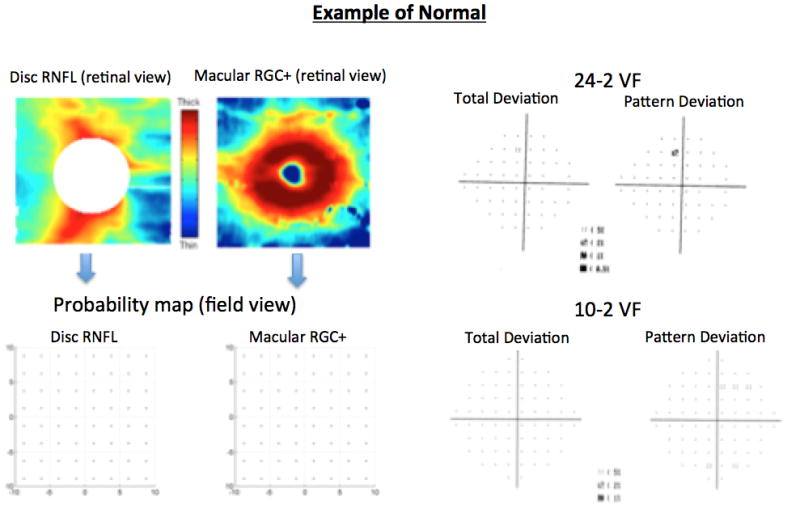
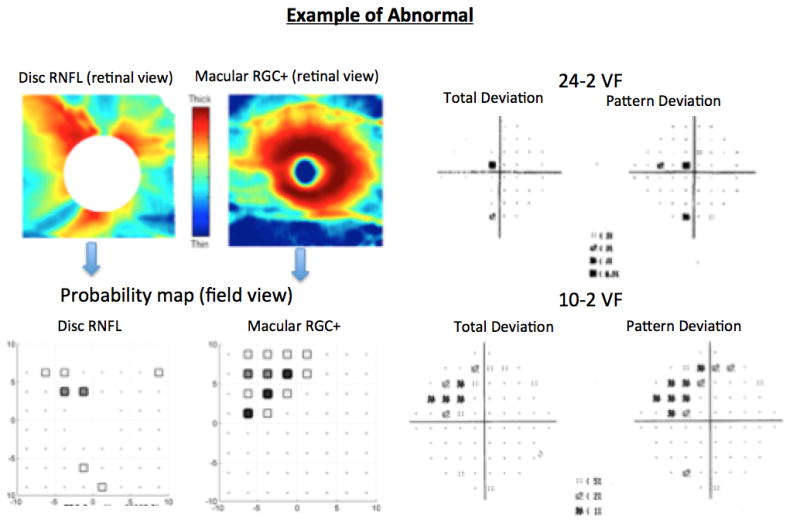
Examples of each group: normal (OCT and VFs normal); abnormal (both the 24-2VF and disc cube OCT and/or both 10-2VF and macula cube OCT were abnormal in the same hemifield).
The association between clinical signs of GON and glaucoma vs. suspect vs. control groups was tested with multivariate regression, which is a technique that estimates a single regression model with more than one outcome variable. It differs from multiple (or multivariable) regression in that several dependent variables are jointly regressed on the same independent variables. Statistical analyses were performed using STATA version 14.0 (StataCorp, College Station, Texas). The alpha level (type I error) was set at 0.05 (two-tailed).
RESULTS
As expected, the average mean deviation (MD) of the 38 eyes in the glaucoma group was poorer (−2.38 dB), than that of the 23 eyes in the suspects group (−0.14dB, P<0.001). Table 1 shows the percent of the eyes with the GON features described by the two graders.
Table 1.
