Abstract
We reviewed 301 patients with newly diagnosed therapy-related acute myeloid leukemia (t-AML) who presented from January 2000 to January 2014 (183, t-AML without antecedent hematologic disorders [AHD]; 118, t-AML with AHD). Overall, median follow-up was 44 months. The primary malignancy was non-Hodgkin lymphoma in 92 (31%); breast cancer in 80 (27%); and prostate cancer in 49 (16%). Median relapse-free survival (RFS) in t-AML without or with AHD was 10 months, and 29 months, respectively (p=0.032): median overall survival (OS) was 8 months, and 8 months, respectively (p=0.53). Multivariate analysis for OS identified older age, poor performance status, thrombocytopenia, non-favorable cytogenetics, and lack of response as adverse factors. The favorable risk cohort had better RFS and OS as compared to the outcomes of patients in the intermediate and adverse risk cohorts; the RFS and OS did not differ between intermediate and adverse cohorts. The presence of AHD did not affect OS.
Keywords: therapy-related acute myeloid leukemia, antecedent myelodysplastic syndrome, relapse-free survival, overall survival
INTRODUCTION
Therapy-related myeloid neoplasms (t-MN) are well-recognized hematopoietic stem cell malignant neoplasms caused by mutational events provoked by previous exposure to cytotoxic therapy and/or radiation therapy for solid tumors or other hematologic malignancies.1–4 The incidence of t-MN in patients with previous exposure to cytotoxic agents varies owing to different types of primary cancer, cytotoxic agents, and timing of exposure.5 Recent reports suggest that t-MN accounts for 5% to 20% of patients with newly diagnosed myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML).6, 7 Cytotoxic agents implicated in the development of t-MN include alkylating agents (e.g., melphalan, cyclophosphamide, busulfan, carboplatin, and dacarbazine); topoisomerase II inhibitors (e.g., etoposide, doxorubicin, epirubicin, and mitoxantrone); and antimetabolites or antitubulin agents in combination with other cytotoxic agents including alkylating agents, and topoisomerase II inhibitors.6, 8–11 Compared with patients with de novo MDS/AML, t-MN are more frequently associated with an adverse karyotype, and resistance to conventional cytotoxic therapies.9, 12, 13 Indeed, patients with t-MN have worse relapse-free survival (RFS) and overall survival (OS) than do those with de novo MDS/AML.8, 14 The presence of dysplasia at diagnosis of de novo AML does not impact on outcomes when cytogenetic risk is taking into account.15, 16 However, whether history of an AHD is associated with additional risk in patients with t-AML has not been examined.
The available data on the outcome of patients with t-AML is limited. Patients with therapy-related acute promyelocytic leukemia (t-APL) respond as well as those with de novo APL to all-trans retinoic acid plus chemotherapy, and outcomes of t-APL seem similar to those of de novo APL.17, 18 On the other hand, patients with therapy-related core-biding factor AML (CBF AML) have worse outcomes than do those with de novo CBF AML.19 However, data on the differences between the clinical outcomes of patients with t-AML and various cytogenetic abnormalities is limited.
The objective of our study was to evaluate whether t-AML was preceded with prior diagnosed AHD (typically MDS) had an impact on the outcome, and to evaluate the significance of cytogenetic risk on outcome as related to the prior cytotoxic or radiation exposure.
METHODS
Patients
We reviewed records of patients with newly diagnosed AML who presented to our tertiary care center between January 2000 and January 2014. We included all the patients with newly diagnosed AML with available cytogenetic test results at diagnosis, and excluded patients with APL from this study. Patients with de novo AML were excluded, and patients with t-AML with or without AHD were the main focus in this study. AML was defined as the presence of at least 20% blasts in the peripheral blood or bone marrow according to World Health Organization 2008 criteria.20 T-AML was defined as the diagnosis of AML with a history of previous cytotoxic chemotherapy or radiation therapy. Patients with prior history of surgery for solid malignancy were not included in this study.
Prior cytotoxic agents were classified by the mechanism of action; this classification was modified according to Smith et al.12 All the patients received age-adjusted induction chemotherapy, which included idarubicin plus high-dose cytarabine, clofarabine plus low-dose cytarabine, decitabine, azacitidine, sapacitabine, cladribine plus cytarabine, fludarabine plus cytarabine twice daily, and fludarabine plus high-dose cytarabine with growth factor; all patients also received subsequent consolidation chemotherapy after confirmation of complete response (CR). Patients less than 60 years of age with adequate organ function and performance status less than 2 received cytarabine-based induction chemotherapy; patients older than 60 years or patients less than 60 years with other significant medical comorbidities received hypomethylating agent-based regimen or low-intensity chemotherapy. Patients with adequate organ functions and performance status less than 2 were eligible for investigational agents regardless of age. Medically eligible patients were referred for the consideration of allogeneic stem cell transplantation (ASCT) when patients achieved response. All the patients gave informed consent as approved by institutional review board according to the Declaration of Helsinki for participation in various clinical trials.
Cytogenetic and molecular analysis
Chromosomal abnormalities at the time of AML diagnosis were reviewed; chromosome G-banding was performed using standard techniques, and patients’ karyotypes were described according to the International System for Human Cytogenetic Nomenclature.21 We followed the criteria recommended by the European LeukemiaNet to classify patients into favorable, intermediate, and unfavorable risk groups.22 Monosomal karyotype was as previously characterized, i.e. two or more distinct autosomal chromosome monosomies or one single autosomal monosomy in addition to structural abnormalities.23 To detect internal tandem duplications (ITD) and D835 point mutations in the FLT3 gene, FLT3 analysis was performed with a multiplex polymerase chain reaction (PCR) and the restriction digestion method, followed by capillary electrophoresis. PCR-based DNA sequencing was performed to examine codon 11 of the NPM1 oncogene.
