Abstract
Chemiluminescence probes are considered to be among the most sensitive diagnostic tools that provide high signal-to-noise ratio for various applications such as DNA detection and immunoassays. We have developed a new molecular methodology to design and foresee light-emission properties of turn-ON chemiluminescence dioxetane probes suitable for use under physiological conditions. The methodology is based on incorporation of a substituent on the benzoate species obtained during the chemiexcitation pathway of Schaap’s adamantylidene–dioxetane probe. The substituent effect was initially evaluated on the fluorescence emission generated by the benzoate species and then on the chemiluminescence of the dioxetane luminophores. A striking substituent effect on the chemiluminescence efficiency of the probes was obtained when acrylate and acrylonitrile electron-withdrawing groups were installed. The chemiluminescence quantum yield of the best probe was more than 3 orders of magnitude higher than that of a standard, commercially available adamantylidene–dioxetane probe. These are the most powerful chemiluminescence dioxetane probes synthesized to date that are suitable for use under aqueous conditions. One of our probes was capable of providing high-quality chemiluminescence cell images based on endogenous activity of β-galactosidase. This is the first demonstration of cell imaging achieved by a non-luciferin small-molecule probe with direct chemiluminescence mode of emission. We anticipate that the strategy presented here will lead to development of efficient chemiluminescence probes for various applications in the field of sensing and imaging.
Short abstract
A new molecular methodology to design and foresee light-emission properties of turn-ON chemiluminescence dioxetane probes is presented. The probes are suitable for use under physiological conditions and thus could provide exceptional live cell images.
Introduction
Chemiluminescence assays are among the most sensitive methods for determination of enzyme activity and analyte concentrations due to their high signal-to-noise ratio.1 Hence, chemiluminescence probes are utilized in a broad range of analytical applications such as immunoassays and assays involving DNA.2 Most chemiluminescence probes produce light emission following reaction with an oxidizing agent. Such probes usually undergo an oxidation step to form an unstable strained peroxide, which rapidly decomposes to generate an emissive species in its excited state that decays to its ground state through emission of light. The oxidation-based mechanism is utilized for activation of common chemiluminescence substrates such as luminol3 and oxalate esters.4 In addition, oxidation-activated chemiluminescence has been used to detect and image reactive oxygen species (ROS) in vitro and in vivo.5−16
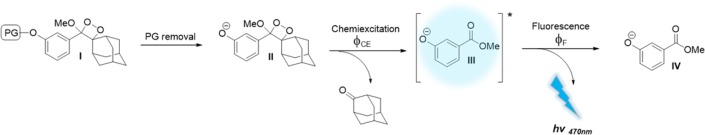
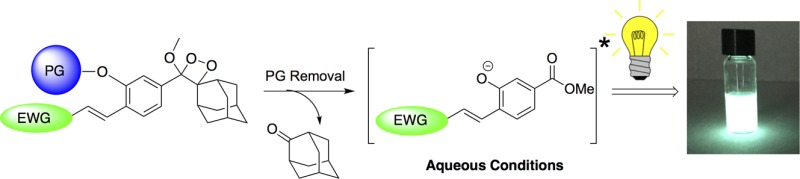
Schaap’s adamantylidene–dioxetane (Figure 1, structure I) is the only known chemiluminescence probe that contains a stable dioxetane moiety.17−19 Therefore, this probe does not require an oxidation step to trigger its chemiluminescence and, thus, can detect a wide range of chemical and biological activities. A schematic diagram illustrating the adamantylidene–dioxetane chemiluminescence pathway is depicted in Figure 1. Schaap’s dioxetane I is equipped with an analyte-responsive protecting group (PG), which is used to mask the phenol moiety of the probe. Removal of the protecting group by the analyte of interest generates an unstable phenolate–dioxetane species II, which decomposes through a chemiexcitation process to produce the excited intermediate benzoate ester III and adamantanone. The excited intermediate decays to its ground state (benzoate ester IV) through an emission of a blue photon.
Figure 1.
Activation pathway of Schaap’s dioxetane (PG, protecting group).
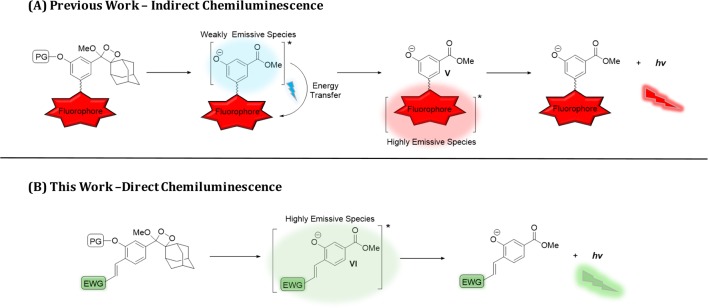
In order to achieve bright chemiluminescence, the resulting excited species must be an efficient emitter (i.e., it must have high fluorescence quantum yield). Benzoate ester III emits strong light in organic solvents like DMSO, but it is a weakly emissive fluorophore under aqueous conditions.20 Therefore, the direct chemiluminescence generated by emission of the corresponding dioxetane probe in water is very weak. The weak emission nature of benzoate ester III under aqueous conditions motivated us to search for new methods to amplify its chemiluminescent emission that would enable biological use. We have recently reported a practical synthetic route to adamantylidene–dioxetane conjugated with fluorescent dyes.21 The dioxetane–fluorophore conjugate decomposes upon its activation to generate the typical excited benzoate. The latter can transfer its energy to the attached fluorophore yielding a highly emissive intermediate (Figure 2A, compound V). The chemiluminescent emission of such conjugates was significantly amplified under physiological conditions through the energy transfer mechanism.
Figure 2.
(A) In previous work, indirect chemiluminescence amplification was obtained by energy transfer to a fluorogenic dye. (B) Here, direct chemiluminescence amplification was obtained by a substituent effect.
The conjugation to a fluorescent dye resulted in signal amplification through an indirect chemiluminescence pathway. We reasoned that it should be possible to amplify the chemiluminescence emission through a direct mode of action. In order to achieve direct amplification, the emissive character of the originally formed benzoate had to be improved. We, therefore, sought to introduce an electron-withdrawing group (EWG) at a conjugated position to the phenolate donor of benzoate ester III (Figure 2B). Such a donor–acceptor pair design (VI) should increase the emissive nature the benzoate species.22 To the best of our knowledge, the influence of a conjugated electron-withdrawing group on the aromatic moiety of Schaap’s chemiluminescence probes has not been studied before under physiologically relevant pHs.23−27 Here we report an extraordinary enhancement effect of chemiluminescence emission under physiological conditions, which results from distinct substituents installed on Schaap’s adamantylidene–dioxetane probe. In addition, by utilizing one of our extremely bright chemiluminescence probes, we were able to obtain chemiluminescence cell images with unprecedented quality.