Percent of eyes in Healthy, Suspects, Glaucoma, and Other groups with features of glaucomatous optic neuropathy.
| Groups | N | VDCR>0.6 | Focal Rim Loss | Diffuse Rim Loss | bPPA | Violation of ISNT Rule | Focal RNFL Loss | Diffuse RNFL Loss | Nasal Cupping | DH |
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | 62 | 3 | 0 | 0 | 11 | 5 | 0 | 0 | 0 | 0 |
| Suspects | 23 | 69 | 91 | 9 | 17 | 87 | 48 | 26 | 9 | 0 |
| Glaucoma | 38 | 92 | 66 | 53 | 68 | 84 | 26 | 61 | 24 | 8 |
| Other | 39 | 87 | 82 | 33 | 44 | 9 | 38 | 31 | 18 | 0 |
| Total | 162 | 43 | 37 | 18 | 30 | 44 | 17 | 23 | 9 | 2 |
Abbreviations: N: number of eyes; VDCR: vertical disc cup ratio; bPPA: beta-zone peripapillary atrophy; ISNT:inferior-superior-nasal-temporal; RNFL: retinal nerve fiber layer; DH: disc hemorrhage
In general, for all 162 eyes, the violation of the “ISNT” rule, VCDR>0.6, focal neuroretinal rim thinning, and bPPA were the most common features observed on stereophotographs (44%, 43%, 37%, and 30%, respectively). Notably, the most common features seen in the control group (i.e.: false-positives) were bPPA (11%), violation of “ISNT” rule (5%), and VCDR>0.6 (3%).
The results of the multivariate analysis are shown in Tables 2 and 3. The Breusch-Pagan test of independence was significant at P<0.001, suggesting the combination of GON features are highly correlated. The overall combination of clinical features for the suspects (n=23) and glaucoma (n=38) eyes was statistically different from controls (n=62, P<0.001, Table 2). Moreover, these GON features explained most of the variance when classifying eyes based upon VF and OCT results, ranging from 13% for nasal cupping up to 68% for VCDR>0.6.
Table 2.
Goodness-of-fit statistics for the multivariate regression for differentiating glaucoma and suspects from healthy subjects.
| Parameter | RMSE | R2 | P |
|---|---|---|---|
| VCDR > 0.6 | 0.28 | 0.68 | <0.0001 |
| Focal neuroretinal rim thinning | 0.29 | 0.63 | <0.0001 |
| Diffuse neuroretinal rim thinning | 0.30 | 0.37 | <0.0001 |
| Beta-zone PPA | 0.38 | 0.31 | <0.0001 |
| Violation of ISNT Rule | 0.29 | 0.65 | <0.0001 |
| Diffuse RNFL loss | 0.33 | 0.24 | <0.0001 |
| Focal RNFL loss | 0.30 | 0.40 | <0.0001 |
| Nasal cupping | 0.26 | 0.13 | 0.0002 |
Table 3.
Multivariate regression testing the association between clinical parameters and the classification groups” glaucoma, suspects, and healthy subjects. Healthy subjects were set as reference.
| Coefficient | P | 95% Confidence Interval | ||
|---|---|---|---|---|
| VCDR > 0.6 | ||||
| Suspect | 0.66 | <0.001 | 0.52 | 0.79 |
| Glaucoma | 0.88 | <0.001 | 0.77 | 1.00 |
| Focal neuroretinal rim thinning | ||||
| Suspect | 0.91 | <0.001 | 0.77 | 1.05 |
| Glaucoma | 0.65 | <0.001 | 0.53 | 0.77 |
| Diffuse neuroretinal rim thinning | ||||
| Suspect | 0.08 | NA* | −0.06 | 0.23 |
| Glaucoma | 0.52 | <0.001 | 0.40 | 0.65 |
| Beta-zone PPA | ||||
| Suspect | 0.06 | 0.517 | −0.12 | 0.24 |
| Glaucoma | 0.57 | <0.001 | 0.41 | 0.72 |
| Violation of ISNT Rule | ||||
| Suspect | 0.82 | <0.001 | 0.67 | 0.96 |
| Glaucoma | 0.79 | <0.001 | 0.67 | 0.91 |
| Diffuse RNFL loss | ||||
| Suspect | 0.26 | 0.002 | 0.09 | 0.42 |
| Glaucoma | 0.60 | <0.001 | 0.46 | 0.74 |
| Focal RNFL loss | ||||
| Suspect | 0.47 | <0.001 | 0.31 | 0.63 |
| Glaucoma | 0.26 | <0.001 | 0.12 | 0.39 |
| Nasal cupping | ||||
| Suspect | 0.08 | NA* | −0.04 | 0.21 |
| Glaucoma | 0.23 | <0.001 | 0.12 | 0.34 |
The number of eyes in the suspect and healthy groups was too small for analysis.