Statistical analysis
Response criteria, complete remission, OS duration, RFS duration, cumulative incidence of relapse, and cumulative incidence of death in patients with complete remission were defined according to the revised International Working Group criteria.24 We defined RFS as the time from achieving complete remission to relapse or death. The Kaplan-Meier method was utilized to develop survival curves, and the log-rank test was used for survival analysis by cytogenetic risk factors.25 We used a Cox model to identify prognostic variables for univariate and multivariate analysis.26 Cox proportional hazards model with time-dependent covariates was used to assess the impact of allogeneic stem cell transplantation. All statistical analyses were performed with SPSS statistical software (version 22). P values less than 0.05 were considered statistically significant.
RESULTS
Patient characteristics
Of the 1677 patients with newly diagnosed AML during the study period, 301 (18%) had t-AML (183 without AHD; 118 with AHD), and 1268 (76%) had AML with no prior cytotoxic or radiation therapy (including 842 with de novo AML and 426 with non t-AML but with AHD). 108 (6%) were excluded due to unavailable karyotype at diagnosis (insufficient metaphases for analysis). Baseline patient characteristics of 301 patients with t-AML with and without AHD are described in Table I. Overall, the median follow-up was 44 months (range, 0.2–130.6 months). The median follow-up in patients with t-AML without AHD was 38 months compared to 62 months in those with t-AML with AHD (p=0.894). Median age at diagnosis of t-AML without AHD was 63 years compared to 69 years in t-AML with AHD (p <0.001). Male gender was less common in t-AML without AHD compared to t-AML with AHD (42% in t-AML without AHD; 66% in t-AML with AHD; p <0.001). Overall, median latency from previous exposure to either chemotherapy or radiation therapy was 76 months (range, 6.0–502.0 months). The median latency in t-AML without AHD was 74 months compared to 77 months in t-AML with AHD (p=0.512).
Table I.
Patient characteristics
| t-AML N=301 |
t-AML without AHD N= 198 |
t-AML with AHD N= 103 |
P | |
|---|---|---|---|---|
| Patient demographic at diagnosis, No. (%) / median (range) | ||||
| Age (years) | 66 (21–89) | 63 (21–89) | 69 (25–88) | <0.001 |
| Age > 60 years | 211 (70%) | 116 (63) | 95 (81) | 0.002 |
| Male | 154 (51) | 76 (42) | 78 (66) | <0.001 |
| Performance Status 0–1 | 234 (78) | 147 (80) | 87 (74) | 0.286 |
| Diagnosis year | ||||
| 2000–2007 | 114 (38) | 67 (37) | 47 (40) | 0.574 |
| 2008–2014 | 187 (62) | 116 (63) | 71 (60) | |
| Laboratory data at diagnosis, median (range) | ||||
| WBC, (×103/μL) | 3.3 (0.2–191) | 3.3 (0–191) | 3.2 (0.2–106.8) | 0.866 |
| Hemoglobin, (g/dL) | 9.1 (4.5–12.9) | 8.9 (4.5–12.9) | 9.2 (5.5–12.3) | 0.739 |
| Platelet, (×103/μL) | 34 (2–542) | 33 (4–454) | 34 (2–542) | 0.794 |
| LDH, (IU/L) | 658 (210–42000) | 645 (210–22090) | 681 (301–42000) | 0.945 |
| PB blast, (%) | 8 (0–99) | 8 (0–98) | 9 (0–91) | 0.913 |
| PB ANC, (×103/μL) | 0.8 (0.0–81.7) | 0.7 (0.0–81.7) | 0.8 (0.0–39.5) | 0.267 |
| PB AMC, (×103/μL) | 0.15 (0.0–38.0) | 0.14 (0.0–38.0) | 0.15 (0–27.8) | 0.984 |
| BM blast, (%) | 40 (0–96) | 40.5 (0–96) | 32 (3–95) | 0.162 |
| BM monocyte, (%) | 2 (0–50) | 2 (0–45) | 2 (0–50) | 0.954 |
| Latency, (months) | 75.5 (6.0–502.0) | 73.7 (8.6–502.0) | 76.5 (6.0–411.8) | 0.512 |
Abbreviations: t-AML, therapy-related acute myeloid leukemia; w/o, without; AHD, antecedent history of myelodysplasia; WBC, white blood cell; ANC, absolute neutrophil count; AMC, absolute monocyte count; BM, bone marrow; LDH, lactate dehydrogenase; PB, peripheral blood.
Primary type of cancer
The original diagnoses of primary cancer in the 301 patients with t-AML are described in Table II. Common types of primary cancer were non-Hodgkin lymphoma in 92 (31%); breast cancer in 80 (27%); and prostate cancer in 49 (16%).
Table II.
Original diagnosis of primary cancer in patients with therapy-related acute myeloid leukemia
| Primary Disease, No. (%) | t-AML* N=301 |
t-AML without AHD* N=183 |
t-AML with AHD* N=118 |
P |
|---|---|---|---|---|
| Solid cancers | 204 (68) | 121 (66) | 83 (70) | 0.445 |
| Breast | 80 (27) | 61 (33) | 19 (16) | 0.001 |
| Prostate | 49 (16) | 8 (4) | 41 (35) | <0.001 |
| Sarcoma | 13 (4) | 11 (6) | 2 (2) | 0.072 |
| Head and Neck | 11 (4) | 6 (3) | 5 (4) | 0.665 |
| Colon | 11 (4) | 9 (5) | 2 (2) | 0.146 |
| Lung | 8 (3) | 5 (3) | 3 (3) | 0.920 |
| Bladder | 8 (3) | 6 (3) | 2 (2) | 0.404 |
| Other | 24 (8) | 18 (10) | 6 (5) | 0.137 |
| Hematologic malignancies | 120 (40) | 77 (42) | 43 (36) | 0.330 |
| Non-Hodgkin lymphoma | 92 (31) | 61 (33) | 31 (26) | 0.194 |
| Hodgkin lymphoma | 12 (4) | 5 (3) | 7 (6) | 0.166 |
| Multiple myeloma | 5 (2) | 3 (2) | 2 (2) | 0.971 |
| Other | 8 (3) | 4 (2) | 4 (3) | 0.526 |
Abbreviations: t-AML, therapy-related acute myeloid leukemia; AHD, antecedent history of dysplasia
: ≥ 3% of patients with t-AML with or without AHD were described in the table
Of these 183 patients with t-AML without AHD, 61 (33%) had a history of non-Hodgkin lymphoma (NHL); 61 (33%) had breast cancer; 11 (6%) had sarcoma; 9 (5%) had colon cancer; 8 (4%) had prostate cancer; and 25 (14%) had other malignancies. Of 118 patients with t-AML with AHD, 41 (35%) had prostate cancer; 31 (26%) had NHL; 19 (16%) had breast cancer; 7 (6%) had Hodgkin lymphoma; 5 (4%) had head and neck cancer; 15 (13%) had other malignancies. The percentages of patients with breast cancer and prostate cancer were significantly different between the t-AML without AHD and the t-AML with AHD cohort (p=0.001; p <0.001).