Results and Discussion
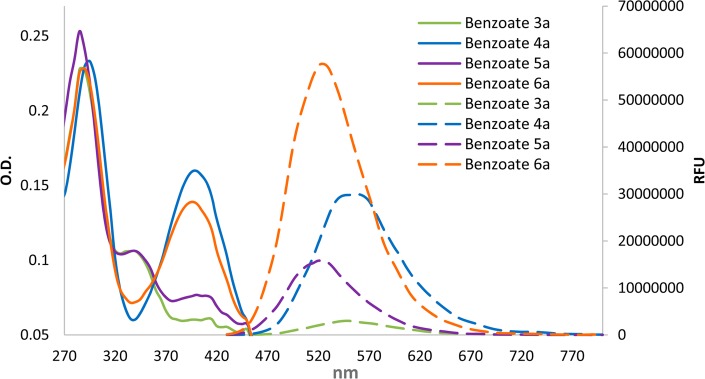
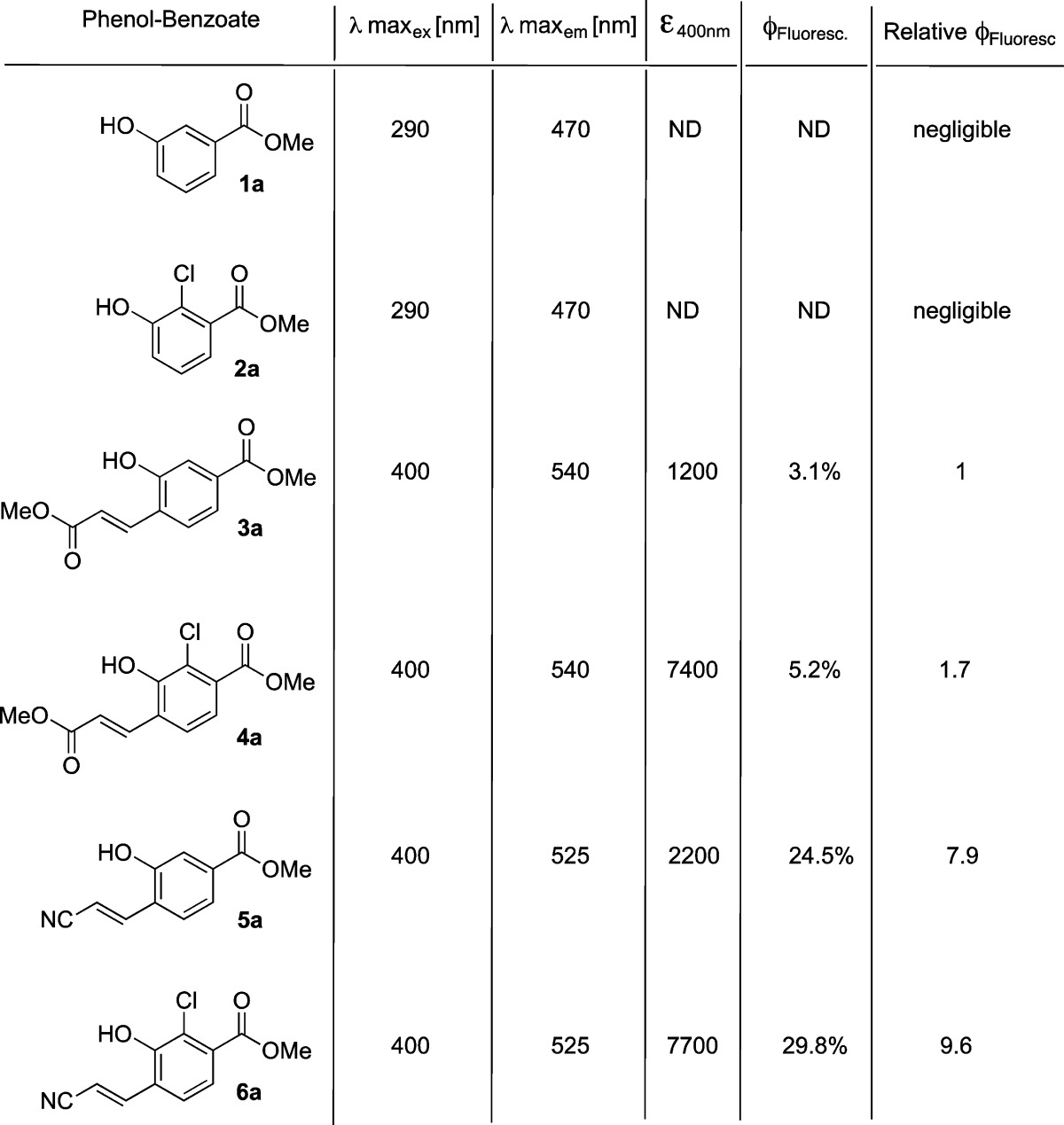
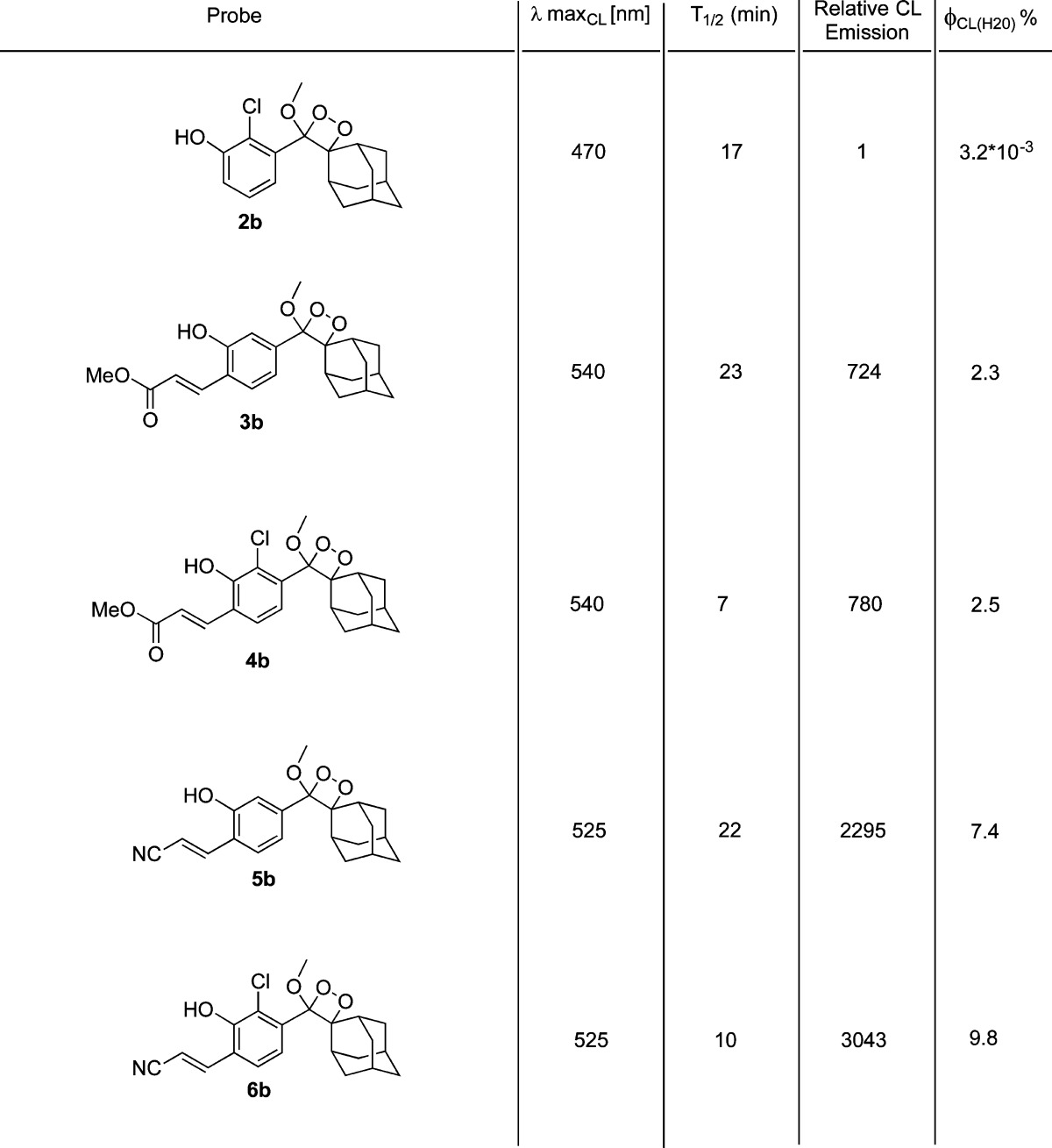
To evaluate the substituent effect, we synthesized numerous phenol–benzoate derivatives with acceptor substituents at ortho and para positions of the phenol and measured their fluorescence emission in PBS buffer at pH 7.4 (see the Supporting Information for synthetic procedures). The most significant effect was obtained when an acceptor was incorporated at the ortho position of the phenol. Following a screen of several electron-withdrawing groups, we chose to focus on methyl acrylate and acrylonitrile substituents. In addition, we also examined the effect of incorporation of chlorine substituent at the ortho position of the phenol. Chlorine substituent was previously used in Schaap’s chemiluminescent probes to reduce the phenol’s pKa.28 The reduced pKa enriches the relative concentration of the phenolate, thus enhancing the rate of the chemiluminescent decomposition in physiological pH. The absorbance and fluorescence spectra of selected phenol–benzoate derivatives are shown in Figure 3, and their molecular structures and spectroscopic parameters are summarized in Table 1.
Figure 3.
Absorbance (solid line) and fluorescence (dashed line) spectra of benzoates 3a, 4a, 5a, and 6a [50 μM] in PBS [100 mM], pH 7.4, 5% DMSO, (excitation wavelength = 400 nm).
Table 1. Spectroscopic Fluorescence Parameters Measured for Selected Phenol–Benzoate Derivatives in PBS, pH 7.4.
The emissive species generated by the chemiexcitation of commercially available adamantylidene–dioxetane probes are eventually the excited states of benzoates 1a or 2a. These benzoates do not present any measurable fluorescence under physiological conditions. However, incorporation of methyl acrylate or acrylonitrile substituents at the ortho position of the phenol (benzoates 3a and 5a) resulted in highly fluorogenic phenol–benzoate derivatives (quantum yields 3.1% and 24.5%, respectively) with maximum emission wavelengths of 540 and 525 nm, respectively. Insertion of an additional chlorine substituent at the other ortho position (benzoates 4a and 6a) resulted in an increase of the extinction coefficient (λ = 400 nm) in comparison to parent benzoates (3a and 5a), and thus enhanced the brightness of the fluorophores. This rise of the extinction coefficient is attributed to the increased concentration of the phenolate species under physiological conditions, produced by the electron-withdrawing effect of the chlorine substituent. However, it did not change the emission wavelength, and also had only minor effect on fluorescence quantum yield.