Most GON features were able to discriminate the glaucoma and suspect groups from the control group, with exception of bPPA (P=0.517), which was statistically as frequent in suspects as in controls (Table 3). Compared to eyes in the suspect group, the 38 eyes classified as glaucoma (sdOCT and SAP abnormal) were more likely to have VCDR > 0.6 (β=0.22; 95% CI=0.07 to 0.37; P=0.003), diffuse rim thinning (β=0.43; 95% CI=0.27 to 0.59; P<0.001), diffuse RNFL loss (β=0.34; 95% CI=0.16 to 0.51; P<0.001), bPPA (β=0.51; 95% CI=0.30 to 0.71; P<0.001), and nasal cupping (β=0.14; 95% CI=0.01 to 0.29; P=0.037) on stereophotographs. Interestingly, eyes in the suspect group were more likely to have focal rim thinning and focal RNFL loss than those classified as glaucoma (β=0.25; 95% CI=0.10 to 0.40; P=0.001 and β=0.21; 95% CI=0.04 to 0.38; P=0.015; respectively).
Ninety-one percent of the 23 eyes classified as suspects (sdOCT and SAP within normal limits) had a focal neuroretinal rim thinning. All of these eyes had areas of focal thinning in the inferior sector. Ten eyes (47.6%) of these patients with focal rim thinning also had thinning in the superior sector. Approximately half (47.6%) of the 21 patients with focal rim thinning also had focal RNFL loss on stereophotos that matched the location of neuroretinal rim thinning.
There were three patients with disc hemorrhages; all of them were classified glaucoma. Two patients had disc hemorrhages in the inferior-temporal sector and one had it in the superior-temporal sector.
Thirty-nine eyes (39%) had sdOCT and/or SAP abnormalities in non-matching hemifields (other). Only 6 eyes (15.38%) showed pre-perimetric glaucoma (i.e.: sdOCT cluster criteria abnormal and SAP VFs within normal limits). Interestingly, 28 eyes (71.8%) of this group had at least one abnormal SAP hemifield but the sdOCT was normal.
DISCUSSION
We investigated whether the classification of GON features seen on stereophotography can differentiate healthy from glaucoma and suspect eyes when glaucoma was defined based on the presence of abnormal sdOCT and SAP results. We found that a combination of clinical signs suggestive of GON can accurately identify eyes with abnormal test results.
Our study showed that VCDR>0.6 was one of the most common features observed in both groups. While it was present in 92% of the eyes classified as glaucoma, it was also present in 70% of the eyes classified as suspects and had a false-positive rate of 3% (among healthy controls). Even though optic disc cupping is one diagnostic feature of glaucoma, non-pathological optic disc cupping is common among healthy subjects. There is a high-variability of optic disc sizes and physiologic cupping among ophthalmologically healthy subjects.[26] A recent study found that large discs were more likely to be falsely considered glaucomatous than those with normal or small sizes. [27] One possible reason is that, assuming the total number of axons in healthy eyes remains relatively constant, larger discs are expected to have larger VCDR. Therefore, although it is a sensitive indicator of glaucoma, relying solely on an increased VCDR for glaucoma diagnosis can lead to a high false-positive rates.