Of 183 patients with t-AML without AHD, 27 (15%) had a second primary cancer before the diagnosis of t-AML; NHL in 9 (5%); breast cancer in 5 (3%); sarcoma in 3 (2%); thyroid cancer, colon cancer, and non-melanoma skin cancer in 2 each (1%); esophageal cancer, melanoma, prostate cancer, renal cell carcinoma in 1 each (1%). Of 118 patients with t-AML with AHD, 13 (11%) had a second primary cancer before the diagnosis of t-AML; 4 (3%), prostate cancer; 2 (2%), NHL; 1 each (1%), thyroid cancer, sarcoma, renal cell carcinoma, lung cancer, colon, breast, and essential thrombocytosis.
Prior exposure to cytotoxic agents, radiation, and stem cell transplantation
Prior exposure to cytotoxic agents, radiation, and autologous stem cell transplantation are described in Table III. Overall, 240 (80%) patients received cytotoxic agents before the diagnosis of t-AML including alkylating agents in 160 (66%) and topoisomerase inhibitors in 46 (19%). 180 (60%) patients received radiation therapy previously, and 20 (7%) underwent stem cell transplantation. The percentage of prior cytotoxic exposure was significantly higher in the t-AML without AHD cohort compared to the t-AML with AHD cohort (p<0.001).
Table III.
Prior exposure to cytotoxic agents, radiation, and stem cell transplantation in patients with therapy-related acute myeloid leukemia
| Type of therapy, No. (%) | t-AML N=301* |
t-AML without AHD N=183* |
t-AML with AHD N=118* |
P |
|---|---|---|---|---|
| Prior chemotherapy | 240 (80) | 178 (97) | 62 (53) | <0.001 |
| Alkylating agents | 160 (66) | 121 (68) | 39 (62) | 0.381 |
| Cyclophosphamide | 124 (52) | 97 (55) | 27 (43) | 0.112 |
| Ifosfamide | 33 (14) | 28 (16) | 5 (8) | 0.122 |
| Cisplatin | 41 (17) | 36 (20) | 5 (8) | 0.026 |
| Antiangiogenic agents | 12 (5) | 11 (6) | 1 (2) | 0.193 |
| Anthracyclines | 138 (57) | 113 (64) | 25 (40) | 0.001 |
| Doxorubicin | 126 (52) | 102 (57) | 24 (38) | 0.009 |
| Antimetabolites | 75 (31) | 56 (32) | 19 (30) | 0.848 |
| 5-fluorouracil | 31 (13) | 28 (16) | 3 (5) | 0.025 |
| Cytarabine | 34 (14) | 29 (16) | 5 (8) | 0.102 |
| Immunotherapies | 66 (27) | 44 (25) | 22 (35) | 0.119 |
| Rituximab | 61 (25) | 40 (23) | 21 (33) | 0.088 |
| Taxanes | 58 (24) | 53 (30) | 5 (8) | <0.001 |
| Paclitaxel | 36 (15) | 35 (20) | 1 (2) | 0.001 |
| Topoisomerase inhibitors | 46 (19) | 35 (20) | 11 (18) | 0.702 |
| Etoposide | 44 (18) | 33 (19) | 11 (18) | 0.849 |
| Vinca alkaloids | 80 (33) | 58 (33) | 22 (35) | 0.735 |
| Vincristine | 73 (30) | 54 (30) | 19 (30) | 0.979 |
| Prior radiation | 180 (60) | 109 (60) | 71 (60) | 0.917 |
| Prior stem cell transplant | 20 (7) | 16 (9) | 4 (3) | 0.096 |
Abbreviations: t-AML, therapy-related acute myeloid leukemia; AHD, antecedent history of dysplasia
Each cytotoxic agent exposure in less than 10% of patients with t-AML was excluded from Table 3. The percentages of cytotoxic agent exposure were derived from patients who received cytotoxic agents.
Of the 183 patients with t-AML without AHD, 178 (97%) were previously treated with alkylating agents, including cyclophosphamide (97 patients, 55%), ifosfamide (28 patients, 16%), and cisplatin (36 patients, 20%). Thirty-five patients (20%) were previously treated with topoisomerase inhibitors. One hundred nine patients (60%) were previously treated with radiation therapy. Sixteen (9%) had received stem cell transplant: 14 patients with lymphoma (2 had Hodgkin lymphoma; 12 had NHL) had undergone autologous stem cell transplant, and 2 patients with ovarian cancer had undergone autologous stem cell transplant.