These results suggest that incorporation of the methyl acrylate and acrylonitrile substituents (with or without the chlorine) in the dioxetane chemiluminescent luminophores could strengthen the emissive nature of the released benzoate. Such a substituent effect would lead to a significant increase in chemiluminescence quantum yield of the dioxetane under physiological conditions. To test this hypothesis, we synthesized five different adamantylidene–dioxetane luminophores (see Supporting Information for synthetic procedures) containing unmasked phenol groups (Table 2). Upon deprotonation of the phenol, the luminophores underwent chemiexcitation decomposition to release the benzoates (Table 1) in their excited state. Next, we measured the chemiluminescence emission spectra and total light emission of the luminophores under physiological conditions. The molecular structure of the dioxetane luminophores and their chemiluminescence parameters are summarized in Table 2. Predictably, the chemiluminescence emission spectra of the dioxetane luminophores overlapped with the fluorescence emission spectra of their corresponding benzoates (Figure 3).
Table 2. Molecular Structure and Chemiluminescence Parameters of Adamantylidene–Dioxetane Luminophores with Different Ortho Substituents (Luminophores 2b–6b [1 μM] in PBS [100 mM], pH 7.4, 5% DMSO, 37 °C).
The dioxetane luminophores exhibited chemiluminescent exponential decay kinetic profiles with varied half-lives (T1/2; see the Supporting Information for kinetic plots). Dioxetane 2b was used as a reference compound since its chemiluminescence quantum yield under aqueous conditions is known (3.2 × 10–3%).29,30 The chemiluminescence emission of dioxetane 2a in water was extremely weak; however, dioxetane luminophores 3b, 4b, 5b, and 6b exhibited remarkably strong chemiluminescence emission signals upon their deprotonation in PBS, pH 7.4. Luminophore 3b (with the methyl acrylate substituent) had an emission signal about 700-fold stronger than that of dioxetane 2b. The chemiluminescence quantum yield of 3b was 2.3%. Luminophore 4b (with the methyl acrylate substituent and an additional chlorine substituent) showed a similar signal enhancement with a shorter T1/2 (7 min) relative to luminophore 3b (T1/2 of 23 min). Luminophore 6b (with acrylonitrile and an additional chlorine) had a chemiluminescence quantum yield of 9.8% and showed the highest enhancement of chemiluminescence emission, about 3000-fold higher than that of dioxetane 2a. A similar faster kinetic profile was observed when the chlorine substituent was present on the luminophore (T1/2 of 10 min for probe 6b vs 22 min for 5b).