Violation of the “ISNT” rule and focal neuroretinal rim thinning were also observed in most of the eyes, 84% of those classified as glaucoma, 87% of those classified as suspects, and 5% of controls. Violation of the “ISNT” rule also occurs in large optic disc cups of non-glaucomatous origin. The clinical observation of focal rim loss caused by glaucoma depends on the stage of the disease. In fact, we found that signs of focal injury seen on stereophotos (i.e.: focal rim thinning and RNFL loss) tended to be more common in the suspect group (with normal SAP and sdOCT) than the glaucoma group (both tests abnormal). Moreover, in eyes with early glaucomatous damage, the focal rim loss is usually located in the inferior-temporal and superior-temporal sectors. Eyes with advanced glaucomatous damage can also show a temporal and nasal thinning, while a complete notch is often related to a focal SAP defect.[15] It is intriguing that many eyes with apparent focal neuroretinal rim thinning did not show abnormalities on the combined SAP and sdOCT results. The actual amount of neuroretinal rim tissue can be difficult to estimate; hence it is possible that these results reflect either false-positives or pre-clinical defects. Whether these signs represent false-positive results or signs of early glaucoma missed by diagnostic techniques was not possible to be tested in our sample and warrant further investigation. To define the rim area clinicians often rely on the clinical margins of the optic disc. Recent findings suggests that such margins often do not match the disc margins defined with sdOCT using Bruch’s membrane opening (BMO) as reference.[28,29] The inability to accurately estimate the BMO on stereophotos may help explain cases of incongruence between clinical findings and sdOCT results.
Diffuse neuroretinal rim and RNFL thinning and bPPA were the features that best differentiated glaucoma from suspect eyes (based on the combination of SAP and sdOCT findings). Although, bPPA is seen in 15 to 20% of healthy subjects (in our sample this number was 11%), it is more frequent and wide-ranging in eyes with glaucoma. It is usually related to focal rim loss and focal SAP defect. [30] In addition, the enlargement of bPPA has been suggested to be associated with progressive visual field loss.[31,32]
Although disc hemorrhage was observed in only 3 fundus photographs, all of these eyes were classified as glaucoma and in 2 of them the disc hemorrhage was located in the inferior-temporal sector. Optic disc hemorrhage is extremely uncommon in non-glaucomatous patients, even though it can be related to diabetes, systemic hypertension, papillitis and other ophthalmic conditions.[33] Disc hemorrhage is most common seen in the inferior-temporal sector and is usually related to other findings like notches, focal rim defect or focal RNFL defect. [34] Because accurate disc hemorrhage detection depends upon follow-up with multiple photographs, and the present study looked at single photographs performed at the time of OCT and VF testing, this variable was not included in the model. Nonetheless, it is not surprising that all eyes with disc hemorrhage were classified as having glaucoma.
Interestingly, the GON parameters related to focal damage were more common in the suspect than the glaucoma group. There are some possible explanations for this observation. First, it is likely that the glaucoma group had more diffuse damage (as suggested by their worse SAP MD value), and hence focal parameters would have a poorer diagnostic ability in these eyes. Second, the definition of focal rim thinning and its location is extremely subjective – as discussed above - and false-positive results are likely to occur due to regional variations in the neuroretinal rim contour, particularly due to differences between clinically invisible vs. sdOCT detected optic disc margin.[28,29] Finally, small focal defects on the RNFL or disc may not show up on VF testing, even when using a combination of 24-2 and 10-2 strategies, especially if they fall in the inferior-nasal and superior-nasal sectors of the disc. This can happen due to their relationship with the location of main blood vessels, low-density of testing outside the central degrees, and the fact that these defects fall largely outside the 24-2 test grid.[35] This may help explain, at least in part, why not all eyes with RNFL defects seen on disc photos or sdOCT had a matching VF abnormality. Future studies ought to assess better methods to demonstrate functional damage in these eyes.
Our results are in consonance with the study by Jonas et al. [36] in which the authors evaluated optic disc variables assessed by optic disc photography and compared their performance to identify patients with RNFL and SAP defects. The authors ranked GON parameters for detection of glaucomatous optic nerve damage based on their sensitivity and specificity. Similar to our results, despite using different definition of abnormality, they found that the vertical cup-to-disc ratio and total neuroretinal rim area loss (here called diffuse rim loss) were the most valuable optic disc variables for early detection of glaucomatous optic nerve damage. They also found that the diagnostic power was lower for rim area defects in the inferior temporal and superior temporal disc sectors (here called focal rim loss). In addition, the ratios of neuroretinal rim width and rim area comparing various optic disc sectors with each other, which in our study we defined as break in ISNT rule and focal rim loss, revealed poor predictive ability.[36]
In sum, the classification of GON features on stereophotographs can differentiate healthy eyes from those with or suspected glaucoma as defined based upon SAP and sdOCT abnormalities consistent with glaucoma. However, clinicians should be aware that signs of focal damage on stereophographs (e.g.: neuretinal rim thinning and RNFL loss) could be due to normal inter-subject variability and limitations of stereophotography to define the optic disc margin. Further follow-up of these eyes will help determine if these clinical findings are indeed false-positives or very early signs of the disease.