Of 118 patients with t-AML with AHD, 39 (53%) were previously treated with alkylating agents, including cyclophosphamide (27 patients, 43%), ifosfamide (5 patients, 8%), and cisplatin 5 patients, 8%). Eleven (18%) were previously treated with topoisomerase inhibitors. Seventy one (60%) patients were previously treated with radiation therapy. Four (3%) had received autologous stem cell transplant: 2, Hodgkin lymphoma; 1, NHL; 1, multiple myeloma.
Cytogenetic and molecular abnormalities
Cytogenetic and molecular abnormalities at diagnosis of t-AML are described in Table IV. Overall, a favorable cytogenetic abnormality was observed in 22 (7%); intermediate, 118 (39%); and adverse, 161 (54%). Favorable cytogenetic risk included t(8;21) in 9 (3%), and inv(16) in 13 (4%); intermediate risk, normal karyotype in 58 (19%), and t(9;11) in 19 (6%); adverse risk, complex in 125 (42%), and monosomal karyotype in 100 (33%). FLT3-ITD and NPM mutations were detected in 31 (12%), and 17 (12%), respectively. T(9;11) was more commonly seen in the t-AML without AHD cohort 16 (9%) than t-AML with AHD, 3 (3%); p=0.031. Normal karyotype was more common in the t-AML with AHD; 28 (15%) in t-AML without AHD, and 30 (25%) in t-AML with AHD; p=0.030. Of 231 patients tested for RAS mutations (t-AML with AHD, 92; t-AML without AHD, 139), 25 patients (11%) were found to have RAS mutations at diagnosis (t-AML with AHD, 7; t-AML without AHD, 18) (p=0.201); of 70 patients tested for JAK2 mutation (t-AML with AHD, 35; t-AML without AHD, 35), 8 patients (11%) were found to have JAK2 mutations at diagnosis (t-AML with AHD, 5; t-AML without AHD, 3) (p=0.452).
Table IV.
Cytogenetic and molecular abnormalities in therapy-related acute myeloid leukemia
| Cytogenetic abnormalities | t-AML N=301 |
t-AML without AHD N=183 |
t-AML with AHD N=118 |
P |
|---|---|---|---|---|
| Favorable | 22 (7) | 15 (8) | 7 (6) | 0.461 |
| t(8;21) | 9 (3) | 5 (3) | 4 (3) | 0.744 |
| inv(16) | 13 (4) | 10 (6) | 3 (3) | 0.223 |
| Intermediate | 118 (39) | 69 (38) | 49 (42) | 0.507 |
| t(9;11) | 19 (6) | 16 (9) | 3 (3) | 0.031 |
| Normal | 58 (19) | 28 (15) | 30 (25) | 0.030 |
| Other | 41 (14) | 25 (14) | 16 (14) | 0.980 |
| Adverse | 161 (54) | 99 (54) | 62 (53) | 0.792 |
| inv(3) or t(3;3) | 7 (2) | 6 (3) | 1 (1) | 0.172 |
| t(6;9) | 1 (0) | 1 (1) | 0 | 1.000 |
| 11q23 rearrangement | 18 (6) | 13 (7) | 5 (4) | 0.306 |
| -5 or 5q- | 113 (38) | 68 (37) | 45 (38) | 0.864 |
| -7 | 65 (22) | 42 (23) | 23 (20) | 0.476 |
| abnl(17p) | 21 (7) | 10 (6) | 11 (9) | 0.200 |
| Complex | 125 (42) | 75 (41) | 50 (42) | 0.811 |
| Monosomal | 100 (33) | 60 (33) | 40 (34) | 0.842 |
| FLT3-ITD mutation | 31/250 (12) | 19/151 (13) | 12/99 (12) | 0.914 |
| NPM1 mutation | 17/147 (12) | 8/89 (9) | 9/58 (16) | 0.455 |
| European LeukemiaNet Risk Classification | ||||
| Favorable | 30 (10) | 17 (9) | 13 (11) | 0.883 |
| Intermediate | 110 (37) | 67 (37) | 43 (36) | |
| Adverse | 161 (54) | 99 (54) | 62 (53) | |
Response and survival
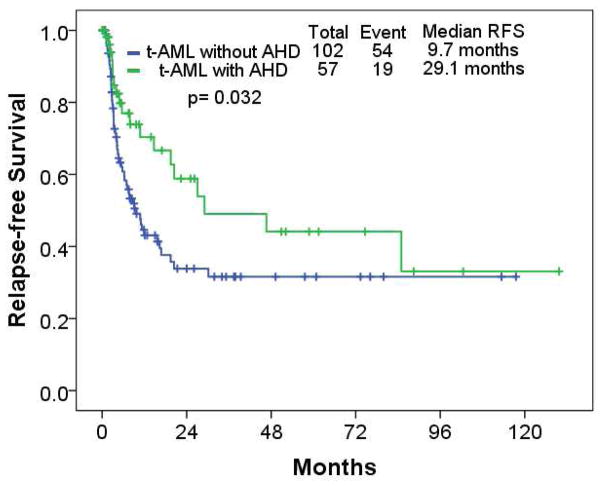
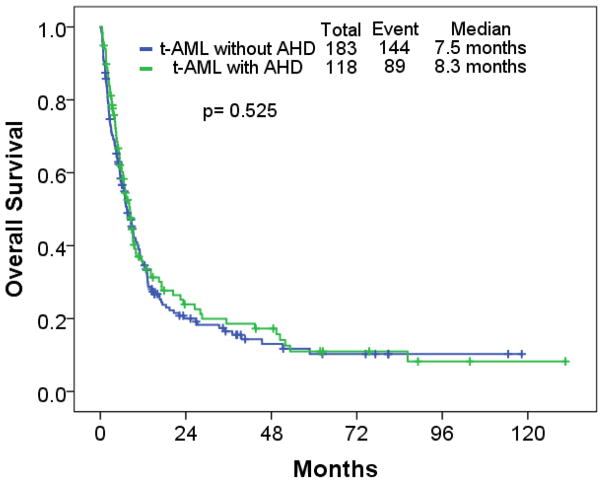
Type of induction therapy, response, and RFS and OS are described in Table V. Overall, 121 (40%), 91 (30%), 56 (17%), and 33 (11%) received cytarabine-based, hypomethylating agent (HMA)-based induction therapy, low intensity therapy, and investigational agents, respectively. Of 301 patients with t-AML, 126 (42%), and 33 (11%) achieved CR, and CR with incomplete platelet recovery (CRp), respectively. Thirty-two (11%) patients underwent ASCT. The type of induction therapy was different between cohorts (p<0.001): cytarabine-based, 89 (49%) in the t-AML without AHD cohort, and 32 (27%) in the t-AML with AHD cohort; HMA-based, 41 (22%) in t-AML without AHD, and 50 (42%) in the t-AML with AHD cohort. A higher CR rate was seen in the t-AML without AHD cohort compared to the t-AML with AHD cohort (p=0.047). Median RFS was 9.7 months, and 29.1 months in the t-AML without AHD, and t-AML with AHD cohort, respectively (p=0.032) (Figure 1); median OS was 7.5 months, and 8.3 months in t-AML without AHD, and t-AML with AHD, respectively (p=0.525) (Figure 2).