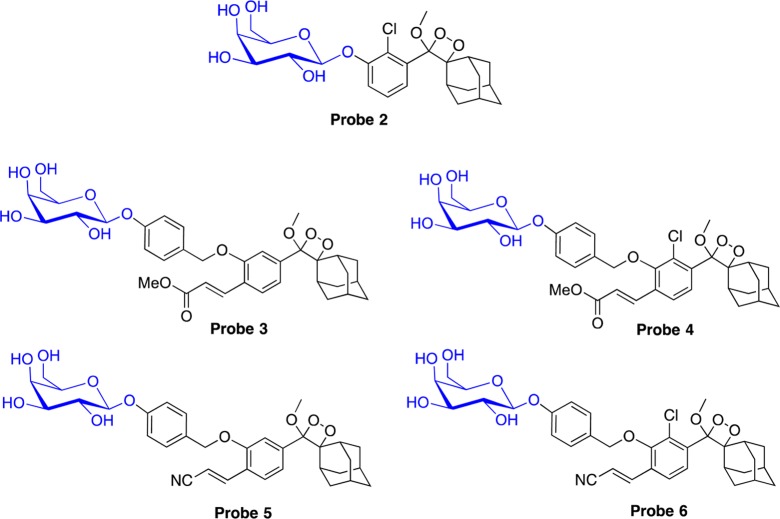
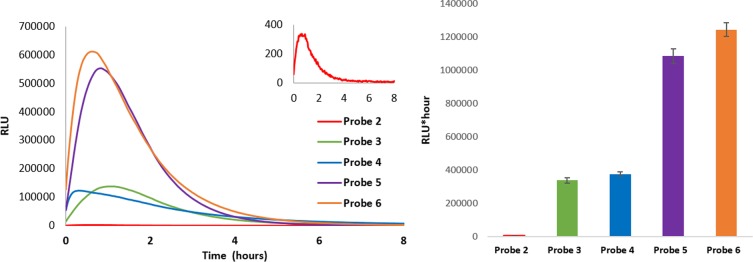
Turn-ON chemiluminescence probes can be simply prepared by masking the phenol functional group of the dioxetane luminophores with an enzyme-responsive substrate. To evaluate this option, we synthesized five different adamantylidene–dioxetane probes (2–6) based on dioxetane luminophores 2b–6b, where the phenols were masked with a triggering substrate suitable for activation by β-galactosidase (Figure 4; see the Supporting Information for synthetic procedures). To avoid steric interference (as a result of the ortho substituent) at the enzyme cleavage site, a short self-immolative spacer was installed between the phenolic oxygen and the galactose substrate as previously described.31−33 Next, we measured the chemiluminescence emission of the probes, as a function of time, in the presence and in the absence of β-galactosidase. The kinetic profiles of the chemiluminescence signals and their relative emission intensities are shown in Figure 5.
Figure 4.
Molecular structures of chemiluminescence probes for detection of β-galactosidase.
Figure 5.
(Left) Chemiluminescence kinetic profiles of probes 2, 3, 4, 5, and 6 [1 μM] in PBS [100 mM], pH 7.4, 10% DMSO in the presence of 1.5 units/mL β-galactosidase at room temperature. The inset focuses on the kinetic profile of probe 2. (Right) Total light emitted from each probe.
The probes exhibited a typical chemiluminescent kinetic profile in the presence of β-galactosidase with an initial signal increase to a maximum followed by a slow decrease to zero. Probes 3, 4, 5, and 6 exhibited remarkably strong chemiluminescence emission signal under aqueous conditions in the presence of β-galactosidase; however, probe 2 produced extremely weak emission (Figure 5, inset). Probe 3 showed an emission signal about 500-fold stronger than that of probe 2. Probe 4 (with the chlorine substituent) showed similar signal enhancement with a faster kinetic profile than probe 3 (the deviation in the kinetic profile observed for probe 4 might be attributed to low solubility at room temperature). Probe 6 showed the highest enhancement of chemiluminescence emission: about 1800-fold higher than that of probe 2. No light emission was observed from the probes in the absence of β-galactosidase.
The striking enhancement of chemiluminescence emission obtained by the new dioxetane luminophores encouraged us to compare the signal intensity of probe 6 to that of commercial chemiluminescence assays. There are several commercially available chemiluminescence probes based on the adamantylidene–dioxetane. Since the chemiluminescence emission of these probes is very weak under aqueous conditions, a surfactant–dye adduct (enhancer) is usually added in order to amplify the signal of the assay.34 The surfactant reduces water-induced quenching by providing a hydrophobic environment for the chemiluminescent reaction that transfers the emitted light to excite the nearby fluorogenic dye. Consequently, the low-efficiency luminescence process is amplified significantly in aqueous medium in the presence of surfactant.35 Commercially available Emerald-II enhancer (10%) was added to probe 2 in the presence of β-galactosidase (in PBS, pH 7.4), and its chemiluminescence emission was compared to that of probe 6 without enhancer. The obtained results are presented in Figure 6. Emerald-II enhancer amplified the chemiluminescence emission of probe 2 by 247-fold (Figure 6A). Remarkably, the chemiluminescence emission signal from probe 6 without enhancer was more than 8-fold stronger than that of probe 2 with the Emerald-II enhancer (Figure 6B). This unprecedented result suggests that a simple small-molecule dioxetane compound like probe 6 can produce chemiluminescence emission that is about an order of magnitude stronger than the signal produced by a two-component system (dioxetane 2 and Emerald-II enhancer). Since our probes produce relatively highly emissive benzoate species under aqueous conditions, addition of the Emerald-II enhancer had only a slight effect on their chemiluminescence emission (see Supporting Information).
Figure 6.
(A) Chemiluminescence kinetic profiles of probe 2 [1 μM] in the presence of 1.5 units/mL β-galactosidase with and without Emerald-II enhancer (10%) in PBS [100 mM], pH 7.4, 10% DMSO, and count of total light emitted. (B) Chemiluminescence kinetic profiles of probe 2 [1 μM] with Emerald-II enhancer [10%] and probe 6 [1 μM] in PBS [100 mM], pH 7.4, 10% DMSO, in the presence of 1.5 units/mL β-galactosidase, and count of total light emitted.
