The availability of bright fluorescent dyes has positioned fluorescence microscopy to dominate live cell molecular imaging for more than a hundred years. Now, a team lead by Doron Shabat offers a potential alternative in the form of elegantly designed small molecule scaffolds that open the gates for live cell chemiluminescence microscopy.1
Fluorescence microscopy offers a high throughput technique that can be used to image cellular structures, enzyme activities, and chemical species. Core fluorescent scaffolds like fluorescein, rhodamine, and boron-dipyrromethene (BODIPY) have been widely adopted to expand the scope of microscopy with new imaging techniques constantly emerging.2 Despite wide success, problems such as photobleaching, light scattering, and autofluorescence have plagued microscopy and stymie translation of the technology to thicker specimens and animal models. Chemiluminescence, the direct generation of light from a chemical reaction, overcomes these problems by eliminating the need for an excitation light source. Indeed, organisms engineered to express luciferase and emit bioluminescence have become a critical tool with new innovative applications in continual development.3 The need for genetic modification, however, can be a drawback in many experimental systems. For this reason efforts have been made to develop nonenzymatic chemiluminescent imaging agents, but up until now low light emission in water has necessitated stabilization with specialized supramolecular systems.4,5
Sterically stabilized 1,2-dioxetanes, initially disclosed in the 1980s by Paul Schaap, are versatile scaffolds that remain inert until triggered in a chemically initiated electron exchange luminescence mechanism to emit light.6−8 This design has been used for chemiluminescent agents to detect β-galactosidase, phosphatase, and other analytes in vitro. More recently, chemiluminescent probes based on this scaffold have been engineered for whole animal imaging of parameters like tissue oxygenation in wild-type tumor models.9 Still, low light emission levels require the use of polymeric “enhancer” solutions that can prevent cell uptake and have cellular toxicity that makes them incompatible with longitudinal studies. In order to overcome the complications of these polymeric systems, previous work from Doron Shabat’s laboratory demonstrated that tethered fluorophores could enhance chemiluminescence efficiency for whole animal imaging, but chemiluminescent microscopy was only achieved in fixed cell samples.10
In their latest work published in ACS Central Science,1 the authors have systematically investigated modifications and uncovered a key structural motif that unlocks high chemiluminescent emission, even in water (Figure 1). Hypothesizing that increasing the fluorescent quantum yield would ultimately translate into increased chemiluminescent emission, the authors discovered that appending an acrylonitrile group at the appropriate position provides a dramatic increase in fluorescence quantum yield and a remarkable 3 order of magnitude increase in chemiluminescence emission. The versatility of this bright chemilumiphore allowed rapid preparation of responsive chemiluminescent imaging agents for hydrogen peroxide, glutathione, and β-galactosidase. Importantly, this enhanced brightness was instrumental in allowingthe team to perform live cell chemiluminescent imaging of HEK293-LacZ cells.
Figure 1.
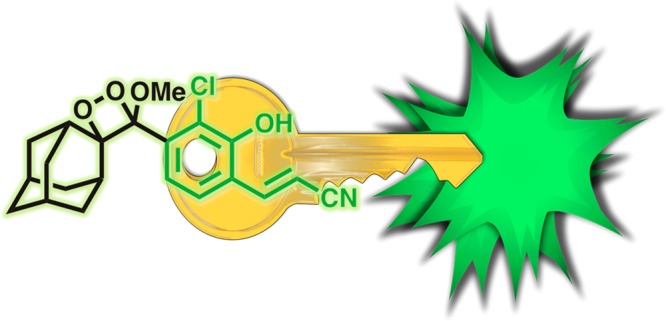
An acrylonitrile substituted phenol endows a spiroadmantane 1,2-dioxetane with high chemiluminescent emission in water, unlocking chemiluminescence microscopy in living cells.
This key result clears the path for small molecule chemiluminescence microscopy (Figure 2). Only recently has the appropriate instrumentation become commercially available, and thus far it has been limited to bioluminescence microscopy of luciferase expressing cell lines. The reported chemiluminescent structure may play a similar role for chemiluminescence microscopy as fluorescein has played for fluorescence microscopy: a highly emissive compound that can be tailored to different types of imaging experiments. The tractable synthetic approach and simplicity of the system will make it straightforward for other researchers to adopt these techniques. Although it remains to be seen what advantages chemiluminescent cellular imaging will have over more traditional fluorescence microscopy, the low background and increased imaging depth may be particularly useful for imaging tissue slices without a reliance on two-photon absorption techniques. At present, imaging in living cells requires overexpression of a marker enzyme, β-galactosidase, but the gates have been opened and a new day of chemiluminescent microscopy probes can be seen on the horizon.
Figure 2.