Table V.
Response to therapy and survival of patients with acute myeloid leukemia
| t-AML N=301 |
t-AML without AHD N= 183 |
t-AML with AHD N= 118 |
P | |
|---|---|---|---|---|
| Type of induction chemotherapy, No. (%) | ||||
| Cytarabine-based | 121 (40) | 89 (49) | 32 (27) | <0.001 |
| Hypomethylating agent-based | 91 (30) | 41 (22) | 50 (42) | |
| Investigational agent | 33 (11) | 17 (9) | 16 (14) | |
| Low intensity chemotherapy | 56 (17) | 36 (20) | 20 (17) | |
| Response, No. (%) | ||||
| CR | 126 (42) | 86 (47) | 40 (34) | 0.047 |
| CRp | 33 (11) | 16 (9) | 17 (14) | |
| PR | 3 (1) | 3 (2) | 0 (0) | |
| HI | 6 (2) | 2 (1) | 4 (3) | |
| No response/death | 133 (44) | 76 (42) | 57 (48) | |
| 1-year RFS, (%) | 52 | 43 | 70 | 0.032 |
| 2-year RFS, (%) | 42 | 34 | 59 | |
| 1-year OS, (%) | 35 | 34 | 35 | 0.525 |
| 2-year OS, (%) | 21 | 20 | 24 | |
| ASCT, No. (%) | 32 (11) | 23 (13) | 9 (8) | 0.175 |
Abbreviations: t-AML, therapy-related acute myeloid leukemia; AHD, antecedent history of myelodysplasia; CR, complete response; CRp, complete response without platelet recovery; HI, hematologic improvement; RFS, relapse-free survival; OS, overall survival; ASCT, allogeneic stem cell transplantation.
Figure 1.
Relapse-free survival in patients with therapy-related acute myeloid leukemia
Figure 2.
Overall survival in patients with therapy-related acute myeloid leukemia
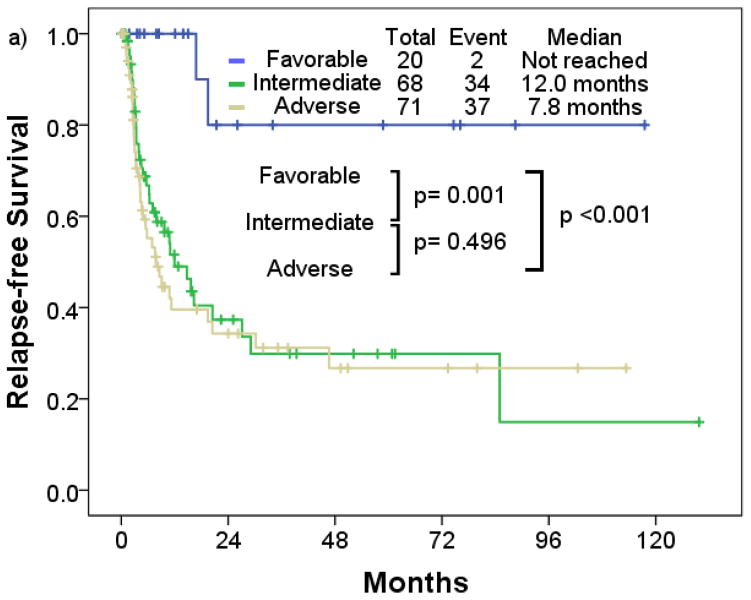
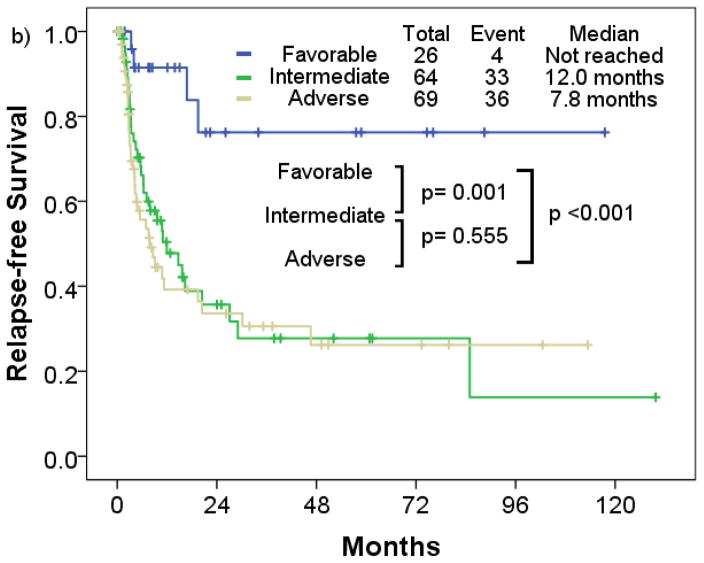
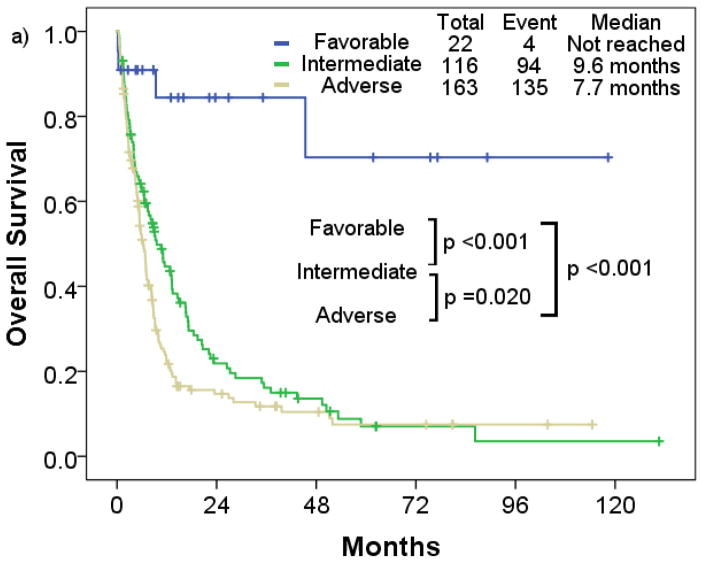
Patients with favorable cytogenetic risk had a significantly longer median RFS compared to patients with intermediate and adverse cytogenetic risk (p=0.001; p<0.001) (Figure 3a). Patients with t-AML with intermediate risk had similar median RFS compared to those with adverse risk (intermediate, 12.0 months; adverse, 7.8 months; p=0.496). Median OS in the favorable risk cohort was significantly longer compared to the intermediate and adverse risk cohort (p=0.001; p<0.001) (Figure 4a). Patients with t-AML with intermediate risk had longer median OS compared to those with adverse risk (intermediate, 9.6 months; adverse, 7.7 months; p=0.020).