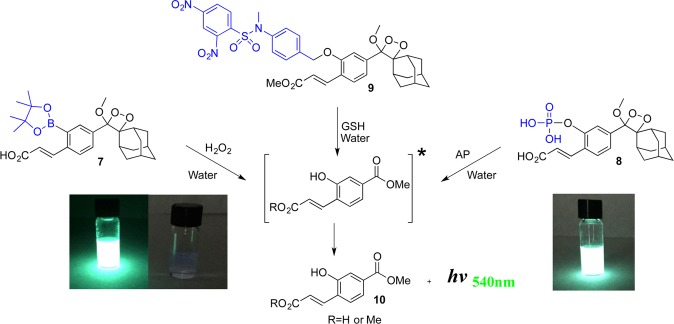
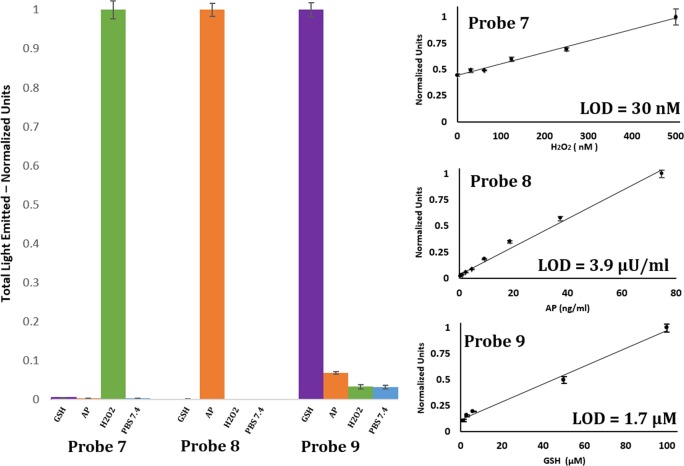
The activation of our chemiluminescence probes is based on removal of a protecting group from the phenolic moiety. Therefore, different phenol protecting groups could be incorporated as triggering substrates for various analytes or enzymes.36 To demonstrate this modular feature, we synthesized three additional probes for detection of the analytes hydrogen peroxide37 and glutathione (GSH) and the enzyme alkaline phosphatase (AP)35 (see Supporting Information for synthetic procedures). Probe 7 was equipped with a boronic ester substrate for hydrogen peroxide, probe 8 with a phosphate group as a substrate for AP, and probe 9 with dinitro–benzene–sulfonyl group as a substrate for GSH (Figure 7). The probes were prepared with an acrylic acid or methyl acrylate substituent at the ortho position of the phenolic oxygen. The presence of an ionizable carboxylic acid group significantly increased the aqueous solubility of probes 7 and 8 and enabled us to conduct evaluations at relatively high concentrations. At a concentration of 1 mM (pH 10), probes 7 and 8 produced bright green luminescence upon reaction with their analyte/enzyme. As described above, the probes decomposed upon activation to release the excited state of the corresponding benzoate. The acrylate substituent efficiently increased the emissive nature of the released benzoate to produce strong light emission, clearly visible to the naked eye (Figure 7). Probe 9 has relatively moderate aqueous solubility with an applicable concertation range between 1 and 10 μM.
Figure 7.
Water-soluble chemiluminescence probes for detection of hydrogen peroxide (probe 7) and alkaline phosphatase (probe 8) produce visible bright green luminescence under aqueous conditions. Left photograph: Comparison between light emission observed by 1 mM probe 7 (left vial) to that of 1 mM luminol (right vial) upon incubation with hydrogen peroxide under aqueous conditions at pH 10. Probe 9 is a chemiluminescence probe for detection of GSH.
To evaluate the sensitivity and selectivity of probes 7, 8, and 9 to detect analyte/enzyme, we determined limits of detection (LOD). The probes exhibited very good selectivity for their analytes of choice under physiological conditions (Figure 8). Probe 7 detected hydrogen peroxide with an LOD value of 30 nM. Probe 8 detected alkaline phosphatase with an LOD of 3.9 μU/ml, and probe 9 detected GSH with an LOD of 1.7 μM.
Figure 8.
(Left) Total light emitted from probe 7 [100 μM], probe 8 [10 μM], and probe 9 [10 μM] in the presence of hydrogen peroxide [1 mM, green], alkaline phosphatase [1.5 EU/mL, orange], and glutathione [1 mM, purple]. Measurements were conducted in PBS [100 mM], pH 7.4, with 10% DMSO at room temperature. (Right) Total light emitted from probe 7 [500 μM], probe 8 [500 μM], and probe 9 [10 μM] in PBS [100 mM], pH 7.4 with 10% DMSO, over a period of 1 h over a range of stimulus concentrations. We determined a detection limit (blank + 3 SD) for each probe.
Since in chemiluminescence each molecule cannot emit more than one photon, signal intensity in absolute values is often weaker than fluorescence signals. Therefore, to localize and quantify chemiluminescent and bioluminescent probes at single-cell resolution, a suitable microscope (like LV200 by Olympus) is required. So far, luminescence cell imaging was achieved solely by luciferin and its derivatives. We sought to evaluate the ability of our probes to image cells overexpressing β-galactosidase using the LV200 microscope. After initial screening for cell permeability, probe 4 was selected for the imaging evaluation. HEK293 cells stably transfected with a vector that encodes LacZ gene and HEK293-WT (control) cells were incubated with probe 4 and then imaged using the LV200 (Figure 9). The obtained images clearly show that the probe is activated by the expressed β-galactosidase. Probe 4 was able to produce high-quality chemiluminescence images of the HEK293-LacZ cells at a 40 s exposure time (Figure 9b). No chemiluminescence signal was observed in the HEK293-WT cells, which do not express β-galactosidase (Figure 9d). Almost 100% of the cell population was imaged by the probe. These are the first chemiluminescence microscopy cell images obtained by a small molecule probe with the direct emission mode that is other than luciferin.
Figure 9.
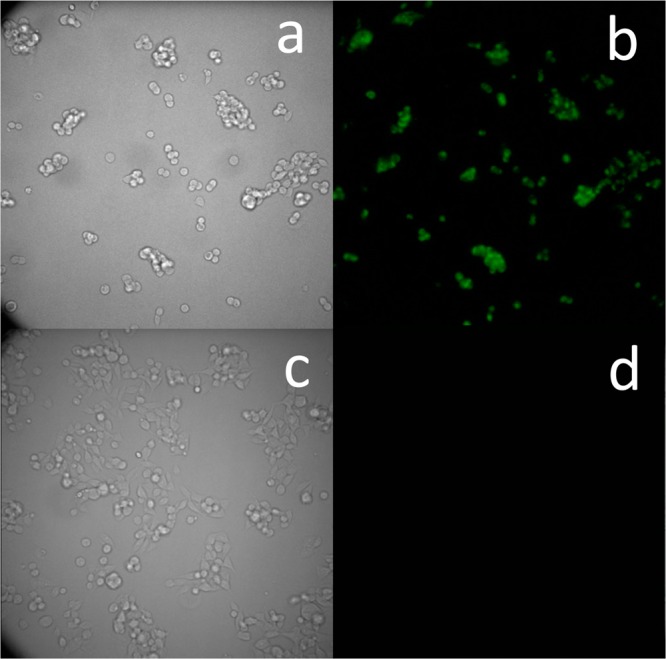
(a) Transmitted light image and (b) chemiluminescence microscopy image of HEK293-LacZ cells. (c) Transmitted light image and (d) chemiluminescence microscopy image of HEK293-WT cells. Images were obtained following 20 min incubation with cell culture medium containing probe 4 (5 μM). Images were taken using the LV200 Olympus microscope using a 60× objective and 40 s exposure time.
Over the past 30 years or so, numerous examples of chemiluminescence probes based on Schaap’s dioxetane have been reported in the literature.28,38−45 In addition, Matsumoto has also explored other triggerable dioxetane probes with alternative groups in place of the phenols.46 These probes were designed to release benzoate upon activation; such benzoate is a weakly emissive species under aqueous conditions. In this study, we aimed to redesign Schaap’s dioxetane in order to develop chemiluminescence probes that are highly emissive in the biological environment. As explained in Figure 2, the chemiluminescence efficiency of Schaap’s dioxetane essentially depends on the emissive nature of the obtained excited-state benzoate. Thus, we assumed that if a substituent increased the benzoate’s fluorescent emission in water, it would similarly be able to intensify the chemiluminescence emission of the corresponding dioxetane probe under physiological conditions. Both acrylate and the acrylonitrile substituents at the ortho position of the phenolic oxygen resulted in about 3 orders of magnitude enhancement under aqueous conditions relative to the unsubstituted probe. Interestingly, introduction of the acrylate substituent at the para position of the phenolic oxygen resulted in only moderate effects on the fluorescence of the benzoate and on the chemiluminescence of the adamantylidene–dioxetane (see Supporting Information). In our previous dioxetane–fluorophore conjugate probes, we used indirect chemiluminescence mode of action and were able to achieve up to a 100-fold signal amplification.21 Remarkably, the direct chemiluminescence mode of action has produced probes with signal amplification greater than 1000-fold.