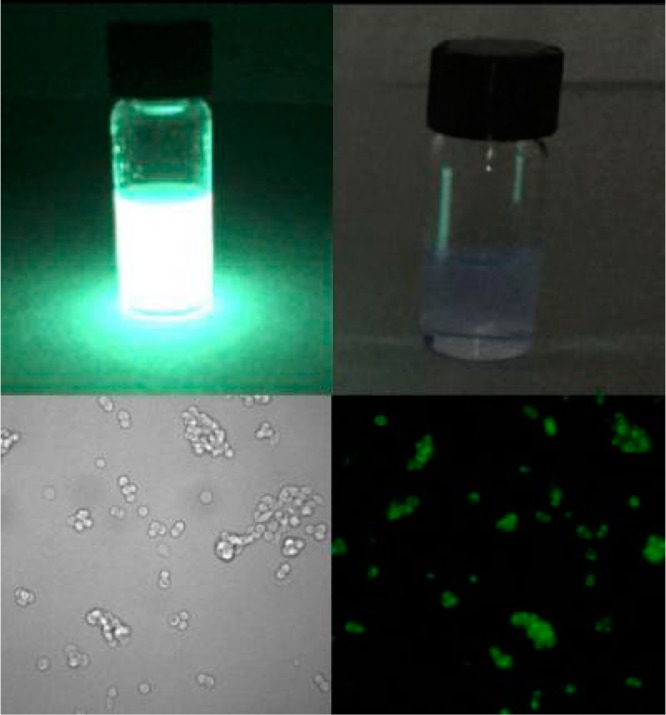
Chemiluminescent probes (top left) are 3000 times brighter than luminol (top right), allowing for the first time nonenzymatic chemiluminescent microcopy (bottom right, bright field image bottom left). Reproduced with permission from ref (1). Copyright 2017 American Chemical Society.
The increased tissue imaging depth granted by chemiluminescence will also have distinct advantages for imaging at the whole animal level, where problems with tissue autofluorescence are particularly limiting. The molecular designs lend themselves to further red-shifting of emission wavelengths, and eliminating the need for polymeric encapsulation dramatically simplifies experiments and helps to alleviate toxicity concerns. Much of the extensive progress in responsive fluorescent molecules can be readily translated to chemiluminescent designs. While the focus of this work has been on biological imaging, the ability to make bright, tunable, and responsive chemiluminescent molecules could find applicability in a range of disciplines from optical displays to photonic logic gates, portending the empowering centrality of this important chemical advance.
References
- Green O.; Eilon T.; Hananya N.; Gutkin S.; Bauer C. R.; Shabat D. Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes. ACS Cent. Sci. 2017, 10.1021/acscentsci.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavis L. D.; Raines R. T. Bright Building Blocks for Chemical Biology. ACS Chem. Biol. 2014, 9, 855–866. 10.1021/cb500078u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A.; Porterfield W. B.; Rathbun C. M.; McCutcheon D. C.; Paley M. A.; Prescher J. A. Orthogonal Luciferase–Luciferin Pairs for Bioluminescence Imaging. J. Am. Chem. Soc. 2017, 139, 2351–2358. 10.1021/jacs.6b11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.; Khaja S.; Velasquez-Castano J. C.; Dasari M.; Sun C.; Petros J.; Taylor W. R.; Murthy N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. 10.1038/nmat1983. [DOI] [PubMed] [Google Scholar]
- Baumes J. M.; Gassensmith J. J.; Giblin J.; Lee J. J.; White A. G.; Culligan W. J.; Leevy W. M.; Kuno M.; Smith B. D. Storable, thermally activated, near-infrared chemiluminescent dyes and dye-stained microparticles for optical imaging. Nat. Chem. 2010, 2, 1025–1030. 10.1038/nchem.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap A. P.; Chen T.-S.; Handley R. S.; DeSilva R.; Giri B. P. Chemical and enzymatic triggering of 1,2-dioxetanes. 2: Fluoride-induced chemiluminescence from tert-butyldimethylsilyloxy-substituted dioxetanes. Tetrahedron Lett. 1987, 28, 1155–1158. 10.1016/S0040-4039(00)95313-9. [DOI] [Google Scholar]
- Matsumoto M.; Watanabe N. Structural Aspects of 1,2-Dioxetanes Active toward Intramolecular Charge-Transfer-Induced Chemiluminescent Decomposition. Bull. Chem. Soc. Jpn. 2005, 78, 1899–1920. 10.1246/bcsj.78.1899. [DOI] [Google Scholar]
- Augusto F. A.; de Souza G.; de Souza Júnior S. P.; Khalid M.; Baader W. J. Efficiency of Electron Transfer Initiated Chemiluminescence. Photochem. Photobiol. 2013, 89, 1299–1317. 10.1111/php.12102. [DOI] [PubMed] [Google Scholar]
- Cao J.; Campbell J.; Liu L.; Mason R. P.; Lippert A. R. In Vivo Chemiluminescent Imaging Agents for Nitroreductase and Tissue Oxygenation. Anal. Chem. 2016, 88, 4995–5002. 10.1021/acs.analchem.6b01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hananya N.; Eldar Boock A.; Bauer C. R.; Satchi-Fainaro R.; Shabat D. Remarkable Enhancement of Chemiluminescent Signal by Dioxetane–Fluorophore Conjugates: Turn-ON Chemiluminescence Probes with Color Modulation for Sensing and Imaging. J. Am. Chem. Soc. 2016, 138, 13438–13446. 10.1021/jacs.6b09173. [DOI] [PubMed] [Google Scholar]