Figure 3.
Relapse-free survival rate of therapy-related acute myeloid leukemia by a) cytogenetic risk, b) European LeukemiaNet risk
Figure 4.
Overall survival rate of patients with therapy-related acute myeloid leukemia a) by cytogenetic risk, b) European LeukemiaNet risk
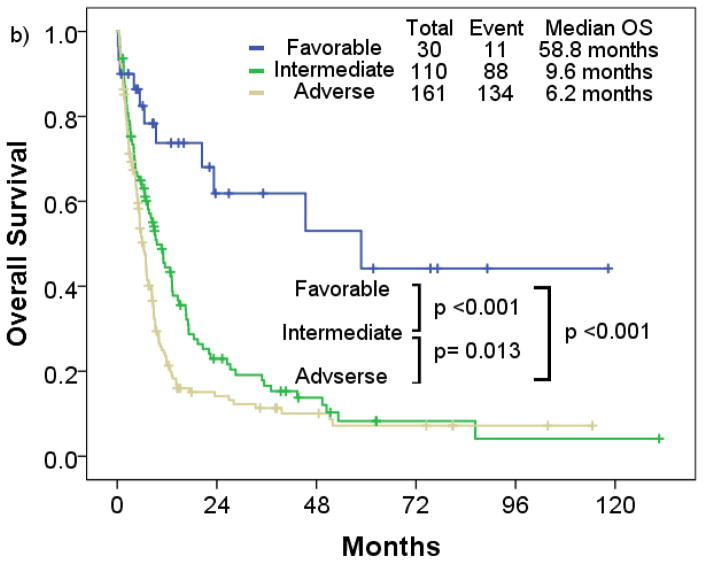
By European LeukemiaNet risk classification, the favorable risk cohort consistently had longer median RFS and OS (median RFS, not reached; median OS, 58.8 months) compared to the intermediate (median RFS, 12.0 months; median OS, 9.6 months) (p<0.001; p=0.001), and adverse cytogenetic risk cohort (median RFS, 6.2 months; median OS, 7.8 months) (p<0.001; p< 0.001) (Figure 3b; Figure 4b). No significant differences in RFS were observed between the intermediate and adverse risk cohort (p=0.555). Median RFS was similar between the intermediate and adverse risk cohort, and median OS was slightly longer in the intermediate cohort (p=0.013).
Univariate and multivariate analysis for survival
Univariate and multivariate analyses for OS are described in Table VI. On multivariate analysis, age over 60 years (p<0.001; hazard ratio [HR], 2.238; 95% confidence interval [CI], 1.551–3.228), performance status ≥2 (p=0.010; HR, 1.569; 95% CI, 1.115–2.208), thrombocytopenia below 30 × 103/μL (p<0.001; HR, 1.717; 95% CI, 1.303–2.263), non-favorable cytogenetic abnormalities (p=0.003; HR, 4.558; 95% CI, 1.650–12.589), and the absence of CRp or better (CRp or CR, p<0.001; HR, 2.840; 95% CI, 2.106–3.830: CR, p<0.001; HR, 1.923; 95% CI, 1.431–2.585) were factors that indicated a poor prognosis. There was a tendency for better OS with ASCT (p=0.171; HR, 0.625; 95% CI, 0.319–1.224). The presence of AHD did not result in worse OS by univariate analysis (p=0.525; HR, 0.918; 95% CI, 0.704–1.196).
Table VI.