In commercial chemiluminescence assays, signal enhancement is achieved indirectly by energy transfer to a fluorescent dye (confined in micelles formed by an added surfactant). Due to high toxicity, such multicomponent probe systems are not suitable for living cell imaging.47 Our new probes are composed of a single component, a small molecule with a direct mode of chemiluminescence emission and reasonable aqueous solubility. Such characteristics make theses probes ideal for cell imaging applications. Probe 4 was able to provide excellent chemiluminescence cell images based on endogenous β-galactosidase activity. As explained above, in chemiluminescence, signal intensity in absolute values is often weaker than fluorescence signals since each molecule emits only one photon. Thus, only highly efficient bio- or chemiluminescence small molecule probes, like luciferin and its derivatives,48−50 could be used for cell imaging.51 As far as we know, in this work we present the first live cell images obtained using a non-luciferin small-molecule-based probe with a direct chemiluminescence mode of emission.
The modular synthetic strategy used enables installation of different triggering substrates on the dioxetane probe, allowing preparation of chemiluminescence probes triggered by various analytes or enzymes of interest. We have demonstrated this option by synthesis and evaluation of new chemiluminescence probes for detection of β-galactosidase, alkaline phosphatase, hydrogen peroxide, and ubiquitous thiols. The high chemiluminescence efficiency observed under aqueous conditions should make these probes ideal substrates for biochemical tests in the field of immunoassays.
Conclusions
In summary, we have developed a new molecular methodology to obtain chemiluminescence probes with high emission efficiency under physiological conditions. The methodology is based on incorporation of a substituent on the benzoate species obtained during the chemiexcitation pathway of Schaap’s adamantylidene–dioxetane probe. The substituent effect was initially evaluated on the fluorescence emission generated by the benzoate species and then on the chemiluminescence of the dioxetane luminophores. A striking substituent effect on the chemiluminescence efficiency of the probes was obtained when acrylate and acrylonitrile electron-withdrawing groups were installed. The chemiluminescence quantum yield of the best probe was more than 3 orders of magnitude higher than that of the standard commercially available adamantylidene–dioxetane probe. Currently, bio- and chemiluminescence cell imaging is limited to luciferin-related probes. One of our new probes was able to provide high-quality chemiluminescence cell images based on endogenous activity of β-galactosidase. To date, this is the first demonstration of cell-imaging achieved by a non-luciferin small-molecule probe with a direct chemiluminescence mode of emission. We anticipate that the notion presented in this study will lead to a major transformation in the molecular design of small molecules that function as chemiluminescence-based probes.
Acknowledgments
D.S. thanks the Israel Science Foundation (ISF), the Binational Science Foundation (BSF), and the German Israeli Foundation (GIF) for financial support. This work is supported in part by a grant from the Israeli National Nanotechnology Initiative in the Focal Technology Area: Nanomedicines for Personalized Theranostics.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00058.
Synthetic procedures, NMR and MS characterization and spectra, CL kinetic measurements, spectral data, light-stability evaluation, and cell images (PDF)
Author Contributions
‡ O.G., T.E., and N.H. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Dodeigne C.; Thunus L.; Lejeune R. Chemiluminescence as diagnostic tool. A review. Talanta 2000, 51, 415–439. 10.1016/S0039-9140(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Kricka L. J. Clinical applications of chemiluminescence. Anal. Chim. Acta 2003, 500, 279–286. 10.1016/S0003-2670(03)00809-2. [DOI] [Google Scholar]
- Merényi G.; Lind J.; Eriksen T. E. Luminol chemiluminescence: Chemistry, excitation, emitter. J. Biolumin. Chemilumin. 1990, 5, 53–56. 10.1002/bio.1170050111. [DOI] [PubMed] [Google Scholar]
- Silva S. M.; Casallanovo F.; Oyamaguchi K. H.; Ciscato L. F. L. M.; Stevani C. V.; Baader W. J. Kinetic studies on the peroxyoxalate chemiluminescence reaction: determination of the cyclization rate constant. Luminescence 2002, 17, 313–320. 10.1002/bio.693. [DOI] [PubMed] [Google Scholar]
- Han J.; Jose J.; Mei E.; Burgess K. Chemiluminescent Energy-Transfer Cassettes Based on Fluorescein and Nile Red. Angew. Chem., Int. Ed. 2007, 46, 1684–1687. 10.1002/anie.200603307. [DOI] [PubMed] [Google Scholar]
- Lee D.; Khaja S.; Velasquez-Castano J. C.; Dasari M.; Sun C.; Petros J.; Taylor W. R.; Murthy N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. 10.1038/nmat1983. [DOI] [PubMed] [Google Scholar]
- Gross S.; Gammon S. T.; Moss B. L.; Rauch D.; Harding J.; Heinecke J. W.; Ratner L.; Piwnica-Worms D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. 10.1038/nm.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.-K.; Lee Y.-D.; Na J.; Oh J. M.; Her S.; Kim K.; Choi K.; Kim S.; Kwon I. C. Chemiluminescence-Generating Nanoreactor Formulation for Near-Infrared Imaging of Hydrogen Peroxide and Glucose Level in vivo. Adv. Funct. Mater. 2010, 20, 2644–2648. 10.1002/adfm.201000780. [DOI] [Google Scholar]