Acknowledgments
Funding sources: Supported by National Institutes of Health Grant R01-EY-02115 (DCH); R01-EY-025253; Research to Prevent Blindness, New York, NY
Footnotes
Declaration of competing/conflicts of interest: P. Alhadeff, None; C.G. de Moraes, None; A.S. Raza, None; M. Chen, None; R. Ritch, None; D.C. Hood, Topcon Medical Systems, Inc. (F,C)
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 3.Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol. 2013;156:724–30. doi: 10.1016/j.ajo.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A, Miller NR, Pollack I, Maumenee AE, George T. The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol. 1977;95:2149–56. doi: 10.1001/archopht.1977.04450120055003. [DOI] [PubMed] [Google Scholar]
- 6.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–46. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CA, Sample PA, Zangwill LM, et al. Structure and function evaluation (SAFE): II. Comparison of optic disk and visual field characteristics. Am J Ophthalmol. 2003;135:148–54. doi: 10.1016/s0002-9394(02)01930-x. [DOI] [PubMed] [Google Scholar]
- 8.Kuang TM, Zhang C, Zangwill LM, Weinreb RN, Medeiros FA. Estimating Lead Time Gained by Optical Coherence Tomography in Detecting Glaucoma before Development of Visual Field Defects. Ophthalmology. 2015;122(10):2002–9. doi: 10.1016/j.ophtha.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer A, Katz J, Quigley HA, Miller NR, Robin AL, Richter RC, Witt KA. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 10.Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99(1):19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 11.Bowd C, Weinreb RN, Zangwill LM. Evaluating the optic disc and retinal nerve fiber layer in glaucoma. I: Clinical examination and photographic methods. Semin Ophthalmol. 2000;15(4):194–205. doi: 10.3109/08820530009037871. [DOI] [PubMed] [Google Scholar]
- 12.Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992 Feb;99(2):215–21. doi: 10.1016/s0161-6420(92)31990-6. [DOI] [PubMed] [Google Scholar]
- 13.Abrams LS, Scott IU, Spaeth GL, Quigley HA, Varma R. Agreement among optometrists, ophthalmologists, and residents in evaluating the optic disc for glaucoma. Ophthalmology. 1994;101(10):1662–7. doi: 10.1016/s0161-6420(94)31118-3. [DOI] [PubMed] [Google Scholar]
- 14.Jampel HD, Friedman D, Quigley H, Vitale S, Miller R, Knezevich F, Ding Y. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am J Ophthalmol. 2009;147(1):39–44. doi: 10.1016/j.ajo.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Susanna R, Jr, Vessani RM. New findings in the evaluation of the optic disc in glaucoma diagnosis. Curr Opin Ophthalmol. 2007;18(2):122–8. doi: 10.1097/ICU.0b013e328040bfe0. Review. [DOI] [PubMed] [Google Scholar]
- 16.Kiernan DF, Mieler WF, Hariprasad SM. Spectral-domain optical coherence tomography: a comparison of modern high-resolution retinal imaging systems. Am J Ophthalmol. 2010;149(1):18–31. doi: 10.1016/j.ajo.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Mwanza JC, Oakley JD, Budenz DL, Anderson DR. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118(2):241–8. doi: 10.1016/j.ophtha.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisboa R, Leite MT, Zangwill LM, Tafreshi A, Weinreb RN, Medeiros FA. Diagnosing preperimetric glaucoma with spectral domain optical coherence tomography. Ophthalmology. 2012;119(11):2261–9. doi: 10.1016/j.ophtha.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao HL, Addepalli UK, Chaudhary S, Kumbar T, Senthil S, Choudhari NS, Garudadri CS. Ability of different scanning protocols of spectral domain optical coherence tomography to diagnose preperimetric glaucoma. Invest Ophthalmol Vis Sci. 2013;54(12):7252–7. doi: 10.1167/iovs.13-12731. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg DM, Vaswani R, Nong E, Al-Aswad L, Cioffi GA. A comparative effectiveness analysis of visual field outcomes after projected glaucoma screening using SD-OCT in African American communities. Invest Ophthalmol Vis Sci. 2014;55(6):3491–500. doi: 10.1167/iovs.14-14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Reisman CA, Wang Z, Fukuma Y, Hangai M, Yoshimura N, Tomidokoro A, Araie M, Raza AS, Hood DC, Chan K. Automated layer segmentation of macular oct images using dual-scale gradient information. Opt Express. 2010;18:21293–307. doi: 10.1364/OE.18.021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood DC, Cho J, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88(1):113–23. doi: 10.1097/OPX.0b013e3181fc3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hood DC, Slobodnick A, Raza AS, De Moraes CG, Teng CC, Ritch R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest Ophthalmol Vis Sci. 2014;55:632–49. doi: 10.1167/iovs.13-13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raza AS, Zhang X, De Moraes CG, Reisman CA, Liebmann JM, Ritch R, Hood DC. Improving glaucoma detection using spatially correspondent clusters of damage and by combining standard automated perimetry and optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:612–24. doi: 10.1167/iovs.13-12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Moraes CG, Liebmann JM, Ritch R, Hood DC. Understanding disparities among diagnostic technologies in glaucoma. Arch Ophthalmol. 2012;130(7):833–40. doi: 10.1001/archophthalmol.2012.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47(3):1008–15. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 27.Garway-Heath DF, Ruben ST, Viswanathan A, Hitchings RA. Vertical cup/disc ratio in relation to optic disc size: its value in the assessment of the glaucoma suspect. Br J Ophthalmol. 1998;82(10):1118–24. doi: 10.1136/bjo.82.10.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis AS, Sharpe GP, Yang H, Nicolela MT, Burgoyne CF, Chauhan BC. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography. Ophthalmology. 2012;119(4):738–47. doi: 10.1016/j.ophtha.2011.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis AS, O’Leary N, Yang H, Sharpe GP, Nicolela MT, Burgoyne CF, Chauhan BC. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci. 2012;53(4):1852–60. doi: 10.1167/iovs.11-9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kono Y, Zangwill L, Sample PA, Jonas JB, Emdadi A, Gupta N, Weinreb RN. Relationship between parapapillary atrophy and visual field abnormality in primary open-angle glaucoma. Am J Ophthalmol. 1999;127(6):674–80. doi: 10.1016/s0002-9394(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 31.Jonas JB, Martus P, Horn FK, Jünemann A, Korth M, Budde WM. Predictive factors of the optic nerve head for development or progression of glaucomatous visual field loss. Invest Ophthalmol Vis Sci. 2004;45(8):2613–8. doi: 10.1167/iovs.03-1274. [DOI] [PubMed] [Google Scholar]
- 32.Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998;105(8):1541–5. doi: 10.1016/S0161-6420(98)98044-7. [DOI] [PubMed] [Google Scholar]
- 33.Drance SM. Disc hemorrhages in the glaucomas. Surv Ophthalmol. 1989;33(5):331–7. doi: 10.1016/0039-6257(89)90010-6. Review. [DOI] [PubMed] [Google Scholar]
- 34.Piltz-Seymour J. Disc hemorrhages and glaucoma management. J Glaucoma. 2000;9(3):273–7. doi: 10.1097/00061198-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Hood DC, Wang DL, Raza AS, de Moraes CG, Liebmann JM, Ritch R. The locations of circumpapillary glaucomatous defects seen on frequency-domain OCT scans. Invest Ophthalmol Vis Sci. 2013;54:7338–43. doi: 10.1167/iovs.13-12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jonas JB, Bergua A, Schmitz-Valckenberg P, Papastathopoulos KI, Budde WM. Ranking of optic disc variables for detection of glaucomatous optic nerve damage. Invest Ophthalmol Vis Sci. 2000;41(7):1764–73. [PubMed] [Google Scholar]