Main results of univariate (UVA) and multivariate analysis (MVA) of overall survival in patients with therapy-related acute myeloid leukemia
| UVA | MVA | |||
|---|---|---|---|---|
| P | P | HR | 95% CI | |
| Age at diagnosis: ≤60 vs. >60 years | <0.001 | <0.001 | 2.238 | 1.551–3.228 |
| Performance status: 0–1 vs. ≥2 | <0.001 | 0.010 | 1.569 | 1.115–2.208 |
| Platelet (×103/μL): ≥30 vs. <30 | 0.001 | <0.001 | 1.717 | 1.303–2.263 |
| Karyotype: favorable vs. non-favorable | <0.001 | 0.003 | 4.558 | 1.650–12.589 |
| FLT3-ITD mutation: pos vs. neg | 0.488 | |||
| NPM1 mutation: pos vs. neg | 0.963 | |||
| Response*: CRp or CR vs. non-CRp | <0.001 | <0.001 | 2.840 | 2.106–3.830 |
| Response*: CR vs. non-CR | <0.001 | <0.001 | 1.923 | 1.431–2.585 |
| Ara-C-based vs. non-Ara-C-based | <0.001 | 0.126 | 1.347 | 0.920–1.972 |
| HMA-based vs. non-HMA-based | <0.001 | 0.401 | 0.863 | 0.613–1.217 |
| Low intensity chemotherapy vs. other | 0.521 | |||
| Presence of AHD: pos vs. neg | 0.525 | |||
| ASCT vs. non-ASCT | <0.001 | 0.171 | 0.625 | 0.319–1.224 |
Abbreviations: UVA, univariate analysis; MVA, multivariate analysis; HR, hazard ratio; CI, confidence interval; CR, complete response; CRp, complete response with incomplete platelet recovery; HMA, hypomethylating agent; AHD, antecedent history of myelodysplasia; ASCT, allogeneic stem cell transplantation.
CRp and CR were calculated individually for MVA. CRp was used as a variable to describe other MVA results.
DISCUSSION
We demonstrated that although overall survival of patients with t-AML without or with a preceding AHD are similar, RFS in patients with t-AML without AHD was shorter compared to those with t-AML with AHD. As regards to therapy, 22% of the t-AML without AHD cohort received HMA-based induction therapy compared to 42% of t-AML with AHD. Prior studies have demonstrated the benefit of azacitidine and decitabine in elderly patients with newly diagnosed AML not fit for intensive cytotoxic chemotherapy. Indeed, in the AZA-001 trial, the median OS in the azacitidine cohort was 24.5 months compared to 15.0 months in the conventional care group (HR, 0.58; 95% CI, 0.43–0.77; p=0.0001) in patients with high-risk myelodysplastic syndromes.27 Similarly, in the AZA-AML-001 study, the median OS was 24.5 months in the azacitidine arm compared to 16.0 months for the conventional care regimens (HR, 0.47; 95% CI, 0.28–0.79; p=0.005) in elderly patients with AML.28 Furthermore, the DACO-016 study showed improved CR plus CRp rate of 17.8% in the decitabine arm versus 7.8% in the treatment of choice arm, and showed a trend for longer median OS with decitabine (median OS, 7.7 months; 95% CI, 6.2–9.2) compared to the treatment of choice (median OS, 5.0 months; 95% CI, 4.3–6.3) (p=0.108; HR, 0.85; 95% CI, 0.69–1.04). In our study, the higher rates of HMA-based therapy in the t-AML with AHD cohort may have contributed to the improved RFS rates compared to those seen in the t-AML without AHD cohort. However, the difference in the rates of HMA-based therapy did not translate to improved OS rates for the cohorts with and without AHD. The multivariate Cox proportional hazards analysis showed the survival benefit was limited to patients with response. Overall, the presence of AHD in the setting of t-AML did not lead to worse outcome (p=0.525; HR, 0.918; 95% CI, 0.704–1.196).
Cytogenetic risk classification used in de novo AML remains applicable in the setting of t-AML. Patients with favorable cytogenetics had significantly better survival compared to those with intermediate and adverse cytogenetics. However, the difference in OS between the intermediate and adverse cytogenetic risk was only by a median of 3.4 months (intermediate, 9.6 months; adverse, 6.2 months; p=0.013). Prior exposure to cytotoxic agents or radiation therapy leads to TP53 mutations which are associated with genetic instability leading to deletion 5q and/or complex karyotype in patients with t-MN.29 Shih et al. reported 21% of patients with t-MN carried a TP53 mutation, which was the most common mutations in t-MN, and patients with a TP53 mutation or loss of the TP53 locus had a worse OS compared to those who had wild-type TP53 (8.8 months vs. 37.4 months; p=0.0035).30 Patients with t-AML with normal karyotype may have had TP53 or other molecular mutations leading to a worse prognosis compared to the OS seen in de novo AML with a normal karyotype.
FLT3-ITD and NPM1 mutations were less frequently observed in patients with t-AML (FLT3-ITD, 12%; NPM1 mutations, 12%). These results are consistent with previous publications which have shown the incidence of FLT3-ITD and NPM1 mutations of 12%, and 16%, respectively in patients with t-AML.8 In that study, the presence of NPM1 and FLT3-ITD mutations impacted the OS (NPM1; p<0.001; HR, 0.78: FLT3-ITD; p<0.001; HR, 1.51). However, in our study and given the relatively small numbers of patients who had NPM1 (17 patients) or FLT3-ITD mutations (31 patients), the presence of NPM1 or FLT3-ITD mutations did not have an impact on the outcomes.
There was a trend for better OS in patents who underwent ASCT (p=0.107; HR, 0.576; 95% CI, 0.294–1.127). Anderson et al. reported the successful treatment with ASCT showing a 5-year actuarial disease-free survival rate of 24.4%.31 Given only 32 patients received ASCT in our study, early intervention with ASCT should be considered in patients with t-AML if feasible. There are several limitation to our study. First, patients with t-AML without AHD might have had an undiagnosed period of AHD before the diagnosis of t-AML. However, patients were regularly followed by an oncologist after the diagnosis of primary cancer. A common clinical presentation of t-MN is pancytopenia which would facilitate bone marrow study for definitive diagnosis given the patients had prior cytotoxic agent or radiation exposure. Given the long latency period with median of 75.5 months, patients with t-AML without AHD had a relatively acute presentation of pancytopenia or related symptoms. Second, molecular abnormalities were not comprehensively tested in our study. The true clinical impact of molecular abnormalities remains unclear.