- Cho S.; Hwang O.; Lee I.; Lee G.; Yoo D.; Khang G.; Kang P. M.; Lee D. Chemiluminescent and Antioxidant Micelles as Theranostic Agents for Hydrogen Peroxide Associated-Inflammatory Diseases. Adv. Funct. Mater. 2012, 22, 4038–4043. 10.1002/adfm.201200773. [DOI] [Google Scholar]
- Lee Y.-D.; Lim C.-K.; Singh A.; Koh J.; Kim J.; Kwon I. C.; Kim S. Dye/peroxalate aggregated nanoparticles with enhanced and tunable chemiluminescence for biomedical imaging of hydrogen peroxide. ACS Nano 2012, 6, 6759–6766. 10.1021/nn3014905. [DOI] [PubMed] [Google Scholar]
- Zhang N.; Francis K. P.; Prakash A.; Ansaldi D. Enhanced detection of myeloperoxidase activity in deep tissues through luminescent excitation of near-infrared nanoparticles. Nat. Med. 2013, 19, 500–505. 10.1038/nm.3110. [DOI] [PubMed] [Google Scholar]
- Shuhendler A. J.; Pu K.; Cui L.; Uetrecht J. P.; Rao J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat. Biotechnol. 2014, 32, 373–380. 10.1038/nbt.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. S.; Deepagan V. G.; You D. G.; Jeon J.; Yi G. R.; Lee J. Y.; Lee D. S.; Suh Y. D.; Park J. H. Nanoparticles based on quantum dots and a luminol derivative: implications for in vivo imaging of hydrogen peroxide by chemiluminescence resonance energy transfer. Chem. Commun. (Cambridge, U. K.) 2016, 52, 4132–4135. 10.1039/C5CC09850E. [DOI] [PubMed] [Google Scholar]
- Li P.; Liu L.; Xiao H.; Zhang W.; Wang L.; Tang B. A New Polymer Nanoprobe Based on Chemiluminescence Resonance Energy Transfer for Ultrasensitive Imaging of Intrinsic Superoxide Anion in Mice. J. Am. Chem. Soc. 2016, 138, 2893–2896. 10.1021/jacs.5b11784. [DOI] [PubMed] [Google Scholar]
- Seo Y. H.; Singh A.; Cho H.-J.; Kim Y.; Heo J.; Lim C.-K.; Park S. Y.; Jang W.-D.; Kim S. Rational design for enhancing inflammation-responsive in vivo chemiluminescence via nanophotonic energy relay to near-infrared AIE-active conjugated polymer. Biomaterials 2016, 84, 111–118. 10.1016/j.biomaterials.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Zhen X.; Zhang C.; Xie C.; Miao Q.; Lim K. L.; Pu K. Intraparticle Energy Level Alignment of Semiconducting Polymer Nanoparticles to Amplify Chemiluminescence for Ultrasensitive In Vivo Imaging of Reactive Oxygen Species. ACS Nano 2016, 10, 6400–6409. 10.1021/acsnano.6b02908. [DOI] [PubMed] [Google Scholar]
- Schaap A. P.; Chen T.-S.; Handley R. S.; DeSilva R.; Giri B. P. Chemical and enzymatic triggering of 1,2-dioxetanes. 2: fluoride-induced chemiluminescence from tert-butyldimethylsilyloxy-substituted dioxetanes. Tetrahedron Lett. 1987, 28, 1155. 10.1016/S0040-4039(00)95313-9. [DOI] [Google Scholar]
- Schaap A. P.; Handley R. S.; Giri B. P. Chemical and enzymatic triggering of 1,2-dioxetanes. 1: Aryl esterase-catalyzed chemiluminescence from a naphthyl acetate-substituted dioxetane. Tetrahedron Lett. 1987, 28, 935–938. 10.1016/S0040-4039(00)95878-7. [DOI] [Google Scholar]
- Schaap A. P.; Sandison M. D.; Handley R. S. Chemical and enzymatic triggering of 1,2-dioxetanes. 3: alkaline phosphatase-catalyzed chemiluminescence from an aryl phosphate-substituted dioxetane. Tetrahedron Lett. 1987, 28, 1159–1162. 10.1016/S0040-4039(00)95314-0. [DOI] [Google Scholar]
- Matsumoto M.; Mizoguchi Y.; Motoyama T.; Watanabe N. Base-induced chemiluminescence of 5-tert-butyl-1-(4-hydroxybenz[d]oxazol-6-yl)-4,4-dimethyl-2,6,7-trioxabicyclo[3.2.0]heptanes: chemiluminescence–chemiexcitation profile in aqueous medium. Tetrahedron Lett. 2001, 42, 8869–8872. 10.1016/S0040-4039(01)01947-5. [DOI] [Google Scholar]
- Hananya N.; Eldar Boock A.; Bauer C. R.; Satchi-Fainaro R.; Shabat D. Remarkable Enhancement of Chemiluminescent Signal by Dioxetane–Fluorophore Conjugates: Turn-ON Chemiluminescence Probes with Color Modulation for Sensing and Imaging. J. Am. Chem. Soc. 2016, 138, 13438–13446. 10.1021/jacs.6b09173. [DOI] [PubMed] [Google Scholar]
- Karton-Lifshin N.; Albertazzi L.; Bendikov M.; Baran P. S.; Shabat D. ″Donor-Two-Acceptor″ Dye Design: A Distinct Gateway to NIR Fluorescence. J. Am. Chem. Soc. 2012, 134, 20412–20420. 10.1021/ja308124q. [DOI] [PubMed] [Google Scholar]
- Hagiwara H.; Watanabe N.; Ijuin H. K.; Yamada M.; Matsumoto M. Synthesis of bicyclic dioxetanes bearing a 4-(benzimidazol-2-yl)-3-hydroxyphenyl group and their base-induced chemiluminescent decomposition in an aprotic medium and in an aqueous medium. Heterocycles 2013, 87, 65–78. 10.3987/COM-12-12602. [DOI] [Google Scholar]
- Matsumoto M.; Akimoto T.; Matsumoto Y.; Watanabe N. Bicyclic dioxetanes bearing a 4-(benzoazol-2-yl)-3-hydroxyphenyl moiety: chemiluminescence profile for base-induced decomposition in aprotic medium and in aqueous medium. Tetrahedron Lett. 2005, 46, 6075–6078. 10.1016/j.tetlet.2005.07.008. [DOI] [Google Scholar]
- Matsumoto M.; Arai N.; Watanabe N. 3-(4-Acyl-3-hydroxyphenyl)-1,2-dioxetanes as a chemiluminescent substrate with high efficiency in an aqueous system. Tetrahedron Lett. 1996, 37, 8535–8538. 10.1016/0040-4039(96)01985-5. [DOI] [Google Scholar]
- Matsumoto M.; Sakuma T.; Watanabe N. Design and synthesis of chemiluminescent substrates with high luminescent efficiency in an aqueous system: 5-tert-butyl-4,4-dimethyl-2,6,7-trioxabicyclo[3.2.0]heptanes bearing a 3-hydroxy-4-(1-iminoethyl)phenyl moiety at the 1-position. Luminescence 2001, 16, 275–280. 10.1002/bio.650.abs. [DOI] [PubMed] [Google Scholar]
- Matsumoto M.; Sakuma T.; Watanabe N. Synthesis of bicyclic dioxetanes bearing a 3-hydroxy-4-isoxazolylphenyl moiety: new CIEEL-active dioxetanes emitting light with remarkable high-efficiency in aqueous medium. Tetrahedron Lett. 2002, 43, 8955–8958. 10.1016/S0040-4039(02)02162-7. [DOI] [Google Scholar]
- Cao J.; Lopez R.; Thacker J. M.; Moon J. Y.; Jiang C.; Morris S. N.; Bauer J. H.; Tao P.; Mason R. P.; Lippert A. R. Chemiluminescent Probes for Imaging H2S in Living Animals. Chem. Sci. 2015, 6, 1979–1985. 10.1039/C4SC03516J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimov A. V.; Mielke K.; Vasil’ev R. F.; Adam W. Chemically Initiated Electron Exchange Luminescence of Silyloxyaryl-Substituted Spiroadamantyl Dioxetanes: Kinetics and Excited State Yields. Photochem. Photobiol. 1996, 63, 463–467. 10.1111/j.1751-1097.1996.tb03070.x. [DOI] [Google Scholar]
- Edwards B.; Sparks A.; Voyta J. C.; Bronstein I. In Bioluminescence and chemiluminescence: fundamentals and applied aspects; Campbell A. K., Kricka L. J., Stanley P. E., Eds.; Wiley: Chichester, 1994; p 56. [Google Scholar]
- Amir R. J.; Pessah N.; Shamis M.; Shabat D. Self-immolative dendrimers. Angew. Chem., Int. Ed. 2003, 42, 4494–4637. 10.1002/anie.200351962. [DOI] [PubMed] [Google Scholar]
- Gnaim S.; Shabat D. Quinone-Methide Species, A Gateway to Functional Molecular Systems: From Self-Immolative Dendrimers to Long-Wavelength Fluorescent Dyes. Acc. Chem. Res. 2014, 47, 2970–2984. 10.1021/ar500179y. [DOI] [PubMed] [Google Scholar]
- Sagi A.; Weinstain R.; Karton N.; Shabat D. Self-immolative polymers. J. Am. Chem. Soc. 2008, 130, 5434–5435. 10.1021/ja801065d. [DOI] [PubMed] [Google Scholar]
- Liu L.; Mason R. P. Imaging beta-Galactosidase Activity in Human Tumor Xenografts and Transgenic Mice Using a Chemiluminescent Substrate. PLoS One 2010, 5, e12024. 10.1371/journal.pone.0012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap A. P.; Akhavan H.; Romano L. J. Chemiluminescent substrates for alkaline phosphatase: application to ultrasensitive enzyme-linked immunoassays and DNA probes. Clin. Chem. 1989, 35, 1863–1864. [PubMed] [Google Scholar]
- Redy-Keisar O.; Kisin-Finfer E.; Ferber S.; Satchi-Fainaro R.; Shabat D. Synthesis and use of QCy7-derived modular probes for the detection and imaging of biologically relevant analytes. Nat. Protoc. 2014, 9, 27–36. 10.1038/nprot.2013.166. [DOI] [PubMed] [Google Scholar]
- Karton-Lifshin N.; Segal E.; Omer L.; Portnoy M.; Satchi-Fainaro R.; Shabat D. A Unique Paradigm for a Turn-ON Near-Infrared Cyanine-Based Probe: Noninvasive Intravital Optical Imaging of Hydrogen Peroxide. J. Am. Chem. Soc. 2011, 133, 10960–10965. 10.1021/ja203145v. [DOI] [PubMed] [Google Scholar]
- Bronstein I.; Edwards B.; Voyta J. C. 1,2-Dioxetanes: Novel chemiluminescent enzyme substrates. Applications to immunoassays. J. Biolumin. Chemilumin. 1989, 4, 99–111. 10.1002/bio.1170040116. [DOI] [PubMed] [Google Scholar]
- Stevenson J. D.; Dietel A.; Thomas N. R. A chemiluminescent catalytic antibody. Chem. Commun. 1999, 2105–2106. 10.1039/a906566k. [DOI] [Google Scholar]
- Sabelle S.; Renard P.-Y.; Pecorella K.; de Suzzoni-Dézard S.; Créminon C.; Grassi J.; Mioskowski C. Design and Synthesis of Chemiluminescent Probes for the Detection of Cholinesterase Activity. J. Am. Chem. Soc. 2002, 124, 4874–4880. 10.1021/ja0171299. [DOI] [PubMed] [Google Scholar]
- Richard J.-A.; Jean L.; Romieu A.; Massonneau M.; Noack-Fraissignes P.; Renard P.-Y. Chemiluminescent Probe for the in Vitro Detection of Protease Activity. Org. Lett. 2007, 9, 4853–4855. 10.1021/ol702190y. [DOI] [PubMed] [Google Scholar]
- Richard J.-A.; Jean L.; Schenkels C.; Massonneau M.; Romieu A.; Renard P.-Y. Self-cleavable chemiluminescent probes suitable for protease sensing. Org. Biomol. Chem. 2009, 7, 2941–2957. 10.1039/b905725k. [DOI] [PubMed] [Google Scholar]
- Turan I. S.; Akkaya E. U. Chemiluminescence Sensing of Fluoride Ions Using a Self-Immolative Amplifier. Org. Lett. 2014, 16, 1680–1683. 10.1021/ol5003412. [DOI] [PubMed] [Google Scholar]
- Cao J.; Campbell J.; Liu L.; Mason R. P.; Lippert A. R. In Vivo Chemiluminescent Imaging Agents for Nitroreductase and Tissue Oxygenation. Anal. Chem. 2016, 88, 4995–5002. 10.1021/acs.analchem.6b01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. M.; Balan A.; van Daal T. L. J.; Sijbesma R. P. Probing Force with Mechanobase-Induced Chemiluminescence. Angew. Chem., Int. Ed. 2016, 55, 1445–1449. 10.1002/anie.201508840. [DOI] [PubMed] [Google Scholar]
- Matsumoto M.; Watanabe N.; Hoshiya N.; Ijuin H. K. Color modulation for intramolecular charge-transfer-induced chemiluminescence of 1, 2-dioxetanes. Chem. Rec. 2008, 8, 213–228. 10.1002/tcr.20150. [DOI] [PubMed] [Google Scholar]
- Partearroyo M. A.; Ostolaza H.; Goñi F. M.; Barberá-Guillem E. Surfactant-induced cell toxicity and cell lysis. Biochem. Pharmacol. 1990, 40, 1323–1328. 10.1016/0006-2952(90)90399-6. [DOI] [PubMed] [Google Scholar]
- Jones K. A.; Li D. J.; Hui E.; Sellmyer M. A.; Prescher J. A. Visualizing cell proximity with genetically encoded bioluminescent reporters. ACS Chem. Biol. 2015, 10, 933–938. 10.1021/cb5007773. [DOI] [PubMed] [Google Scholar]
- Porterfield W. B.; Jones K. A.; McCutcheon D. C.; Prescher J. A. A ″Caged″ Luciferin for Imaging Cell-Cell Contacts. J. Am. Chem. Soc. 2015, 137, 8656–8659. 10.1021/jacs.5b02774. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. C.; Rathbun C. M.; Krull B. T.; Yu J. M.; Yang Y.; Nguyen B. D.; Kwon J.; McCutcheon D. C.; Jones K. A.; Furche F.; et al. Brominated Luciferins Are Versatile Bioluminescent Probes. ChemBioChem 2017, 18, 96–100. 10.1002/cbic.201600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Bittner G. C.; Bertozzi C. R.; Chang C. J. Strategy for Dual-Analyte Luciferin Imaging: In Vivo Bioluminescence Detection of Hydrogen Peroxide and Caspase Activity in a Murine Model of Acute Inflammation. J. Am. Chem. Soc. 2013, 135, 1783–1795. 10.1021/ja309078t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.