In conclusion, patients with t-AML have a poor prognosis, and the presence of AHD did not affect their survival. The European LeukemiaNet classification appears applicable to patients with t-AML although the intermediate risk cohort had only a slightly better outcome compared to that seen in the adverse risk cohort.
Clinical practice points.
The available data on the outcome of patients with therapy-related acute myeloid leukemia (t-AML) is limited. The common primary malignancies were non-Hodgkin lymphoma, breast cancer, and prostate cancer before the diagnosis of t-AML. The presence of antecedent hematologic disorders at diagnosis of t-AML did not affect overall survival. The European LeukemiaNet classification appears applicable to patients with t-AML although the intermediate risk cohort had only a slightly better outcome compared to that seen in the adverse risk cohort.
Acknowledgments
The authors would like to acknowledge Osamu Miura at Tokyo Medical and Dental University for providing intellectual input for this project.
Footnotes
Authorship contributions
K.S. collected data, designed the study, analyzed the data, and wrote the manuscript. E.J. designed the study, and treated the patients. J.C., G.G.M., G.B., P.J., N.D., S.O., and H.K. treated the patients. S.P. managed the data. F.R. designed the study, and wrote the manuscript. All authors provided significant intellectual input, and reviewed and approved the final version of the manuscript.
Conflicts of interests
E.J. received consultancy for Ariad, BMS, and Pfizer, and research grants from Ariad, BMS, TEVA, and Pfizer. J.C. received research support from Ariad, BMS, Novartis, Pfizer, and Teva, and is a consultant for Ariad, BMS, Novartis and Pfizer. N.D. received research funding from BMS, Novartis, Sunesis, Incyte, and Bioline. H.K. received research grants from Novartis, BMS, Pfizer, and Ariad. Other authors have nothing to disclose. F.R. received research funding from Novartis and BMS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swerdlow SH International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Godley LA, Larson RA. Therapy-related myeloid leukemia. Seminars in oncology. 2008;35:418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borthakur G, Estey AE. Therapy-related acute myelogenous leukemia and myelodysplastic syndrome. Current oncology reports. 2007;9:373–377. doi: 10.1007/s11912-007-0050-z. [DOI] [PubMed] [Google Scholar]
- 4.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nature reviews Cancer. 2005;5:943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 5.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92:1389–1398. doi: 10.3324/haematol.11034. [DOI] [PubMed] [Google Scholar]
- 7.Pagana L, Pulsoni A, Tosti ME, et al. Clinical and biological features of acute myeloid leukaemia occurring as second malignancy: GIMEMA archive of adult acute leukaemia. British journal of haematology. 2001;112:109–117. doi: 10.1046/j.1365-2141.2001.02527.x. [DOI] [PubMed] [Google Scholar]
- 8.Kayser S, Dohner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 9.Larson RA. Etiology and management of therapy-related myeloid leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2007:453–459. doi: 10.1182/asheducation-2007.1.453. [DOI] [PubMed] [Google Scholar]
- 10.Carney DA, Westerman DA, Tam CS, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24:2056–2062. doi: 10.1038/leu.2010.218. [DOI] [PubMed] [Google Scholar]
- 11.Czader M, Orazi A. Therapy-related myeloid neoplasms. American journal of clinical pathology. 2009;132:410–425. doi: 10.1309/AJCPD85MCOHHCOMQ. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 13.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 14.Hosing C, Munsell M, Yazji S, et al. Risk of therapy-related myelodysplastic syndrome/acute leukemia following high-dose therapy and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2002;13:450–459. doi: 10.1093/annonc/mdf109. [DOI] [PubMed] [Google Scholar]
- 15.Goasguen JE, Matsuo T, Cox C, Bennett JM. Evaluation of the dysmyelopoiesis in 336 patients with de novo acute myeloid leukemia: major importance of dysgranulopoiesis for remission and survival. Leukemia. 1992;6:520–525. [PubMed] [Google Scholar]
- 16.Haferlach T, Schoch C, Loffler H, et al. Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies. J Clin Oncol. 2003;21:256–265. doi: 10.1200/JCO.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Pulsoni A, Pagano L, Lo Coco F, et al. Clinicobiological features and outcome of acute promyelocytic leukemia occurring as a second tumor: the GIMEMA experience. Blood. 2002;100:1972–1976. doi: 10.1182/blood-2001-12-0312. [DOI] [PubMed] [Google Scholar]
- 18.Beaumont M, Sanz M, Carli PM, et al. Therapy-related acute promyelocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:2123–2137. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 19.Borthakur G, Lin E, Jain N, et al. Survival is poorer in patients with secondary core-binding factor acute myelogenous leukemia compared with de novo core-binding factor leukemia. Cancer. 2009;115:3217–3221. doi: 10.1002/cncr.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 21.Simons A, Shaffer LG, Hastings RJ. Cytogenetic and genome research. 2013. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. [DOI] [PubMed] [Google Scholar]
- 22.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 23.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4791–4797. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.DRC Regression models and life tables (with discussion) J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 27.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 29.Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001;19:1405–1413. doi: 10.1200/JCO.2001.19.5.1405. [DOI] [PubMed] [Google Scholar]
- 30.Shih AH, Chung SS, Dolezal EK, et al. Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia. Haematologica. 2013;98:908–912. doi: 10.3324/haematol.2012.076729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JE, Gooley TA, Schoch G, et al. Stem cell transplantation for secondary acute myeloid leukemia: evaluation of transplantation as initial therapy or following induction chemotherapy. Blood. 1997;89:2578–2585. [PubMed] [Google Scholar]